Visible-Light-Driven Ag-Doped BiOBr Nanoplates with an Enhanced Photocatalytic Performance for the Degradation of Bisphenol A
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Ag-BiOBr Photocatalysts
2.2. Characterizations
2.3. Electrochemical Measurements
2.4. Photocatalytic Activity Evaluation
3. Results and Discussion
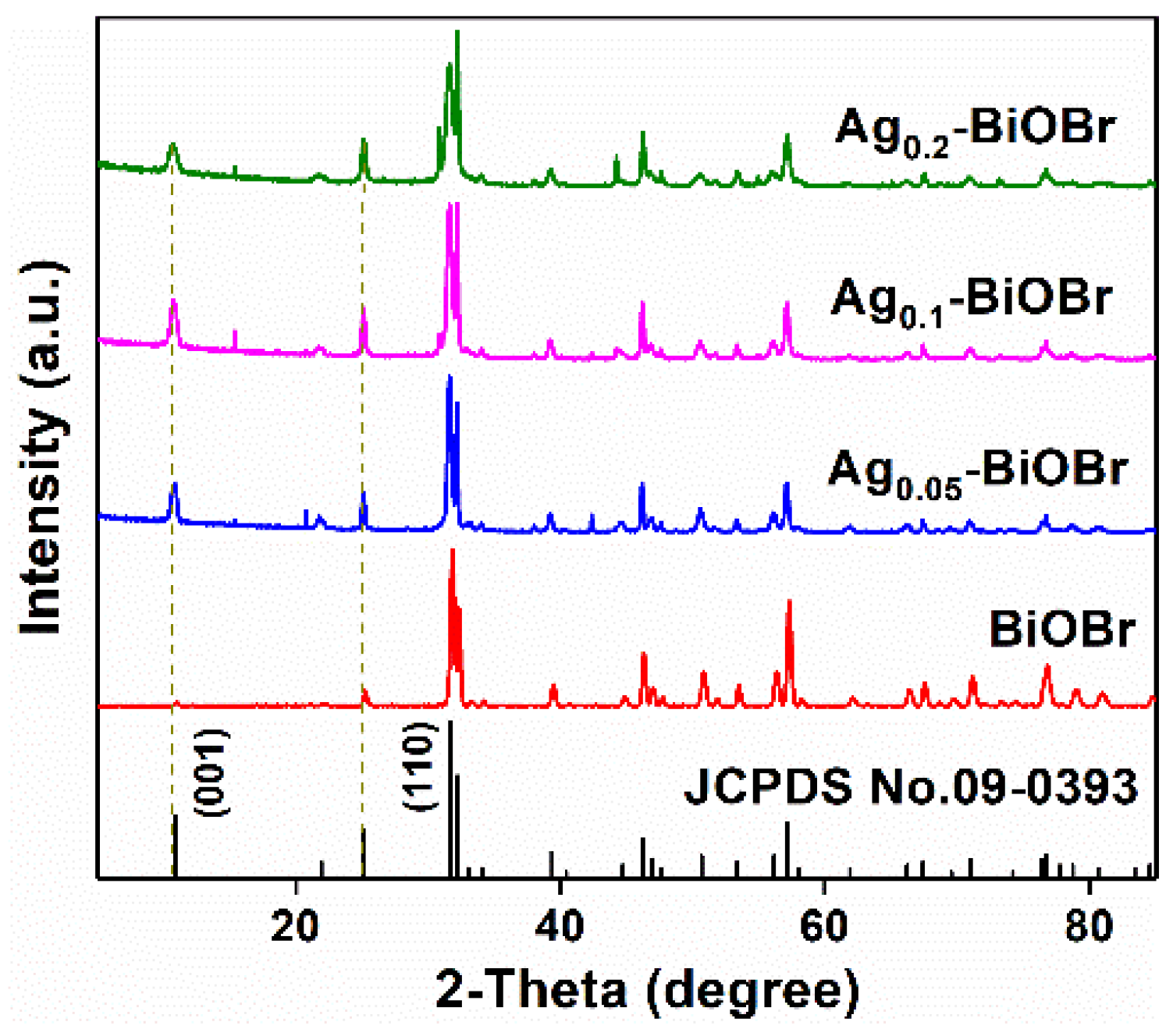
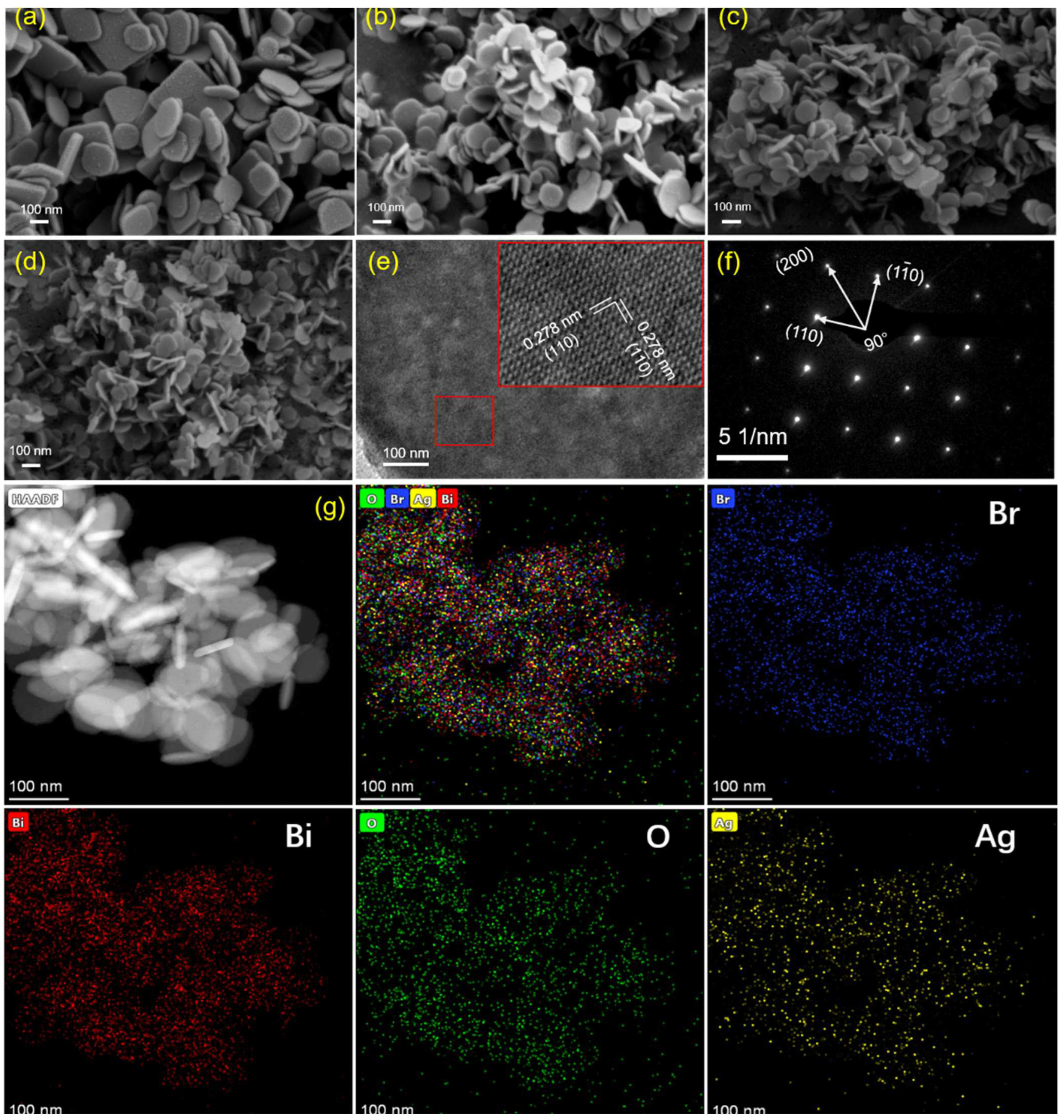
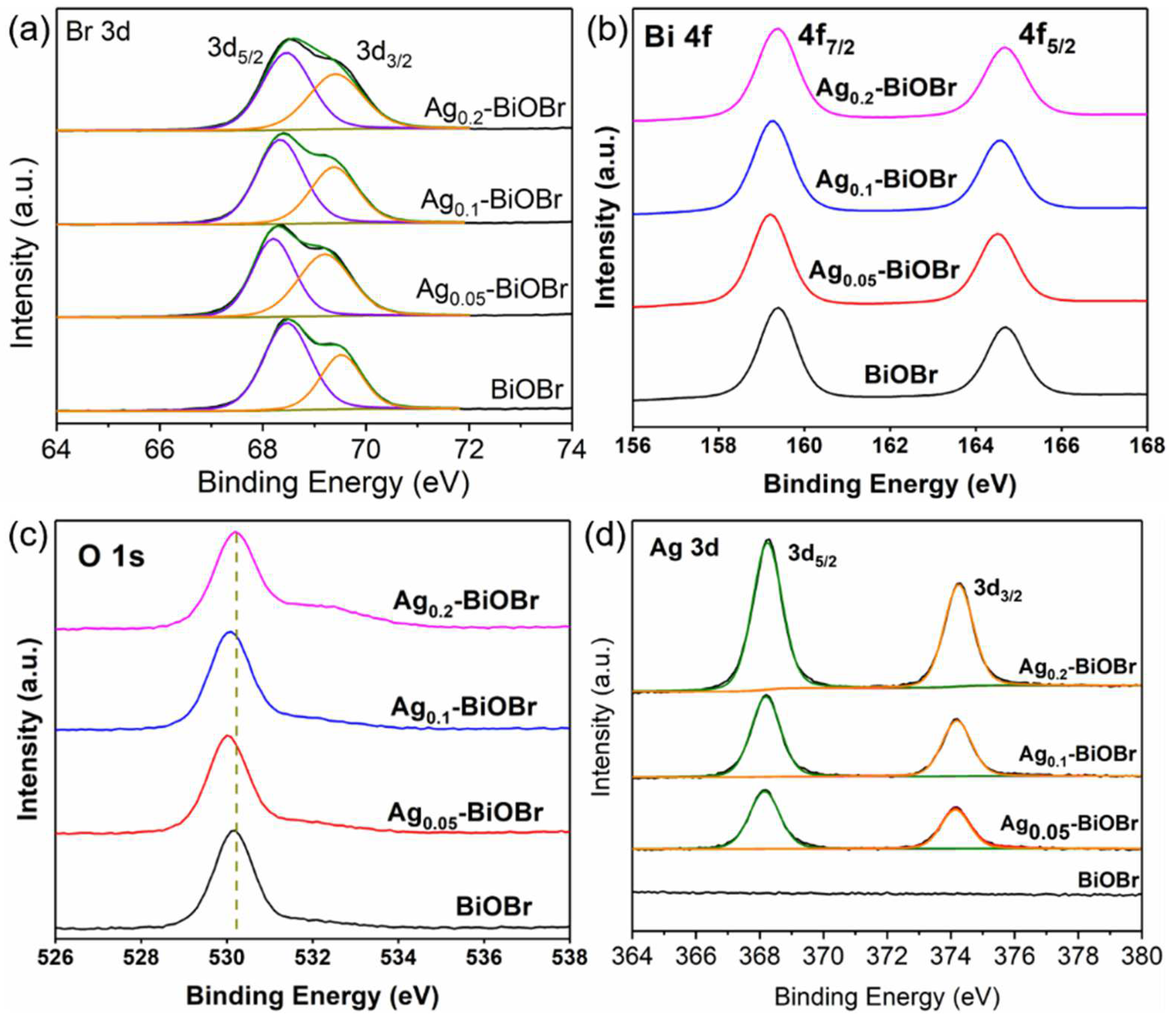
3.1. Characterizations
3.2. Band Structures and Electrochemical Properties
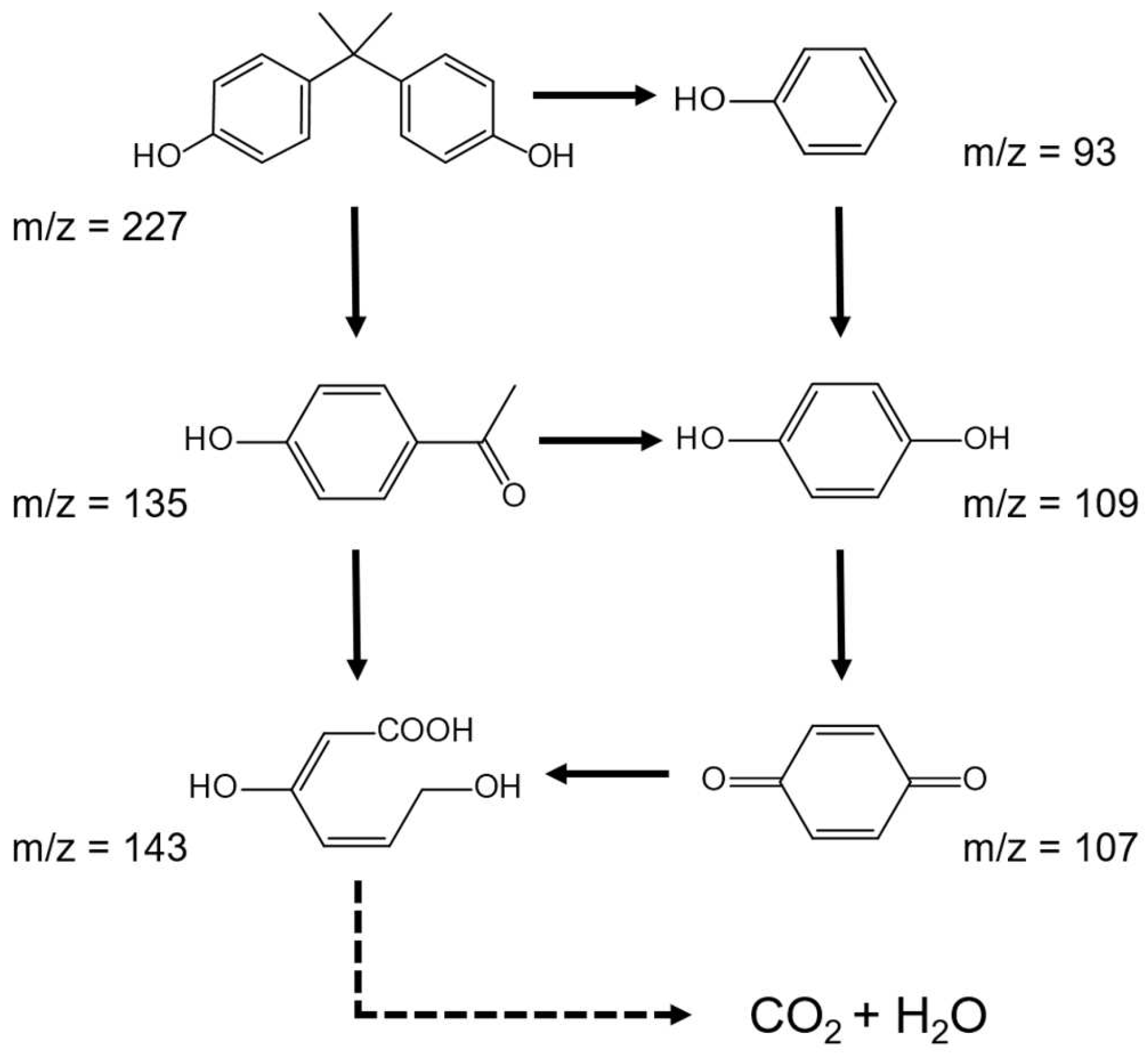
3.3. Photocatalytic Degradation of BPA
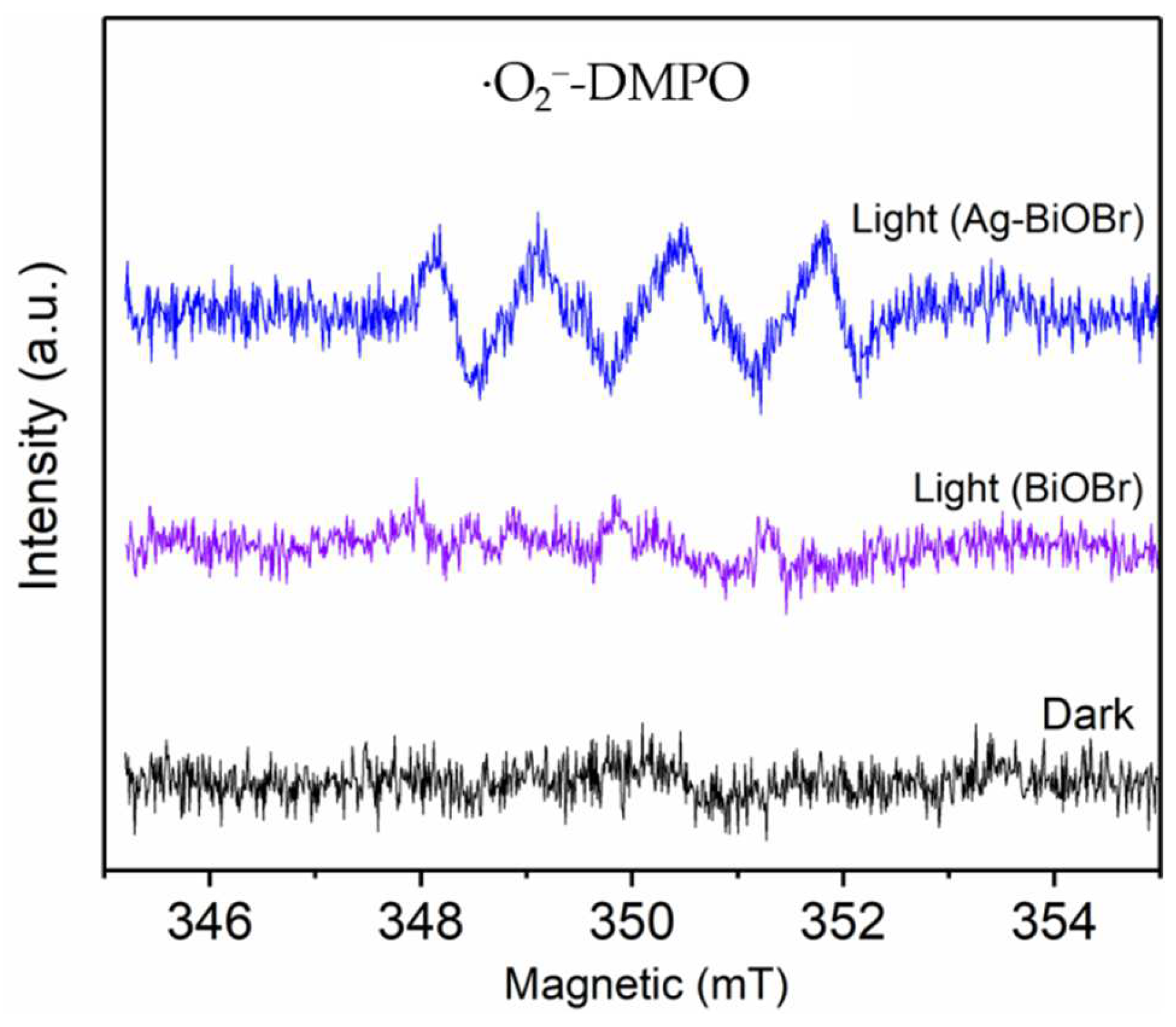
3.4. Mechanism of BPA Photocatalytic Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morin-Crini, N.; Lichtfouse, E.; Fourmentin, M.; Ribeiro, A.R.L.; Noutsopoulos, C.; Mapelli, F.; Fenyvesi, E.; Vieira, M.G.A.; Picos-Corrales, L.A.; Moreno-Pirajan, J.C.; et al. Removal of emerging contaminants from wastewater using advanced treatments. A review. Environ. Chem. Lett. 2022, 20, 1333–1375. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.F.; Huang, B.B.; Dai, Y. Engineering BiOX (X = Cl, Br, I) Nanostructures for highly efficient photocatalytic applications. Nanoscale 2014, 6, 2009–2026. [Google Scholar] [CrossRef]
- Wang, Z.W.; Chen, M.; Huang, D.L.; Zeng, G.M.; Xu, P.; Zhou, C.Y.; Lai, C.; Wang, H.; Cheng, M.; Wang, W.J. Multiply structural optimized strategies for bismuth oxyhalide photocatalysis and their environmental application. Chem. Eng. J. 2019, 374, 1025–1045. [Google Scholar] [CrossRef]
- Lei, H.; Zhang, H.H.; Zou, Y.; Dong, X.P.; Jia, Y.M.; Wang, F.F. Synergetic photocatalysis/piezocatalysis of bismuth oxybromide for degradation of organic pollutants. J. Alloy. Compd. 2019, 809, 151840. [Google Scholar] [CrossRef]
- Qu, S.Y.; Xiong, Y.H.; Zhang, J. Graphene oxide and carbon nanodots co-modified BiOBr nanocomposites with enhanced photocatalytic 4-chlorophenol degradation and mechanism insight. J. Colloid Interf. Sci. 2018, 527, 78–86. [Google Scholar] [CrossRef]
- Ye, L.Q.; Su, Y.R.; Jin, X.L.; Xie, H.Q.; Zhang, C. Recent advances in BiOX (X = Cl, Br and I) Photocatalysts: Synthesis, modification, facet effects and mechanisms. Environ. Sci. Nano 2014, 1, 90–112. [Google Scholar] [CrossRef]
- Wei, X.X.; Chen, C.M.; Guo, S.Q.; Guo, F.; Li, X.M.; Wang, X.X.; Cui, H.T.; Zhao, L.F.; Li, W. Advanced visible-light-driven photocatalyst BiOBr-TiO2-graphene composite with graphene as a nano-filler. J. Mater. Chem. A 2014, 2, 4667–4675. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zeng, Q.; Zhu, G.C. Novel S-doped BiOBr nanosheets for the enhanced photocatalytic degradation of bisphenol a under visible light irradiation. Chemosphere 2021, 268, 128854. [Google Scholar] [CrossRef]
- Huang, W.M.; Hua, X.; Zhao, Y.P.; Li, K.; Tang, L.P.; Zhou, M.; Cai, Z.S. Enhancement of visible-light-driven photocatalytic performance of BiOBr nanosheets by Co2+ doping. J. Mater. Sci. Mater. Electron. 2019, 30, 14967–14976. [Google Scholar] [CrossRef]
- Wu, Z.H.; Liu, J.; Tian, Q.Y.; Wu, W. Efficient visible light formaldehyde oxidation with 2D p-n heterostructure of BiOBr/BiPO4 nanosheets at room temperature. ACS Sustain. Chem. Eng. 2017, 5, 5008–5017. [Google Scholar] [CrossRef]
- Dong, Y.M.; Feng, C.Y.; Zhang, J.J.; Jiang, P.P.; Wang, G.L.; Wu, X.M.; Miao, H.Y. A new p-metal-n structure AgBr-Ag-BiOBr with superior visible-light-responsive catalytic performance. Chem. Asian J. 2015, 10, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, Z.B.; Li, M.J.; Hou, Y.P.; Sun, L.; Wang, L.; Peng, Z.B.; Chen, D.M.; Liu, Y.X. Fabrication of Ag/AgBr/Ga2O3 Heterojunction Composite with Efficient Photocatalytic Activity. Mol. Catal. 2017, 432, 57–63. [Google Scholar] [CrossRef]
- Javaid, R.; Qazi, U.Y.; Ikhlaq, A.; Zahid, M.; Alazmi, A. Subcritical and supercritical water oxidation for dye decomposition. J. Environ. Manag. 2021, 290, 112605. [Google Scholar] [CrossRef]
- Javaid, R.; Qazi, U.Y.; Kawasaki, S.I. Highly efficient decomposition of remazol Brilliant Blue R using tubular reactor coated with thin layer of PdO. J. Environ. Manag. 2016, 180, 551–556. [Google Scholar] [CrossRef]
- Chen, Q.L.; Zhang, Y.L.; Zhang, D.D.; Yang, Y.Q. Ag and N Co-doped TiO2 nanostructured photocatalyst for printing and dyeing wastewater. J. Water Process Eng. 2017, 16, 14–20. [Google Scholar] [CrossRef]
- Zhai, Y.J.; Chen, X.Y.; Li, J.H.; Chu, X.Y.; Xu, M.Z.; Jin, F.J.; Li, X.; Fang, X.; Wei, Z.; Wang, X.H. Preparation and properties of Ag doped Zno nanorods with N plasmon treatment. Ferroelectrics 2016, 505, 43–51. [Google Scholar] [CrossRef]
- Zhang, W.D.; Dong, F.; Xiong, T.; Zhang, Q. Synthesis of BiOBr-graphene and BiOBr-graphene oxide nanocomposites with enhanced visible light photocatalytic performance. Ceram. Int. 2014, 40, 9003–9008. [Google Scholar] [CrossRef]
- Phu, N.D.; Hoang, L.H.; Hai, P.V.; Huy, T.Q.; Chen, X.B.; Chou, W.C. Photocatalytic activity enhancement of Bi2WO6 Nanoparticles by Ag doping and Ag nanoparticles modification. J. Alloy. Compd. 2020, 824, 153914. [Google Scholar] [CrossRef]
- Wu, D.; Ye, L.Q.; Yip, H.Y.; Wong, P.K. Organic-free synthesis of {001} facet dominated BiOBr nanosheets for selective photoreduction of CO2 to CO. Catal. Sci. Technol. 2017, 7, 265–271. [Google Scholar] [CrossRef]
- Wu, X.Y.; Zhang, K.K.; Zhang, G.K.; Yin, S. Facile Preparation of BiOX (X = Cl, Br, I) nanoparticles and up-conversion phosphors/BiOBr composites for efficient degradation of NO gas: Oxygen vacancy effect and near infrared light responsive mechanism. Chem. Eng. J. 2017, 325, 59–70. [Google Scholar] [CrossRef]
- Di, J.; Chen, C.; Zhu, C.; Song, P.; Xiong, J.; Ji, M.; Zhou, J.; Fu, Q.; Xu, M.; Hao, W.; et al. Bismuth vacancy-tuned bismuth oxybromide ultrathin nanosheets toward photocatalytic CO2 reduction. ACS Appl. Mater. Inter. 2019, 11, 30786–30792. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.L.; Ma, D.K.; Zhou, Z.L.; Xu, C.L.; Cao, C.; Zhao, P.Y.; Huang, Q.L. Efficient photocatalytic degradation of herbicide glyphosate in water by magnetically separable and recyclable BiOBr/Fe3O4 nanocomposites under visible light irradiation. Chem. Eng. J. 2019, 368, 212–222. [Google Scholar] [CrossRef]
- Patil, S.S.; Mali, M.G.; Hassan, M.A.; Patil, D.R.; Kolekar, S.S.; Ryu, S.W. One-pot in situ hydrothermal growth of BiVO4/Ag/rGo hybrid architectures for solar water splitting and environmental remediation. Sci. Rep. 2017, 7, 8404. [Google Scholar] [CrossRef] [Green Version]
- Baby, B.H.; Thomas, A.M.; Amrutha, E.G.; Mohan, D.B. Enhancement of optoelectronic properties via substitutional doping of Cu, in and Ag in SnS nanorods for thin film photovoltaics. Sol. Energy 2020, 205, 446–455. [Google Scholar] [CrossRef]
- Patil, S.S.; Mali, M.G.; Tamboli, M.S.; Patil, D.R.; Kulkarni, M.V.; Yoon, H.; Kim, H.; Al-Deyab, S.S.; Yoon, S.S.; Kolekar, S.S.; et al. Green approach for hierarchical nanostructured Ag-Zno and their photocatalytic performance under sunlight. Catal. Today 2016, 260, 126–134. [Google Scholar] [CrossRef]
- Meng, X.C.; Jiang, L.Y.; Wang, W.W.; Zhang, Z.S. Enhanced photocatalytic activity of BiOBr/ZnO heterojunction semiconductors prepared by facile hydrothermal method. Int. J. Photoenergy 2015, 2015, 747024. [Google Scholar] [CrossRef] [Green Version]
- Shang, J.; Hao, W.C.; Lv, X.J.; Wang, T.M.; Wang, X.L.; Du, Y.; Dou, S.X.; Xie, T.F.; Wang, D.J.; Wang, J.O. Bismuth oxybromide with reasonable photocatalytic reduction activity under visible light. ACS Catal. 2014, 4, 954–961. [Google Scholar] [CrossRef]
- Khachatryan, L.; Vejerano, E.; Lomnicki, S.; Dellinger, B. Environmentally persistent free radicals (EPFRs). 1. generation of reactive oxygen species in aqueous solutions. Environ. Sci. Technol. 2011, 45, 8559–8566. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Wang, C.Y.; Xu, B.X.; Han, J.Y.; Fang, X.; Zhu, G.C. Electron-level mechanistic insights into Ce doping for enhanced efficiency degradation of bisphenol a under visible light irradiation. Nanomaterials 2022, 12, 1382. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yue, S.T.; Wang, W.; An, T.C.; Li, G.Y.; Yip, H.Y.; Zhao, H.J.; Wong, P.K. Boron doped BiOBr nanosheets with enhanced photocatalytic inactivation of Escherichia coli. Appl. Catal. B Environ. 2016, 192, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, Y.; Shi, Z.; Cao, W.; Zhang, L.; Liu, J.; Chen, Z. BiOBr/Ag/AgBr heterojunctions decorated carbon fiber cloth with broad-spectral photoresponse as filter-membrane-shaped photocatalyst for the efficient purification of flowing wastewater. J. Colloid Inter. Sci. 2021, 587, 633–643. [Google Scholar] [CrossRef]
- Carolina, O.N.; Yuri, P.; Varsha, S.; Netzahualpille, H.; Jürgen, M.; Mika, S.; Nancy, O.S. Gd3+ doped BiVO4 and visible light-emitting diodes (LED) for photocatalytic decomposition of bisphenol A, bisphenol S and bisphenol AF in water. J. Environ. Chem. Eng. 2021, 9, 105842. [Google Scholar]
- Yuan, T.; Xiaohong, Y.; Manman, M.; Yue, J.; Xiaoli, L.; Hao, Z.; Tianwei, O. Anatase TiO2@MIL-101(Cr) nanocomposite for photocatalytic degradation of bisphenol A. Colloid Surface A 2020, 596, 124745. [Google Scholar]
- Ye, C.; Hu, K.; Niu, Z.; Lu, Y.; Zhang, L.; Yan, K. Controllable synthesis of rhombohedral α-Fe2O3 efficient for photocatalytic degradation of bisphenol A. J. Water Process Eng. 2019, 27, 205–210. [Google Scholar] [CrossRef]
- Wang, C.Y.; Xing, Z.; Qiu, H.B.; Wang, W.K.; Huang, G.X.; Jiang, J.; Yu, H.Q. Photocatalytic degradation of bisphenol A by oxygen-rich and highly visible-light responsive Bi12O17Cl2 nanobelts. Appl. Catal. B Environ. 2017, 200, 659–665. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-Y.; Zeng, Q.; Wang, L.-X.; Fang, X.; Zhu, G. Visible-Light-Driven Ag-Doped BiOBr Nanoplates with an Enhanced Photocatalytic Performance for the Degradation of Bisphenol A. Nanomaterials 2022, 12, 1909. https://doi.org/10.3390/nano12111909
Wang C-Y, Zeng Q, Wang L-X, Fang X, Zhu G. Visible-Light-Driven Ag-Doped BiOBr Nanoplates with an Enhanced Photocatalytic Performance for the Degradation of Bisphenol A. Nanomaterials. 2022; 12(11):1909. https://doi.org/10.3390/nano12111909
Chicago/Turabian StyleWang, Chu-Ya, Qi Zeng, Li-Xia Wang, Xin Fang, and Guangcan Zhu. 2022. "Visible-Light-Driven Ag-Doped BiOBr Nanoplates with an Enhanced Photocatalytic Performance for the Degradation of Bisphenol A" Nanomaterials 12, no. 11: 1909. https://doi.org/10.3390/nano12111909
APA StyleWang, C.-Y., Zeng, Q., Wang, L.-X., Fang, X., & Zhu, G. (2022). Visible-Light-Driven Ag-Doped BiOBr Nanoplates with an Enhanced Photocatalytic Performance for the Degradation of Bisphenol A. Nanomaterials, 12(11), 1909. https://doi.org/10.3390/nano12111909







