Polypyrrole Modified MoS2 Nanorod Composites as Durable Pseudocapacitive Anode Materials for Sodium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
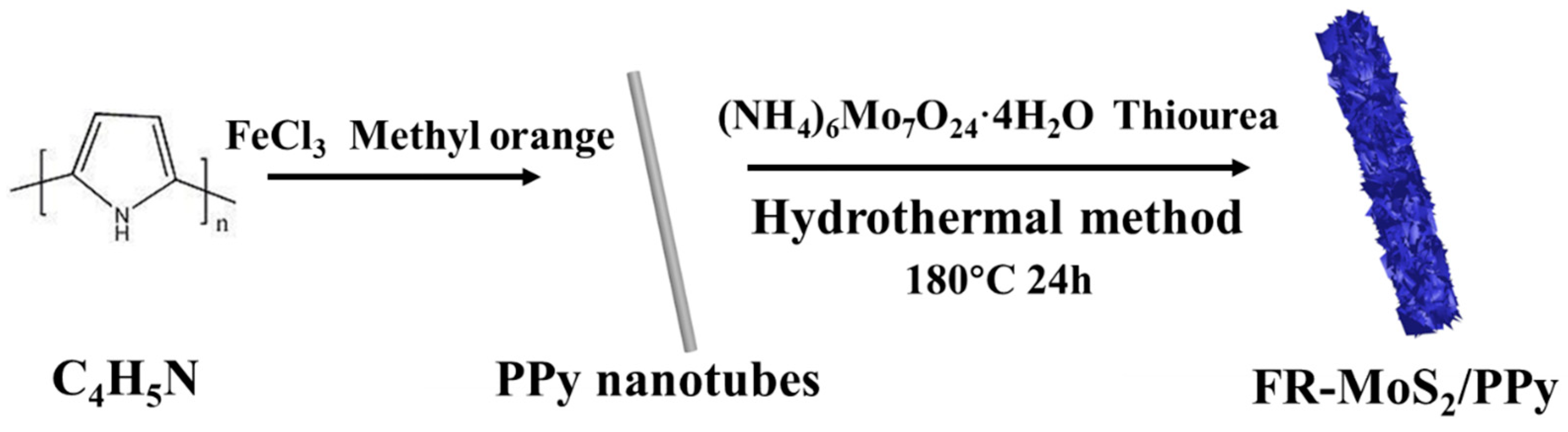
2.1. Materials Synthesis
2.1.1. Synthesis of PPy Nanotubes
2.1.2. Synthesis of FR-MoS2/PPy
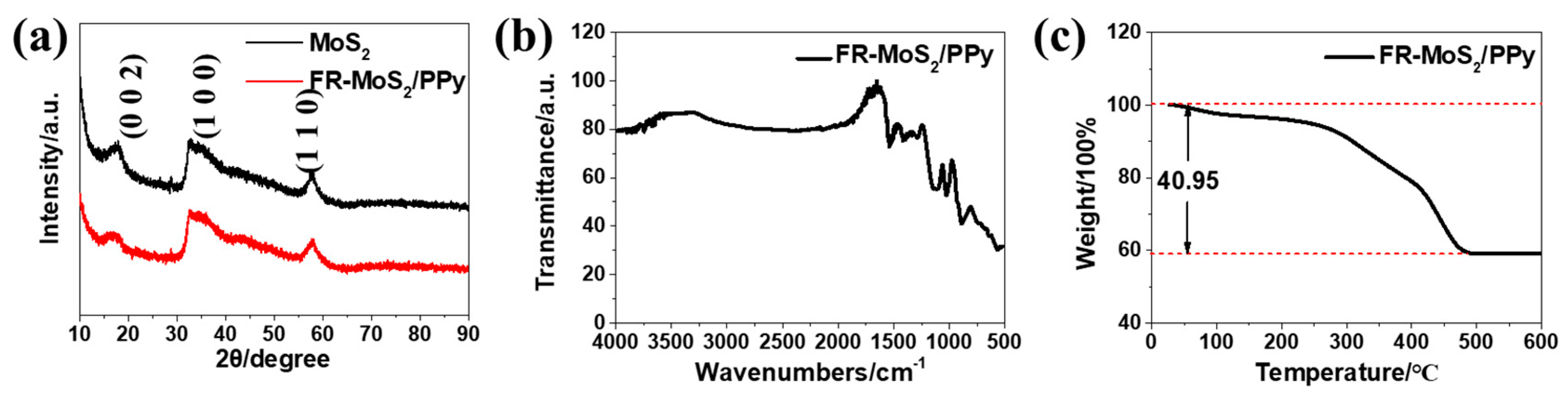
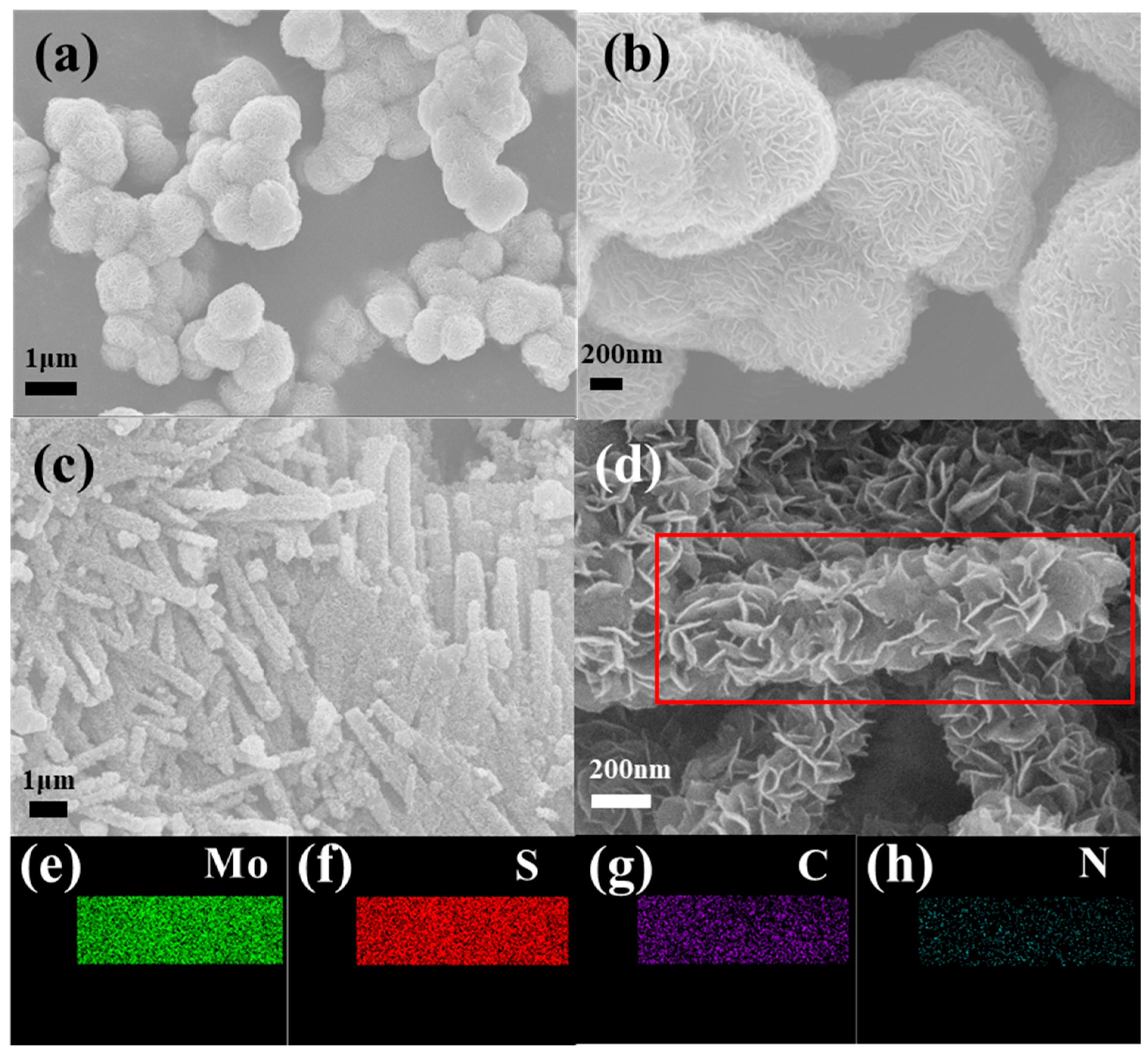
2.2. Materials Characterization
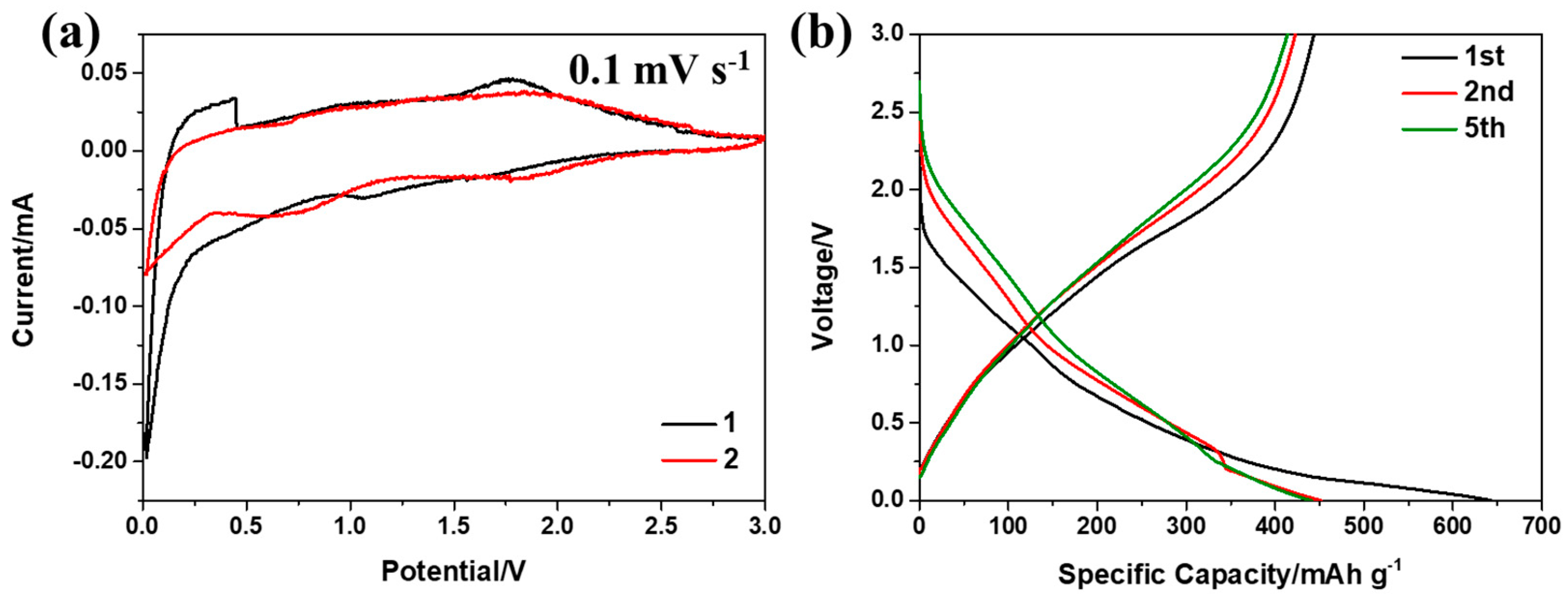
2.3. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Han, M.; Zhou, Z.; Xia, X.; Chen, Q.; Chen, M. Topological Insulator-Assisted MoSe2/Bi2Se3 Heterostructure: Achieving Fast Reaction Kinetics Toward High Rate Sodium-Ion Batteries. ChemElectroChem 2021, 8, 697–704. [Google Scholar] [CrossRef]
- Lim, Y.; Li, X.; Yang, H. Recent Tactics and Advances in the Application of Metal Sulfides as High-Performance Anode Materials for Rechargeable Sodium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2006761. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, P.; Liu, J.; Zhou, J.; Wang, W.; Li, K.; Wu, J.; Lei, Y.; Chen, L. Hierarchical NiCo2Se4 nanoneedles/nanosheets with N-doped 3D porous graphene architecture as free-standing anode for superior sodium ion batteries. J. Colloid Interface Sci. 2021, 587, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Men, S.; Lin, J.; Zhou, Y.; Kang, X. N-doped porous carbon wrapped FeSe2 nanoframework prepared by spray drying: A potential large-scale production technique for high-performance anode materials of sodium ion batteries. J. Power Source 2021, 485, 229310. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Shao, S.i.; Ma, Y.; Zhang, J.; Kang, W.; Xu, J. Transformation of Two-Dimensional Iron Sulfide Nanosheets from FeS2 to FeS as High-Rate Anodes for Pseudocapacitive Sodium Storage. ACS Appl. Energy Mater. 2020, 3, 12672–12681. [Google Scholar] [CrossRef]
- Park, J.; Kang, Y. Multicomponent (Mo, Ni) metal sulfide and selenide microspheres with empty nanovoids as anode materials for Na-ion batteries. J. Mater. Chem. A 2017, 5, 8616–8623. [Google Scholar] [CrossRef]
- Park, J.; Park, G.; Kang, Y. Exploration of cobalt selenite–carbon composite porous nanofibers as anode for sodium-ion batteries and unveiling their conversion reaction mechanism. J. Mater. Sci. Technol. 2021, 89, 24–35. [Google Scholar] [CrossRef]
- Park, J.; Lee, A.; Park, G.; Kang, Y. Synthesis of MnSe@C yolk-shell nanospheres via a water vapor-assisted strategy for use as anode in sodium-ion batteries. Int. J. Energy Res. 2022, 46, 2500–2511. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y.; Zhang, H.; Diemant, T.; Behm, R.; Varzi, A.; Passerini, S. Metal–Organic Framework Derived Fe7S8 Nanoparticles Embedded in Heteroatom-Doped Carbon with Lithium and Sodium Storage Capability. Small 2020, 4, 2000637. [Google Scholar] [CrossRef]
- Dogrusoz, M.; Cakan, R. Mechanochemical synthesis of SnS anodes for sodium ion batteries. Energy Res. 2020, 44, 10809–10820. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, S.; Li, C.; Liu, Z.; Li, D. Hollow CoS2@C nanocubes for high-performance sodium storage. Appl. Surf. Sci. 2020, 519, 146268. [Google Scholar] [CrossRef]
- Xiao, Y.; Lee, S.; Sun, Y. The Application of Metal Sulfides in Sodium Ion Batteries. Adv. Energy Mater. 2017, 7, 1601329. [Google Scholar] [CrossRef]
- Huang, Y.; Xiong, D.; Li, X.; Sari, H.; Peng, J.; Li, Y.; Li, Y.; Li, D.; Sun, Q.; Sun, X. Recent Advances of Bimetallic Sulfide Anodes for Sodium Ion Batteries. Front. Chem. 2020, 8, 353. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, D.; Ma, H.; Li, M.; Pan, Z.; Jiang, Z.Y. Tian, Ionic liquid assisted hydrothermal synthesis of MoS2 double-shell polyhedral cages with enhanced catalytic hydrogenation activities. RSC Adv. 2017, 7, 23523–23529. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Jung, Y.; Kim, D. High performance of MoS2 microflowers with a water-based binder as an anode for Na-ion batteries. RSC Adv. 2015, 5, 79845–79851. [Google Scholar] [CrossRef]
- Yao, K.; Xu, Z.; Li, Z.; Liu, X.; Shen, X.; Cao, L.; Huang, J. Synthesis of Grain-like MoS2 for High-Performance Sodium-Ion Batteries. ChemSusChem 2018, 11, 2130–2137. [Google Scholar] [CrossRef]
- Wang, J.; Han, L.; Li, X.; Zeng, L.; Wei, M. MoS2 hollow spheres in ether-based electrolyte for high performance sodium ion battery. J. Colloid Interface Sci. 2019, 548, 20–24. [Google Scholar] [CrossRef]
- Fan, B.; Fan, H.; Chen, X.; Gao, X.; Chen, S.; Tang, Q.; Luo, W.; Deng, Y.; Hu, A.; Hu, W. Metallic-State MoS2 Nanosheets with Atomic Modification for Sodium Ion Batteries with a High Rate Capability and Long Lifespan. ACS Appl. Mater. Interfaces 2021, 13, 19894–19903. [Google Scholar] [CrossRef]
- Meng, A.; Huang, T.; Li, H.; Cheng, H.; Lin, Y.; Zhao, J.; Li, Z. Sulfur vacancies and morphology dependent sodium storage properties of MoS2-x and its sodiation/desodiation mechanism. J. Colloid Interface Sci. 2021, 589, 147–156. [Google Scholar] [CrossRef]
- Yue, X.; Wang, J.; Patil, A.; An, X.; Xie, Z.; Hao, X.; Jiang, Z.; Abudula, A.; Guan, G. A novel vanadium-mediated MoS2 with metallic behavior for sodium ion batteries: Achieving fast Na+ diffusion to enhance electrochemical kinetics. Chem. Eng. J. 2021, 417, 128107. [Google Scholar] [CrossRef]
- He, C.; Yin, W.; Li, X.; Zheng, J.; Tang, B.; Rui, Y. Molybdenum disulfide synthesized by molybdenum-based metal organic framework with high activity for sodium ion battery. Electrochim. Acta 2021, 365, 137353. [Google Scholar] [CrossRef]
- Liu, M.; Chen, S.; Jin, Y.; Fang, Z. MoS2 encapsulated in three-dimensional hollow carbon frameworks for stable anode of sodium ion batteries. CrystEngComm 2021, 23, 5214–5225. [Google Scholar] [CrossRef]
- Wu, X.; Wu, H.; Xie, B.; Wang, R.; Wang, J.; Wang, D.; Shi, Q.; Diao, G.; Chen, M. Atomic Welded Dual-Wall Hollow Nanospheres for Three-in-One Hybrid Storage Mechanism of Alkali Metal Ion Batteries. ACS Nano 2021, 15, 14125–14136. [Google Scholar] [CrossRef]
- Dai, Z.; Ke, H.; Long, Z.; Li, R.; Shi, C.; Su, X.; Qiao, H.; Wang, K.; Liu, K. Facile synthesis and high lithium storage properties of mesoporous polypyrrole coated CoFe2O4 nanofibers. J. Alloys Compd. 2021, 858, 158324. [Google Scholar] [CrossRef]
- Jyothibasu, J.; Kuo, D.; Lee, R. Flexible and freestanding electrodes based on polypyrrole/carbon nanotube/cellulose composites for supercapacitor application. Cellulose 2019, 26, 4495–4513. [Google Scholar] [CrossRef]
- Li, Q.; Jiao, Q.; Zhou, W.; Feng, X.; Shi, Q.; Dai, Z.; Gu, T.; Zhao, Y.; Li, H.; Feng, C. Controllable construction of core–shell CuCo2S4@polypyrrole nanocomposites as advanced anode materials for high-performance sodium ion half/full batteries. Mater. Chem. Front. 2021, 5, 293–303. [Google Scholar] [CrossRef]
- Fang, Y.; Yu, X.; Lou, X. Formation of Polypyrrole-Coated Sb2Se3 Microclips with Enhanced Sodium-Storage Properties. Angew. Chem. Int. Ed. 2018, 57, 9859–9863. [Google Scholar] [CrossRef]
- He, Q.; Rui, K.; Chen, C.; Yang, J.; Wen, Z. Interconnected CoFe2O4–Polypyrrole Nanotubes as Anode Materials for High Performance Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 36927–36935. [Google Scholar] [CrossRef]
- Liao, Q.; Hou, H.; Duan, J.; Liu, S.; Yao, Y.; Dai, Z.; Yu, C.; Li, D. Composite sodium p-toluenesulfonate/polypyrrole/TiO2 nanotubes/Ti anode for sodium ion battery. Int. J. Hydrogen Energy 2017, 42, 12414–12419. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Han, L.; Liu, K.; Hao, B.; Wu, X. Soft-templated synthesis of core–shell heterostructured Ni3S2@polypyrrole nanotube aerogels as anode materials for high-performance lithium ion batteries. New J. Chem. 2021, 45, 13127–13136. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, J.; Song, X.; Zhao, L.; Zhang, P.; Gao, L. Interface guide: In-situ integrating MoS2 nanosheets into highly ordered polypyrrole film for high performance flexible supercapacitor electrodes. Compos. Sci. Technol. 2020, 197, 108263. [Google Scholar] [CrossRef]
- Hao, J.; Liu, H.; Han, S.; Lian, J. MoS2 Nanosheet-Polypyrrole Composites Deposited on Reduced Graphene Oxide for Supercapacitor Applications. ACS Appl. Nano Mater. 2021, 4, 1330–1339. [Google Scholar] [CrossRef]
- Wang, J.; Sun, L.; Gong, Y.; Wu, L.; Sun, C.; Zhao, X.; Shi, X.; Lin, Y.; Wang, K.; Zhang, Y. A CNT/MoS2@PPy composite with double electron channels and boosting charge transport for high-rate lithium storage. Appl. Surf. Sci. 2021, 566, 150693. [Google Scholar] [CrossRef]
- Lei, J.; Lu, X.; Nie, G.; Jiang, Z.; Wang, C. One-Pot Synthesis of Algae-Like MoS2/PPy Nanocomposite: A Synergistic Catalyst with Superior Peroxidase-Like Catalytic Activity for H2O2 Detection. Part. Part. Syst. Charact. 2015, 32, 886–892. [Google Scholar] [CrossRef]
- Casamachin, D.; Rosa, J.; Ortiz, C.; Rio, D.; Vargas, D.; Escamilla, G.; Guzman, N.; Medina, V.; Velazquez, E. Visible-light photocatalytic degradation of acid violet 7 dye in a continuous annular reactor using ZnO/PPy photocatalyst: Synthesis, characterization, mass transfer effect evaluation and kinetic analysis. Chem. Eng. J. 2019, 373, 325–337. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, W.; Cai, K.; Yang, X.; Huang, C. In Situ Growth of Polypyrrole onto Three-Dimensional Tubular MoS2 as an Advanced Negative Electrode Material for Supercapacitor. Electrochim. Acta 2017, 246, 615–624. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, X.; Yang, X. Synthesis of core-shell NiSe/C nanospheres as anodes for lithium and sodium storage. Electrochim. Acta 2016, 208, 238–243. [Google Scholar] [CrossRef]
- Geng, X.; Jiao, Y.; Han, Y.; Mukhopadhyay, A.; Yang, L.; Zhu, H. Freestanding Metallic 1T MoS2 with Dual Ion Diffusion Paths as High Rate Anode for Sodium-Ion Batteries. Adv. Funct. Mater. 2017, 27, 1702998. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Z.; Ni, J.; Li, L. Freestanding nanosheets of 1T-2H hybrid MoS2 as electrodes for efficient sodium storage. J. Mater. Sci. Technol. 2021, 67, 237–242. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, H.; Wu, Y.; Yang, L.; Sun, Y.; Chen, G.; Liu, Y.; Wu, Z.; Zhang, C.; Guo, X. Unveiling the abnormal capacity rising mechanism of MoS2 anode during long-term cycling for sodium-ion batteries. RSC Adv. 2021, 11, 28488–28495. [Google Scholar] [CrossRef]
- Wang, T.; Xi, Q.; Wang, K.; Zeng, Z.; Du, Z.; Xu, Z.; Xie, L.; Ai, W.; Huang, W. Covalently binding ultrafine MoS2 particles to N, S co-doped carbon renders excellent Na storage performances. Carbon 2021, 184, 177–185. [Google Scholar] [CrossRef]
- Jia, M.; Jin, Y.; Zhao, C.; Zhao, P.; Jia, M. ZnSe nanoparticles decorated with hollow N-doped carbon nanocubesfor high-performance anode material of sodium ion batteries. J. Alloys Compd. 2020, 831, 154749. [Google Scholar] [CrossRef]
- Wang, S.; Cao, F.; Li, Y.; Zhang, Z.; Zhou, D.; Yang, Y.; Tang, Z. MoS2-Coupled Carbon Nanosheets Encapsulated on Sodium Titanate Nanowires as Super-Durable Anode Material for Sodium-Ion Batteries. Adv. Sci. 2019, 6, 1900028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Yu, K.; Fu, H.; Guo, B.; Lei, X.; Zhu, Z. MoS2/Graphene Hybrid Nanoflowers with Enhanced Electrochemical Performances as Anode for Lithium-Ion Batteries. J. Phys. Chem. C 2015, 119, 7959–7968. [Google Scholar] [CrossRef]
- Ren, W.; Zhou, W.; Zhang, H.; Cheng, C. ALD TiO2-Coated Flower-like MoS2 Nanosheets on Carbon Cloth as Sodium Ion Battery Anode with Enhanced Cycling Stability and Rate Capability. ACS Appl. Mater. Interfaces 2017, 9, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Wang, T.; Fu, X.; Fan, L.; Peng, Z. Coherent SnS2/NiS2 hetero-nanosheet arrays with fast charge transfer for enhanced sodium-ion storage. Appl. Surf. Sci. 2020, 508, 145241. [Google Scholar] [CrossRef]
- Fang, G.; Wu, Z.; Zhou, J.; Zhu, C.; Cao, X.; Lin, T.; Chen, Y.; Wang, C.; Pan, A.; Liang, S. Observation of Pseudocapacitive Effect and Fast Ion Diffusion in Bimetallic Sulfides as an Advanced Sodium-Ion Battery Anode. Adv. Energy Mater. 2018, 8, 1703155. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, N.; Zhang, Y.; Xue, P.; Guo, M.; Tang, B.; Bai, Z.; Dou, S. Pyrite FeS2@C nanorods as smart cathode for sodium ion battery with ultra-long lifespan and notable rate performance from tunable pseudocapacitance. Electrochim. Acta 2018, 260, 755–761. [Google Scholar] [CrossRef]


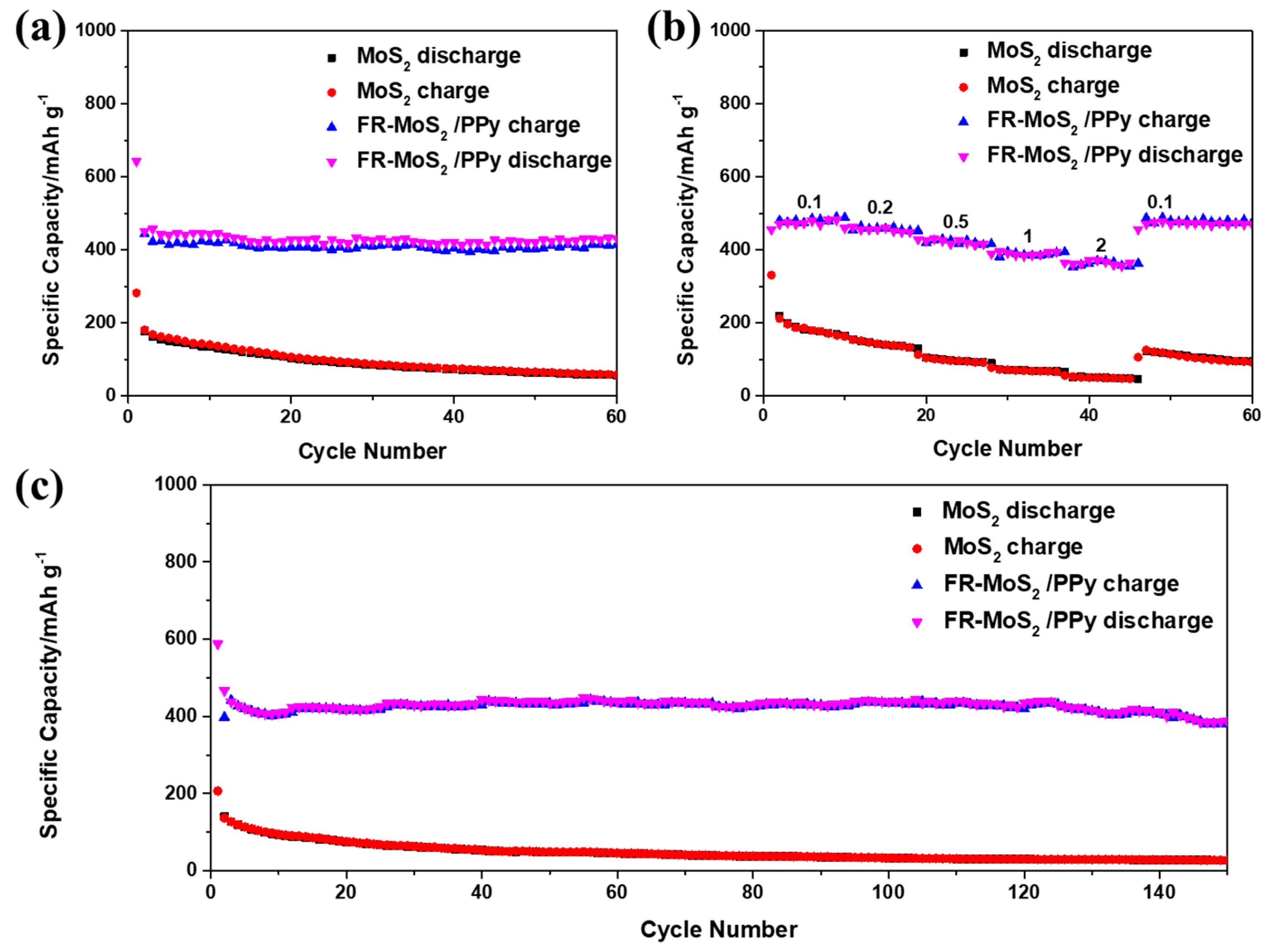
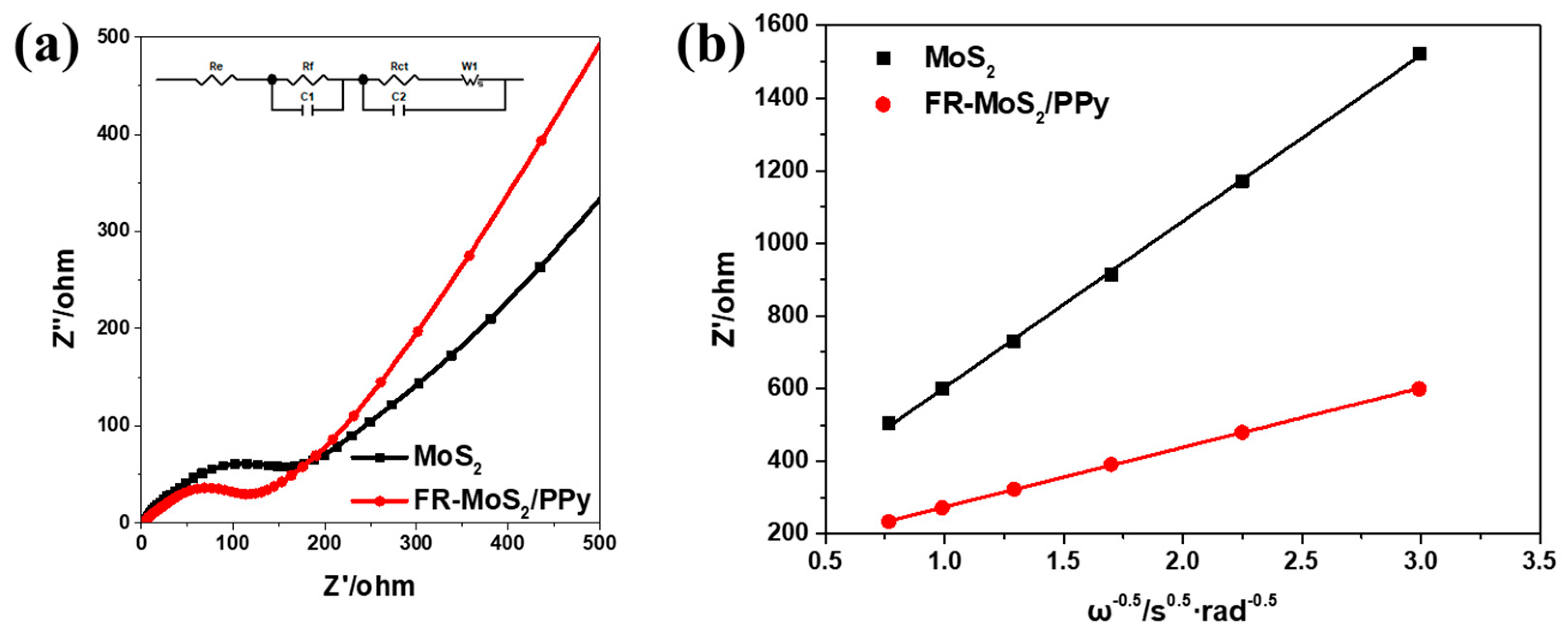
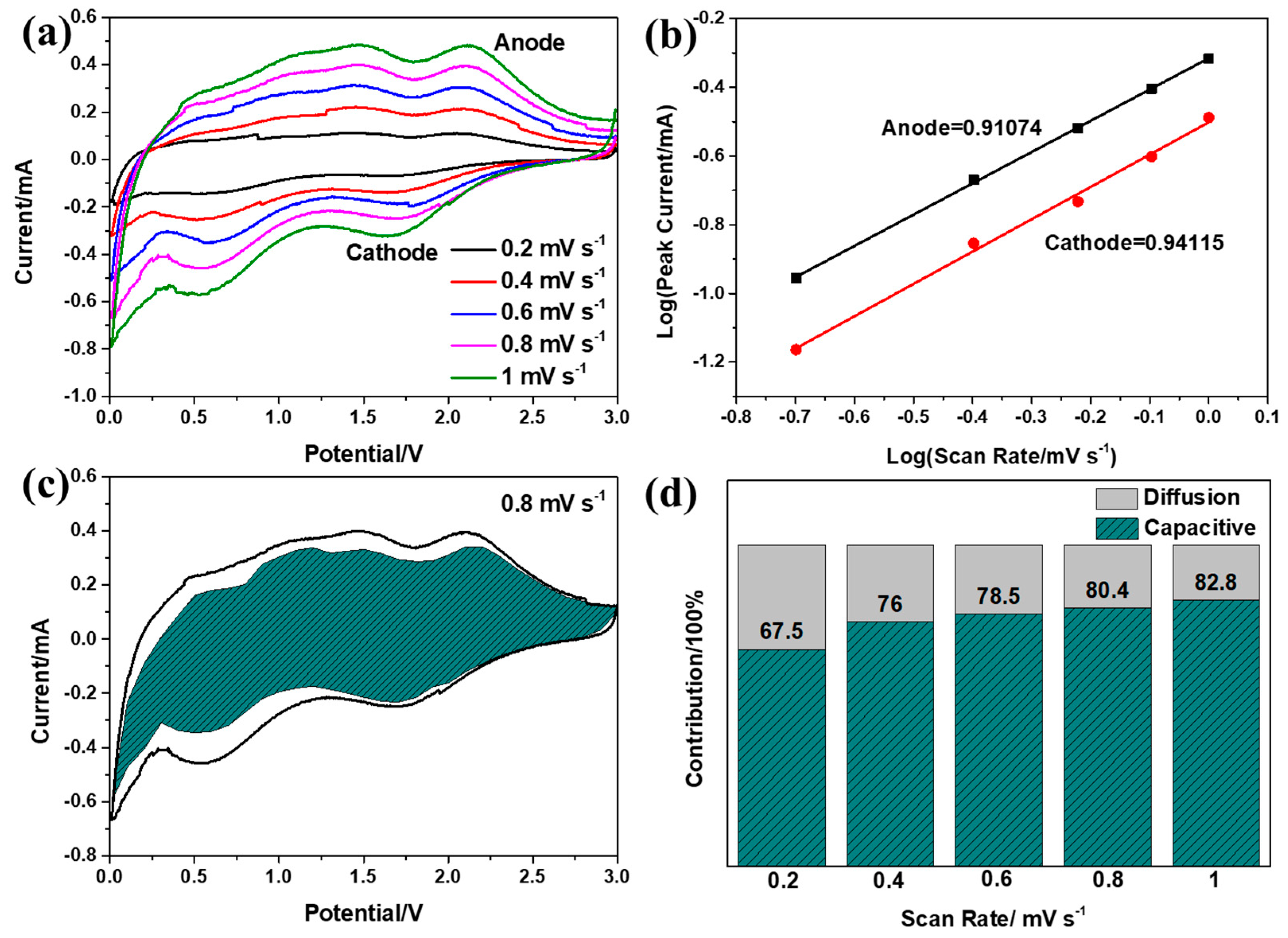
| Samples | Re/Ω | Rf/Ω |
|---|---|---|
| MoS2 | 5.13 | 25.14 |
| FR-MoS2/PPy | 5.54 | 18.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, M.; Qi, T.; Yuan, Q.; Zhao, P.; Jia, M. Polypyrrole Modified MoS2 Nanorod Composites as Durable Pseudocapacitive Anode Materials for Sodium-Ion Batteries. Nanomaterials 2022, 12, 2006. https://doi.org/10.3390/nano12122006
Jia M, Qi T, Yuan Q, Zhao P, Jia M. Polypyrrole Modified MoS2 Nanorod Composites as Durable Pseudocapacitive Anode Materials for Sodium-Ion Batteries. Nanomaterials. 2022; 12(12):2006. https://doi.org/10.3390/nano12122006
Chicago/Turabian StyleJia, Miao, Tong Qi, Qiong Yuan, Peizhu Zhao, and Mengqiu Jia. 2022. "Polypyrrole Modified MoS2 Nanorod Composites as Durable Pseudocapacitive Anode Materials for Sodium-Ion Batteries" Nanomaterials 12, no. 12: 2006. https://doi.org/10.3390/nano12122006
APA StyleJia, M., Qi, T., Yuan, Q., Zhao, P., & Jia, M. (2022). Polypyrrole Modified MoS2 Nanorod Composites as Durable Pseudocapacitive Anode Materials for Sodium-Ion Batteries. Nanomaterials, 12(12), 2006. https://doi.org/10.3390/nano12122006





