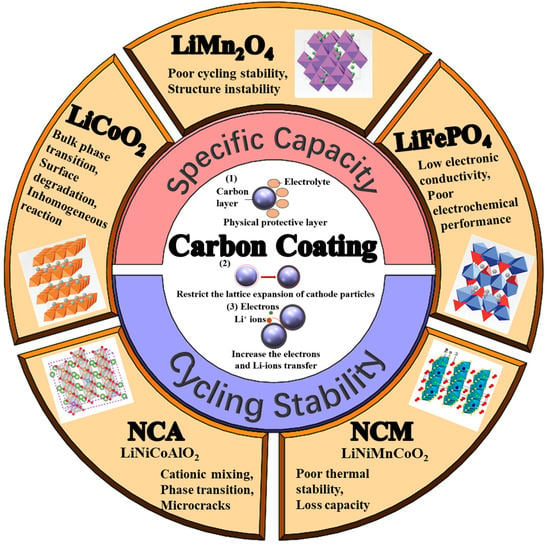
Carbon-Coatings Improve Performance of Li-Ion Battery
Abstract
:1. Introduction
2. Impact of Carbon Coating on Cathode
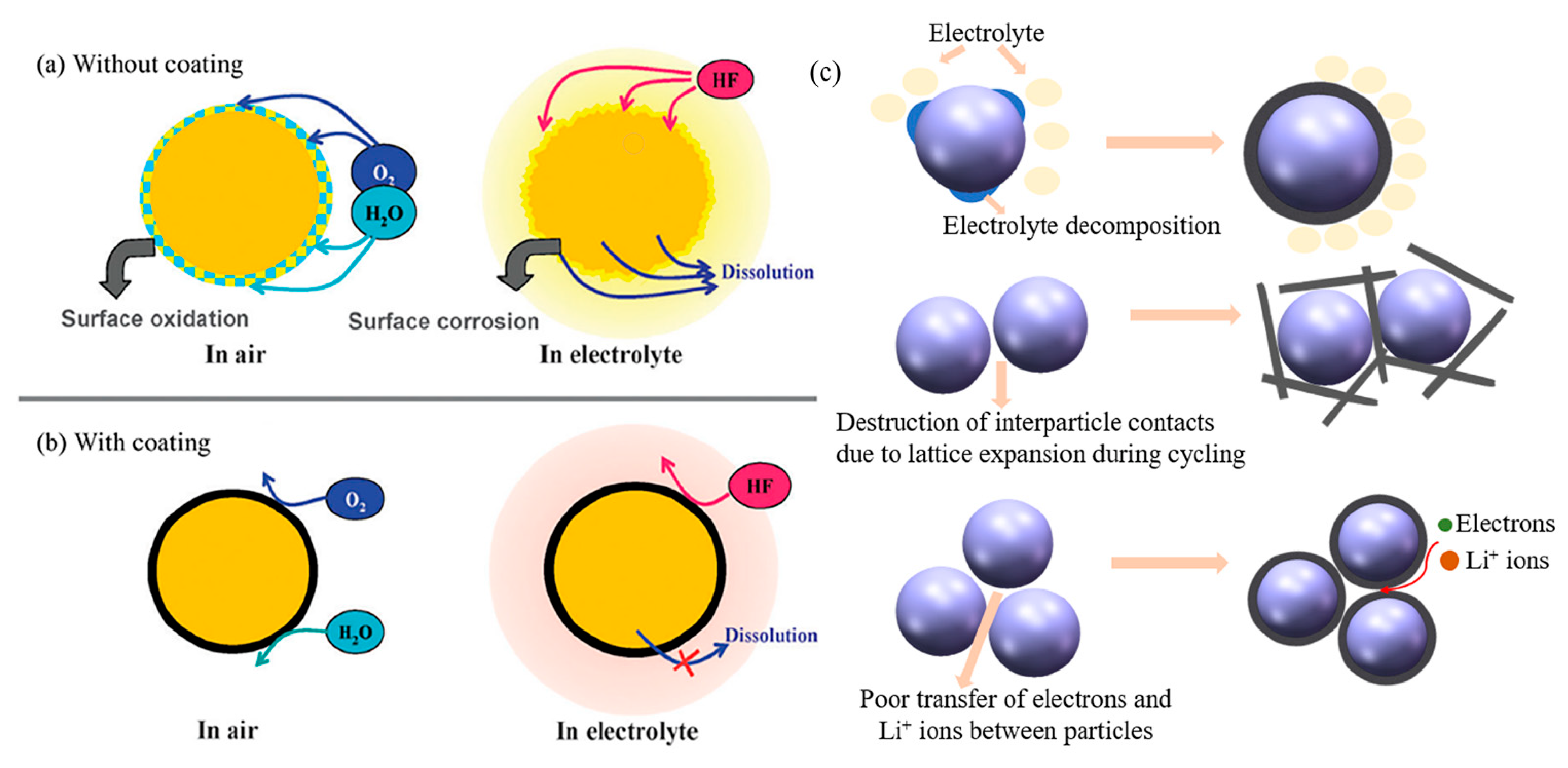
2.1. Modifying Surface Chemistry
2.2. Enhancing Structural Stability
2.3. Improving Li-Ions Diffusion
3. Carbon Coating Method
3.1. Wet Chemical Methods
3.1.1. Hydrothermal/Solvothermal
3.1.2. Sol-Gel Method
3.1.3. The Chemical Polymerization Routes
3.2. Dry Coating
3.2.1. High-Temperature Solid-State Method
3.2.2. Chemical Vapor Deposition
3.2.3. Physical Vapor Deposition
3.2.4. Atomic Layer Deposition
4. Performance of Carbon Coating on Cathode Materials in LIB
4.1. Carbon Coating on Olivine Structure Cathode (LiFePO4)
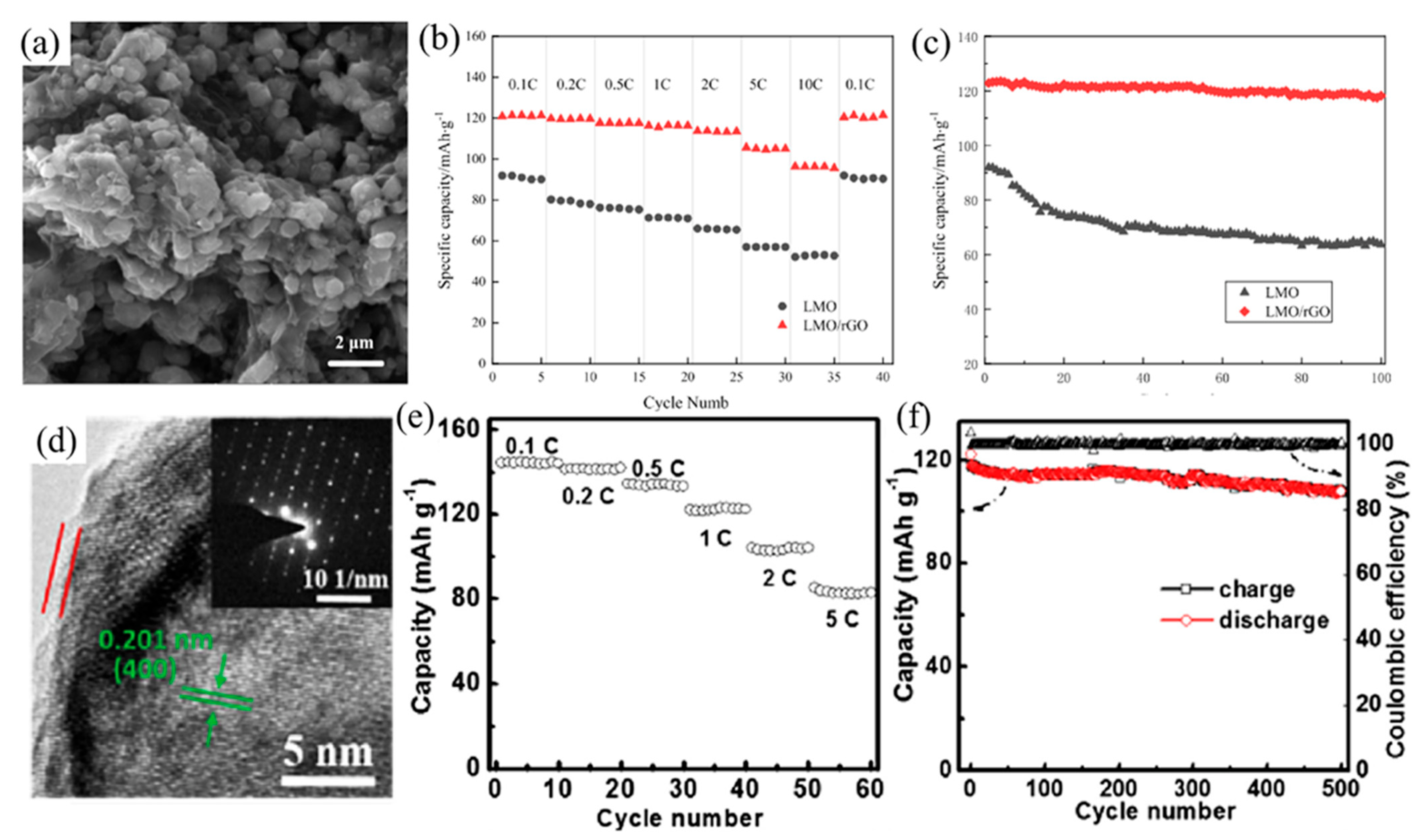
4.2. Carbon Coating on Spinels Structure Cathode (LiMn2O4)
4.3. Carbon Coating on Layered Oxide Structure Cathode
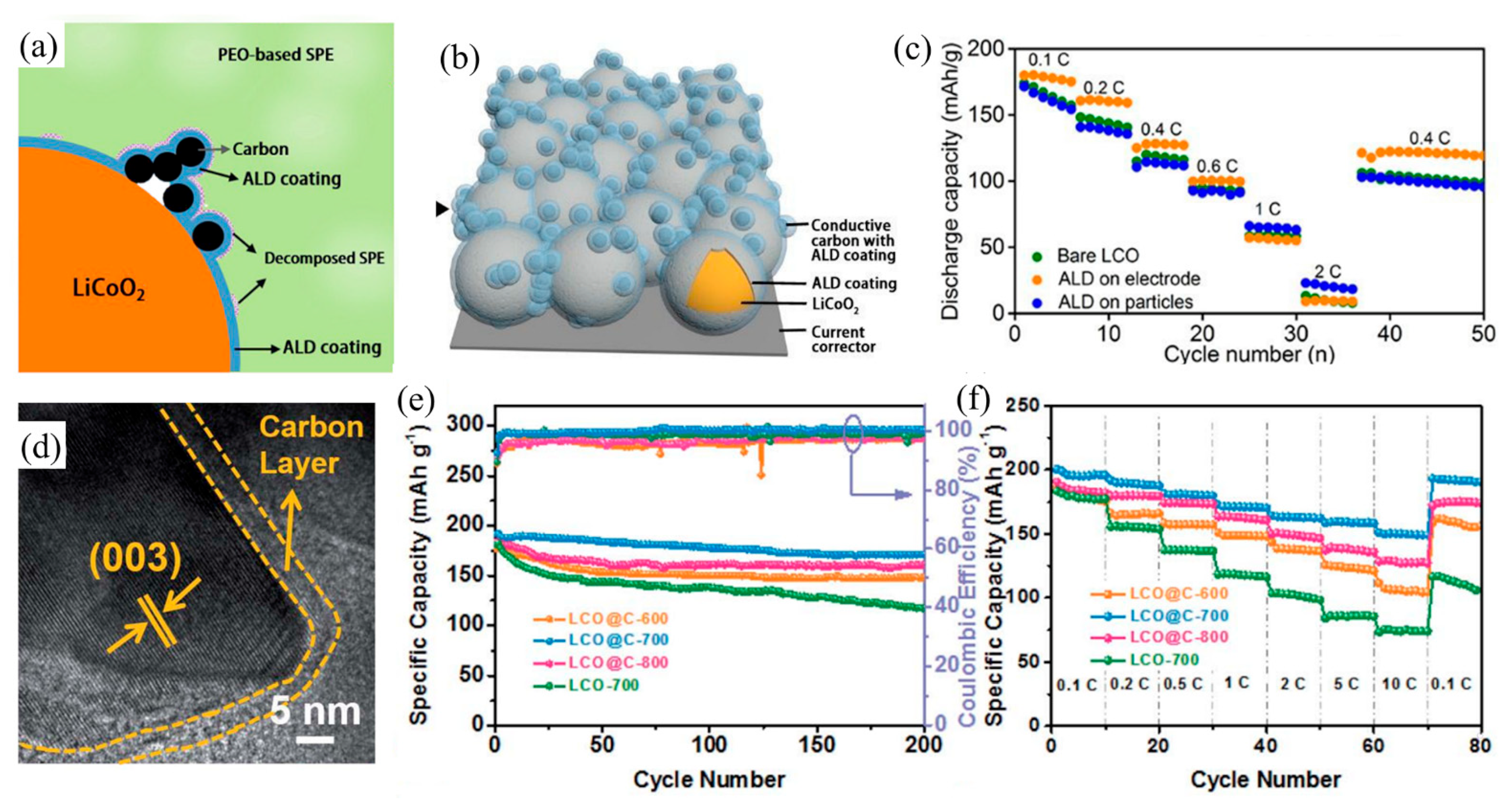
4.3.1. Carbon Coating on LiCoO2 Cathode
4.3.2. Carbon Coating on LiNiO2 Cathode
4.3.3. Carbon Coating on NCM (LiNixCoyMn1−x−yO2) Cathode
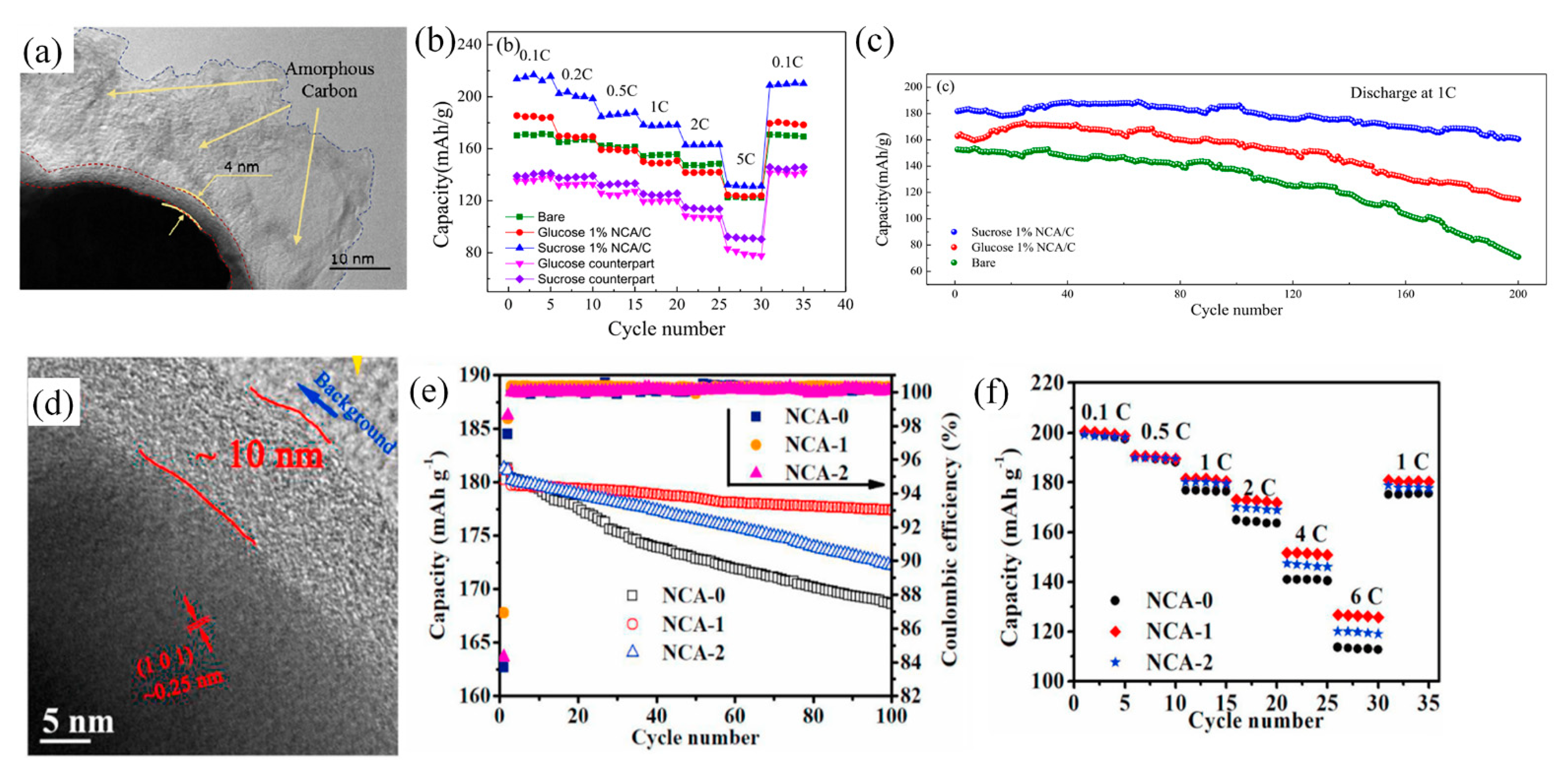
4.3.4. Carbon Coating on NCA (LiNixCoyAl1-x-yO2) Cathode
5. Limitation of Carbon Coating Method
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Liang, Q.; Liao, Q.; Yi, F.; Zheng, X.; Ma, M.; Gao, F.; Zhang, Y. Service behavior of multifunctional triboelectric nanogenerators. Adv. Mater. 2017, 29, 1606703. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Yan, X.; Liao, X.; Cao, S.; Zheng, X.; Si, H.; Lu, S.; Zhang, Y. Multi-unit hydroelectric generator based on contact electrification and its service behavior. Nano Energy 2015, 16, 329–338. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, H.; Xie, J.; Lin, Y.; Yu, J.; Chen, L. Preparation, lithium storage performance and thermal stability of nickel-rich layered LiNi0.815 Co0.15 Al0.035 O2/RGO composites. ChemElectroChem 2018, 5, 3176–3182. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, Q.; Nandakumar, D.K.; Qu, H.; Shi, Q.; Alzakia, F.I.; Tay, D.J.J.; Yang, L.; Zhang, X.; Suresh, L.; et al. Shadow enhanced self-charging power system for wave and solar energy harvesting from the ocean. Nat. Commun. 2021, 12, 616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, Z.; Liang, Q.; Shi, Q.; Zhu, M.; Lee, C. All in one, self-powered bionic artificial nerve based on a triboelectric nanogenerator. Adv. Sci. 2021, 8, 2004727. [Google Scholar] [CrossRef]
- Deng, D. Li-ion batteries: Basics, progress, and challenges. Energy Sci. Eng. 2015, 3, 385–418. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, Q.; Nandakumar, D.K.K.; Ravi, S.K.; Qu, H.; Suresh, L.; Zhang, X.; Zhang, Y.; Yang, L.; Wee, A.T.S.; et al. Energy harvesting from shadow-effect. Energy Environ. Sci. 2020, 13, 2404–2413. [Google Scholar] [CrossRef]
- Zheng, X.; Yan, X.; Sun, Y.; Bai, Z.; Zhang, G.; Shen, Y.; Liang, Q.; Zhang, Y. Au-embedded ZnO/NiO hybrid with excellent electrochemical performance as advanced electrode materials for supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 2480–2485. [Google Scholar] [CrossRef]
- Meng, Y.; Nie, C.; Guo, W.; Liu, D.; Chen, Y.; Ju, Z.; Zhuang, Q. Inorganic Cathode Materials for Potassium Ion Batteries. Mater. Today Energy 2022, 25, 100982. [Google Scholar] [CrossRef]
- Lu, S.; Qi, J.; Liu, S.; Zhang, Z.; Wang, Z.; Lin, P.; Liao, Q.; Liang, Q.; Zhang, Y. Piezotronic interface engineering on ZnO/Au-based schottky junction for enhanced photoresponse of a flexible self-powered UV detector. ACS Appl. Mater. Interfaces 2014, 6, 14116–14122. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on Lithium-Ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Demirocak, D.E.; Srinivasan, S.S.; Stefanakos, E.K. A review on nanocomposite materials for rechargeable Li-Ion batteries. Appl. Sci. 2017, 7, 731. [Google Scholar] [CrossRef] [Green Version]
- Masias, A.; Marcicki, J.; Paxton, W.A. Opportunities and challenges of Lithium Ion batteries in automotive applications. ACS Energy Lett. 2021, 6, 621–630. [Google Scholar] [CrossRef]
- Chaudhary, M.; Tyagi, S.; Gupta, R.K.; Singh, B.P.; Singhal, R. Surface modification of cathode materials for energy storage devices: A review. Surf. Coatings Technol. 2021, 412, 127009. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, Q.; Gou, J.; Song, T.T.; Arramel; Chen, H.; Yang, M.; Lim, S.X.; Wang, Q.; Zhu, R.; et al. Performance improvement by ozone treatment of 2D PdSe2. ACS Nano 2020, 14, 5668–5677. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, Q.; Zhao, X.; Liu, M.; Wee, A.T.S. Defect engineering of two-dimensional transition-metal dichalcogenides: Applications, challenges and opportunities. ACS Nano 2021, 15, 2165–2181. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, H. Enhancing the performances of Li-Ion batteries by carbon-coating: Present and future. Chem. Commun. 2011, 48, 1201–1217. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Gou, J.; Arramel; Zhang, Q.; Zhang, W.; Wee, A.T.S. Oxygen-induced controllable p-type doping in 2D semiconductor transition metal dichalcogenides. Nano Res. 2020, 13, 3439–3444. [Google Scholar] [CrossRef]
- Inagaki, M. Carbon coating for enhancing the functionalities of materials. Carbon 2011, 50, 3247–3266. [Google Scholar] [CrossRef]
- Su, L.; Jing, Y.; Zhou, Z. Li Ion battery materials with core–shell nanostructures. Nanoscale 2011, 3, 3967–3983. [Google Scholar] [CrossRef]
- Kwon, N.H.; Conder, J.; Srout, M.; Fromm, K.M. Surface modifications of positive-electrode materials for Lithium-Ion batteries. CHIMIA 2019, 73, 880–893. [Google Scholar] [CrossRef] [Green Version]
- Pagot, G.; Bertasi, F.; Nawn, G.; Negro, E.; Delpeuch, A.B.; Vezzù, K.; Cristofori, D.; Di Noto, V. Effect of graphite and copper oxide on the performance of high potential Li[Fe1/3Ni1/3Co1/3]PO4 olivine cathodes for Lithium batteries. Electrochim. Acta 2017, 225, 533–542. [Google Scholar] [CrossRef]
- Pagot, G.; Bandiera, M.; Vezzù, K.; Migliori, A.; Bertoncello, R.; Negro, E.; Morandi, V.; Di Noto, V. High valence transition metal-doped olivine cathodes for superior energy and fast cycling lithium batteries. J. Mater. Chem. A 2020, 8, 25727–25738. [Google Scholar] [CrossRef]
- Chen, Z.; Qin, Y.; Amine, K.; Sun, Y.-K. Role of surface coating on cathode materials for lithium-ion batteries. J. Mater. Chem. 2010, 20, 7606–7612. [Google Scholar] [CrossRef]
- Zhang, W.-M.; Hu, J.-S.; Guo, Y.-G.; Zheng, S.-F.; Zhong, L.-S.; Song, W.-G.; Wan, L.-J. Tin-nanoparticles encapsulated in elastic hollow carbon spheres for high-performance anode material in Lithium-Ion batteries. Adv. Mater. 2008, 20, 1160–1165. [Google Scholar] [CrossRef]
- Wu, M.; Xu, B.; Ouyang, C. Physics of electron and lithium-ion transport in electrode materials for Li-Ion batteries. Chin. Phys. B 2016, 25, 018206. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B.; Stenina, I.A. Carbon coating of electrode materials for lithium-ion batteries. Surf. Innov. 2021, 9, 92–110. [Google Scholar] [CrossRef]
- Qi, M.; Liu, Y.; Xu, M.; Feng, M.; Gu, J.; Liu, Y.; Wang, L. Improved electrochemical performances of carbon-coated LiFePO4 microspheres for Li-Ion battery cathode. Mater. Res. Express 2019, 6, 115520. [Google Scholar] [CrossRef]
- Kalluri, S.; Yoon, M.; Jo, M.; Park, S.; Myeong, S.; Kim, J.; Dou, S.X.; Guo, Z.; Cho, J. Surface engineering strategies of layered LiCoO2 cathode material to realize high-energy and high-voltage Li-Ion cells. Adv. Energy Mater. 2016, 7, 1601507. [Google Scholar] [CrossRef]
- Yilmaz, E.; Soylak, M. 15-Functionalized nanomaterials for sample preparation methods. In Handbook of Nanomaterials in Analytical Chemistry; Mustansar Hussain, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–413. ISBN 978-0-12-816699-4. [Google Scholar]
- Cao, J.; Guo, S.; Yan, R.; Zhang, C.; Guo, J.; Zheng, P. Carbon-coated single-crystalline LiMn2O4 nanowires synthesized by high-temperature solid-state reaction with high capacity for Li-Ion battery. J. Alloys Compd. 2018, 741, 1–6. [Google Scholar] [CrossRef]
- Xia, L. 2-Importance of nanostructured surfaces. In Bioceramics; Osaka, A., Narayan, R., Eds.; Elsevier Series on Advanced Ceramic Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 5–24. ISBN 978-0-08-102999-2. [Google Scholar]
- Tian, R.; Liu, H.; Jiang, Y.; Chen, J.; Tan, X.; Liu, G.; Zhang, L.; Gu, X.; Guo, Y.; Wang, H.; et al. Drastically enhanced high-rate performance of carbon-coated LiFePO4 nanorods using a green Chemical Vapor Deposition (CVD) method for Lithium Ion battery: A selective carbon coating process. ACS Appl. Mater. Interfaces 2015, 7, 11377–11386. [Google Scholar] [CrossRef] [PubMed]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On coating techniques for surface protection: A review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Behera, A.; Mallick, P.; Mohapatra, S.S. Chapter 13—Nanocoatings for anticorrosion: An introduction. In Corrosion Protection at the Nanoscale; Rajendran, S., Nguyen, T.A.N.H., Kakooei, S., Yeganeh, M., Li, Y., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 227–243. ISBN 978-0-12-819359-4. [Google Scholar]
- Goutam, S.; Omar, N.; Van Den Bossche, P.; Van Mierlo, J. Chapter two—Review of nanotechnology for anode materials in batteries. In Emerging Nanotechnologies in Rechargeable Energy Storage Systems; Rodriguez-Martinez, L.M., Omar, N., Eds.; Micro and Nano Technologies; Elsevier: Boston, MA, USA, 2017; pp. 45–82. ISBN 978-0-323-42977-1. [Google Scholar]
- Liu, Y.; Jiang, L.; Wang, H.; Wang, H.; Jiao, W.; Chen, G.; Zhang, P.; Hui, D.; Jian, X. A brief review for fluorinated carbon: Synthesis, properties and applications. Nanotechnol. Rev. 2019, 8, 573–586. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, Q.-Y.; Du, P.-P.; Wang, L.-Z.; Zhang, A.-Q.; Song, Y.-H.; Lv, Y.; Li, G.-Y. Advances in new cathode material LiFePO4 for Lithium-Ion batteries. Synth. Met. 2012, 162, 1315–1326. [Google Scholar] [CrossRef]
- Gu, M.; Shi, W.; Zheng, J.; Yan, P.; Zhang, J.-G.; Wang, C. Probing the failure mechanism of nanoscale LiFePO4 for Li-Ion batteries. Appl. Phys. Lett. 2015, 106, 203902. [Google Scholar] [CrossRef]
- Luo, W.-B.; Wen, L.; Luo, H.-Z.; Song, R.-S.; Zhai, Y.-C.; Liu, C.; Li, F. Carbon nanotube-modified LiFePO4 for high rate Lithium Ion batteries. New Carbon Mater. 2014, 29, 287–294. [Google Scholar] [CrossRef]
- Fei, H.; Peng, Z.; Yang, Y.; Li, L.; Raji, A.-R.O.; Samuel, E.L.G.; Tour, J.M. LiFePO4 nanoparticles encapsulated in graphene nanoshells for high-performance Lithium-Ion battery cathodes. Chem. Commun. 2014, 50, 7117–7119. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Bu, X.; Xu, L.; Liu, J.; Zhang, C. A novel LiFePO4/graphene/carbon composite as a performance-improved cathode material for Lithium-Ion batteries. Electrochim. Acta 2012, 64, 190–195. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, F.; Zhu, Y.; Liu, Z. Graphene modified LiFePO4 cathode materials for high power lithium ion batteries. J. Mater. Chem. 2011, 21, 3353–3358. [Google Scholar] [CrossRef]
- Bao, L.; Li, L.; Xu, G.; Wang, J.; Zhao, R.; Shen, G.; Han, G.; Zhou, S. Olivine LiFePO4 nanocrystallites embedded in carbon-coating matrix for high power Li-Ion batteries. Electrochim. Acta 2016, 222, 685–692. [Google Scholar] [CrossRef]
- Wang, J.; Gu, Y.-J.; Kong, W.-L.; Liu, H.-Q.; Chen, Y.-B.; Liu, W. Effect of carbon coating on the crystal orientation and electrochemical performance of nanocrystalline LiFePO4. Solid State Ion. 2018, 327, 11–17. [Google Scholar] [CrossRef]
- Jiang, G.; Hu, Z.; Xiong, J.; Zhu, X.; Yuan, S. Enhanced performance of LiFePO4 originating from the synergistic effect of graphene modification and carbon coating. J. Alloys Compd. 2018, 767, 528–537. [Google Scholar] [CrossRef]
- Okada, D.; Sugiki, T.; Uematsu, K.; Itadani, A.; Toda, K.; Sato, M.; Yamaguchi, T.; Arimitsu, N.; Nishikawa, S. Electrochemical properties of LiFePO4 cathode materials coated with newly developed carbon black. Electrochemistry 2015, 83, 858–860. [Google Scholar] [CrossRef] [Green Version]
- Pratheeksha, P.M.; Rajeshwari, J.S.; Daniel, P.J.; Rao, T.N.; Anandan, S. Investigation of In situ carbon coated LiFePO4 as a superior cathode material for Lithium-Ion batteries. J. Nanosci. Nanotechnol. 2019, 19, 3002–3011. [Google Scholar] [CrossRef] [PubMed]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The Lithium-Ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, J.; Wang, C.; Yuan, J.; Yang, Y.; Song, G. Crystal structure and electrochemical performance of lithium-ion battery cathode materials. J. Prog. Chem. 2010, 22, 9–18. [Google Scholar]
- Lee, M.-J.; Lee, S.; Oh, P.; Kim, Y.; Cho, J. High performance LiMn2O4 cathode materials grown with epitaxial layered nanostructure for Li-Ion batteries. Nano Lett. 2014, 14, 993–999. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Higher, stronger, better a review of 5 volt cathode materials for advanced Lithium-Ion batteries. Adv. Energy Mater. 2012, 2, 922–939. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, X.; Tang, Z. Improving the electrochemical performance of LiMn2O4 by amorphous carbon coating. Fuller. Nanotub. Carbon Nanostruct. 2014, 23, 676–679. [Google Scholar] [CrossRef]
- Li, A.; Shao, Z.; Yang, S.; Li, X.; Zhang, A. Precipitation synthesis and enhanced electrochemical performance of graphene-modified LiMn2O4 for Lithium-Ion batteries. Ionics 2020, 26, 3231–3238. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Zhao, L.; Liu, X.Q. Synthesis and electrochemical performance of spinel LiMn2O4 modified by CNTs. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2013; Volume 734, pp. 2523–2527. [Google Scholar]
- Zhuo, H.; Wan, S.; He, C.; Zhang, Q.; Li, C.; Gui, D.; Zhu, C.; Niu, H.; Liu, J. Improved electrochemical performance of spinel LiMn2O4 In Situ coated with graphene-like membrane. J. Power Sources 2014, 247, 721–728. [Google Scholar] [CrossRef]
- Liang, X.; Zeng, S.; Liu, Y.; Shi, L.; Ye, C. Study on carbon black coated LiMn2O4 doped with Cr2O3. High Temp. Mater. Process 2015, 34, 515–518. [Google Scholar] [CrossRef]
- Michalska, M.; Buchberger, D.; Jasiński, J.; Thapa, A.; Jain, A. Surface modification of nanocrystalline LiMn2O4 using graphene oxide flakes. Materials 2021, 14, 4134. [Google Scholar] [CrossRef]
- Park, J.S.; Park, Y.J. Electrochemical performance of carbon coated LiMn2O4 nanoparticles using a new carbon source. J. Electrochem. Sci. Technol. 2016, 7, 139–145. [Google Scholar] [CrossRef]
- Jiang, C.; Tang, Z.; Deng, S.; Hong, Y.; Wang, S.; Zhang, Z. High-performance carbon-coated mesoporous LiMn2O4 cathode materials synthesized from a novel hydrated layered-spinel lithium manganate composite. RSC Adv. 2017, 7, 3746–3751. [Google Scholar] [CrossRef] [Green Version]
- Chudzik, K.; Lis, M.; Świętosławski, M.; Bakierska, M.; Gajewska, M.; Molenda, M. Improving the performance of sulphur doped LiMn2O4 by carbon coating. J. Power Sources 2019, 434, 226725. [Google Scholar] [CrossRef]
- Wang, K.; Wan, J.; Xiang, Y.; Zhu, J.; Leng, Q.; Wang, M.; Xu, L.; Yang, Y. Recent advances and historical developments of high voltage lithium cobalt oxide materials for rechargeable Li-Ion batteries. J. Power Sources 2020, 460, 228062. [Google Scholar] [CrossRef]
- Lyu, Y.; Wu, X.; Wang, K.; Feng, Z.; Cheng, T.; Liu, Y.; Wang, M.; Chen, R.; Xu, L.; Zhou, J.; et al. An overview on the advances of LiCoO2 cathodes for Lithium-Ion batteries. Adv. Energy Mater. 2020, 11, 2000982. [Google Scholar] [CrossRef]
- Qu, Z.; Liu, S.; Zhang, P.; Wang, R.; Wang, H.; He, B.; Gong, Y.; Jin, J.; Li, S. Enhanced eletrochemical performances of LiCoO2 at high cut-off voltage by introducing LiF additive. Solid State Ion. 2021, 365, 115654. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief history of early Lithium-Battery development. Materials 2020, 13, 1884. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, B.; Lee, J.-G.; Cho, J.; Park, B. Direct carbon-black coating on LiCoO2 cathode using surfactant for high-density Li-Ion cell. J. Power Sources 2005, 139, 289–294. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, H.; Wang, G.; Xia, Q.; Wu, Y.; Wu, H. A novel carbon-coated LiCoO2 as cathode material for lithium ion battery. Electrochem. Commun. 2007, 9, 1228–1232. [Google Scholar] [CrossRef]
- Kwon, N.H. The effect of carbon morphology on the LiCoO2 cathode of Lithium-Ion batteries. Solid State Sci. 2013, 21, 59–65. [Google Scholar] [CrossRef]
- Kwon, N.H.; Yin, H.; Brodard, P.; Sugnaux, C.; Fromm, K.M. Impact of composite structure and morphology on electronic and ionic conductivity of carbon contained LiCoO2 cathode. Electrochim. Acta 2014, 134, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Wang, J. Enhancement of electrochemical performance of LiCoO2 cathode material at high cut-off voltage (4.5 V) by partial surface coating with graphene nanosheets. Int. J. Electrochem. Sci. 2020, 9282–9293. [Google Scholar] [CrossRef]
- Sun, Y.; Dong, H.; Wu, K.; Chen, X.; Wang, S.; Gu, W.; Hong, Z.; Liu, M.; Shen, Y.; Lu, W. Graphene quantum dots coated LiCoO2 for improved cycling stability and thermal safety at high voltage. J. Electroanal. Chem. 2020, 866, 114109. [Google Scholar] [CrossRef]
- Lin, J.; Zeng, C.; Chen, Y.; Lin, X.; Xu, C.; Su, C.-Y. In situ construction of a MOF-derived carbon-encapsulated LiCoO2 heterostructure as a superior cathode for elevated-voltage lithium storage: From experimental to theoretical study. J. Mater. Chem. A 2020, 8, 6607–6618. [Google Scholar] [CrossRef]
- Liang, J.; Sun, Y.; Zhao, Y.; Sun, Q.; Luo, J.; Zhao, F.; Lin, X.; Li, X.; Li, R.; Zhang, L.; et al. Engineering the conductive carbon/PEO interface to stabilize solid polymer electrolytes for all-solid-state high voltage LiCoO2 batteries. J. Mater. Chem. A 2020, 8, 2769–2776. [Google Scholar] [CrossRef]
- Luo, S.; Wang, K.; Wang, J.; Jiang, K.; Li, Q.; Fan, S. Binder-free LiCoO2/carbon nanotube cathodes for high-performance Lithium-Ion batteries. Adv. Mater. 2012, 24, 2294–2298. [Google Scholar] [CrossRef]
- Park, M.S.; Hyun, S.H.; Nam, S.C. Characterization of a LiCoO2 thick film by screen-printing for a Lithium-Ion micro-battery. J. Power Sources 2006, 159, 1416–1421. [Google Scholar] [CrossRef]
- Välikangas, J.; Laine, P.; Hietaniemi, M.; Hu, T.; Tynjälä, P.; Lassi, U. Precipitation and calcination of high-capacity LiNiO2 cathode material for Lithium-Ion batteries. Appl. Sci. 2020, 10, 8988. [Google Scholar] [CrossRef]
- Bianchini, M.; Roca-Ayats, M.; Hartmann, P.; Brezesinski, T.; Janek, J. There and back again—The journey of LiNiO2 as a cathode active material. Angew. Chem. Int. Ed. 2018, 58, 10434–10458. [Google Scholar] [CrossRef]
- Murali, N.; Veeraiah, V. Preparation, dielectric and conductivity studies of LiNi1-XMgxO2 cathode materials for Lithium-Ion batteries. Process. Appl. Ceram. 2017, 11, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Vandenberg, A.; Hintennach, A. A comparative microwave-assisted synthesis of carbon-coated LiCoO2 and LiNiO2 for Lithium-Ion batteries. Russ. J. Electrochem. 2015, 51, 310–317. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.; Fu, L.; Liu, H.; Wu, Y.; Rahm, E.; Holze, R.; Wu, H. Cathode materials modified by surface coating for lithium ion batteries. Electrochim. Acta 2006, 51, 3872–3883. [Google Scholar] [CrossRef]
- Jung, C.-H.; Shim, H.; Eum, D.; Hong, S.-H. Challenges and recent progress in LiNixCoyMn1−x−yO2 (NCM) cathodes for Lithium-Ion batteries. J. Korean Ceram. Soc. 2020, 58, 1–27. [Google Scholar] [CrossRef]
- Liu, S.; Xiong, L.; He, C. Long cycle life lithium ion battery with lithium nickel cobalt manganese oxide (NCM) cathode. J. Power Sources 2014, 261, 285–291. [Google Scholar] [CrossRef]
- Kim, H.-J.; Krishna, T.; Zeb, K.; Rajangam, V.; Gopi, C.V.V.M.; Sambasivam, S.; Raghavendra, K.V.G.; Obaidat, I.M. A comprehensive review of Li-Ion battery materials and their recycling techniques. Electronics 2020, 9, 1161. [Google Scholar] [CrossRef]
- Chen, G.; Peng, B.; Han, R.; Chen, N.; Wang, Z.; Wang, Q. A robust carbon coating strategy toward Ni-rich lithium cathodes. Ceram. Int. 2020, 46, 20985–20992. [Google Scholar] [CrossRef]
- Li, X.; Zhao, X.; Wang, M.-S.; Zhang, K.-J.; Huang, Y.; Qu, M.-Z.; Yu, Z.-L.; Geng, D.-S.; Zhao, W.-G.; Zheng, J.-M. Improved rate capability of a LiNi1/3Co1/3Mn1/3O2/CNT/graphene hybrid material for Li-Ion batteries. RSC Adv. 2017, 7, 24359–24367. [Google Scholar] [CrossRef] [Green Version]
- Hou, Q.; Cao, G.; Wang, P.; Zhao, D.; Cui, X.; Li, S.; Li, C. Carbon coating nanostructured-LiNi1/3Co1/3Mn1/3O2 cathode material synthesized by chemical vapor deposition method for high performance lithium-ion batteries. J. Alloys Compd. 2018, 747, 796–802. [Google Scholar] [CrossRef]
- Yang, S.; Fan, Q.; Shi, Z.; Liu, L.; Liu, J.; Ke, X.; Liu, J.; Hong, C.; Yang, Y.; Guo, Z. Superior stability secured by a four-phase cathode electrolyte interface on a Ni-rich cathode for Lithium-Ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 36742–36750. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Huang, M.; Huang, J.; Fang, Z. Preparation and rate capability of carbon coated LiNi1/3Co1/3Mn1/3O2 as cathode material in Lithium-Ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 12408–12415. [Google Scholar] [CrossRef]
- Sim, S.-J.; Lee, S.-H.; Jin, B.-S.; Kim, H.-S. Use of carbon coating on LiNi0.8Co0.1Mn0.1O2 cathode material for enhanced performances of Lithium-Ion batteries. Sci. Rep. 2020, 10, 11114. [Google Scholar] [CrossRef] [PubMed]
- Ban, C.; Li, Z.; Wu, Z.; Kirkham, M.J.; Chen, L.; Jung, Y.S.; Payzant, E.A.; Yan, Y.; Whittingham, M.S.; Dillon, A.C. Extremely durable high-rate capability of a LiNi0.4Mn0.4Co0.2O2 cathode enabled with single-walled carbon nanotubes. Adv. Energy Mater. 2010, 1, 58–62. [Google Scholar] [CrossRef]
- Al-Shroofy, M.; Zhang, Q.; Xu, J.; Chen, T.; Kaur, A.P.; Cheng, Y.-T. Solvent-free dry powder coating process for low-cost manufacturing of LiNi1/3Mn1/3Co1/3O2 cathodes in Lithium-Ion batteries. J. Power Sources 2017, 352, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Son, I.H.; Park, J.H.; Park, S.; Park, K.; Han, S.; Shin, J.; Doo, S.-G.; Hwang, Y.; Chang, H.; Choi, J.W. Graphene balls for lithium rechargeable batteries with fast charging and high volumetric energy densities. Nat. Commun. 2017, 8, 1561. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Ngo, M.D.; Kim, Y.H. Effect of soybean oil as a carbon source on the electrochemical property of LiNi1/3Co1/3Mn1/3O2 cathode material for Lithium Ion battery. Carbon Lett. 2020, 30, 621–626. [Google Scholar] [CrossRef]
- Ahsan, Z.; Ding, B.; Cai, Z.; Wen, C.; Yang, W.; Ma, Y.; Zhang, S.; Song, G.; Javed, M.S.; Bo, D. Recent progress in capacity enhancement of LiFePO4 cathode for Li-Ion batteries. J. Electrochem. Energy Convers. Storage 2020, 18, 1–54. [Google Scholar] [CrossRef]
- Purwanto, A.; Yudha, C.S.; Ubaidillah, U.; Widiyandari, H.; Ogi, T.; Haerudin, H. NCA cathode material: Synthesis methods and performance enhancement efforts. Mater. Res. Express 2018, 5, 122001. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Luo, L.; Fan, Y.; Du, Z. A review on thermal management of lithium-ion batteries for electric vehicles. Energy 2021, 238, 121652. [Google Scholar] [CrossRef]
- Wang, D.; Liu, W.; Zhang, X.; Huang, Y.; Xu, M.; Xiao, W. Review of modified nickel-cobalt lithium aluminate cathode materials for Lithium-Ion batteries. Int. J. Photoenergy 2019, 2019, 2730849. [Google Scholar] [CrossRef] [Green Version]
- Fang, R.; Miao, C.; Nie, Y.; Wang, D.; Xiao, W.; Xu, M.; Wang, C. Degradation mechanism and performance enhancement strategies of LiNixCoyAl1-x-yO2 (x≥0.8) cathodes for rechargeable Lithium-Ion batteries: A review. Ionics 2020, 26, 3199–3214. [Google Scholar] [CrossRef]
- Visbal, H.; Aihara, Y.; Ito, S.; Watanabe, T.; Park, Y.; Doo, S. The effect of diamond-like carbon coating on LiNi0.8Co0.15Al0.05O2 particles for all solid-state lithium-ion batteries based on Li2S–P2S5 glass-ceramics. J. Power Sources 2016, 314, 85–92. [Google Scholar] [CrossRef]
- Yu, J.; Li, H.; Zhang, G.; Li, X.; Huang, J.; Li, C.; Wei, C.; Xiao, C. Carbon nanotubes coating on LiNi0.8Co0.15Al0.05O2 as cathode materials for Lithium battery. Int. J. Electrochem. Sci. 2017, 12, 11892–11903. [Google Scholar] [CrossRef]
- Gao, P.; Jiang, Y.; Zhu, Y.; Hu, H. Improved cycle performance of nitrogen and phosphorus co-doped carbon coatings on lithium nickel cobalt aluminum oxide battery material. J. Mater. Sci. 2018, 53, 9662–9673. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Hu, Y.; Jiang, H.; Li, C. Surface-engineering of layered LiNi0.815Co0.15Al0.035O2 cathode material for high-energy and stable Li-Ion batteries. J. Energy Chem. 2018, 27, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Wang, Z.; Lu, T.; Dai, P.; Gao, P.; Zhu, Y. Modification of LiNi0.8Co0.15Al0.05O2 using nanoscale carbon coating. J. Alloys Compd. 2018, 763, 701–710. [Google Scholar] [CrossRef]
- Lim, J.-M.; Luu, N.S.; Park, K.-Y.; Tan, M.T.Z.; Kim, S.; Downing, J.R.; He, K.; Dravid, V.P.; Hersam, M.C. Enhancing nanostructured Nickel-rich Lithium-Ion battery cathodes via surface stabilization. J. Vac. Sci. Technol. A 2020, 38, 063210. [Google Scholar] [CrossRef]
- Park, K.; Lim, J.; Luu, N.S.; Downing, J.R.; Wallace, S.G.; Chaney, L.E.; Yoo, H.; Hyun, W.J.; Kim, H.; Hersam, M.C. Concurrently approaching volumetric and specific capacity limits of Lithium battery cathodes via conformal pickering emulsion graphene coatings. Adv. Energy Mater. 2020, 10, 2001216. [Google Scholar] [CrossRef]
- Zhao, H.; Law, H.; Liao, S.; Chen, D.; Lin, P. Novel graphitic sheets with ripple-like folds as an NCA cathode coating layer for high-energy-density Lithium-Ion batteries. Nanotechnology 2020, 32, 08LT01. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Liu, Q.; Hu, T.; Chen, Y.; Zeng, T. Boosting cyclability performance of the LiNi0.8Co0.15Al0.05O2 cathode by a polyacrylonitrile-induced conductive carbon surface coating. Ceram. Int. 2021, 47, 12706–12715. [Google Scholar] [CrossRef]
- Park, C.W.; Lee, J.-H.; Seo, J.K.; Jo, W.Y.; Whang, D.; Hwang, S.M.; Kim, Y.-J. Graphene collage on Ni-rich layered oxide cathodes for advanced Lithium-Ion batteries. Nat. Commun. 2021, 12, 2145. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, Y.; Duh, J.-G. Developing a diamine-assisted polymerization method to synthesize nano-LiMnPO4 with n-doped carbon from polyamides for high-performance Li-Ion batteries. ACS Sustain. Chem. Eng. 2018, 6, 13302–13311. [Google Scholar] [CrossRef]
- Chen, X.; Ma, F.; Li, Y.; Liang, J.; Matthews, B.; Sokolowski, J.; Han, J.; Wu, G.; Lu, X.; Li, Q. Nitrogen-doped carbon coated LiNi0.6Co0.2Mn0.2O2 cathode with enhanced electrochemical performance for Li-Ion batteries. Electrochim. Acta 2018, 284, 526–533. [Google Scholar] [CrossRef]



| Carbon Source | Coating Method | Coating (wt%) | Thickness (nm) | LIB Performance | Ref. | |
|---|---|---|---|---|---|---|
| Specific Capacity | Cycling Stability | |||||
| Sucrose | Hydrothermal method and heat treatment | 15.0 | / | 128 mAh g−1 (0.1 C) | No capacity fading (0.1 C, 50 cycles) | [28] |
| Carbon nanotubes and glucose | Ultra-fine ball milling and spray-drying | 5.0 | / | 127.1 mAh g−1 (10.0 C) | 85.3% (10.0 C, 450 cycles) | [40] |
| Graphene nanosheet | Chemical vapor deposition | 5.1 | 3.66 | 145 mAh g−1 (0.1 C) | 95.3% (0.1 C,1000 cycles) | [41] |
| Graphene and sucrose | Solvothermal, drying and calcination | 8.0 | 5 | 163.7 mAh g−1 (0.1 C) 114 mAh g−1 (5.0 C) | 97% (0.1 C, 30 cycles) | [42] |
| Graphene | Spray-drying and annealing process | 5.0 | 2 | 140 mAh g−1 (0.1 C) | 95% (20.0 C, 1000 cycles) | [43] |
| Sucrose | Hydrothermal treatment | / | / | 166 mAh g−1 (0.05 C) | 98% (0.1 C, 100 cycles) | [44] |
| Glucose | Hydrothermal synthesis and annealing process | 1.65 | / | 162 mAh g−1 (0.1 C) | No capacity fading (5.0 C, 50 cycles) | [45] |
| Graphene oxide and sucrose | Solvothermal method and high temperature solid state reaction | 10.0 | 2–4 | 148.3 mAh g−1 (1.0 C) | No capacity fading (10.0 C, 200 cycles) | [46] |
| New carbon black and polystyrene | Ball-milling and heat treatment | 6.0–8.0 | / | 160 mAh g−1 (0.5 C) | / | [47] |
| Fructose | Hydrothermal process | 8.0 | <5 | Fructose: 98 mAh g−1 (0.1 C) Sucrose: 116 mAh g−1 (0.1 C) Glucose: 63 mAh g−1 (0.1 C) | / | [48] |
| Sucrose | ||||||
| Glucose | ||||||
| Carbon Source | Coating Method | Coating (wt%) | Thickness (nm) | LIB Performance | Ref. | |
|---|---|---|---|---|---|---|
| Specific Capacity | Cycling Stability | |||||
| Glucose | High temperature solid-state method | 10.0 | 3 | 132 mAh g−1 (0.1 C) | 90% (1.0 C, 500 cycles) | [31] |
| Glucose | Hydrothermal method and heat treatment | 10.0 | 1.5 | 138.5 mAh g−1 (0.1 C) | 97.76% (0.1 C, 100 cycles) | [53] |
| Reduced graphene oxide | Ball-milling and calcination | 5.0 | / | 127 mAh g−1 (0.1 C) | 96.2% (0.2 C, 100 cycles) | [54] |
| Carbon nanotubes | High temperature solid-state reaction | 5.0 | / | 110.3 mAh g−1 (1.0 C) | 98% (1.0 C, 20 cycles) | [55] |
| Liquid-polyacrylonitrile (LPAN) graphene-like membrane | Solid-state ball-milling | 20.0 | 3 | 131.1 mAh g−1 (0.1 C) | 96% (0.1 C, 50 cycles) | [56] |
| Carbon black | Wet slurry and heat treatment | 4.0 | / | 107 mAh g−1 (0.5 C) | 92.3% (0.5 C, 36 cycles) | [57] |
| Graphene oxide flakes | Wet chemical and heat treatment | 5.0 | / | 98 mAh g−1 (20 mAh g−1 current density) | 91.2% (20 mAh g−1 current density, 100 cycles) | [58] |
| Polydopamine | Polymerization process of dopamine and heat treatment | 0.25 0.65 | / | 113.3 mAh g−1 (70 mAh g−1 current density) 93 mAh g−1 (70 mAh g−1 current density) | 51.7% (140 mAh g−1 current density, 36 cycles) 73.2% (140 mAh g−1 current density, 36 cycles) | [59] |
| Ethanol | Hydrothermal process and annealing treatment | 0.27 | / | 129.4 mAh g−1 (0.5 C) | 90% (30.0 C, 1500 cycles) | [60] |
| Poly (N-vinylformamide) | Mixing in solvent and heat treatment | 5.0 | 2–3 | 121 mAh g−1 (1.0 C) | 74% (5.0 C, 1700 cycles) | [61] |
| Carbon Source | Coating Method | Coating (wt%) | Thickness (nm) | LIB Performance | Ref. | |
|---|---|---|---|---|---|---|
| Specific Capacity | Cycling Stability | |||||
| Carbon black | Sol-gel method | 1.0 | / | 145 mAh g−1 (1.0 C) | / | [66] |
| Sucrose | Milling and calcination | 5.0 | / | 130 mAh g−1 (0.1 C) | / | [67] |
| Plated-shape graphite | Ball-milling and drying | 20.0 | / | 80 mAh g−1 (0.1 C) | / | [68] |
| Graphite | Milling and drying | 10.0 | / | 220 mAh g−1 (0.1 C) | / | [69] |
| Graphene nanosheet | Dispersing in solution and evaporation | 2.1 | / | 180.8 mAh g−1 (0.1 C) | 88.5% (0.1 C, 100 cycles) | [70] |
| Graphene quantum dots | Liquid phase method and filtrating and drying | 1.0 | 10 | 182.7 mAh g−1 (0.1 C) | 82.8% (0.5 C, 100 cycles) | [71] |
| MOF-derived carbon | High temperature solid-state method | 14.03 | 5 | 193.4 mAh g−1 (0.1 C) | 89.1% (0.1 C, 200 cycles) | [72] |
| Carbon black | Mixing solvent and drying | 6.0 | 10 | 170–177 mAh g−1 (0.1 C) | 60.3% (0.1 C, 100 cycles) | [73] |
| Super-aligned Carbon nanotubes | Ultrasonication and co-deposition technique | 5.0 | 20 | 151.4 mAh g−1 (0.1 C) | 98.4% (0.1 C, 50 cycles) | [74] |
| Carbon black | Pyrolysis of resorcinol | 0.88 | 2 | 147 mAh g−1 (0.3 C) | / | [75] |
| Carbon Source | Coating Method | Coating (wt%) | Thickness (nm) | LIB Performance | Ref. | |
|---|---|---|---|---|---|---|
| Specific Capacity | Cycling Stability | |||||
| Polymers | Chemical wetting method and heat treatment | 0.39 | 4 | 191 mAh g−1 (0.5 C) | 98.74% (0.2 C, 100 cycles) | [84] |
| Carbon nanotubes and graphene | Wet chemical method | 10.0 | / | 187 mAh g−1 (0.5 C) | 93.8% (1.0 C, 50 cycles) | [85] |
| Sucrose | Chemical vapor deposition | 2.5 | 6 | 218.2 mAh g−1 (0.1 C) | 94.78% (0.1 C, 100 cycles) | [86] |
| Carbon nanotubes | Wet chemical method | 0.01 | 4 | 202.6 mAh g−1 (0.5 C) | 84.8% (0.5 C, 500 cycles) | [87] |
| Active carbon | Sol-gel route | 4.1 | 10 | 191.2 mAh g−1 (0.5 C) | 90.3% (1.0 C, 100 cycles) | [88] |
| Super-P carbon black | RAM (resonant acoustic mixer) and heat treatment | 0.5 | 0.89–1.23 | 188.6 mAh g−1 (0.5 C) | 87.8% (0.5 C, 80 cycles) | [89] |
| Single-walled carbon nanotubes | Chemical wetting method and heat treatment | 5.0 | 8 | 160 mAh g−1 (0.5 C) 130 mAh g−1 (5.0 C) | 92% (5.0 C, 500 cycles) | [90] |
| Carbon black | Electrostatic spraying | 1.0 | / | 156 mAh g−1 (0.2 C) | 80% (0.2 C, 300 cycles) | [91] |
| Graphene ball | Chemical vapor deposition and wet slurry method | 1.0 | 5 | 191.6 mAh g−1 (0.1 C) | 97.3% (1.0 C, 100 cycles) | [92] |
| Soybean oil | Solid-state method | / | 5 | 159 mAh g−1 | 95% (100 cycles) | [93] |
| Carbon Source | Coating Method | Coating (wt%) | Thickness (nm) | LIB Performance | Ref. | |
|---|---|---|---|---|---|---|
| Specific Capacity | Cycling Stability | |||||
| Diamond-like carbon | Chemical vapor deposition method | 5.0 | 4.3 | 120.7 mAh g−1 (0.05 C) | 90% (0.1 C, 100 cycles) | [99] |
| Multi-walled carbon | high-powder ultrasonic stirring | 0.5 | / | 205.6 mAh g−1 (0.1 C) | 91.7% (2.0 C, 800 cycles) | [100] |
| Aniline and phytic | chemical wetting and heat treatment | 1.0 | 8 | 190 mAh g−1 (1.0 C) | 90.7% (1.0 C, 200 cycles) | [101] |
| Reduced graphene oxide | Mechanical wet ball-milling method | 1.0 | 3.9 | 196 mAh g−1 (0.2 C) | 91.7% (1.0 C, 100 cycles) | [102] |
| Sucrose | Chemical wet and heat treatment | 1.0 | 4 | 250 mAh g−1 (0.1 C) | 88.3% (1.0 C, 200 cycles) | [103] |
| Glucose | 1.0 | 3 | 225 mAh g−1 (0.1 C) | 70.4% (1.0 C, 200 cycles) | ||
| Graphene | Wet slurry and heat treatment | 4.5 | <20 | 190 mAh g−1 (0.1 C) | 60.5% (1.0 C, 200 cycles) | [104] |
| Graphene | Pickering emulsion process | 0.5 | <10 | 191 mAh g−1 (0.1 C) | 70% (1.0 C, 250 cycles) | [105] |
| Graphite sheets | Mixing and cladding process by a mechanical fusing machine | 8 | / | 181 mAh g−1 (0.2 C) | 85% (0.5 C, 400 cycles) | [106] |
| Polyacryloni-trile (PAN) | Chemical wet and high temperature heat treatment | 4 | 5 | 180.2 mAh g−1 (1.0 C) | 98.4% (1.0 C, 100 cycles) | [107] |
| Graphene | Sonication and “collage” technique | 1.0 | 3.1 | 208 mAh g−1 (0.1 C) | 72% (0.5 C,100 cycles) | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Zhang, Q.; Liang, Q. Carbon-Coatings Improve Performance of Li-Ion Battery. Nanomaterials 2022, 12, 1936. https://doi.org/10.3390/nano12111936
Chen Z, Zhang Q, Liang Q. Carbon-Coatings Improve Performance of Li-Ion Battery. Nanomaterials. 2022; 12(11):1936. https://doi.org/10.3390/nano12111936
Chicago/Turabian StyleChen, Ziling, Qian Zhang, and Qijie Liang. 2022. "Carbon-Coatings Improve Performance of Li-Ion Battery" Nanomaterials 12, no. 11: 1936. https://doi.org/10.3390/nano12111936
APA StyleChen, Z., Zhang, Q., & Liang, Q. (2022). Carbon-Coatings Improve Performance of Li-Ion Battery. Nanomaterials, 12(11), 1936. https://doi.org/10.3390/nano12111936