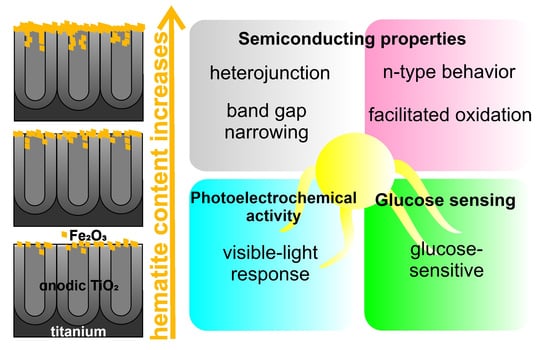
Photoelectrochemical Performance of Nanotubular Fe2O3–TiO2 Electrodes under Solar Radiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Nanostructured Fe2O3–TiO2
2.2. Characterization of Fe2O3–TiO2 Materials
2.3. Electrochemical and Photoelectrochemical Measurements
3. Results and Discussion
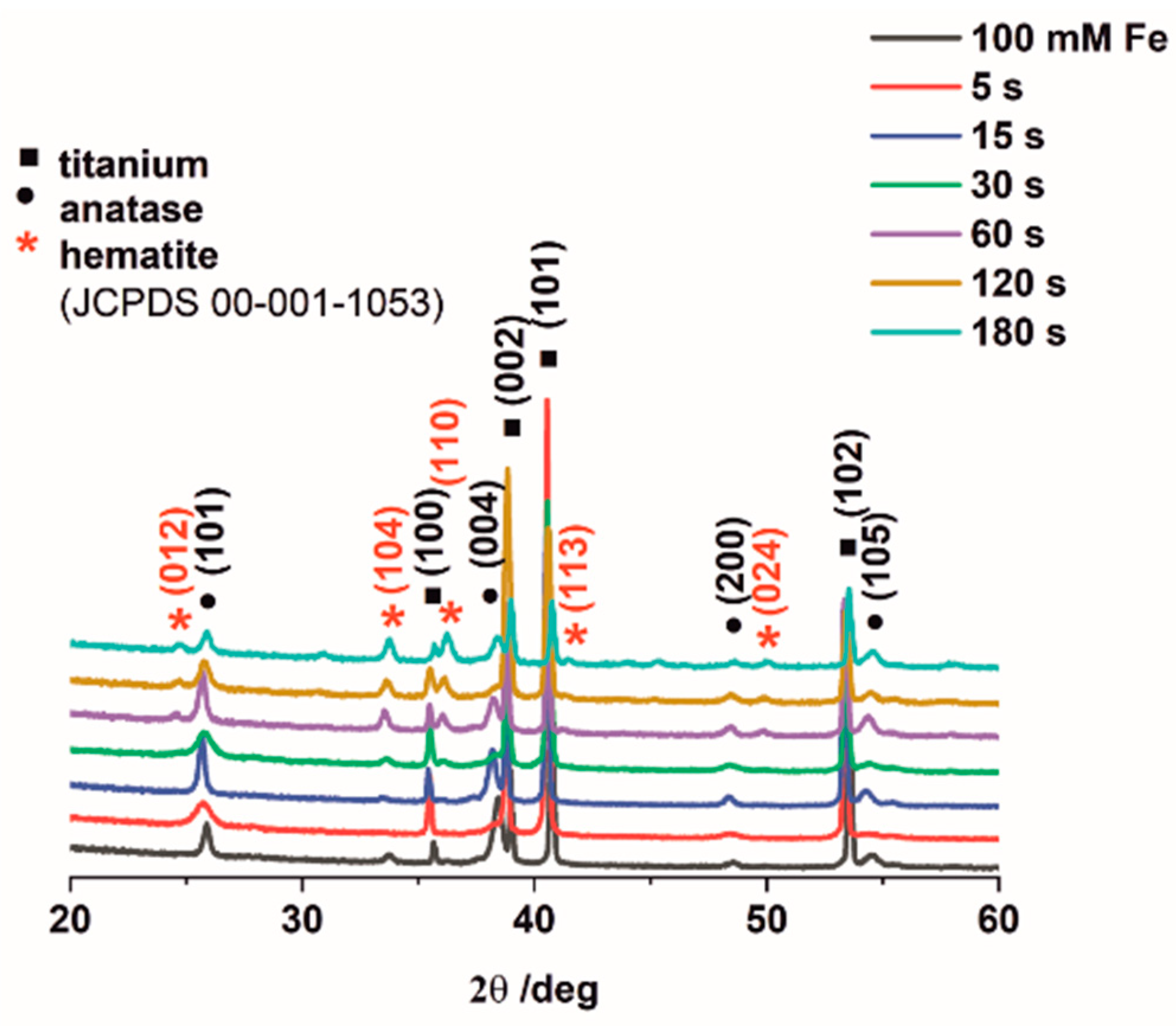
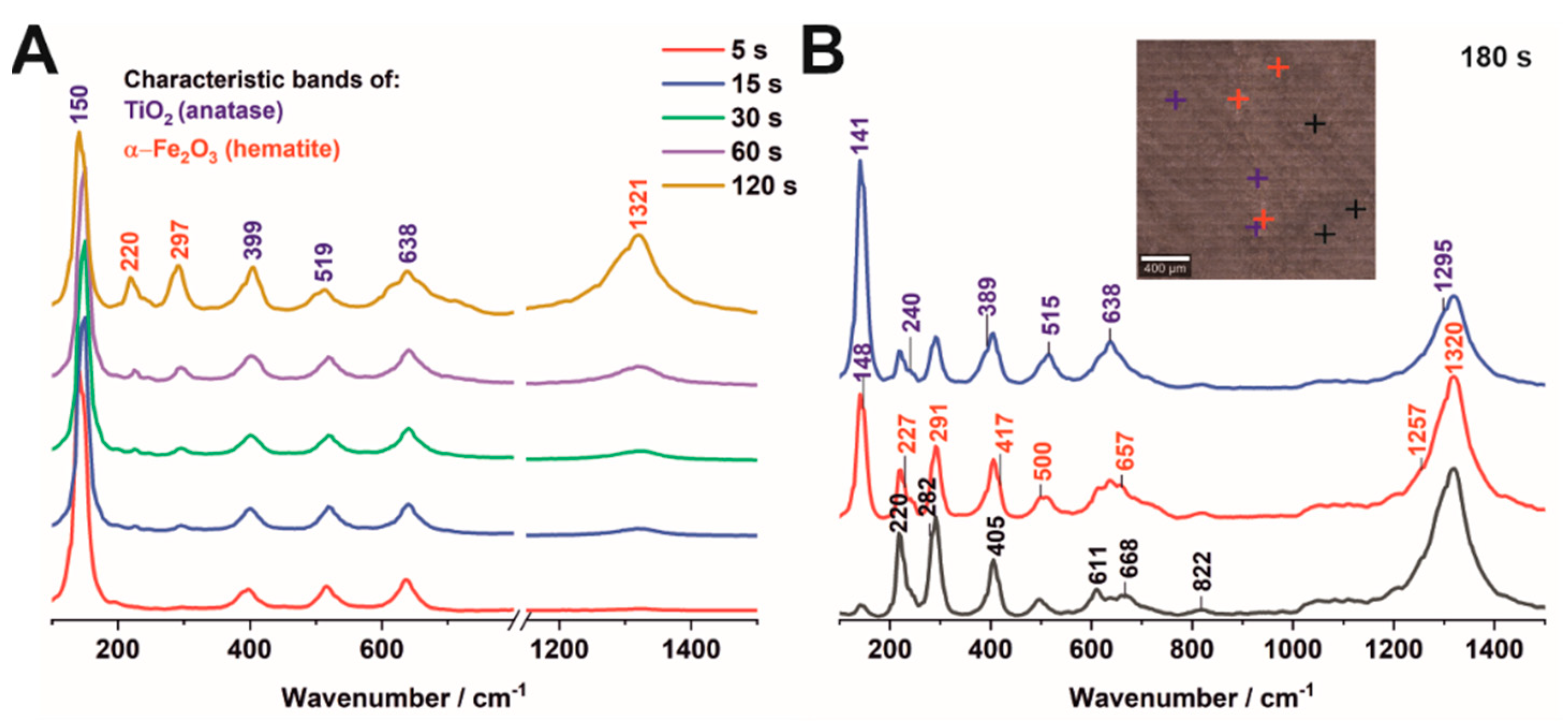
3.1. Morphology and Crystallinity of Fe2O3–TiO2 Nanostructured Materials Synthesized by Electrodeposition
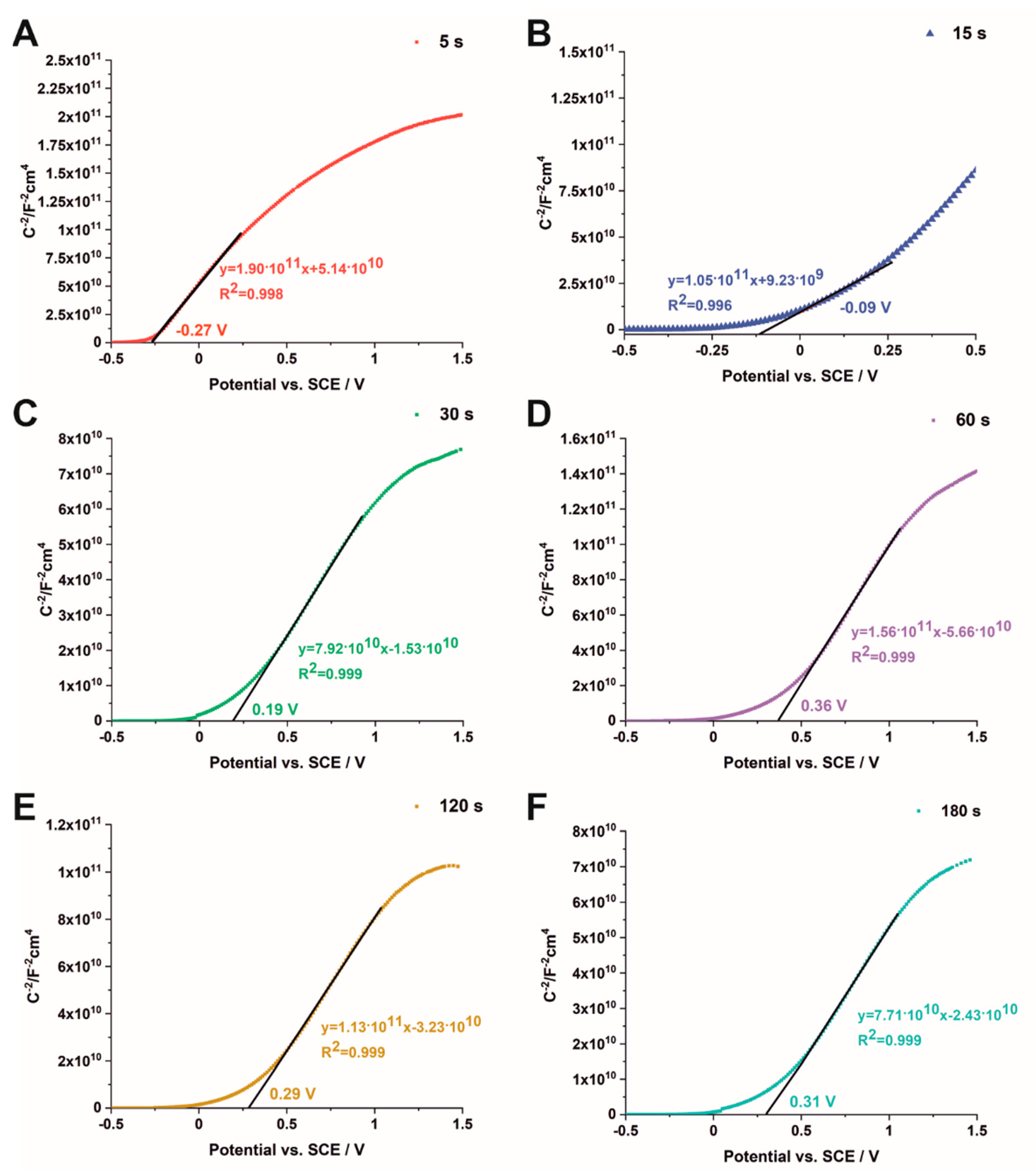
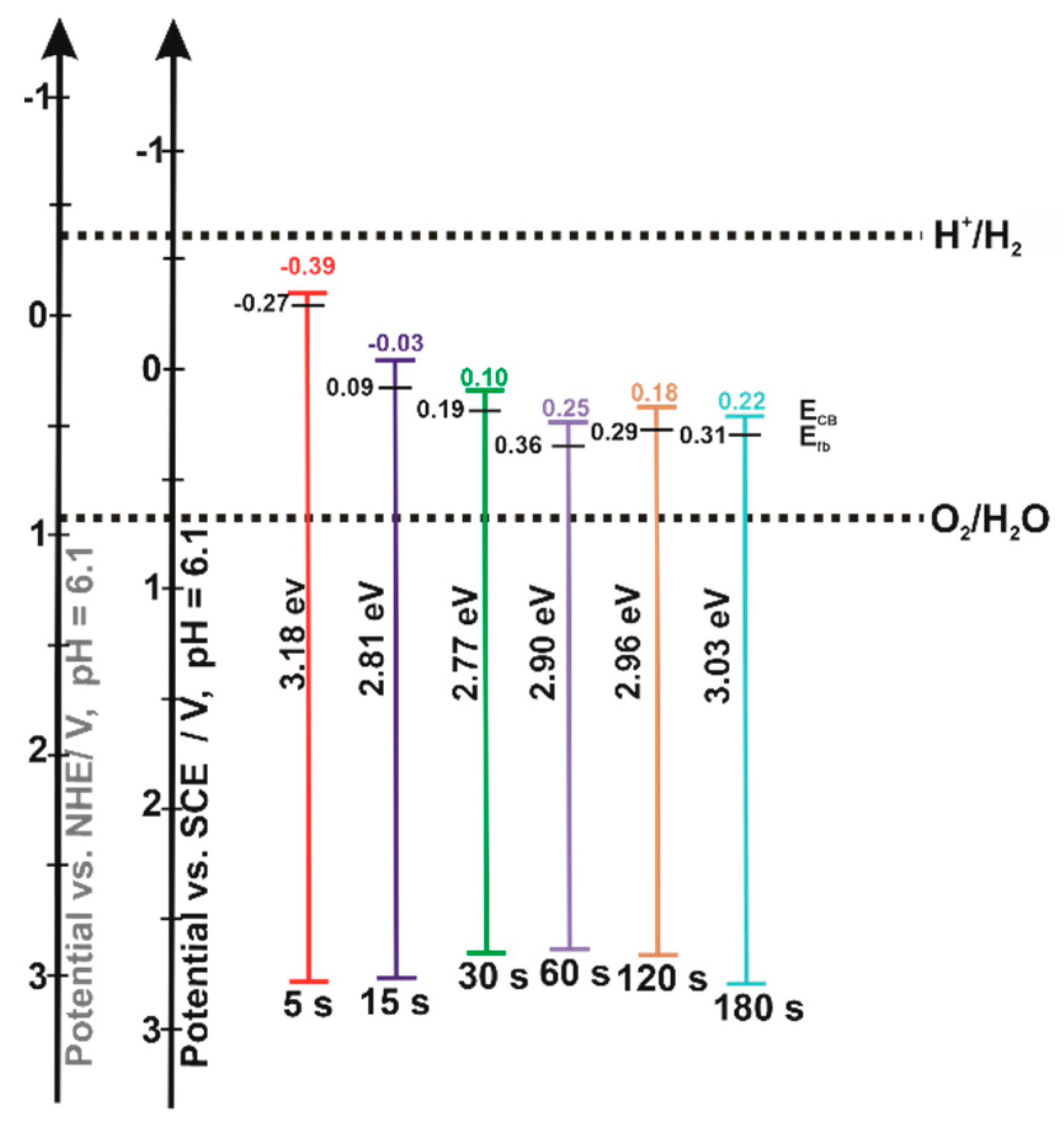
3.2. Semiconducting Properties
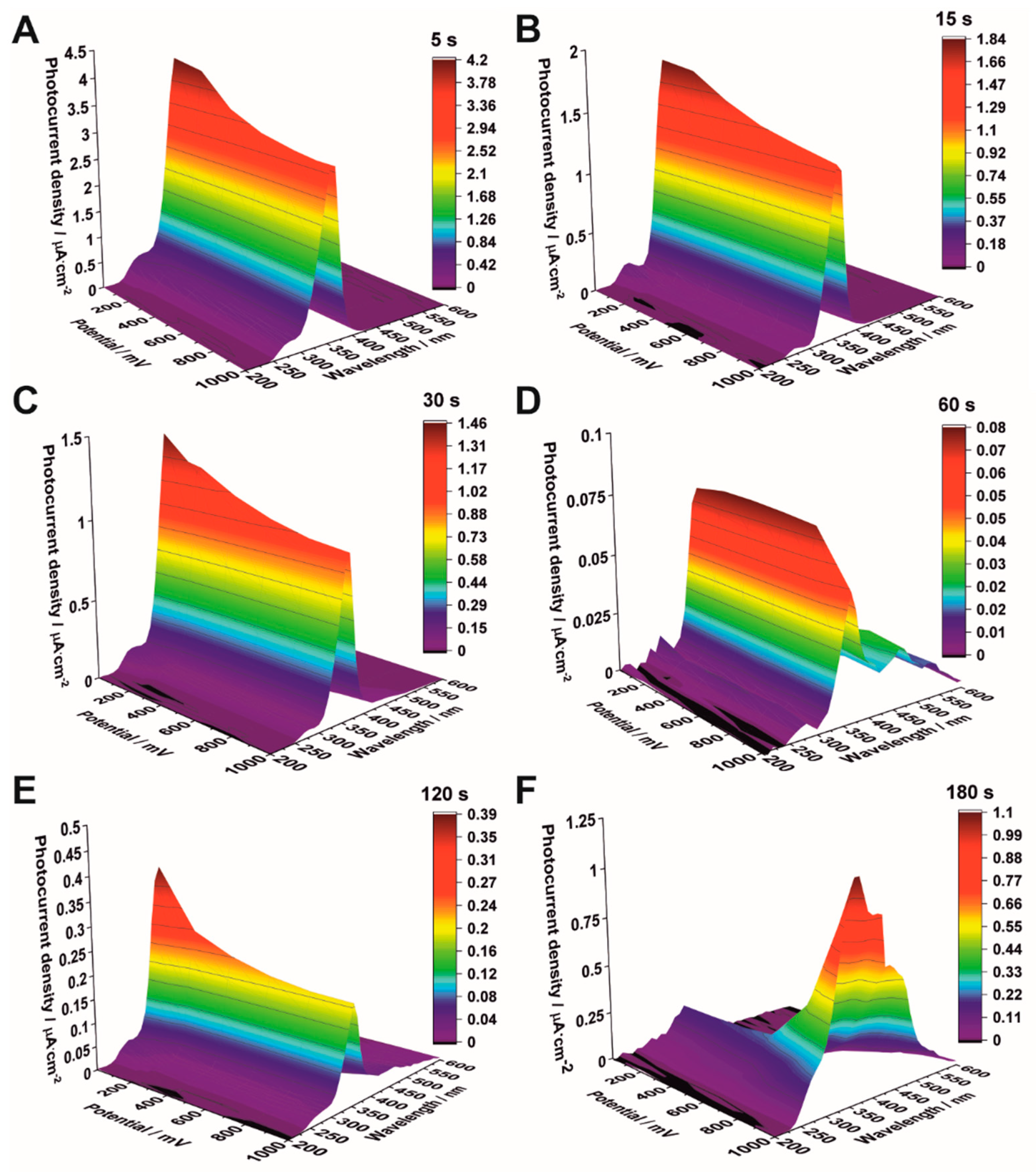
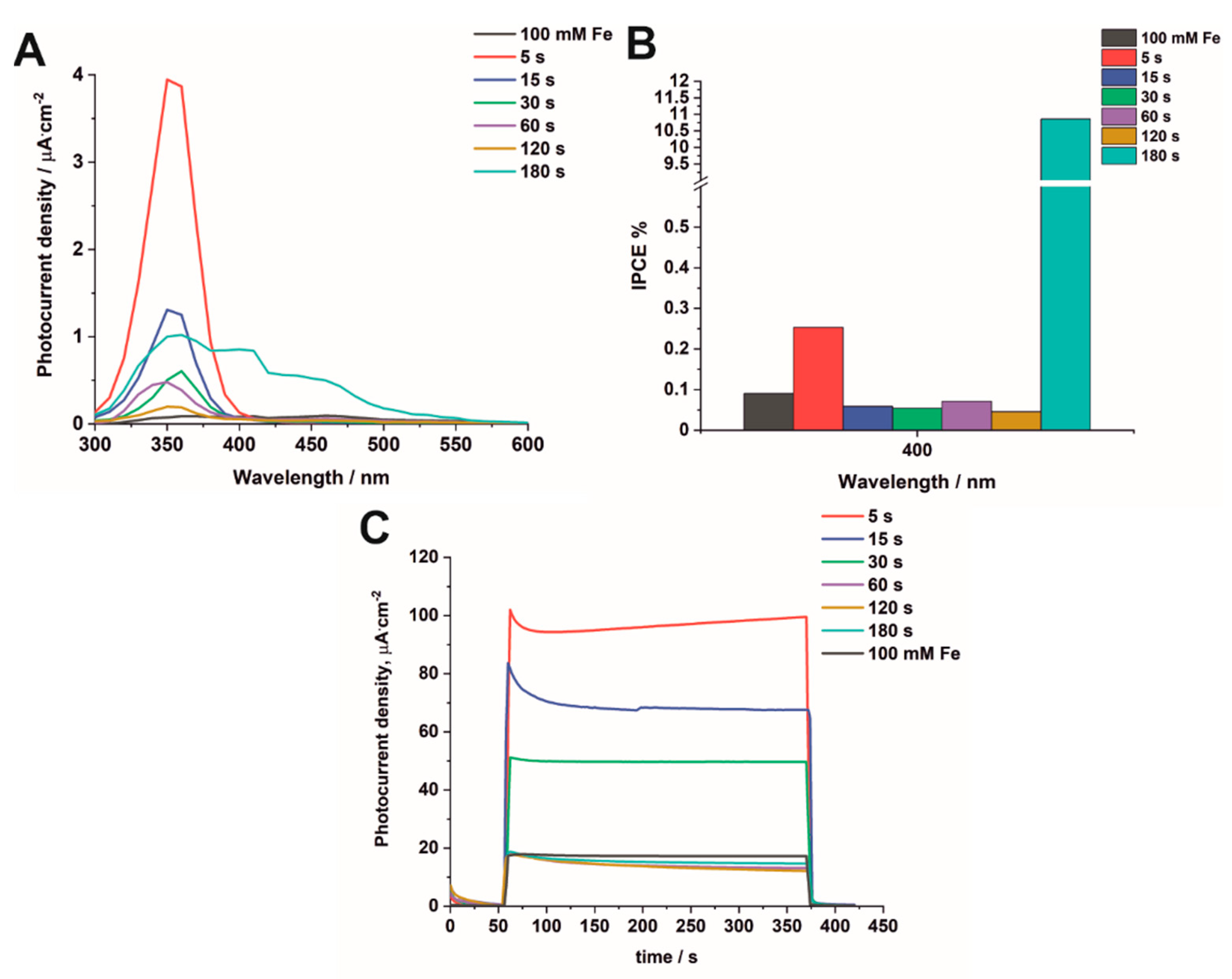
3.3. Photoelectrochemical Measurements
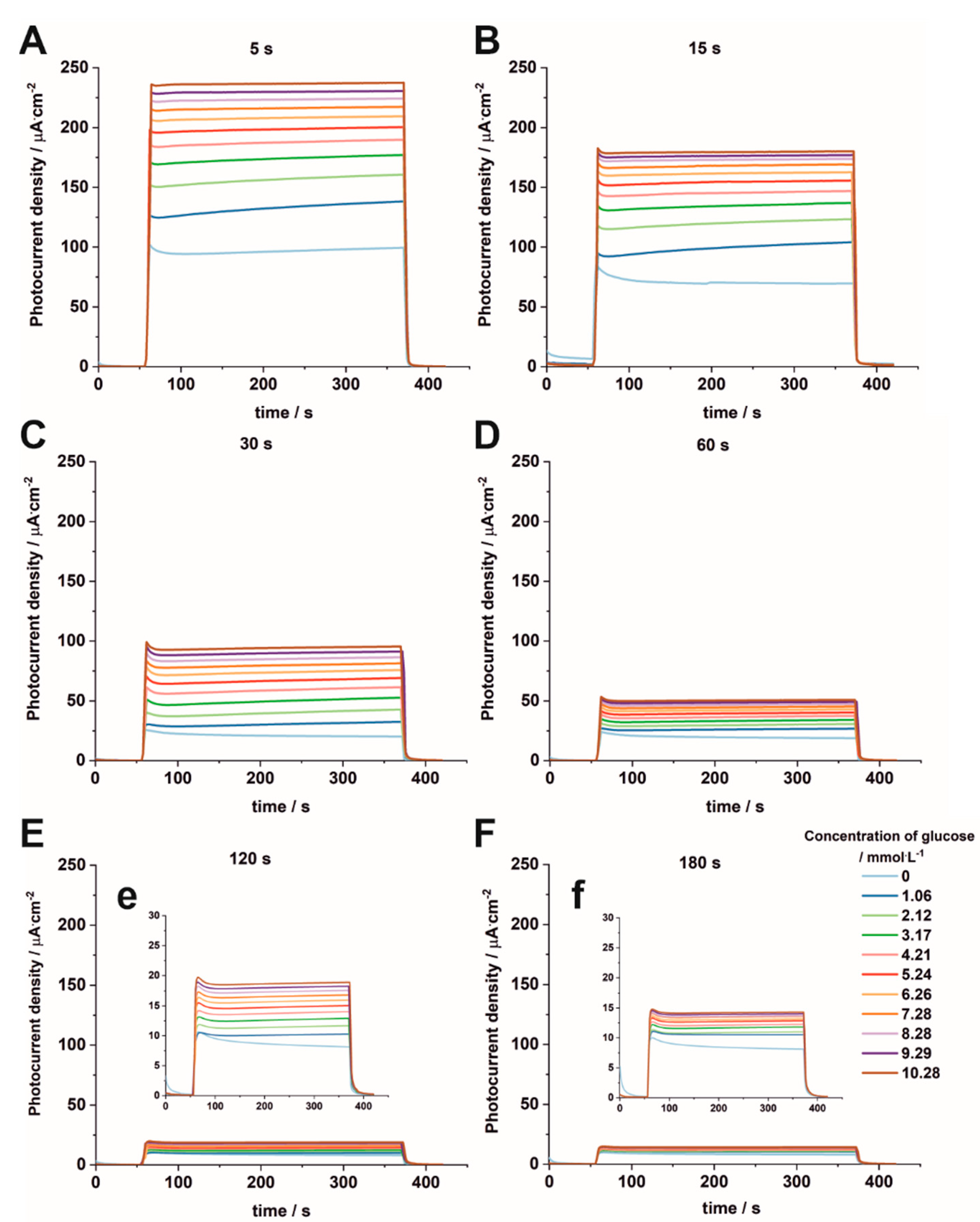
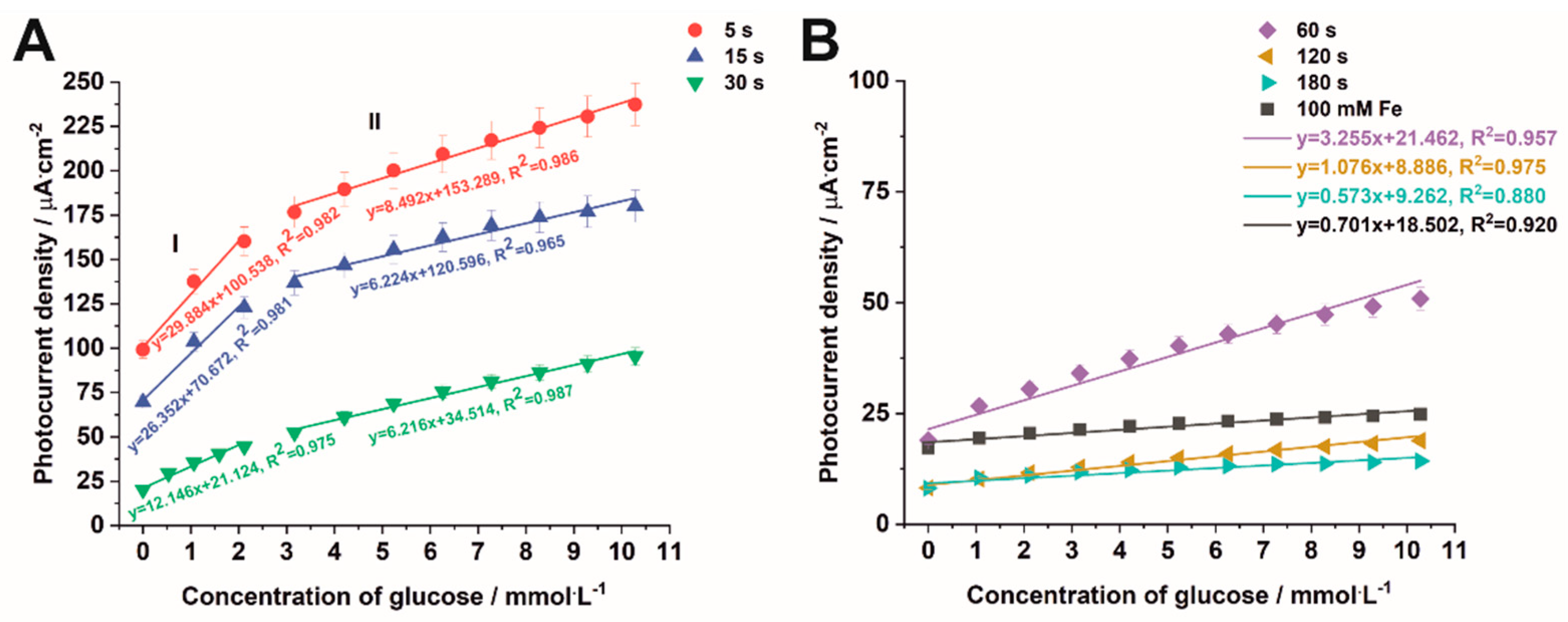
3.4. Non-Enzymatic Glucose Sensing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Chernomordik, B.D.; Russell, H.B.; Cvelbar, U.; Jasinski, J.B.; Kumar, V.; Deutsch, T.; Sunkara, M.K. Photoelectrochemical activity of as-grown, α-Fe2O3 nanowire array electrodes for water splitting. Nanotechnology 2012, 23, 194009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.X.; Yang, Z.Z.; Wang, J.J.; Xie, Z.Y.; Cui, M.Y.; Tian, C.M.; Du, Y.G.; Zhang, K.H.L. Epitaxial growth and band alignment of p-NiO/n-Fe2O3 heterojunction on Al2O3(0001). Appl. Surf. Sci. 2019, 464, 488–493. [Google Scholar] [CrossRef]
- Zhang, D.; Dong, S. Challenges in band alignment between semiconducting materials: A case of rutile and anatase TiO2. Prog. Nat. Sci. Mater. 2019, 29, 277–284. [Google Scholar] [CrossRef]
- Kodan, N.; Agarwal, K.; Mehta, B.R. All-oxide α-Fe2O3/H:TiO2 heterojunction photoanode: A platform for stable and enhanced photoelectrochemical performance through favorable band edge alignment. J. Phys. Chem. C 2019, 123, 3326–3335. [Google Scholar] [CrossRef]
- Danilov, F.I.; Tsurkan, A.V.; Vasil’eva, E.A.; Protsenko, V.S. Electrocatalytic activity of composite Fe/TiO2 electrodeposits for hydrogen evolution reaction in alkaline solutions. Int. J. Hydrogen Energy 2016, 41, 7363–7372. [Google Scholar] [CrossRef]
- Park, H.; Ayala, P.; Deshusses, M.A.; Mulchandani, A.; Choi, H.; Myung, N.V. Electrodeposition of maghemite (γ-Fe2O3) nanoparticles. Chem. Eng. J. 2008, 139, 208–212. [Google Scholar] [CrossRef]
- Kimmich, D.; Taffa, D.H.; Dosche, C.; Wark, M.; Wittstock, G. Photoactivity andscattering behavior of anodically and cathodically deposited hematite photoanodes—A comparison by scanning photoelectrochemical microscopy. Electrochim. Acta 2016, 202, 224–230. [Google Scholar] [CrossRef]
- Schrebler, R.S.; Altamirano, H.; Grez, P.; Herrera, F.V.; Muñz, E.C.; Ballesteros, L.A.; Córdova, R.A.; Gómez, H.; Dalchiele, E.A. The influence of different electrodeposition E/t programs on the photoelectrochemical properties of α-Fe2O3 thin films. Thin Solid Films 2010, 518, 6844–6852. [Google Scholar] [CrossRef]
- Tamboli, S.H.; Rahman, G.; Joo, O.-S. Influence of potential, deposition time and annealing temperature on photoelectrochemical properties of electrodeposited iron oxide thin films. J. Alloys Compd. 2012, 520, 232–237. [Google Scholar] [CrossRef]
- Mohapatra, S.K.; Banerjee, S.; Misra, M. Synthesis of Fe2O3/TiO2 nanorod–nanotube arrays by filling TiO2 nanotubes with Fe. Nanotechnology 2008, 19, 315601. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Q.; Cui, Z.D.; Zhu, S.L.; Yang, X.J. Formation and characterization of iron oxide nanoparticles loaded on self-organized TiO2 nanotubes. Electrochim. Acta 2010, 55, 5245–5252. [Google Scholar] [CrossRef]
- Jeon, T.H.; Choi, W.; Park, H. Photoelectrochemical and photocatalytic behaviors of hematite-decorated titania nanotube arrays: Energy level mismatch versus surface specific reactivity. J. Phys. Chem. C 2011, 115, 7134–7142. [Google Scholar] [CrossRef]
- Cong, Y.-Q.; Li, Z.; Wang, Q.; Zhang, Y.; Xu, Q.; Fu, F.-X. Enhanced photoeletrocatalytic activity of TiO2 nanotube arrays modified with simple transition metal oxides (Fe2O3, CuO, NiO). Acta Phys. Chim. Sin. 2012, 28, 1489–1496. [Google Scholar]
- Cong, Y.; Li, Z.; Zhang, Y.; Wang, Q.; Xu, Q. Synthesis of α-Fe2O3/TiO2 nanotube arrays for photoelectro-Fenton degradation of phenol. Chem. Eng. J. 2012, 191, 356–363. [Google Scholar] [CrossRef]
- Ren, K.; Gan, Y.X.; Young, T.J.; Moutassem, Z.M.; Zhang, L. Photoelectrochemical responses of doped and coated titanium dioxide composite nanotube anodes. Compos. B Eng. 2013, 52, 292–302. [Google Scholar] [CrossRef]
- Tsui, L.-k.; Zangari, G. The influence of morphology of electrodeposited Cu2O and Fe2O3 on the conversion efficiency of TiO2 nanotube photoelectrochemical solar cells. Electrochim. Acta 2013, 100, 220–225. [Google Scholar] [CrossRef]
- Chin, L.Y.; Mustaffa, N.; Ayal, A.K.; Kanakaraju, D.; Pei, L.Y. Structural characterization and visible light-induced photoelectrochemical performance of Fe-sensitized TiO2 nanotube arrays prepared via electrodeposition. Malays. J. Chem. 2021, 23, 173–182. [Google Scholar]
- Radecka, M.; Rekas, M.; Trenczek-Zajac, A.; Zakrzewska, K. Importance of the band gap energy and flat band potential for application of modified TiO2 photoanodes in water photolysis. J. Power Sources 2008, 181, 46–55. [Google Scholar] [CrossRef]
- Muñoz, A.G. Semiconducting properties of self-organized TiO2 nanotubes. Electrochim. Acta 2007, 52, 4167–4176. [Google Scholar] [CrossRef]
- Acevedo-Pena, P.; Carrera-Crespo, J.E.; Gonzalez, F.; Gonzalez, I. Effect of heat treatment on the crystal phase composition, semiconducting properties and photoelectrocatalytic color removal efficiency of TiO2 nanotubes arrays. Electrochim. Acta 2014, 140, 564–571. [Google Scholar] [CrossRef]
- Syrek, K.; Sennik-Kubiec, A.; Rodriguez-Lopez, J.; Rutkowska, M.; Żmudzki, P.; Hnida-Gut, K.E.; Grudzień, J.; Chmielarz, L.; Sulka, G.D. Reactive and morphological trends on porous anodic TiO2 substrates obtained at different annealing temperatures. Int. J. Hydrogen Energy 2020, 45, 4376–4389. [Google Scholar] [CrossRef]
- Sulka, G.D.; Kapusta-Kołodziej, J.; Brzózka, A.; Jaskuła, M. Fabrication of nanoporous TiO2 by electrochemical anodization. Electrochim. Acta 2010, 55, 4359–4367. [Google Scholar] [CrossRef]
- Kapusta-Kołodziej, J.; Syrek, K.; Pawlik, A.; Jarosz, M.; Tynkevych, O.; Sulka, G.D. Effects of anodizing potential and temperature on the growth of anodic TiO2 and its photoelectrochemical properties. Appl. Surf. Sci. 2017, 396, 1119–1129. [Google Scholar] [CrossRef]
- Jarosz, M.; Syrek, K.; Kapusta-Kołodziej, J.; Mech, J.; Małek, K.; Hnida, K.; Łojewski, T.; Jaskula, M.; Sulka, G.D. Heat treatment effect on crystalline structure and photoelectrochemical properties of anodic TiO2 nanotube arrays formed in ethylene glycol and glycerol based electrolytes. J. Phys. Chem. C 2015, 119, 24182–24191. [Google Scholar] [CrossRef]
- Syrek, K.; Kapusta-Kołodziej, J.; Jarosz, M.; Sulka, G.D. Effect of electrolyte agitation on anodic titanium dioxide (ATO) growth and its photoelectrochemical properties. Electrochim. Acta 2015, 180, 801–810. [Google Scholar] [CrossRef]
- Sołtys-Mróz, M.; Syrek, K.; Pierzchała, J.; Wiercigroch, E.; Malek, K.; Sulka, G.D. Band gap engineering of nanotubular Fe2O3-TiO2 photoanodes by wet impregnation. Appl. Surf. Sci. 2020, 517, 146195. [Google Scholar] [CrossRef]
- Sołtys-Mróz, M.; Syrek, K.; Wiercigroch, E.; Małek, K.; Rokosz, K.; Raaen, S.; Sulka, G.D. Enhanced visible light photoelectrochemical water splitting using nanotubular FeOx-TiO2 annealed at different temperatures. J. Power Sources 2021, 507, 230274. [Google Scholar] [CrossRef]
- El Sawy, E.N.; Birss, V.I. Nano-porous iridium and iridium oxide thin films formed by high efficiency electrodeposition. J. Mater. Chem. 2009, 19, 8244–8252. [Google Scholar] [CrossRef]
- Ghicov, A.; Schmuki, P. Self-ordering electrochemistry: A review on growth and functionality of TiO2 nanotubes and other self-aligned MOx structures. Chem. Commun. 2009, 2791–2808. [Google Scholar] [CrossRef]
- Liŭ, D.; Li, Z.; Wang, W.; Wang, G.; Liú, D. Hematite doped magnetic TiO2 nanocomposites with improved photocatalytic activity. J. Alloys Compd. 2016, 654, 491–497. [Google Scholar] [CrossRef]
- Aïnouche, L.; Hamadou, L.; Kadri, A.; Benbrahim, N.; Bradai, D. Interfacial barrier layer properties of three generations of TiO2 nanotube arrays. Electrochim. Acta 2014, 133, 597–609. [Google Scholar] [CrossRef]
- Rahman, G.; Joo, O.-S. Photoelectrochemical water splitting at nanostructured α-Fe2O3 electrodes. Int. J. Hydrogen Energy 2012, 37, 13989–13997. [Google Scholar] [CrossRef]
- Kontos, A.I.; Likodimos, V.; Stergiopoulos, T.; Tsoukleris, D.S.; Falaras, P.; Rabias, I.; Papavassiliou, G.; Kim, D.; Kunze, J.; Schmuki, P. Self-organized anodic TiO2 nanotube arrays functionalized by iron oxide nanoparticles. Chem. Mater. 2009, 21, 662–672. [Google Scholar] [CrossRef]
- Zhu, Z.; Murugananthan, M.; Gu, J.; Zhang, Y. Fabrication of a Z-scheme g-C3N4/Fe-TiO2 photocatalytic composite with enhanced photocatalytic activity under visible light irradiation. Catalysts 2018, 8, 112. [Google Scholar] [CrossRef] [Green Version]
- Iandolo, B.; Zhang, H.; Wickman, B.; Zori, I.; Conibeer, G.; Hellman, A. Correlating flatband and onset potentials for solar water splitting on model hematite photoanodes. RSC Adv. 2015, 5, 61021–61030. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, R.H.; Lima, B.H.R.; Leite, E.R. Magnetite colloidal nanocrystals: A facile pathway to prepare mesoporous hematite thin films for photoelectrochemical water splitting. J. Am. Chem. Soc. 2011, 133, 6012–6019. [Google Scholar] [CrossRef]
- Cesar, I.; Sivula, K.; Kay, A.; Zboril, R.; Grätzel, M. Influence of feature size, film thickness, and silicon doping on the performance of nanostructured hematite photoanodes for solar water splitting. J. Phys. Chem. C 2009, 113, 772–782. [Google Scholar] [CrossRef]
- Sellers, M.C.K.; Seebauer, E.G. Measurement method for carrier concentration in TiO2 via the Mott–Schottky approach. Thin Solid Films 2011, 519, 2103–2110. [Google Scholar] [CrossRef]
- Hu, Y.-S.; Kleiman-Shwarsctein, A.; Forman, A.J.; Hazen, D.; Park, J.-N.; McFarland, E.W. Pt-doped α-Fe2O3 thin films active for photoelectrochemical water splitting. Chem. Mater. 2008, 20, 3803–3805. [Google Scholar] [CrossRef]
- Smith, W.A.; Sharp, I.D.; Strandwitz, N.C.; Bisquert, J. Interfacial band-edge energetics for solar fuels production. Energy Environ. Sci. 2015, 8, 2851–2862. [Google Scholar] [CrossRef] [Green Version]
- Seger, B.; Tilley, S.D.; Pedersen, T.; Vesborg, P.C.K.; Hansen, O.; Gratzel, M.; Chorkendorff, I. Silicon protected with atomic layer deposited TiO2: Conducting versus tunnelling through TiO2. J. Mater. Chem. A 2013, 1, 15089–15094. [Google Scholar] [CrossRef]
- Tamirat, A.G.; Rick, J.; Dubale, A.A.; Sub, W.-N.; Hwang, B.-J. Using hematite for photoelectrochemical water splitting: A review of current progress and challenges. Nanoscale Horiz. 2016, 1, 243–267. [Google Scholar] [CrossRef] [PubMed]
- Venkata Reddy, C.; Neelakanta Reddy, I.; Akkinepally, B.; Raghava Reddy, K.; Shim, J. Synthesis and photoelectrochemical water oxidation of (Y, Cu) codoped α-Fe2O3 nanostructure photoanode. J. Alloys Compd. 2020, 814, 152349. [Google Scholar] [CrossRef]
- Kment, S.; Riboni, F.; Pausova, S.; Wang, L.; Wang, L.; Han, H.; Hubicka, Z.; Krysa, J.; Schmuki, P.; Zboril, R. Photoanodes based on TiO2 and α-Fe2O3 for solar water splitting—Superior role of 1D nanoarchitectures and of combined heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. [Google Scholar] [CrossRef] [PubMed]
- Syrek, K.; Skolarczyk, M.; Zych, M.; Sołtys-Mróz, M.; Sulka, G.D. A photoelectrochemical sensor based on anodic TiO2 for glucose determination. Sensors 2019, 19, 4981. [Google Scholar] [CrossRef] [Green Version]
- Soliveri, G.; Pifferi, V.; Panzarasa, G.; Ardizzone, S.; Cappelletti, G.; Meroni, D.; Sparnaccic, K.; Falciola, L. Self-cleaning properties in engineered sensors for dopamine electroanalytical detection. Analyst 2015, 140, 1486. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, I.; Lim, H.N.; Zawawi, R.M.; Tajudin, A.A.; Ng, Y.H.; Guo, H.; Huang, N.M. A review on visible-light induced photoelectrochemical sensors based on CdS nanoparticles. J. Mater. Chem. B 2018, 6, 4551. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, L.; Sun, X.; Li, F. Biphasic photoelectrochemical sensing strategy based on in situ formation of CdS quantum dots for highly sensitive detection of acetylcholinesterase activity and inhibition. Biosens. Bioelectron. 2016, 75, 359–364. [Google Scholar] [CrossRef]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef]
- Wang, H.; Ye, H.; Zhang, B.; Zhao, F.; Zeng, B. Electrostatic interaction mechanism based synthesis of a Z-scheme BiOI–CdS photocatalyst for selective and sensitive detection of Cu2+. J. Mater. Chem. A 2017, 5, 10599–10608. [Google Scholar] [CrossRef]
- Liu, F.; Wang, P.; Zhang, Q.; Wang, Z.; Liu, Y.; Zheng, Z.; Qin, X.; Zhang, X.; Dai, Y.; Huang, B. α-Fe2O3 film with highly photoactivity for non-enzymatic photoelectrochemical detection of glucose. Electroanalysis 2019, 31, 1809–1814. [Google Scholar] [CrossRef]
- Cao, X.; Wang, N. A novel non-enzymatic glucose sensor modified with Fe2O3 nanowire arrays. Analyst 2011, 136, 4241–4246. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xia, J.; Wang, L.; Qian, J.; Li, H.; Wang, K.; Sun, K.; He, M. α-Fe2O3 cubes with high visible-light-activated photoelectrochemical activity towards glucose: Hydrothermal synthesis assisted by a hydrophobic ionic liquid. Chem. Eur. J. 2014, 20, 2244–2253. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.C.; Tian, J.Q.; Asiri, A.M.; Qusti, A.H.; Al-Youbi, A.O.; Sun, X.P. Two-dimensional hybrid mesoporous Fe2O3–graphene nanostructures: A highly active and reusable peroxidase mimetic toward rapid, highly sensitive optical detection of glucose. Biosens. Bioelectron. 2014, 52, 452–457. [Google Scholar] [CrossRef]
- He, L.; Zhang, Q.; Gong, C.; Liu, H.; Hu, F.; Zhong, F.; Wang, G.; Su, H.; Wen, S.; Xiang, S.; et al. The dual-function of hematite-based photoelectrochemical sensor for solar-to-electricity conversion and self-powered glucose detection. Sens. Actuators B Chem. 2020, 310, 12784. [Google Scholar] [CrossRef]
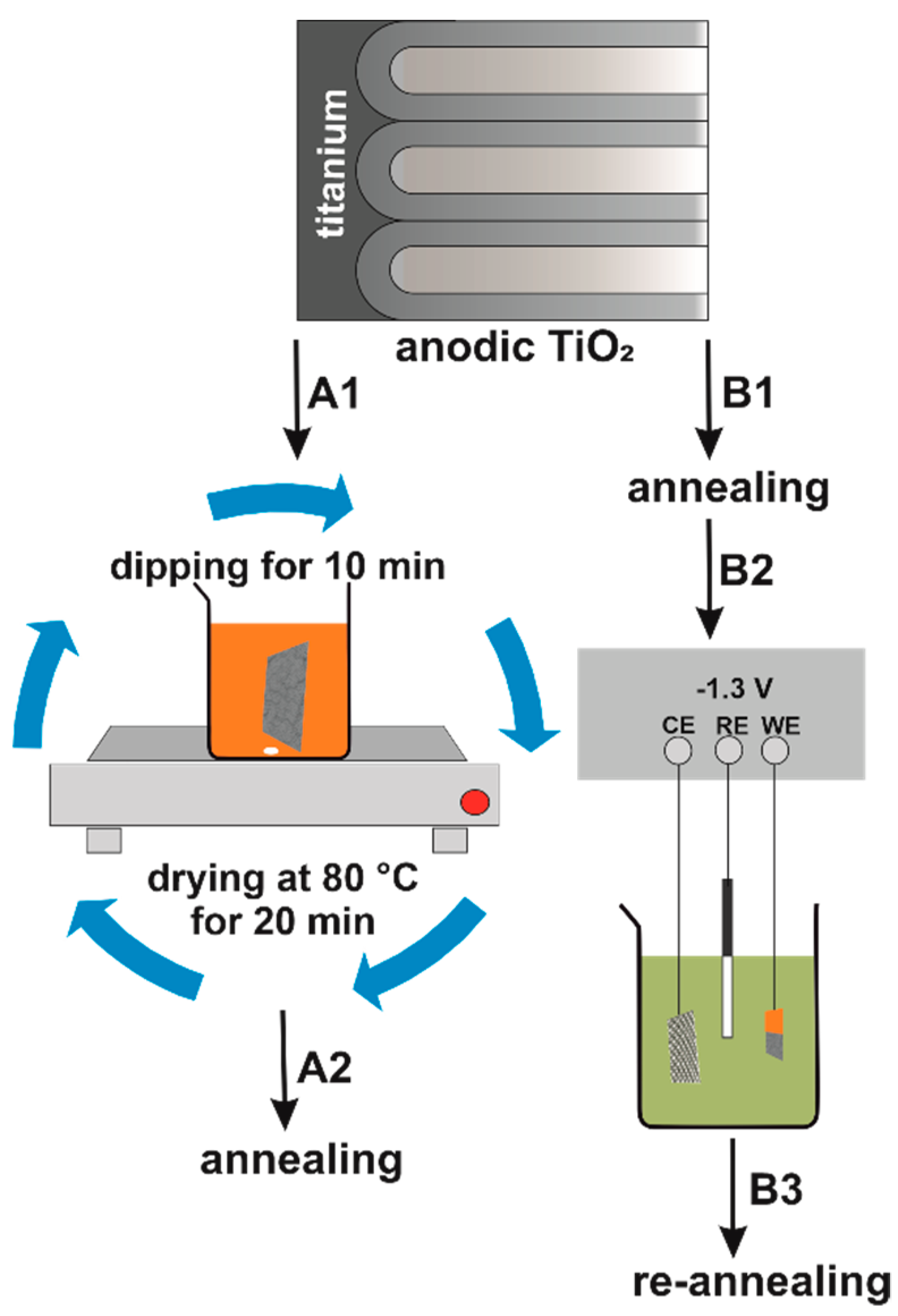


| Morphology of TiO2 NTs [nm] | Pre-Treatment (Immersion) | Electrochemical Deposition Conditions | Post-Treatment | Obtained Material | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Electrolytic Bath | Temperature [°C] | Time [s] | Technique/Conditions | |||||
| Dp = 80 L ≈ 550 W = 15–20 | - | aqueous solution of 60 g FeSO4, 1.5 g C6H8O6, 0.5 g H2NSO3H, 15 g H3BO3 | ambient | 60–300 | P cathodic current pulse: 8 ms of −70 mAcm−2; anodic potential pulse: 2 ms of 3 V | Annealing 500 °C for 6 h in an oxygen atmosphere | α-Fe2O3–TiO2 NTs | [11] |
| - | - | 0.02 M FeCl3 in water:glycerol mixture (volume ratio: 1:1) | ambient | 300–3600 | C potential: −1 V, −3 V, −5 V | Annealing 450 °C for 2 h in air | α-Fe2O3–TiO2 NTs | [12] |
|
Dp = 100 L = 400–500 | 5 mM NaF, 0.1 M NaCl, 1 M H2O2, 5 mM FeCl3 for 1 h, and 24 h | 5 mM NaF, 0.1 M NaCl, 1 M H2O2, 5 mM FeCl3 | - | - | PC potential: −0.52 V to 0.41 V vs. SCE, sweep rate: 0.1 V s−1, number of cycles: 50 | Annealing 500 °C for 30 min in air | Fe2O3@TiO2 NTs | [13] |
|
Dp = 80–100
W = 15 – 20 Dp ≈ 80 Dc ≈ 110 L ≈ 1200 | 0.05 M Fe(NO3)3 for 10 min | 0.1 M Na2SO4 | 85 | 1200 | A potential: 8 V | Electrochemical oxidation of Fe/TiO2-NT at 8 V for 2 min at room temperature in 1 M KOH | Fe2O3/TiO2 NTs | [14,15] |
|
Dc = 270
L = 3300 | - | 0.2 M Fe(NO3)3 | 25 | - | PC potential: 0 to −0.5 V vs. Ag/AgCl, scan rate: 0.01 V s−1, number of cycle: 2 | - | Fe–TiO2 NTs | [16] |
|
Dp ≈ 75
L = 1000 | - | 0.1 M FeSO4, 0.1 M H3BO3 (pH 3.5) | - | 20–160 | C potential: −1.3 V vs. SCE | Annealing 450 °C for 3 h in air | Fe2O3/TiO2-NTs | [17] |
|
Dp = 54 ± 9
Dc = 93 ± 11 W = 18 ± 5 | - | 0.1 M FeCl3 | - | 600 | A potential: 2 V, 2.5 V, 3 V, 4 V | Annealing 500 °C for 2 h | Fe–TiO2 NTs | [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sołtys-Mróz, M.; Syrek, K.; Pięta, Ł.; Malek, K.; Sulka, G.D. Photoelectrochemical Performance of Nanotubular Fe2O3–TiO2 Electrodes under Solar Radiation. Nanomaterials 2022, 12, 1546. https://doi.org/10.3390/nano12091546
Sołtys-Mróz M, Syrek K, Pięta Ł, Malek K, Sulka GD. Photoelectrochemical Performance of Nanotubular Fe2O3–TiO2 Electrodes under Solar Radiation. Nanomaterials. 2022; 12(9):1546. https://doi.org/10.3390/nano12091546
Chicago/Turabian StyleSołtys-Mróz, Monika, Karolina Syrek, Łukasz Pięta, Kamilla Malek, and Grzegorz D. Sulka. 2022. "Photoelectrochemical Performance of Nanotubular Fe2O3–TiO2 Electrodes under Solar Radiation" Nanomaterials 12, no. 9: 1546. https://doi.org/10.3390/nano12091546
APA StyleSołtys-Mróz, M., Syrek, K., Pięta, Ł., Malek, K., & Sulka, G. D. (2022). Photoelectrochemical Performance of Nanotubular Fe2O3–TiO2 Electrodes under Solar Radiation. Nanomaterials, 12(9), 1546. https://doi.org/10.3390/nano12091546