Optimization of Sulfonated Polycatechol:PEDOT Energy Storage Performance by the Morphology Control
Abstract
:1. Introduction
2. Materials and Methods
3. Results
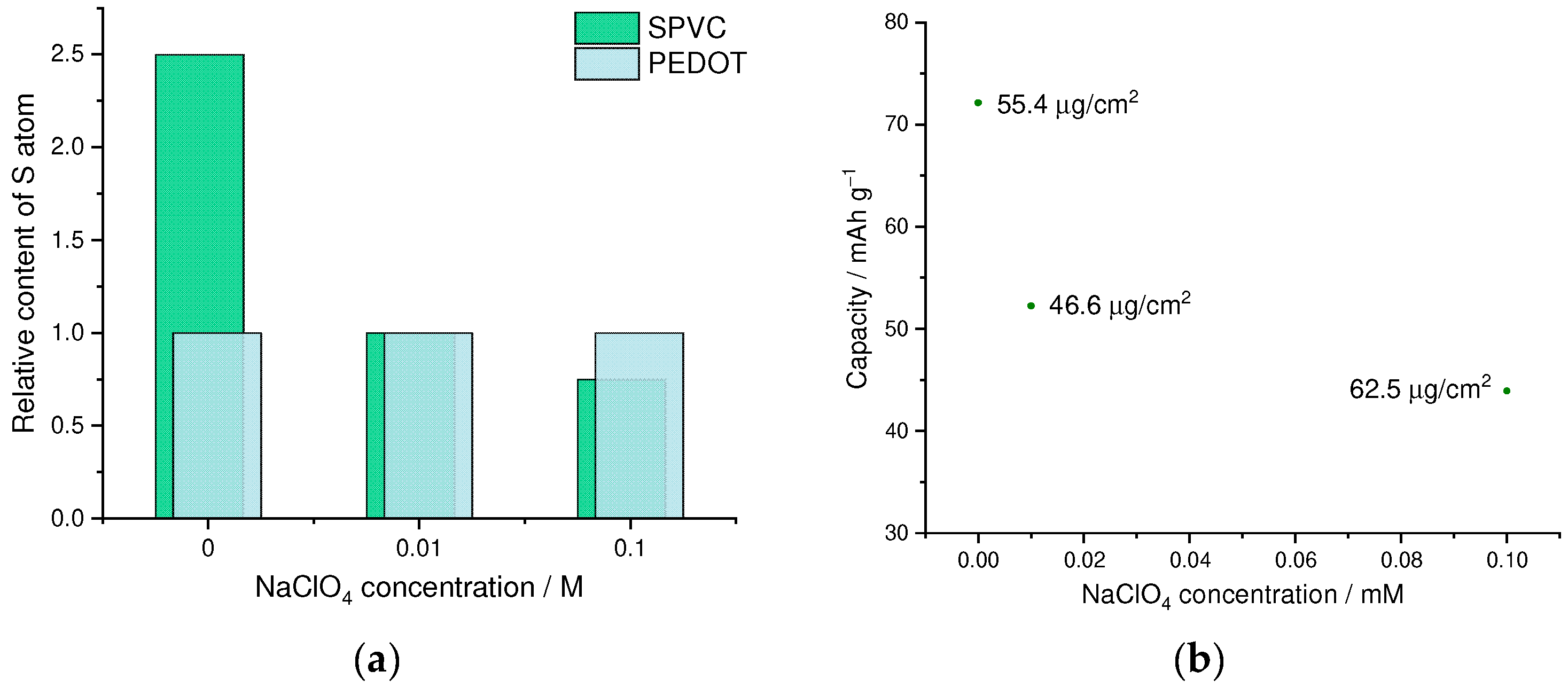
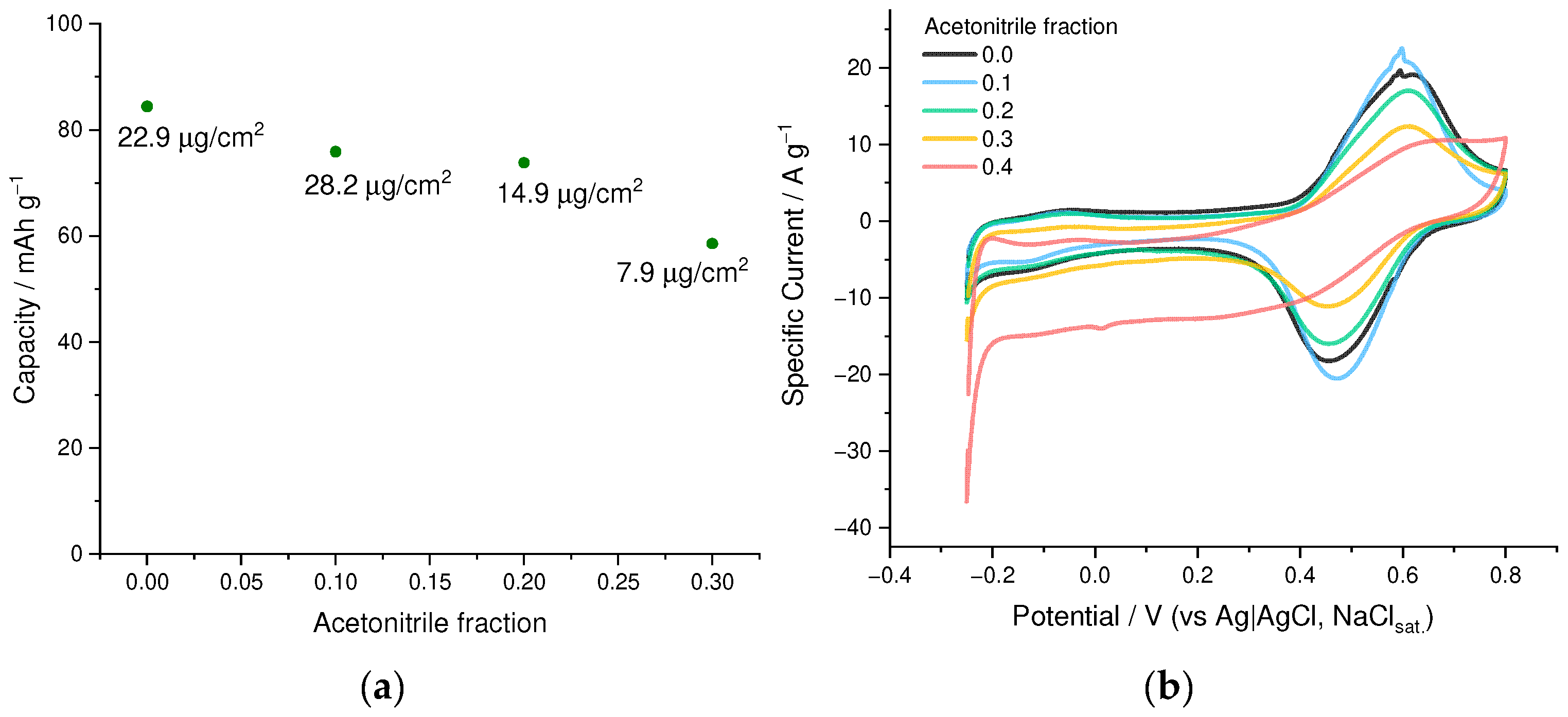
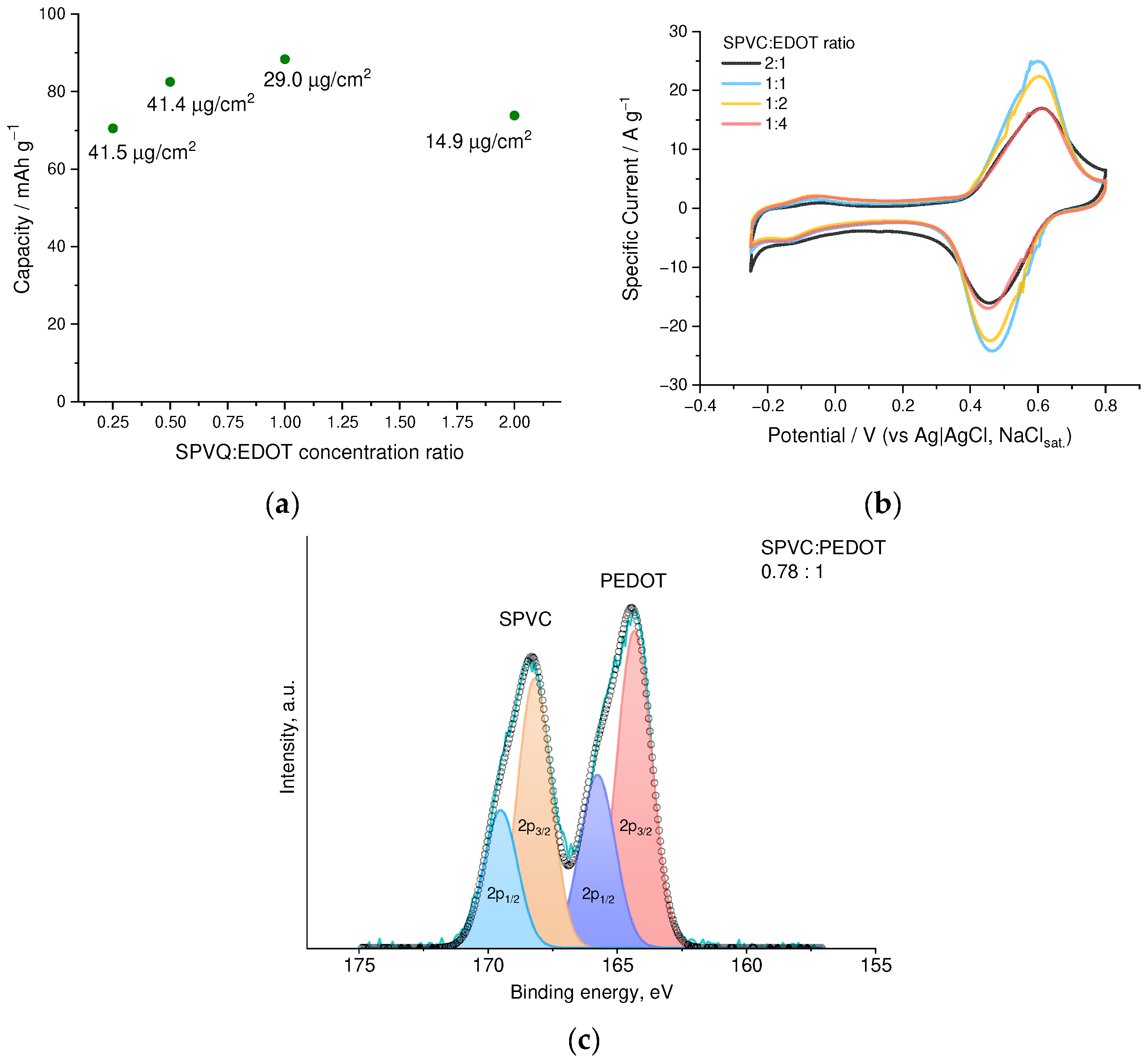
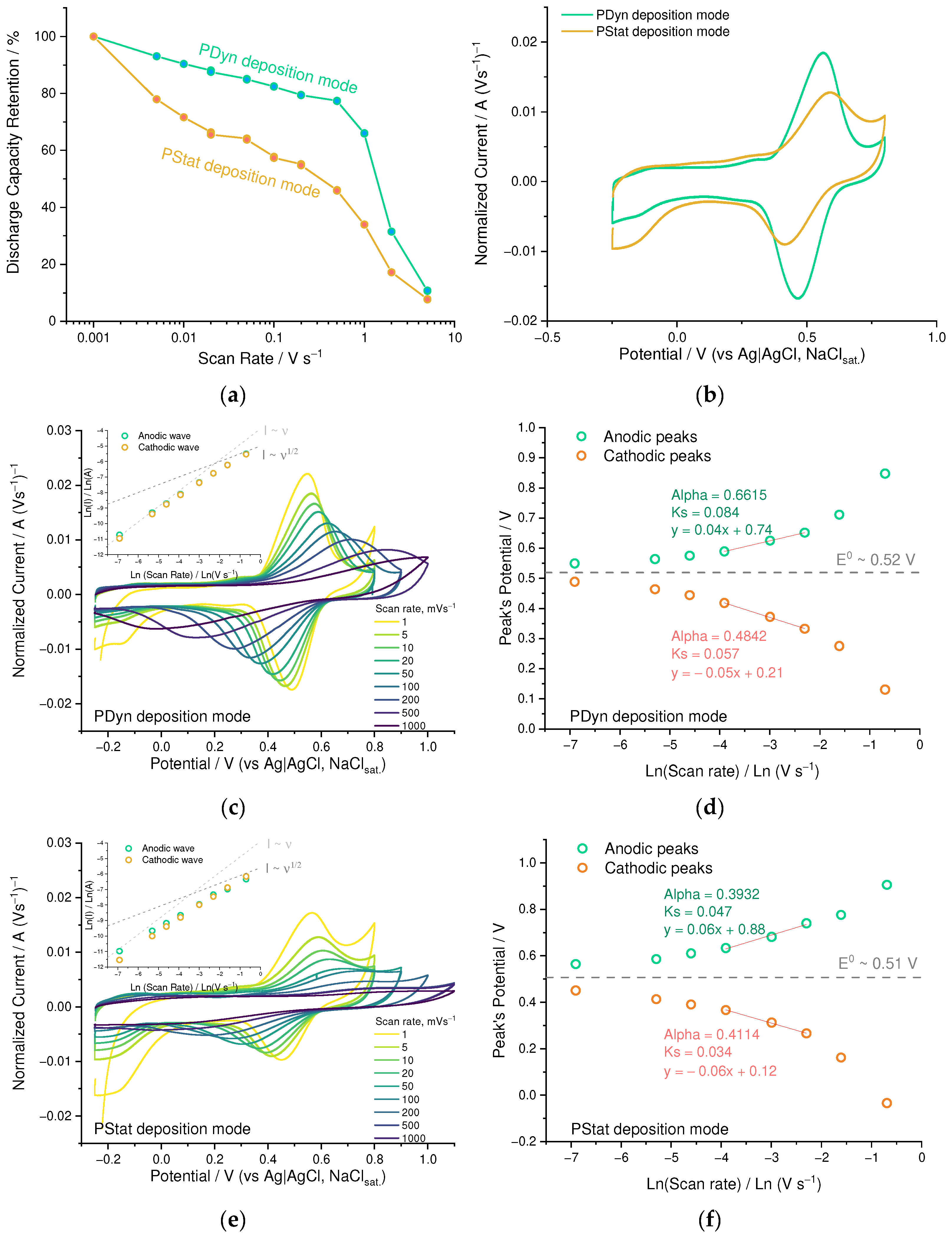
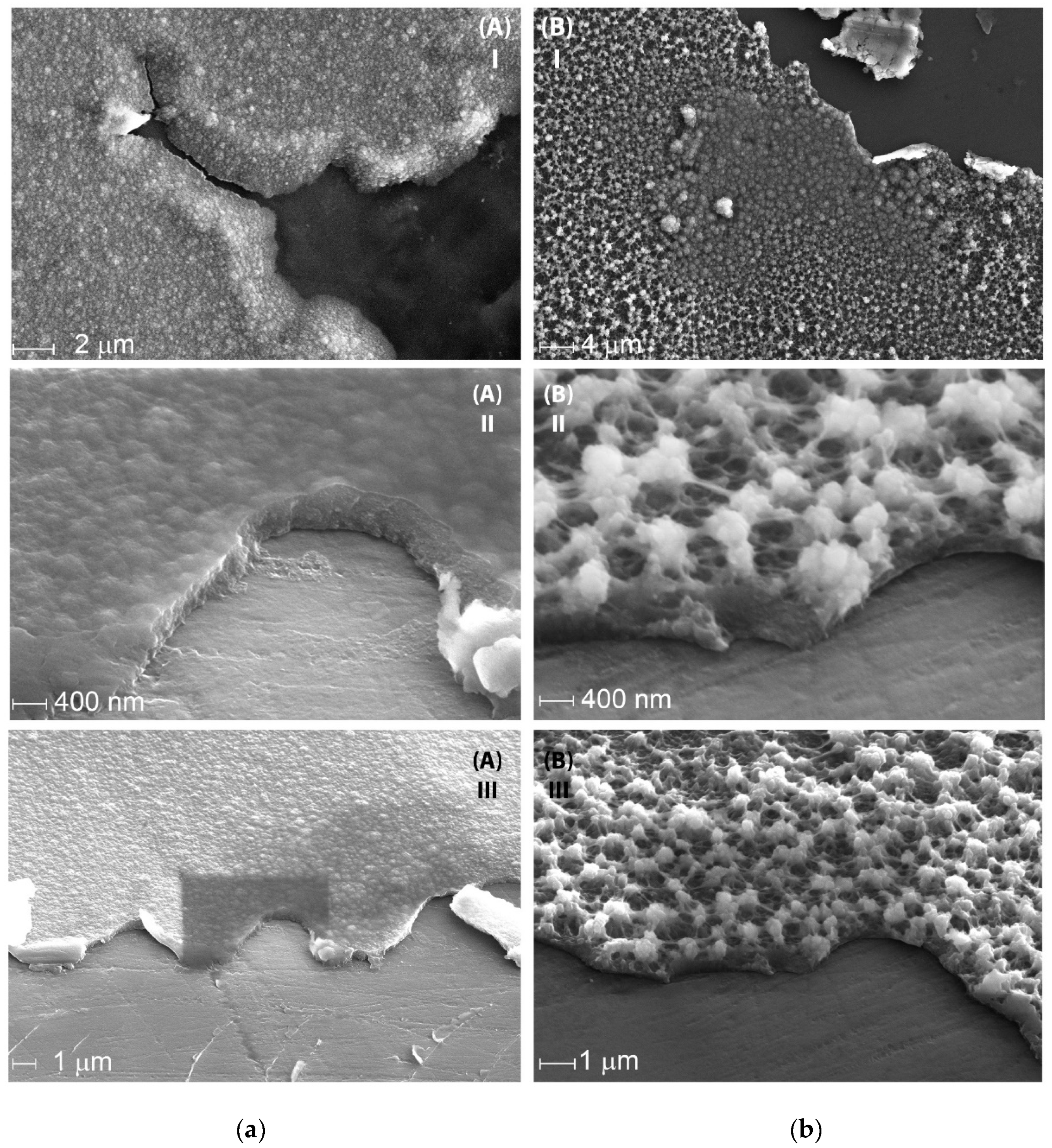
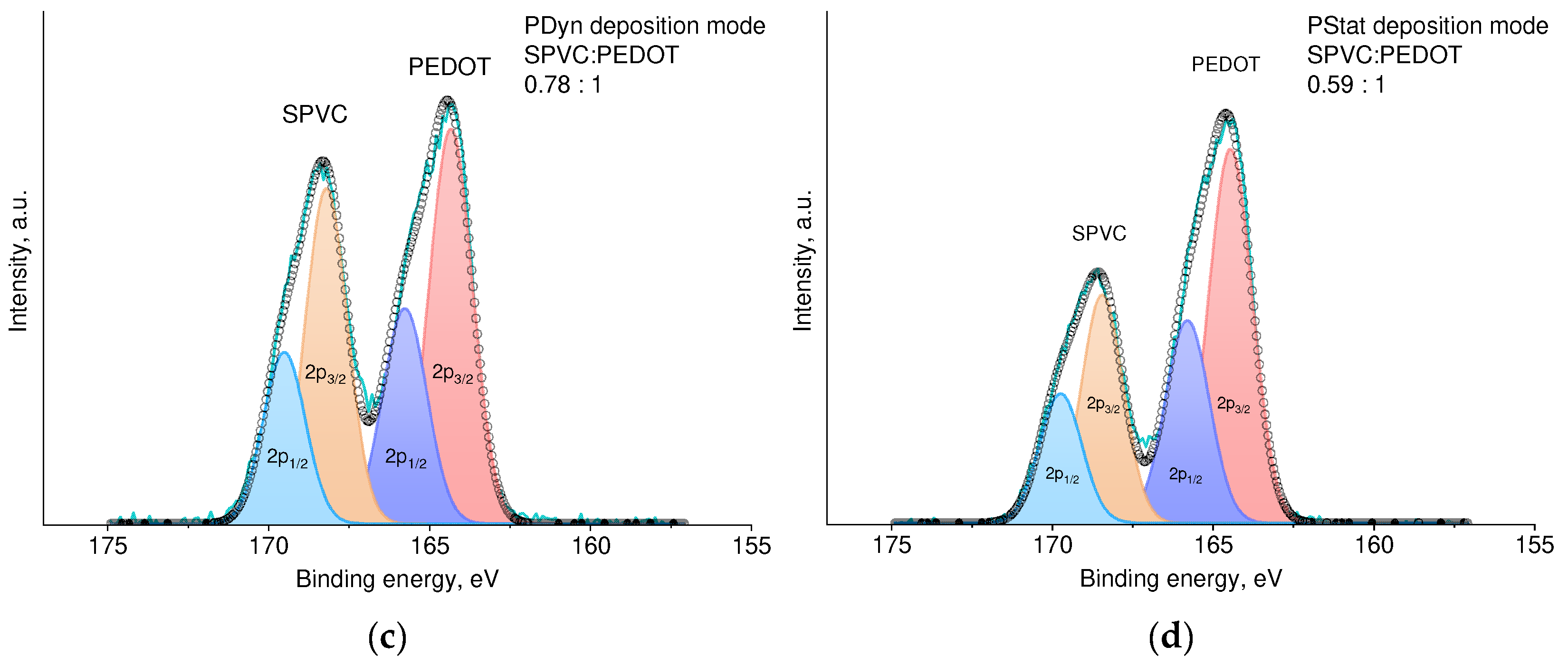
3.1. Optimization of the Electrochemical Deposition
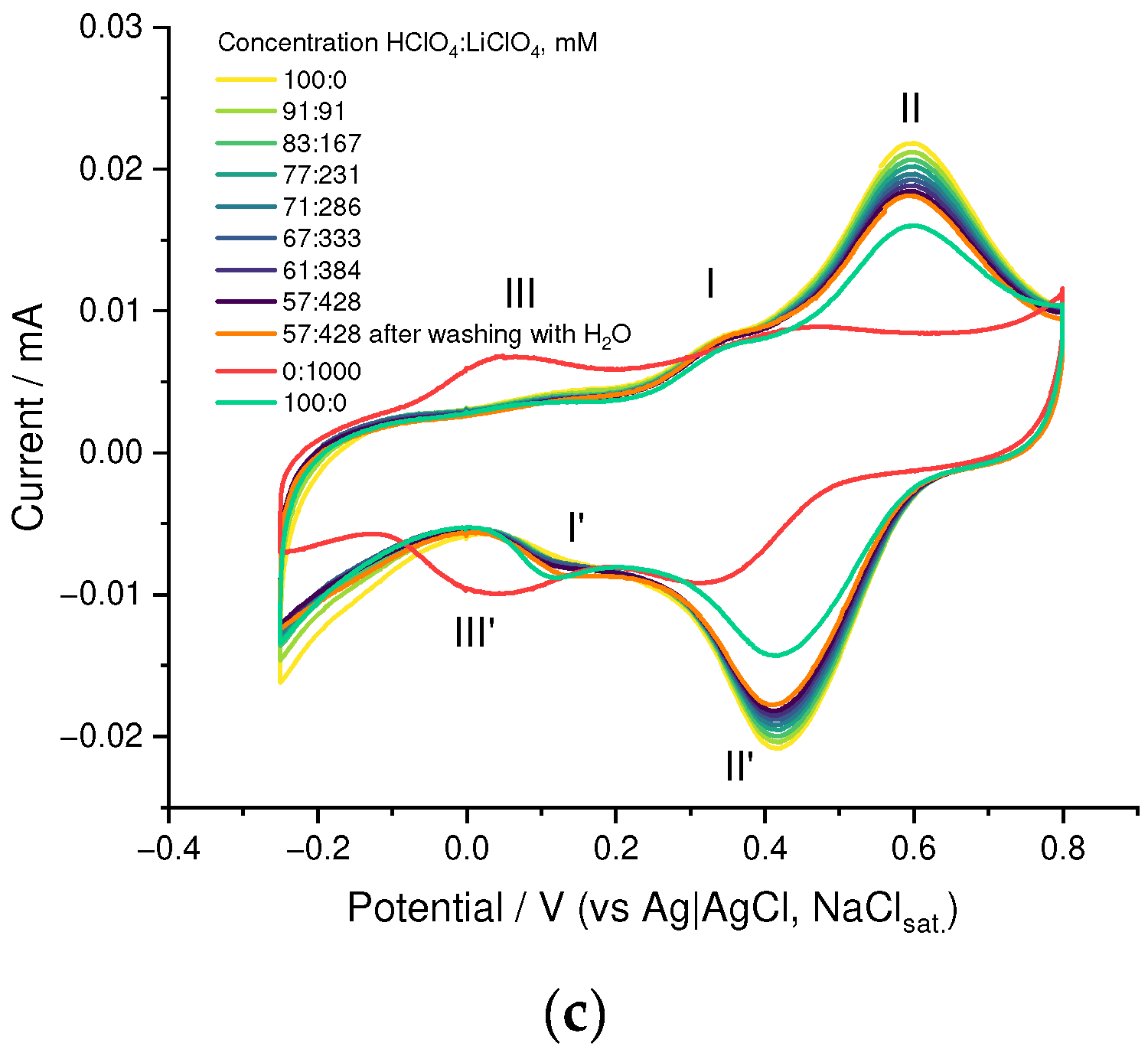
3.2. Ion Transport Studies
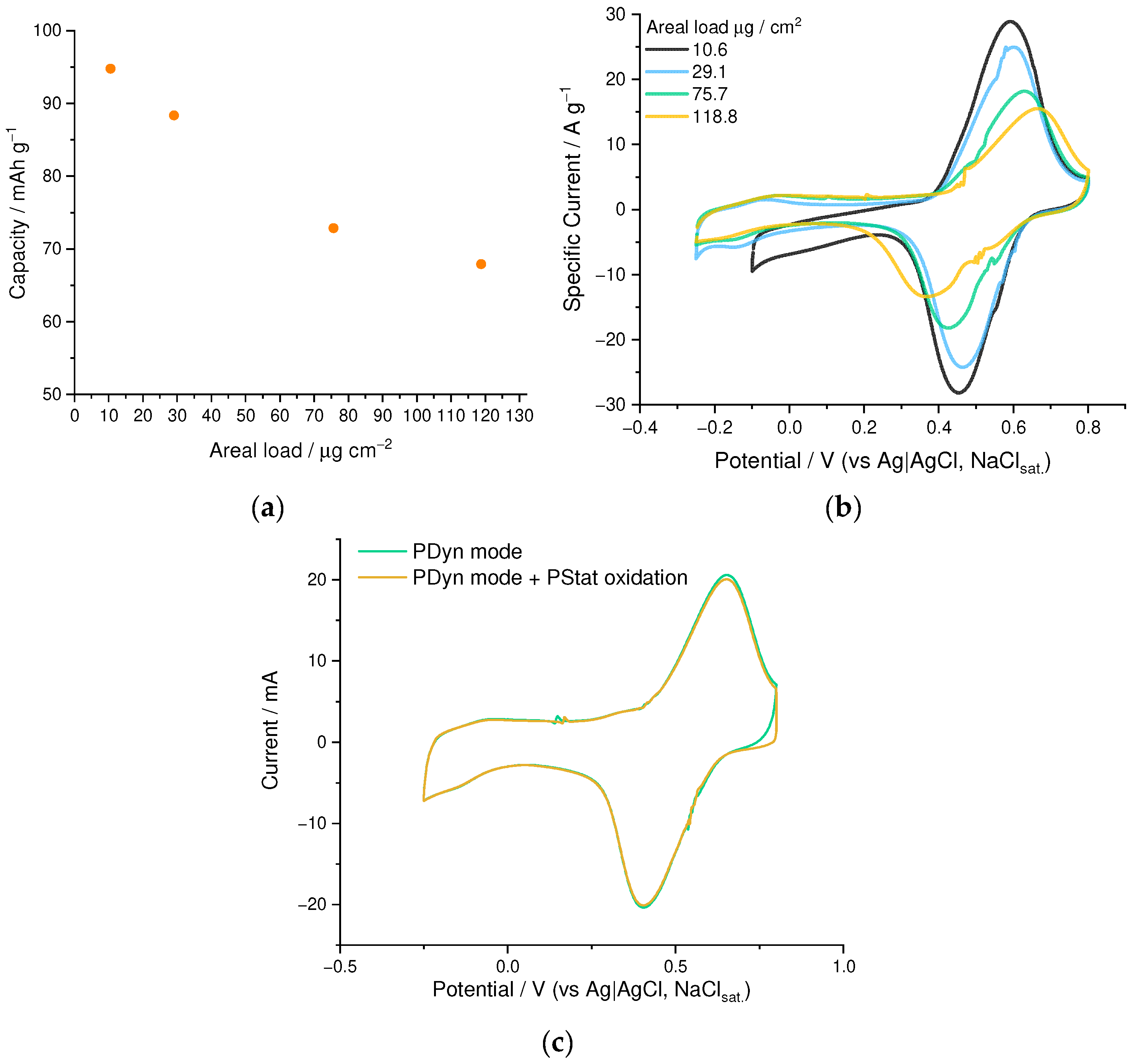
3.3. Thickness Limitation Studies
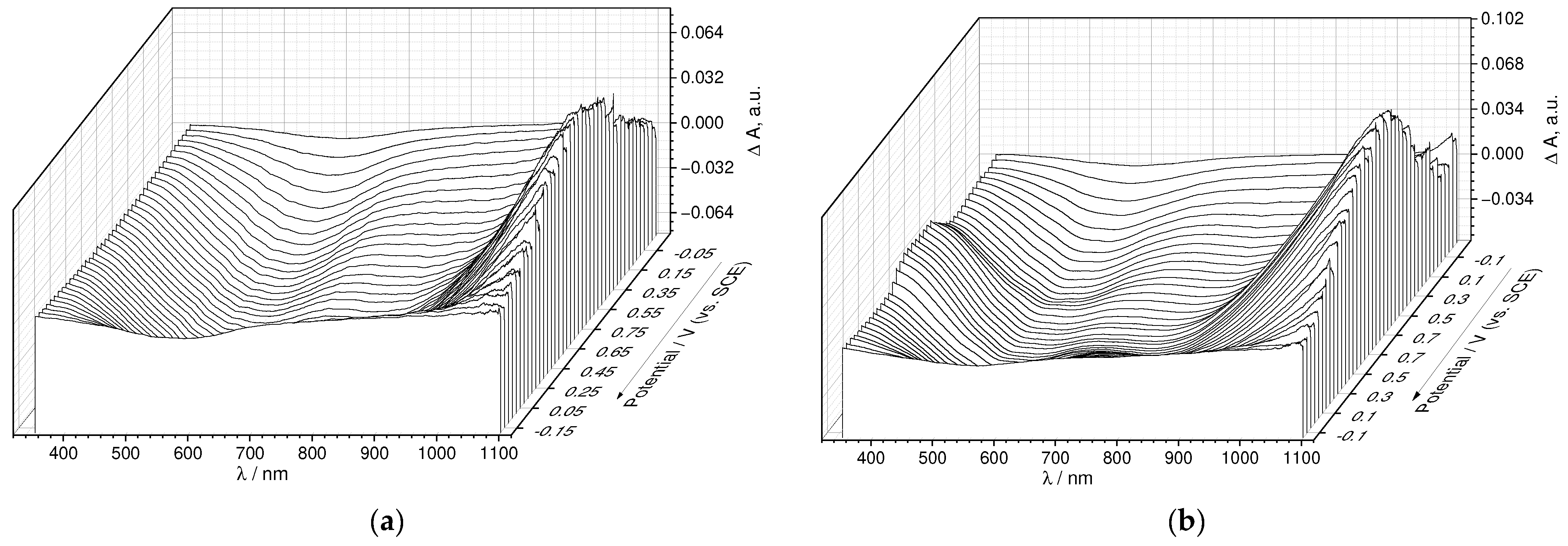
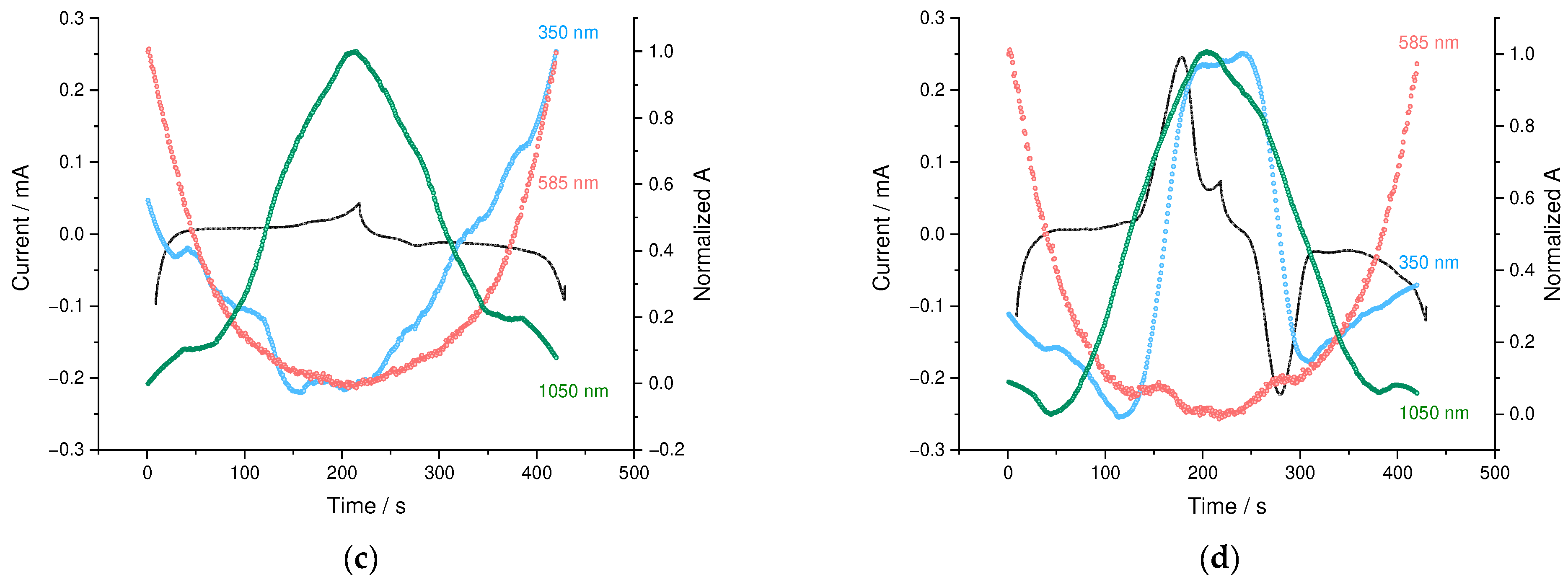
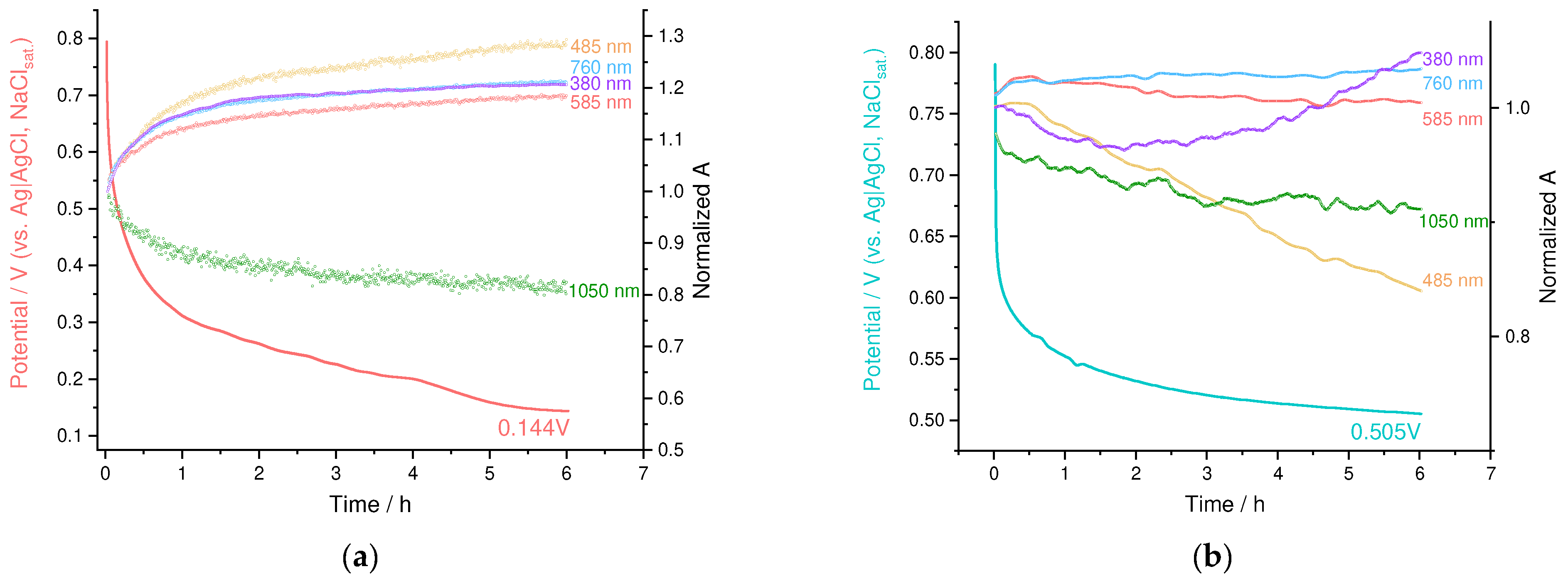
3.4. Operando Spectroelectrochemical Studies
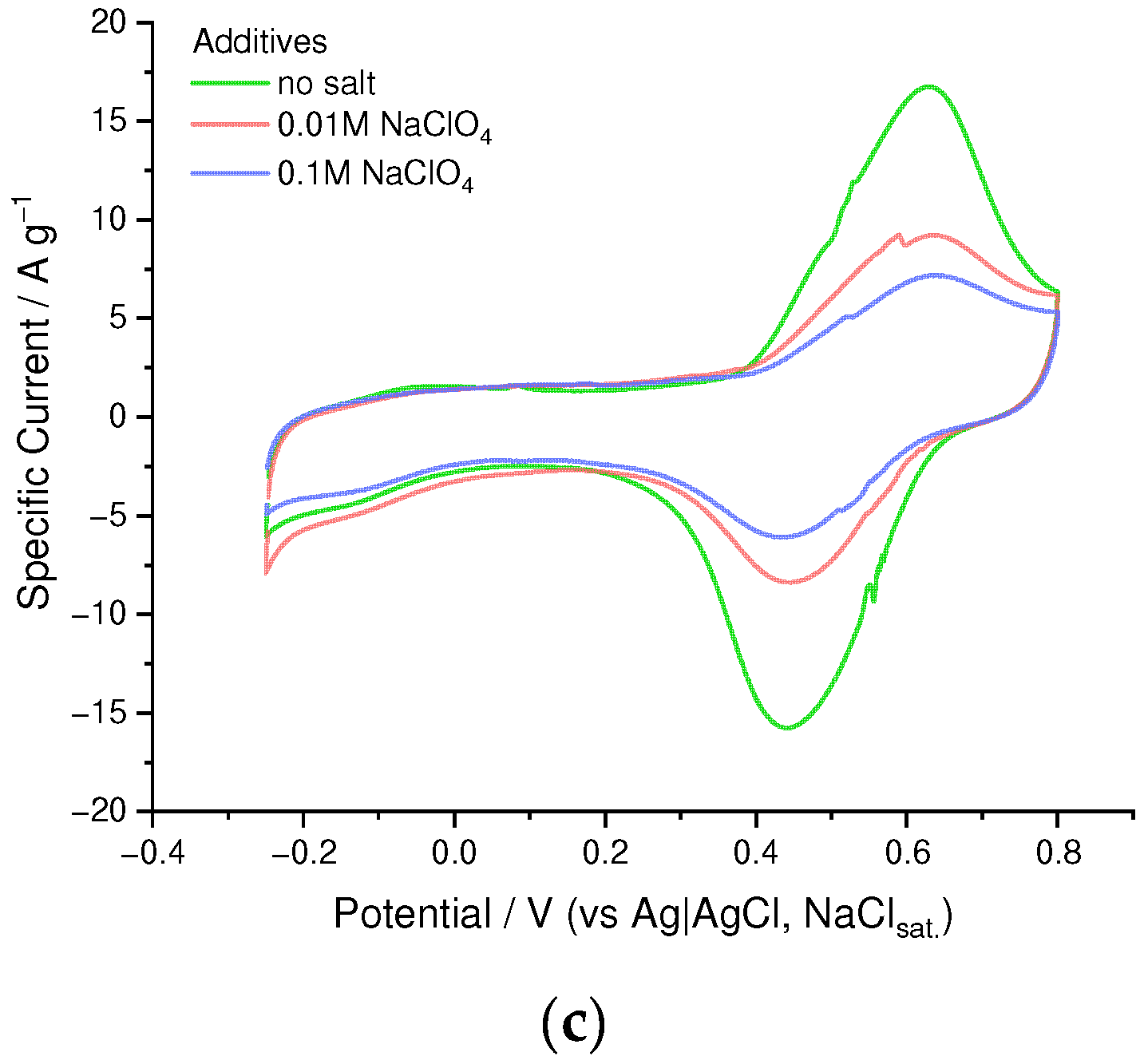
3.5. Electrochemical Stability
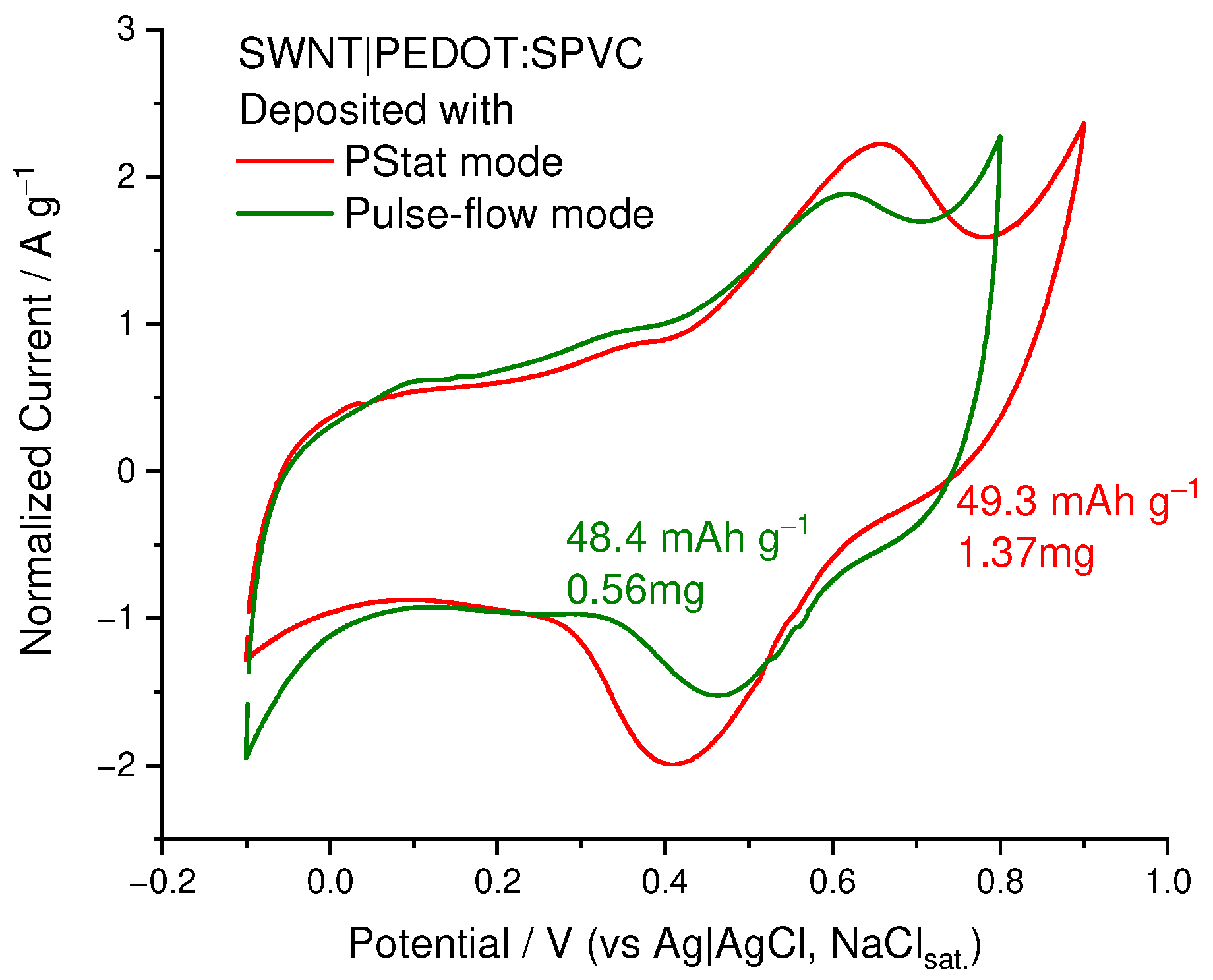
3.6. SWCNT-Supported PEDOT:SPVC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muench, S.; Wild, A.; Friebe, C.; Haupler, B.; Janoschka, T.; Schubert, U.S. Polymer-Based Organic Batteries. Chem. Rev. 2016, 116, 9438–9484. [Google Scholar] [CrossRef] [PubMed]
- Pirnat, K.; Casado, N.; Porcarelli, L.; Ballard, N.; Mecerreyes, D. Synthesis of Redox Polymer Nanoparticles Based on Poly(vinyl catechols) and Their Electroactivity. Macromolecules 2019, 52, 8155–8166. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zhao, W.; Li, H.; Xu, Q. Cross-Conjugated Polycatechol Organic Cathode for Aqueous Zinc-Ion Storage. ChemSusChem 2020, 13, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Lukyanov, D.A.; Apraksin, R.V.; Yankin, A.N.; Vlasov, P.S.; Levin, O.V.; Tolstopjatova, E.G.; Kondratiev, V.V. Synthesis and electrochemical properties of poly(3,4-dihydroxystyrene) and its composites with conducting polymers. Synth. Met. 2019, 256, 116151. [Google Scholar] [CrossRef]
- Patil, N.; Aqil, A.; Ouhib, F.; Admassie, S.; Inganäs, O.; Jérôme, C.; Detrembleur, C. Bioinspired Redox-Active Catechol-Bearing Polymers as Ultrarobust Organic Cathodes for Lithium Storage. Adv. Mater. 2017, 29, 1703373. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, Z.J.; Wang, H.G.; Bao, D.; Meng, F.L.; Zhang, X.B. A Biodegradable Polydopamine-Derived Electrode Material for High-Capacity and Long-Life Lithium-Ion and Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2016, 55, 10662–10666. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Lee, J.; Kim, D.W.; Yoo, C.Y.; Park, S.H.; Yoo, J.J.; Kim, S.; Kim, B.; Cho, W.K.; Yoon, H. Mussel-inspired surface functionalization of porous carbon nanosheets using polydopamine and Fe3+/tannic acid layers for high-performance electrochemical capacitors. J. Mater. Chem. A 2017, 5, 25368–25377. [Google Scholar] [CrossRef]
- Liu, T.; Lee, B.; Kim, B.G.; Lee, M.J.; Park, J.; Lee, S.W. In Situ Polymerization of Dopamine on Graphene Framework for Charge Storage Applications. Small 2018, 14, 1801236. [Google Scholar] [CrossRef]
- Yue, X.; Liu, H.; Liu, P. Polymer grafted on carbon nanotubes as a flexible cathode for aqueous zinc ion batteries. Chem. Commun. 2019, 55, 1647–1650. [Google Scholar] [CrossRef]
- Vereshchagin, A.A.; Vlasov, P.S.; Konev, A.S.; Yang, P.; Grechishnikova, G.A.; Levin, O.V. Novel highly conductive cathode material based on stable-radical organic framework and polymerized nickel complex for electrochemical energy storage devices. Electrochim. Acta 2019, 295, 1075–1084. [Google Scholar] [CrossRef]
- Vereshchagin, A.A.; Lukyanov, D.A.; Kulikov, I.R.; Panjwani, N.A.; Alekseeva, E.A.; Behrends, J.; Levin, O.V. The Fast and the Capacious: A [Ni(Salen)]-TEMPO Redox-Conducting Polymer for Organic Batteries. Batter. Supercaps 2020, 4, 336–346. [Google Scholar] [CrossRef]
- Sterby, M.; Emanuelsson, R.; Huang, X.; Gogoll, A.; Strømme, M.; Sjödin, M. Characterization of PEDOT-Quinone Conducting Redox Polymers for Water Based Secondary Batteries. Electrochim. Acta 2017, 235, 356–364. [Google Scholar] [CrossRef]
- Groenendaal, L.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and Its Derivatives: Past, Present, and Future. Adv. Mater. 2000, 12, 481–494. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Ashraf, S.; Wallace, G.G. Polypyrrole doped with redox-active poly(2-methoxyaniline-5-sulfonic acid) for lithium secondary batteries. RSC Adv. 2013, 3, 5447. [Google Scholar] [CrossRef] [Green Version]
- Patil, N.; Aqil, M.; Aqil, A.; Ouhib, F.; Marcilla, R.; Minoia, A.; Lazzaroni, R.; Jérôme, C.; Detrembleur, C. Integration of Redox-Active Catechol Pendants into Poly(ionic liquid) for the Design of High-Performance Lithium-Ion Battery Cathodes. Chem. Mater. 2018, 30, 5831–5835. [Google Scholar] [CrossRef]
- Son, E.J.; Kim, J.H.; Kim, K.; Park, C.B. Quinone and its derivatives for energy harvesting and storage materials. J. Mater. Chem. A 2016, 4, 11179–11202. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, R.; Nan, J.; Shu, D.; Qiu, Y.; Chou, S.L. Quinone Electrode Materials for Rechargeable Lithium/Sodium Ion Batteries. Adv. Energy Mater. 2017, 7, 1700278. [Google Scholar] [CrossRef]
- Nagaraju, D.H.; Rebis, T.; Gabrielsson, R.; Elfwing, A.; Milczarek, G.; Inganäs, O. Charge storage capacity of renewable biopolymer/conjugated polymer interpenetrating networks enhanced by electroactive dopants. Adv. Energy Mater. 2014, 4, 1300443. [Google Scholar] [CrossRef]
- Ajjan, F.N.; Casado, N.; Rȩbiś, T.; Elfwing, A.; Solin, N.; Mecerreyes, D.; Inganäs, O. High performance PEDOT/lignin biopolymer composites for electrochemical supercapacitors. J. Mater. Chem. A 2016, 4, 1838–1847. [Google Scholar] [CrossRef] [Green Version]
- Che, C.; Vagin, M.; Ail, U.; Gueskine, V.; Phopase, J.; Brooke, R.; Gabrielsson, R.; Jonsson, M.P.; Mak, W.C.; Berggren, M.; et al. Twinning Lignosulfonate with a Conducting Polymer via Counter-Ion Exchange for Large-Scale Electrical Storage. Adv. Sustain. Syst. 2019, 3, 1900039. [Google Scholar] [CrossRef]
- Chhin, D.; Padilla-Sampson, L.; Malenfant, J.; Rigaut, V.; Nazemi, A.; Schougaard, S.B. Conducting Polymers Doped with Bifunctional Copolymers for Improved Organic Batteries. ACS Appl. Energy Mater. 2019, 2, 7781–7790. [Google Scholar] [CrossRef]
- Lukyanov, D.A.; Vereshchagin, A.A.; Soloviova, A.V.; Grigorova, O.V.; Vlasov, P.S.; Levin, O.V. Sulfonated Polycatechol Immobilized in a Conductive Polymer for Enhanced Energy Storage. ACS Appl. Energy Mater. 2021, 4, 5070–5078. [Google Scholar] [CrossRef]
- Apraksin, R.V.; Volosatova, Y.A.; Volkov, A.I.; Vlasov, P.S.; Lukyanov, D.A.; Kulikov, I.R.; Eliseeva, S.N.; Levin, O.V. Electrochemical synthesis and characterization of poly [Ni(CH3Osalen)] with immobilized poly(styrenesulfonate) anion dopants. Electrochim. Acta 2021, 368, 137637. [Google Scholar] [CrossRef]
- Beletskii, E.V.; Volosatova, Y.A.; Eliseeva, S.N.; Levin, O.V. The Effect of Electrode Potential on the Conductivity of Polymer Complexes of Nickel with Salen Ligands. Russ. J. Electrochem. 2019, 55, 339–345. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Für. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Topart, P.A.; Noel, M.A.M. High-Frequency Impedance Analysis of Quartz Crystal Microbalances. 2. Electrochemical Deposition and Redox Switching of Conducting Polymers. Anal. Chem. 2002, 66, 2926–2934. [Google Scholar] [CrossRef]
- Fan, B.; Mei, X.; Ouyang, J. Significant Conductivity Enhancement of Conductive Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) Films by Adding Anionic Surfactants into Polymer Solution. Macromolecules 2008, 41, 5971–5973. [Google Scholar] [CrossRef]
- Elschner, A.; Kirchmeyer, S.; Lovenich, W.; Merker, U.; Reuter, K. PEDOT. Principles and Applications of an Intrinsically Conductive Polymer; CRC Press: Boca Raton, FL, USA, 2010; p. 377. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Kaim, W.; Fiedler, J. Spectroelectrochemistry: The best of two worlds. Chem. Soc. Rev. 2009, 38, 3373–3382. [Google Scholar] [CrossRef]
- Gribkova, O.; Iakobson, O.; Nekrasov, A.; Cabanova, V.; Tverskoy, V.; Tameev, A.; Vannikov, A. Ultraviolet-Visible-Near Infrared and Raman spectroelectrochemistry of poly(3,4-ethylenedioxythiophene) complexes with sulfonated polyelectrolytes. The role of inter- and intra-molecular interactions in polyelectrolyte. Electrochim. Acta 2016, 222, 409–420. [Google Scholar] [CrossRef]
- Victor, T.A.N.Y.; Alexy, A.; Usov, A.A.; Goncharova, A.N.; Leskin, N.A.; Messineva, N.V.; Trusova, M.V. Efimkina, UV/Visible Spectra. In NIST Chemistry WebBook, NIST Standard Reference Database; NIST: Gaithersburg, MD, USA, 2022; Volume 69. [Google Scholar]
- Patil, N.; Mavrandonakis, A.; Jérôme, C.; Detrembleur, C.; Casado, N.; Mecerreyes, D.; Palma, J.; Marcilla, R. High-performance all-organic aqueous batteries based on a poly(imide) anode and poly(catechol) cathode. J. Mater. Chem. A 2021, 9, 505–514. [Google Scholar] [CrossRef]
- Karushev, M.P.; Timonov, A.M. Adsorption-electrochemical modification of nanoporous carbon materials by nickel complexes with Schiff bases. Russ. J. Appl. Chem. 2012, 85, 914–920. [Google Scholar] [CrossRef]
- Essafi, W.; Spiteri, M.-N.; Williams, C.; Boue, F. Hydrophobic Polyelectrolytes in Better Polar Solvent. Structure and Chain Conformation As Seen by SAXS and SANS. Macromolecules 2009, 42, 9568–9580. [Google Scholar] [CrossRef] [Green Version]
- Wijeratne, K.; Ail, U.; Brooke, R.; Vagin, M.; Liu, X.; Fahlman, M.; Crispin, X. Bulk electronic transport impacts on electron transfer at conducting polymer electrode-electrolyte interfaces. Proc. Natl. Acad. Sci. USA 2018, 115, 11899–11904. [Google Scholar] [CrossRef] [PubMed] [Green Version]



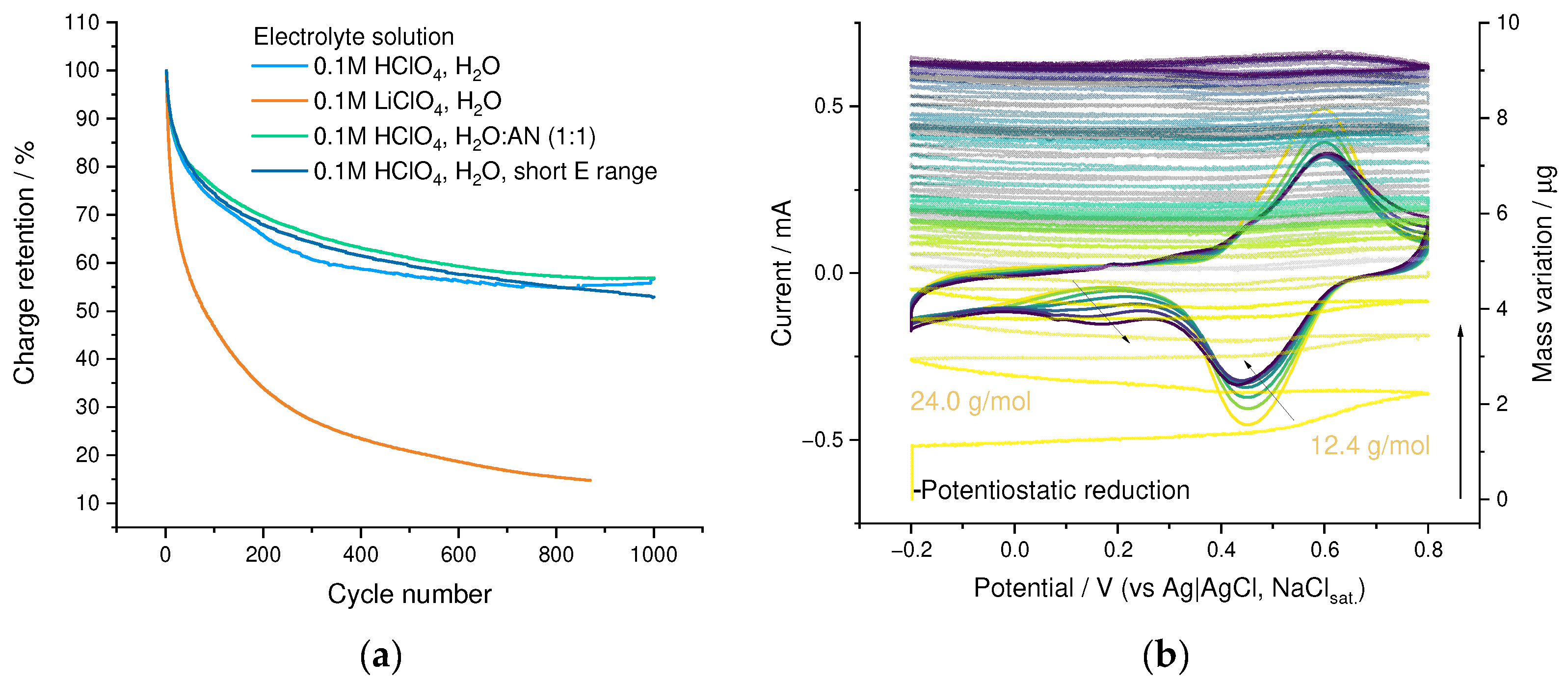
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vereshchagin, A.A.; Potapenkov, V.V.; Vlasov, P.S.; Lukyanov, D.A.; Levin, O.V. Optimization of Sulfonated Polycatechol:PEDOT Energy Storage Performance by the Morphology Control. Nanomaterials 2022, 12, 1917. https://doi.org/10.3390/nano12111917
Vereshchagin AA, Potapenkov VV, Vlasov PS, Lukyanov DA, Levin OV. Optimization of Sulfonated Polycatechol:PEDOT Energy Storage Performance by the Morphology Control. Nanomaterials. 2022; 12(11):1917. https://doi.org/10.3390/nano12111917
Chicago/Turabian StyleVereshchagin, Anatoliy A., Vasiliy V. Potapenkov, Petr S. Vlasov, Daniil A. Lukyanov, and Oleg V. Levin. 2022. "Optimization of Sulfonated Polycatechol:PEDOT Energy Storage Performance by the Morphology Control" Nanomaterials 12, no. 11: 1917. https://doi.org/10.3390/nano12111917
APA StyleVereshchagin, A. A., Potapenkov, V. V., Vlasov, P. S., Lukyanov, D. A., & Levin, O. V. (2022). Optimization of Sulfonated Polycatechol:PEDOT Energy Storage Performance by the Morphology Control. Nanomaterials, 12(11), 1917. https://doi.org/10.3390/nano12111917







