3.2. Design of Inorganic-Magnetic-Organic Nanohybrids
Inorganic-magnetic-organic nanohybrids appear to be very creative because they can produce unlimited sets of known or unknown properties. In this way, nanohybrids were designed by a combination of zero-dimensional nanoparticles of magnetic nanocomposite and two-dimensional nanolayered structures, in addition to long chains of organic acid. This combination was achieved in an order arrangement through building Al/Zn nanolayered structures which have cationic nanolayers. In the presence of n-capric acid (CH
3(CH
2)
8COO
−), the long chains of the aliphatic acid were intercalated among the nanolayers for neutralizing their positive charges. At the same time, the long chains of hydrocarbons of organic compounds were working as pillars for building the nanolayered structures. In addition, these pillars expanded and widened the interlayered spacing among the nanolayers to produce enough space for existing magnetic nanoparticles of cobalt iron oxides nanocomposite. To indicate the positive role of organic species for designing this nanohybrid, a pure Al/Zn nanolayered structure was prepared for comparison. In addition, the Al/Zn nanolayered structure was modified by the nanoparticles of cobalt iron oxides nanocomposite with longer chains of hydrocarbon of stearic acid to study the effect of the organic species. The X-ray diffraction patterns of the prepared nanolayered structures and nanohybrids are displayed in
Figure 2.
Figure 2a shows the x-ray diffraction pattern of the pure Al/Zn nanolayered structure. Sharp and symmetric peaks were observed at 2Ѳ = 11.62°, 23.36°, and 34.54°, aligning with the d-spacing of 0.76 nm, 0.38 nm, and 0.26 nm, respectively. These peaks are due to the reflections of the main planes (003), (006), and (009). The clear arrangement between these reflections (0.76 nm = 2 × 0.38 nm = 3 × 0.26 nm) confirmed formation of the nanolayered structures of the natural hydrotalcite (JCPDS file No. 37–629) and zinc aluminum carbonate hydroxide hydrate (JCPDS file No. 38–486). The other reflections of the planes (012), (015), (110), and (113) of the nanolayered structures of the natural hydrotalcite were observed at 2Ѳ = 39.16°, 46.56°, 60.05°, and 61.44° and matched with the d-spacing of 0.23 nm, 0.19 nm, 0.17 nm, 153, and 0.150 nm, respectively. The crystal parameters (a, c) could be calculated depending on the d-spacing of the planes (003) and (110), respectively. The first parameter was 2 × d
(110) = 0.306 nm. It means that the average distance between Zn-cation and Al-cation is 0.306 nm, agreeing with the previous published data of zinc aluminum carbonate hydroxide hydrate (JCPDS file No. 38–486). The second parameter was assessed by 3 × d
(003) = 2.28 nm. It was similar to that reported for the natural hydrotalcite.
By intercalating the long chains of hydrocarbons of n-capric acid (CH
3(CH
2)
8COO
−) with the Al/Zn nanolayered structures in presence of the nanoparticles of cobalt iron oxides nanocomposite, inorganic-magnetic-organic nanohybrid HZ-1 was formed through a host–guest interaction. The X-ray diffraction pattern of HZ-1, which is displayed in
Figure 2b, shows new peaks at low 2Ѳ in addition to the disappearance of the original peaks of the nanolayered structure of LDH, noting that the peaks of the nanoparticles of magnetic nanocomposite were observed as weak peaks at 2Ѳ = 35.56°, 41.6°, and 62.9°. A sharp peak was observed at 2.2 nm indicating that the interlayered spacing of the nanolayered structure expanded and widened from 0.755 nm to 2.20 nm. This spacing could allow for the nanoparticles of cobalt iron oxides to intercalate among the nanolayers of the nanolayered structure because the peaks of cobalt iron oxides were not clear in
Figure 2b. The crystal parameter (a), which depends on the reflection of the plane (110), has a little shift. At the same time, a large change was observed for the parameter (c) from 2.280 nm to 6.60 nm. It means that the nanohybrid HZ-1 consists of nanolayered structures having organic species and magnetic nanoparticles.
With intercalating longer chains of organic compounds, stearic acid (CH
3(CH
2)
16COO
−) and the nanoparticles of cobalt iron oxides nanocomposite inside the pure Al/Zn nanolayered structures, HZ-2 was formed to build another inorganic-magnetic-organic nanohybrid.
Figure 2c shows the main peaks of the Al/Zn LDH in addition to appearing as new peaks after building the nanohybrid HZ-2. The new peaks of the nanohybrid HZ-2 were observed at 1.6 nm and 1.4 nm, as seen in
Figure 2c (inset). It indicated that the interlayered spacing of the nanolayered structure expanded and widened from 0.755 nm to become higher. This expansion could allow for the nanoparticles of cobalt iron oxides to intercalate among the nanolayers of the nanolayered structure because the characteristic peak of cobalt iron oxides overlaps with the peak of nanolayered structure at 2Ѳ = 35.56°, as shown in
Figure 3c. It means that the nanohybrid HZ-2 consists of nanolayered structures that have organic species and magnetic nanoparticles.
This finding was confirmed by transmission electron microscopy (TEM) and energy dispersive X-ray spectrometry (EDX). TEM images of the nanohybrid HZ-1 are displayed in
Figure 3.
Figure 3a shows that the nanohybrid HZ-2 has nanoplatelets with a size of less than 50 nm. Furthermore, very fine nanoparticles, which are marked by arrows, were observed in
Figure 3c,d, representing the magnetic nanoparticles cobalt iron oxides. Through magnification,
Figure 3c confirmed the presence of the magnetic nanoparticles among the nanolayers of nanohybrid. In addition,
Figure 3d shows one particle started to intercalate with the nanolayered structure. By EDX analysis, the different elements were identified in the nanohybrid HZ-2, as shown in
Figure 3e.
Figure 3e shows sharp peaks for the non-magnetic elements zinc and aluminum. In addition, the magnetic elements cobalt and iron were observed by weak peaks.
In order to identify the function groups of the nanohybrids HZ-1 and HZ-2, the infrared spectra (FT-IR) was used and is displayed in
Figure 4. For the nanohybrid HZ-1, the absorption band was observed at 3434 cm
−1, indicating the stretching mode of hydroxyl groups as seen in
Figure 5a. The presence of long chains of hydrocarbon of n-capric acid was clear in the IR spectrum because the stretch absorption of carbon–hydrogen was observed by sharp peaks at 2924 cm
−1 and 2953 cm
−1. In addition, the bending mode of the carbon–hydrogen was clear through an observing band at 1468 cm
−1. The symmetric stretching vibration of carboxylate, which belonged to the aliphatic acid, was observed at 1554 cm
−1. Furthermore, the absorption at 1411 cm
−1 is assigned to the asymmetric stretching vibration of carboxylate. The bands observed below 1000 cm
−1 could be ascribed to Zn-O and Al-O.
For the nanohybrid HZ-2,
Figure 4b confirms the formation of inorganic-magnetic-organic nanohybrid through observing the main bands of stearic acid. The presence of long chains of hydrocarbon was confirmed by observing sharp peaks at 2922 cm
−1 and 2849 cm
−1, indicating the stretch absorption of carbon–hydrogen. Additionally, the bending mode of the carbon–hydrogen was clear through observing the band at 1466 cm
−1. The symmetric stretching vibration of carboxylate, which belonged to the aliphatic acid, was observed at 1589 cm
−1. Furthermore, the absorption at 1397 cm
−1 is assigned to the asymmetric stretching vibration of carboxylate. In addition, the absorption band of the hydroxyl groups of the nanolayered structure was observed at 3467 cm
−1. In the same trend, the presence of different kinds of hydroxyl groups were confirmed by observing another band for hydroxyl groups at 3694 cm
−1. It indicated that the presence of the nanoparticles of cobalt iron oxides among the nanolayers affect the vibrational mode of hydroxyl groups. It means that the confinement of the nanoparticles of CoFe
2O
4 among the nanolayers affect the hydroxyl groups which are closer to these nanoparticles.
The thermal gravimetric analysis and differential scanning calorimetry (TGA-DSC curves) were used to study the thermal behavior of the prepared nanohybrids.
Figure 5 indicates that the thermal decomposition of both HZ-1 and HZ-2 can give information for the nature of the interlayer species inside the nanohybrids. The DSC curve of the nanohybrid HZ-1 shows two series of peaks, as shown in
Figure 5a. The first series is endothermic peaks at 92 °C and 171 °C, which are ascribed to the removal of the surface and interlayered water. The second series is exothermic peaks at 250 °C, 419 °C, and 552 °C, representing the oxidation reactions of the chains of hydrocarbon of n-capric acid. From the TG curve (
Figure 5c), the weight loss of 18%, which happened up to 222 °C, represents the internal content of water inside the nanohybrid HZ-1. In the same way, the weight loss of 36%, which occurred up to 460 °C, is due to the internal content of organic species inside the nanohybrid HZ-1. The DSC curve of the nanohybrid NHA-2 is similar to that of the nanohybrid HZ-2, as seen in
Figure 5b.
Figure 5b shows endothermic and exothermic peaks, indicating the removal of water and oxidation reactions of the long chains of hydrocarbon of stearic acid. In the same way, similar behavior was observed for the TG curve of HZ-2, as shown in
Figure 5d. The thermal analyses results confirmed formation of the nanohybrids HZ-1 and HZ-2.
3.3. Design of Nanohybrids Based on Oxides
The main reason for designing nanohybrids with organic and inorganic species is directed to produce stable and effective zinc oxides nanohybrids and nanocomposites with distinguished properties. Therefore, the prepared nanohybrids were thermally treated at 500 °C to remove unstable species and create new active sites.
X-ray diffraction has been used to identify the produced structures from the calcination of the nanohybrids.
Figure 6 shows X-ray diffraction patterns of ZOA-500, HZ-1-500, and HZ-2-500. The XRD pattern of ZOA-500 exhibited new weak peaks at 2Ѳ = 32.01°, 34.32°, 36.49°, 47.71°, 7.05°, and 62.81°, in addition to disappearance of the original peaks of the nanolayered structures, as shown in
Figure 6a. By comparing the diffraction lines of the zinc oxide crystal (JCPDS No. 36-1451) and the standard entire diffraction pattern of zincite phase (JCPDS No. 75-576), ZOA-500 has a similar structure for zinc oxide. However, the broad and diffuse peaks of ZOA-500 indicated that the structure of ZOA-500 is not pure because of the presence of the amorphous structure of aluminum oxide inside the zincite phase. In case of the nanohybrid HZ-1-500,
Figure 6b shows clear and sharp peaks at 0.28 nm, 0.26 nm, and 0.24 nm, indicating a crystalline structure. Furthermore, weak peaks were observed at 0.19 nm, 0.16 nm, 0.15 nm, and 0.14 nm. These diffraction lines agree with the peaks of the zinc oxide crystal (JCPDS No. 36-1451) and the standard entire diffraction pattern of the zincite phase (JCPDS No. 75-576). In addition, a weak peak was observed at 0.30 nm and marked with (*) in
Figure 6b. At the same time,
Figure 6b reveals that the characteristic peak of cobalt iron oxides is observed at 0.25 nm and overlaps with the peak of zinc oxide at 0.24 nm.
For the nanohybrid HZ-2-500,
Figure 6c shows that the characteristic peaks of zinc oxide were observed at 0.28 nm, 0.26 nm, and 0.24 nm, agreeing with the crystalline structure of the sample HZ-1-500. This similarity was confirmed by observing weak peaks at 0.19 nm, 0.16 nm, 0.15 nm, and 0.14 nm. These diffraction lines agree with the peaks of the zinc oxide crystal (JCPDS No. 36-1451) and the standard entire diffraction pattern of zincite phase (JCPDS No. 75-576). At the same time,
Figure 6c revealed that the characteristic peak of cobalt iron oxides at 0.25 nm were not clear in the sample NH-2-500. These XRD results can conclude that both HZ-1-500 and HZ-2-500 have a zincite phase doping with aluminum and cobalt iron oxides.
To confirm the presence of magnetic elements inside the ZnO crystals, the chemical composition of HZ-1-500 was measured through scanning electron microscopy (SEM) and energy dispersive X-ray spectrometry (EDX). SEM images showed that HZ-1-500 has one phase, as shown in
Figure 7a. In addition, SEM image indicated that this phase consisted of nanoparticles. The chemical composition of this phase was determined by the EDX equipment, which is attached to SEM. The EDX spectrum confirmed the presence of magnetic elements Co and Fe. Furthermore,
Figure 7b shows that the atomic percentages of cobalt and iron in the nanoparticles of HZ-1-500 are 1.24% and 0.96%; respectively. In addition, the atomic percentage of aluminum was 18.64%. At the same time,
Figure 7b revealed that the highest percentage is due to zinc. It means that HZ-1-500 is composed of zinc oxide doping with Al, Co, and Fe.
TEM images of HZ-2-500 confirmed this finding, as shown in
Figure 8. Clear nanoparticles were observed for HZ-1-500, as seen in
Figure 8a. It indicated that the width of HZ-1-500 is 20 nm. Additionally, very fine white spots were observed and marked by the arrow on the surface of the nanoparticles. These spots represent the cobalt iron oxides nanocomposites. These white spots became clearer by magnification, as seen in
Figure 8b.
Figure 8b shows the combination between the zinc oxide particles with the particles of cobalt iron oxides. Energy dispersive X-ray spectrometry (EDX) analysis of HZ-1-500 confirmed the presence of magnetic elements through observing two weak peaks for cobalt and iron, as seen in
Figure 8c. Furthermore, inorganic elements (zinc, aluminum and oxygen) were also observed by sharp peaks in
Figure 8c.
According to the results of XRD and the images of TEM in addition to SEM-EDX analysis, the zinc oxide nanohybrids were produced from the thermal decomposition of the inorganic-magnetic-organic nanohybrid, as shown in
Figure 9.
Figure 9 shows a schematic representation for transforming the inorganic-magnetic-organic nanohybrid to zinc oxides nanohybrids. It indicates that the presence of the magnetic nanoparticles of cobalt iron oxides nanocomposite among the nanolayers of Al/Zn gave a good chance for incorporation of cobalt iron oxides nanocomposite with the nanoparticles of the Al-doped ZnO, which was produced during the thermal decomposition of organic species. This combination, which happened during the crystallization process of zinc oxide, created new optical active sites for HZ-1-500. According to the similarity between the results of XRD, FIIR, and thermal analyses of both nanohybrids HZ-1 and HZ-2, a similar process happened to produce HZ-2-500.
3.4. Optical Properties
Zinc oxide is familiar for the researchers in the field of optical application as one of the most famous photo-active materials. However, its optical applications are concentrated in the UV-region. Therefore, many studies were published in literature for developing the structure and the morphology of zinc oxide to advance its optical behavior through increasing the range of its absorbance and decreasing its band gap energy
In this way, the optical absorbance and the band gap energy of the prepared nanohybrids were studied and compared by using the UV-Vis absorption technique which considers one of the main means for giving significant details about their optical properties.
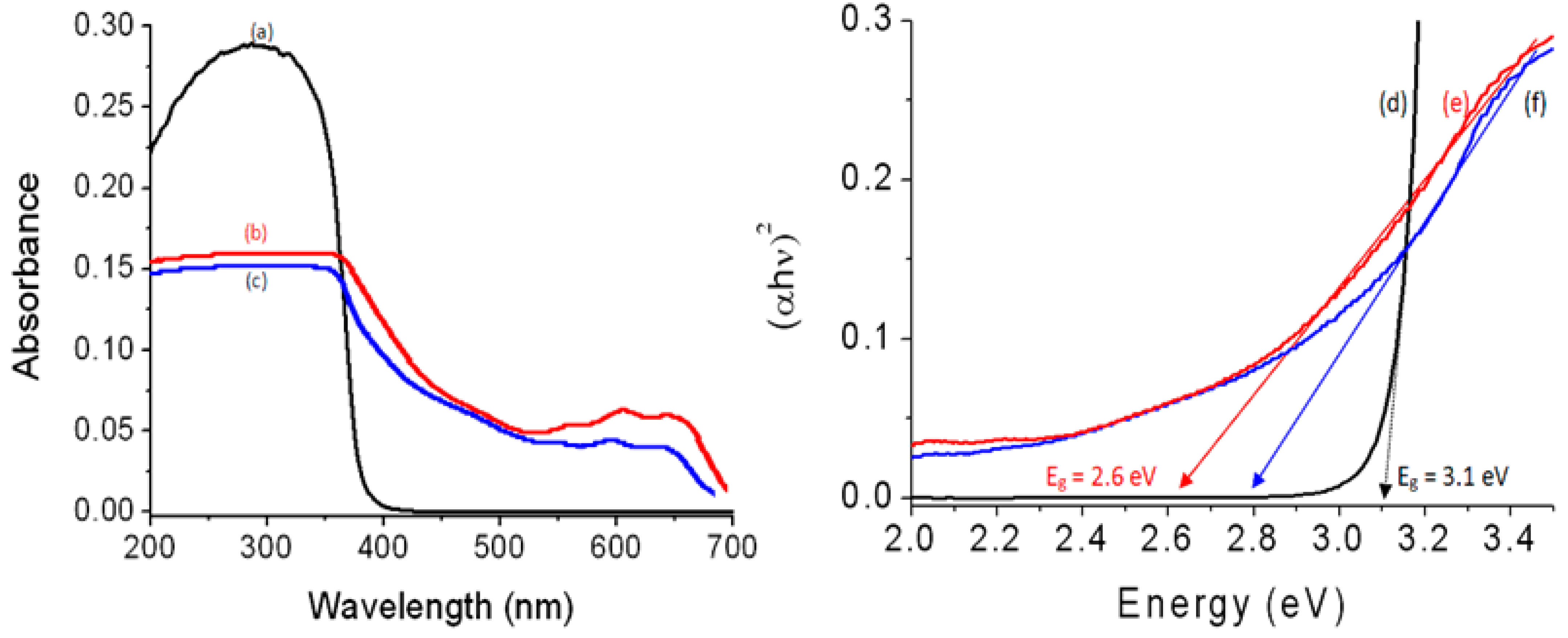
Figure 10 shows the UV-Vis absorbance of ZOA-500, HZ-1-500, and HZ-2-500 in addition to their band gap energy.
Figure 10a indicates that ZOA-500 is active in the UV region because it has absorption in the range of wavelengths of 200–350 nm. At the same time, there is no absorption in the visible region above 400 nm. By modifying the structure of ZOA-500 through building nanohybrid with magnetic nanocomposites and n-capric acid, the optical properties of HZ-1-500 improved, as shown in
Figure 10b. A new absorbance band was observed in the visible region at 630 nm. At the same time, the absorbance edge shifted to higher wavelength at 700 nm. This positive effect was also observed for HZ-2-500, as shown in
Figure 10c.
Figure 10c shows clear absorbance for HZ-2-500, starting from 700 nm to 200 nm with two maxima at 600 nm and 350 nm. It means that the intercalation of magnetic nanoparticles inside the interlayered space of the nanohybrid led to good and ordered dispersion inside the structure of zinc oxide, in addition to creating new optical active centers for ZnO after calcination.
These results were confirmed by calculating their optical band gap energies. The band gap energy was determined through plotting the relation (αhν)
2 against energy (hν), as seen in
Figure 10d–f. The band gap energy E
g of ZOA was calculated by drawing the tangent to the axis of energy to determine the optical band gap energy at (αhν)
2 of 0. It showed 3.10 eV indicating a little shift from the band gap of pure ZnO because of the doping of aluminum inside the zinc oxide structure [
7]. In the case of HZ-1-500, a large change was observed for the band gap energy because
Figure 10e shows 2.60 eV. This strong effect of the nanohybrid structure was also observed for HZ-2-500 as shown in
Figure 10f.
Figure 10f revealed the narrowing of the band gap energy for HZ-2-500 to be 2.79 eV. The comparison with the pure zinc oxide showed strong narrowing for band gap energy because the reduction was from 3.30 eV to 2.60 and 2.79 eV for both the nanohybrids, indicating that the inorganic–magnetic-organic nanohybrids have a strong positive effect on the optical properties of zinc oxide.
3.5. Optical Activity
It is known that the improvement of the optical properties of the products of zinc oxides leads to positive effects for their photo activities. In order to indicate these positive effects, the prepared products have been used as photocatalysts to be appropriate means for increasing the photocatalytic activity of zinc oxide to decompose and remove pollutants by sunlight in short time. In this way, the green dye of naphthol green B was used to be specimen for industrial pollutants. The photo activities of zinc oxides (doped or undoped), and their products based on the nanohybrids structure were studied through photocatalytic degradation of naphthol green B. By measuring the absorbance of the liquid portion after exposure of the green solution of dyes to the sunlight for few minutes in the presence of the one of the prepared photocatalyst, the degradation of the main structure of the pollutant was observed through decreasing the intensity of the absorbance band at a wavelength of 714 nm, as seen in
Figure 11a,b. At the same time, the reduction of the intensity of the absorption peaks at 322 nm, 280 nm, and 230 nm indicated the degradation of the naphthyl rings in the dye.
This blank experiment, which was performed without a photocatalyst, indicated the high stability of naphthol green B in sunlight. The photocatalytic degradation of the green dye was studied as a function of the time of sunlight exposure in the presence of the photocatalyst, as seen in
Figure 11. When the aqueous solution of naphthol green B was mixed with the photocatalyst for 10 min in the dark, an appropriate change was observed, indicating that these photocatalysts have low adsorption power. For reference, it was used as 0 min irradiation.
Figure 11a showed the photo catalytic degradation of NGB under sunlight in the presence of HZ-1-500. By increasing the irradiation time, the photocatalytic degradation of naphthol green B increased. After 10 min of sunlight exposure, the green color was completely removed, indicating high activity for HZ-1-500. In the case of using HZ-2-500, the activity became lower, as shown in
Figure 11b. The photocatalytic degradation of naphthol green B was arrived to 78% after 10 min of sunlight irradiation time. It means that the nanohybrid HZ-2-00 needs more than 10 min to completely remove the colored pollutant. It means that the nanohybrid HZ-1-500 is active and effective in sunlight because it completely destroyed the green dye at shorter time.
The high performance of the zinc oxide nanohybrids HZ-1-500 and HZ-2-500 was clear after comparison with the ZOA-500 and the pure zinc oxide. Where the complete removal of the green dye happened after 360 min of solar energy in presence of ZOA-500, in the case of the pure zinc oxide, the complete removal of the green dyes was achieved after 840 min of sunlight irradiation time. It means that the zinc oxide nanohybrids became very active in sunlight. In order to indicate the effect of the organic species on the optical activity, the kinetics of photocatalytic decolorization and degradation of naphthol green B were studied for both HZ-1-500 and HZ-2-500 through the next relation:
The concentration of naphthol green B at certain times is coded as [C]. In the case of [Co], it represents the concentration of naphthol green B at t = 0. The rate reaction constant is k. To determine kinetically the type of reactions, ln([Co]/[C] was plotted in Y-axis against the irradiation time in minutes on the X-axis.
Figure 12 shows a straight line indicating pseudo-first-order reactions for the reactions of photocatalytic degradation and decolorization of naphthol green B in the case of using both HZ-1-500 and HZ-2-500.
Figure 12b shows that the photo activity of HZ-2-500 led to the rate reaction constant of the photocatalytic degradation of naphthol green B in 0.143 min
−1. By using HZ-1-500,
Figure 12a indicates that the rate reaction constant increased to 0.294 min
−1. The kinetics study concluded that the rate of photocatalytic degradation of naphthol green B in the presence of HZ-1-500 increased to be higher than that of HZ-2-500. It means that the low band gap of HZ-1-500 accelerated the photocatalytic degradation of naphthol green B. In addition, the zinc oxide nanohybrid, which was based on pure nanohybrid HZ-1 is better than the zinc oxide nanohybrid, which was produced from mixed phases between nanohybrid and nanolayered LDH.
3.6. Discussion
The fast photocatalytic degradation of the green dyes in sunlight showed the excellent activity of the prepared zinc oxide nanohybrid HZ-1-500 which was produced from inorganic-magnetic-organic nanohybrids. The high performance of HZ-1-500 can be explained through the novel approach for building the nanohybrid structure of HZ-1-500. The intercalation of the fine nanoparticles of CoFe
2O
4 nanocomposite among the nanolayers of Al/Zn created a good chance for incorporation of this nanocomposite with zinc oxide structures during the crystallization process. Therefore, HZ-1-500 has a good crystalline structure for zinc oxide and there are no peaks for aluminum or cobalt iron oxides. This good incorporation of CoFe
2O
4 nanocomposite with the crystals of zinc oxide partially failed for the sample HZ-2-500 because XRD results showed two mixed phases: nanolayered structure and nanohybrid. It means that the nanoparticles of CoFe
2O
4 nanocomposite could intercalate among the nanolayers of Al/Zn for part of the nanohybrid and support on the external surface of the plates of the nanolayered structure of Al/Zn. The good incorporation of CoFe
2O
4 nanocomposite with the crystals of zinc oxide which doped with aluminum created new optical active centers inside zinc oxide nanohybrid HZ-1-500 and caused reduction for its band gap energy to be very effective in sunlight because of the low band gap energy of CoFe
2O
4 (1.32 eV) [
43]. At the same time, some sites of Zn in zinc oxide are occupied by CoFe2O4 atoms, producing new optical active centers called shallow traps between the valance band and conduction band, leading to decreasing for the band gap energy [
1,
44].
This low band gap energy and the small size of the nanoparticles of the zinc oxide nanohybrid HZ-1-500 have a strong effect on the mechanism of the photocatalytic degradation process of the green dyes. The mechanism of the photocatalytic degradation process is controlled by two critical reactions.
The first one depends on the amount of energy which was absorbed by the photocatalyst as seen in the Reaction (2). The second reaction is the movement and separation of light-induced electrons-holes, as shown in the Reactions (3) and (4). The photo-generated holes, which produced in the conduction band, react with the water molecules to produce highly oxidizing agents free radicals of hydroxyl groups (•OH). At the same time, the photo-generated electrons attack the oxygen molecules, which are adsorbed on the surface of the photocatalyst or dissolved in water to produce strong oxidizing agents superoxide radical anion (•O2−).
In the presence of green dyes, the molecules of NGB adsorbed on the surface of the nanoparticles of zinc oxide nanohybrid. The HZ-1-500 accelerated the first reaction in sunlight to become excited because it has absorbance from the wavelength 700 nm to 200 nm, in addition to low band gap energy as shown in the following Reaction (5).
The band gap of HZ-1-500 is not very small to accelerate the recombination reactions in addition to the shallow traps which help for separating between electrons and holes. Therefore, the degradation reaction continues as shown in Equations (6) and (7). By this way, the colored pollutants disappeared after ten minutes of sunlight exposure. For testing the re-use of the optimum sample, the photocatalytic degradation of HZ-1-500 was repeated two times for the fresh sample of the green dye. The same results were observed after 10 min of sunlight exposure, indicating high recyclability of the photocatalyst.