Nanomaterials with Excellent Adsorption Characteristics for Sample Pretreatment: A Review
Abstract
:1. Introduction
2. Synthesis of Nanomaterials and Their Applications in Sample Pretreatment
2.1. Carbon Nanomaterials
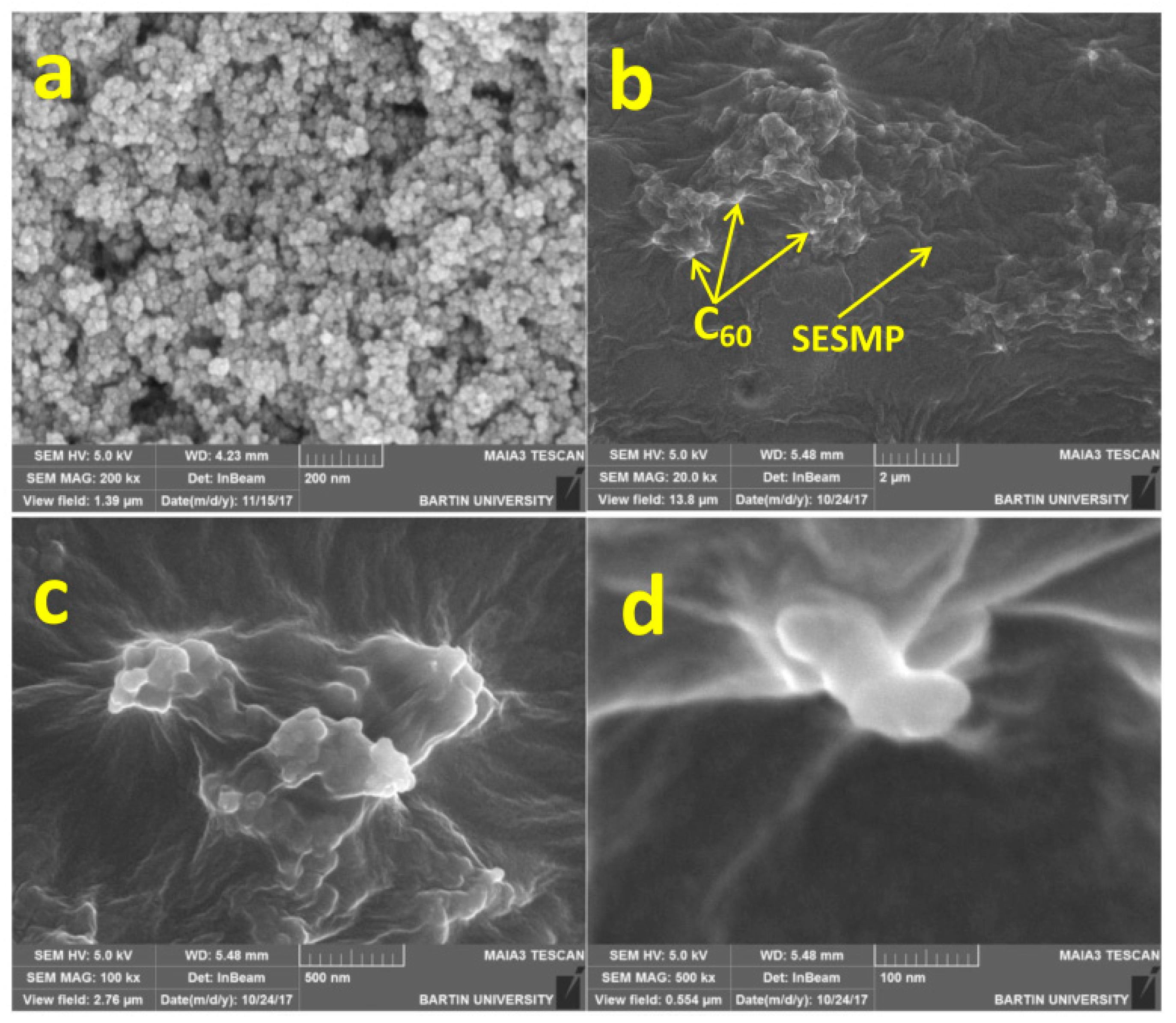
2.1.1. Application of Fullerene in Sample Pretreatment
2.1.2. Synthesis of Carbon Nanotubes and Its Application in Sample Pretreatment
2.1.3. Synthesis of Graphene and Its Application in Pretreatment
2.2. Porous Nanomaterials
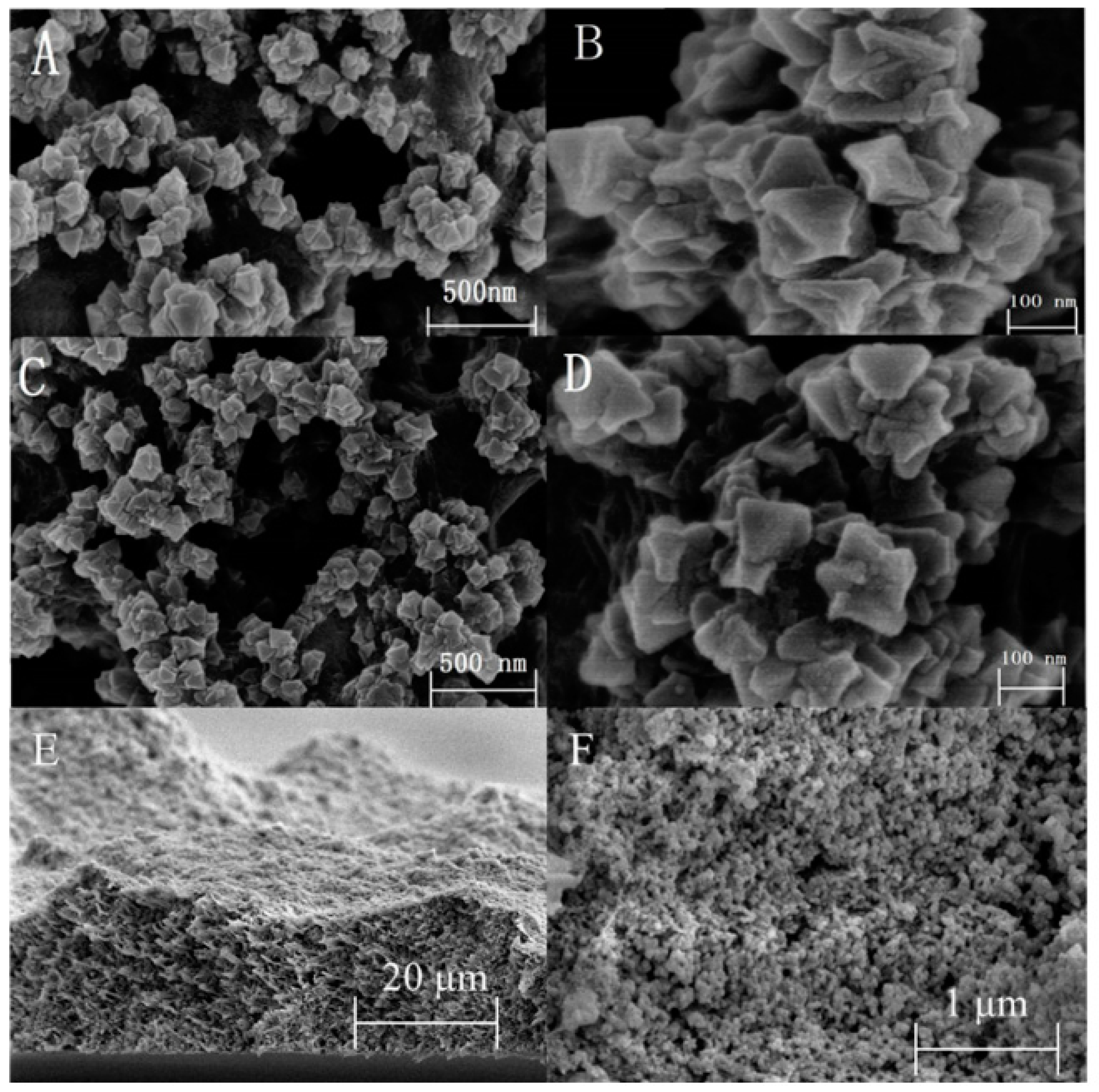
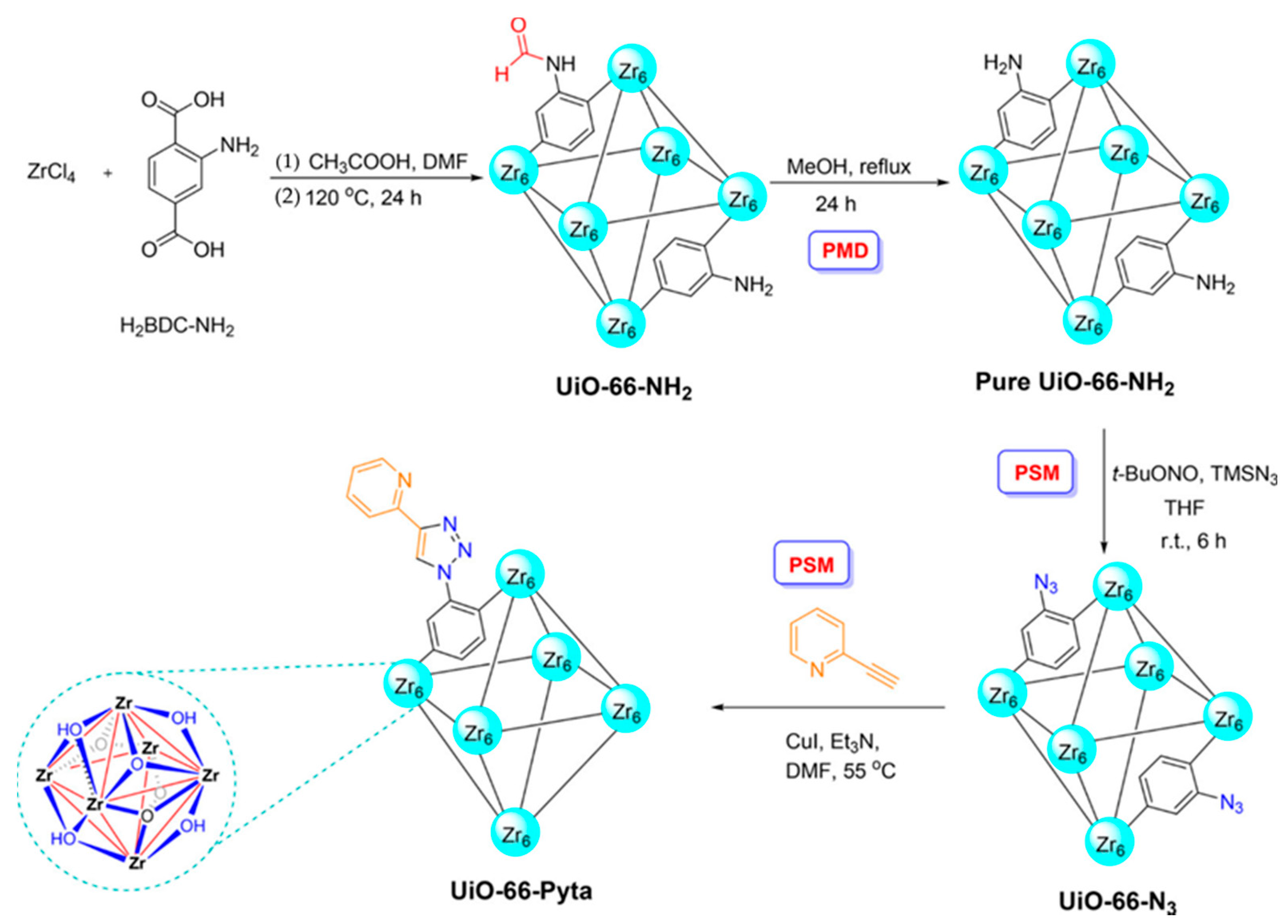
2.2.1. Synthesis of MOF and Its Application in Sample Pretreatment
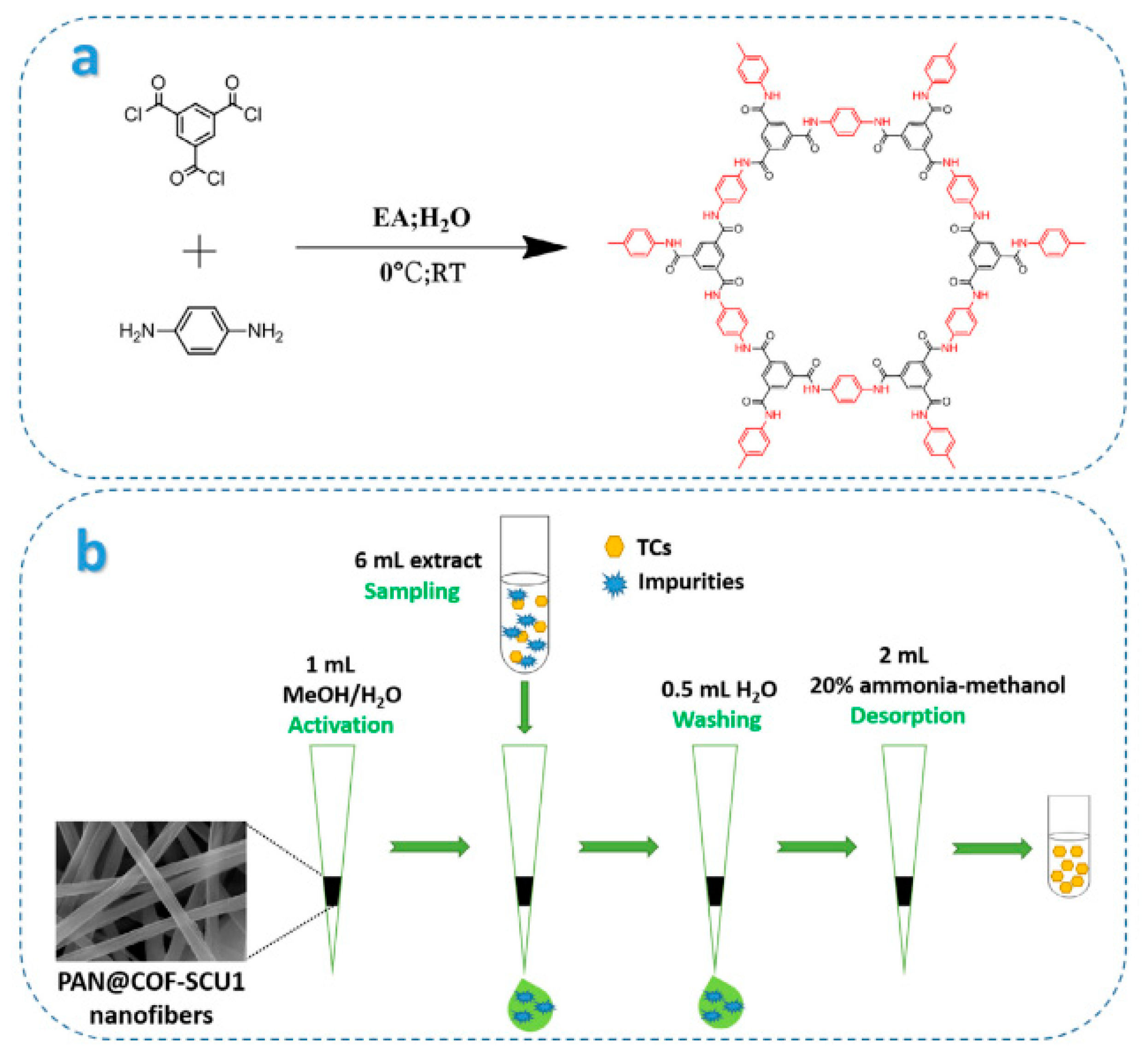
2.2.2. Synthesis of a Covalent Organic Framework (COF) and Its Application in Sample Pretreatment
2.2.3. Synthesis of a Molecularly Imprinted Polymer (MIP) and Its Application in Sample Pretreatment
2.2.4. Synthesis of Porous Hybrid Material and Its Application in Sample Pre-Treatment
2.3. Magnetic Nanomaterials
2.4. Regeneration and Reproducibility of Nanomaterials
3. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CD-IMS | corona discharge ion mobility spectrometry |
| ICP-AES | inductively coupled plasma atomic emission spectrometer |
| PT-SPE | pipette tip solid-phase extraction |
| ox-MWCNTs | oxidized multiwalled carbon nanotubes |
| DMSPE | dispersive micro-solid phase extraction |
| DLLME | dispersive liquid-liquid microextraction |
| SPE | solid-phase extraction |
| HPLC | high-performance liquid chromatography |
| ICP-OES | Inductively Coupled Plasma Optical-Emission Spectrometry |
| ESMP | Eggshell membrane protein |
| SESMP | soluble eggshell membrane protein |
| OPPs | organophosphorus pesticides |
| DIZ | diazinon |
| CPs | chlorpyrifos |
| CD-IMS | corona discharge ion mobility spectrometry |
| HS-SPME | headspace solid-phase microextraction |
| IL-COOH | the hydrophobic carboxyl-functionalized ionic liquid |
| FQs | fluoroquinolone antibiotics |
| Br-PADAP | [2-(5-Bromo-2-pyridylazo)-5-(diethylamino)phenol] |
| PSM | postsynthetic modification |
| SWCNTs | single-walled carbon nanotubes |
| MWCNTs | multi-walled carbon nanotubes |
| CVD | chemical vapor deposition |
| FAAS | flame atomic absorption spectrometry |
| GO | graphene oxide |
| CS | chitosan |
| MoS2/GO | molybdenum disulfide-graphene oxide composite |
| MOFs | metal-organic frameworks |
| MMM | mixed-matrix membrane |
| PCAs | phenoxy carboxylic acids |
| DME | dispersive membrane extraction |
| COF | covalent organic framework |
| NTM-COFs | nano-titania modified covalent organic frameworks |
| MSPE | magnetic solid-phase extraction |
| HPLC | high performance liquid chromatography |
| TCs | Tetracycline antibiotics |
| PT-SPE | Pipette tip solid-phase extraction |
| PAN@COF-SCU1 | polyacrylonitrile@COFs |
| PAN | polyacrylonitrile |
| LC–MS/MS | liquid chromatography-tandem quadrupole mass spectrometry |
| dSPE | dispersive solid phase extraction |
| OSCE | one step cleanup and extraction |
| LODs | limit of detections |
| MIP | molecularly imprinted polymer |
| RA | rosmarinic acid |
| cAMP | cyclic adenosine monophosphate |
| TEOS | tetraethyl orthosilicate |
| PAMAM@Mag-CNTs | polyamidoamine-functionalized magnetic carbon nanotubes |
| GO-Fe3O4 | graphene oxide-Fe3O4 |
| RSD | relative standard deviations |
| PS-IL-COFs | petal-shaped ionic liquids modified covalent organic frameworks |
| DPT-MSPE | double layer pipette tip magnetic dispersive solid phase extraction |
| UFLC-MS/MS | ultra-fast liquid chromatography-tandem quadrupole mass spectrometry |
| PAN/Ni-MOF | electrospun polyacrylonitrile/nickel-based metal–organic framework nanocomposite |
| EH-Mag-GO | enhanced cleanup efficiency hydroxy functionalized-magnetic graphene oxide |
| UPLC-PDA | Ultra-high-performance liquid chromatography with photodiode array detector |
| Cu–MOF/GO | Cu-based metal–organic framework/graphene oxide |
| GC-FID | gas chromatography-flame ionization detection |
| GO/COF-300/PPy | polypyrrole doped GO/COF-300 |
| IDM | indomethacin |
| DCF | diclofenac |
| QA-Mag-CCNTs | quaternary ammonium modified magnetic carboxyl-carbon nanotubes |
| 3D-IL-Fe3O4-GO | three-dimensional ionic liquid-ferrite functionalized graphene oxide nanocomposite |
References
- Azzouz, A.; Kailasa, S.K.; Lee, S.S.; Rascon, A.J.; Ballesteros, E.; Zhang, M.; Kim, K.H. Review of nanomaterials as sorbents in solid-phase extraction for environmental samples. TrAC Trends Anal. Chem. 2018, 108, 347–369. [Google Scholar] [CrossRef]
- Ridgway, K.; Lalljie, S.P.D.; Smith, R.M. Sample preparation techniques for the determination of trace residues and contami-nants in foods. J. Chromatogr. A 2007, 1153, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, M.; Ahmadzadeh, H.; Amiri, A. Sample preparation and extraction methods for pesticides in aquatic environments: A review. TrAC Trends Anal. Chem. 2020, 123, 72–97. [Google Scholar] [CrossRef]
- Płotka–Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Modern trends in solid phase extraction: New sorbent media. TrAC Trends Anal. Chem. 2016, 77, 23–43. [Google Scholar] [CrossRef]
- Kusano, M.; Sakamoto, Y.; Natori, Y.; Miyagawa, H.; Tsuchihashi, H.; Ishii, A.; Zaitsu, K. Development of “Quick-DB forensic”: A total workflow from QuEChERS-dSPE method to GC-MS/MS quantification of forensically relevant drugs and pesticides in whole blood. Forensic Sci. Int. 2019, 300, 125–135. [Google Scholar] [CrossRef]
- Vijayasarathy, S.; Baduel, C.; Hof, C.; Bell, I.; Del Mar Gomez Ramos, M.; Ramos, M.J.G.; Kock, M.; Gaus, C. Multi-residue screening of non-polar hazardous chemicals in green turtle blood from different foraging regions of the Great Barrier Reef. Sci. Total Environ. 2019, 652, 862–868. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, J.; Lee, J.H.; Lee, J.; Kim, E.; Liu, K.H.; Lee, H.S.; Kim, J.H. Validation of a multiresidue analysis method for 379 pesticides in human serum using liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2018, 66, 3550–3560. [Google Scholar] [CrossRef]
- Taliansky-Chamudis, A.; Gomez-Ramirez, P.; Leon-Ortega, M.; Garcia-Fernandez, A.J. Validation of a QuEChERS method for analysis of neonicotinoids in small volumes of blood and assessment of exposure in Eurasian eagle owl (Bubo bubo) nestlings. Sci. Total Environ. 2017, 595, 93–100. [Google Scholar] [CrossRef]
- Ma, J.B.; Qiu, H.W.; Rui, Q.H.; Liao, Y.F.; Chen, Y.M.; Xu, J.; Zhang, Y.; Zhu, Y.; Zhao, Y.G. Enhanced cleanup efficiency hydroxy functionalized-magnetic graphene oxide and its comparison with magnetic carboxyl-graphene for PRiME pass-through cleanup of strychnine and brucine in human plasma samples. Anal. Chim. Acta 2018, 1020, 41–50. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Li, H.; Zhou, S.; Chen, D.; Zhang, Y.; Yao, Q.; Sun, C. A simple and high-throughput analysis of amatoxins and phallotoxins in human plasma, serum and urine using UPLC-MS/MS combined with PRiME HLB μElution platform. Toxins 2016, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Miao, H.; Huang, Y.; Ma, C.; Li, J.; Zhao, Y.; Wu, Y. Ultra-high-performance liquid chromatography-isotope dilution tandem mass spectrometry for the determination of phthalate secondary metabolites in human serum based on solid-phase extraction. J. AOAC Int. 2019, 102, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Wojnicz, A.; Belmonte, C.; Koller, D.; Ruiz-Nuno, A.; Roman, M.; Ochoa, D.; Ruiz-Nuno, A.; Roman, M.; Ochoa, D.; Santos, F.A. Effective phospholipids removing microelution-solid phase extraction LC-MS/MS method for simultaneous plasma quantification of aripiprazole and dehydro-aripiprazole: Application to human pharmacokinetic studies. J. Pharmaceyt. Biomed. 2018, 151, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Soriano, M.L.; Zougagh, M.; Valcarcel, M.; Rios, A. Analytical nanoscience and nanotechnology: Where we are and where we are heading. Talanta 2018, 177, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Soylak, M. 15-Functionalized nanomaterials for sample preparation methods. In Handbook of Nanomaterials in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–413. [Google Scholar]
- Zhang, B.T.; Zheng, X.X.; Li, H.F.; Lin, J.M. Application of carbon-based nanomaterials in sample preparation: A review. Anal. Chim. Acta 2013, 784, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Kumar, B.G.; Rajendran, S.; Qin, J.; Vadivel, S.; Durgalakshmi, D.; Gracia, F.; Soto-Moscoso, M.; Orooji, Y.; Karimi, F. Tuning of metal oxides photocatalytic performance using Ag nanoparticles integration. J. Mol. Liq. 2020, 314, 113588. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Shafieizadeh, T.; Taher, M.A.; Opoku, F.; Kiarii, E.M.; Opoku, F.; Kiarii, E.M.; Govender, P.P.; Ranjbari, S.; Rezapour, M.; et al. The role of magnetite/graphene oxide nano-composite as a high-efficiency adsorbent for removal of phenazopyridine residues from water samples, an experimental/theoretical investigation. J. Mol. Liq. 2020, 298, 112040. [Google Scholar] [CrossRef]
- Ahmadi, M.; Elmongy, H.; Madrakian, T.; Abdel-Rehim, M. Nanomaterials as sorbents for sample preparation in bioanalysis: A review. Anal. Chim. Acta 2017, 958, 1–21. [Google Scholar] [CrossRef]
- Wang, J.L.; Liu, G.H.; Zhang, X.R. Recent developments of nanomaterials as sorbents. Chin. J. Anal. Chem. 2005, 33, 1787–1793. [Google Scholar]
- Zhong, C.J.; Yang, B.; Jiang, X.; Li, J. Current progress of nanomaterials in molecularly imprinted electrochemical sensing. Crit. Rev. Anal. Chem. 2018, 48, 15–32. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.; Hu, Y.; Wang, R.; Chen, M.; Wu, J.; Wang, Y.; Kang, S.; Sun, Y.; Zhu, M. Behavior, remediation effect and toxicity of nanomaterials in water environments. Environ. Res. 2019, 174, 54–60. [Google Scholar] [CrossRef]
- Saleh, T.A. Trends in the sample preparation and analysis of nanomaterials as environmental contaminants. Anal. Chem. 2020, 28, 1–10. [Google Scholar] [CrossRef]
- Khan, W.A.; Arain, M.B.; Soylak, M. Nanomaterials-based solid phase extraction and solid phase microex traction for heavy metals food toxicity. Food Chem. Toxicol. 2020, 145, 111704. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Palomero, C.; Soriano, M.L.; Valcarcel, M. Nanocellulose as analyte and analytical tool: Opportunities and challenges. TrAC Trends Anal. Chem. 2017, 87, 1–18. [Google Scholar] [CrossRef]
- Westerho, P.; Alvarez, P.; Li, Q.; Torresdey, J.G.; Zimmerman, J. Overcoming implementation barriers for nanotechnology in drinking water treatment. Environ. Sci.-Nano 2016, 3, 1241–1253. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.Y.; Qiao, J.Q.; Lian, H.Z.; Chen, H.Y. Solid phase extraction of magnetic carbon doped Fe3O4 nanoparticles. J. Chromatogr. A 2014, 1325, 8–15. [Google Scholar] [CrossRef]
- Zhang, S.; Niu, H.; Zhang, Y.; Liu, J.; Shi, Y.; Zhang, X.; Cai, Y. Biocompatible phosphatidylcholine bilayer coated on magnetic nanoparticles and their application in the extraction of several polycyclic aromatic hydrocarbons from environmental water and milk samples. J. Chromatogr. A 2012, 1238, 38–45. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.L.; Wang, P.F.; Tang, Z.; Niu, H.; Cai, Y.; Wu, F.C.; Wang, H.; Meng, W.; Giesy, J.P. Surfactant-modified flowerlike layered double hydroxide-coated magnetic nanoparticles for preconcentration of phthalate esters from environmental water samples. J. Chromatogr. A 2015, 1414, 22–30. [Google Scholar] [CrossRef]
- Wang, W.D.; Huang, Y.M.; Shu, W.Q.; Cao, J. Multiwalled carbon nanotubes as adsorbents of solid-phase extraction for determination of polycyclic aromatic hydrocarbons in environmental waters coupled with high-performance liquid chromatography. J. Chromatogr. A 2007, 1173, 27–36. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Chen, Q.Q.; Lin, Z.F.; Yin, D.Q. Combined effects of aqueous suspensions of fullerene and humic acid on the availability of polycyclic aromatic hydrocarbons: Evaluated with negligible depletion solid-phase microextraction. Sci. Total Environ. 2014, 493, 12–21. [Google Scholar] [CrossRef]
- Cai, M.Q.; Su, J.; Hu, J.Q.; Wang, Q.; Dong, C.Y.; Pan, S.D.; Jin, M.C. Planar graphene oxide-based magnetic ionic liquid nanomaterial for extraction of chlorophenols from environmental water samples coupled with liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2016, 1459, 38–46. [Google Scholar] [CrossRef]
- Han, Q.; Liang, Q.L.; Zhang, X.Q.; Yang, L.; Ding, M.Y. Graphene aerogel based monolith for effective solid-phase extraction of trace environmental pollutants from water samples. J. Chromatogr. A 2016, 1447, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Abujaber, F.; Zougagh, M.; Jodeh, S.; Ríosb, Á.; Javier, F.; Bernardo, G.; Martín-Doimeadios, R.C.R. Magnetic cellulose nanoparticles coated with ionic liquid as a new material for the simple and fast monitoring of emerging pollutants in waters by magnetic solid phase extraction. Microchem. J. 2018, 137, 490–495. [Google Scholar] [CrossRef]
- Lian, L.; Lv, J.L.; Wang, X.Y.; Lou, D.W. Magnetic solid–phase extraction of tetracyclines using ferrous oxide coated magnetic silica microspheres from water samples. J. Chromatogr. A 2018, 1534, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shahriman, M.S.; Ramachandran, M.R.; Zain, N.N.M.; Mohamad, S.; Manan, N.S.A.; Mohamad, S.; Manan, N.S.A.; Yaman, S.M. Polyaniline-dicationic ionic liquid coated with magnetic nanoparticles composite for magnetic solid phase extraction of polycyclic aromatic hydrocarbons in environmental samples. Talanta 2018, 178, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Lucena, R.; Simonet, B.M.; C’ardenas, S.; Valc´arcel, M. Potential of nanoparticles in sample preparation. J. Chromatogr. A 2011, 1218, 620–637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, H.N.; Yu, Y.B.; Zhang, Y. Application of nanomaterials in proteomics-driven precision medicine. Theranostics 2022, 12, 2674–2686. [Google Scholar] [CrossRef]
- Ma, C.X.; Xie, L.; Wang, X.; Liang, K.; Kong, B. Interfacial assembly of functional mesoporous nanomatrices for laser desorption/ionization mass spectrometry. Nano Today 2022, 42, 101365. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; Obrien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic caron. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.; Chen, L.; Li, J.; Liu, D.; Chen, L. Recent advances in solid-phase sorbents for sample preparation prior to chromatographic analysis. TrAC Trends Anal. Chem. 2014, 59, 26–41. [Google Scholar] [CrossRef]
- Hayashi, T.; Kim, Y.A.; Natsuki, T.; Endo, M. Mechanical properties of carbon nanomaterials. Chem. Phys. Chem. 2007, 8, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, M.; Asadpour, S.; Sarmast, N.; Dinari, M. A review of the synthesis methods, properties, and applications of layered double hydroxides/carbon nanocomposites. J. Mol. Liq. 2022, 348, 118399. [Google Scholar] [CrossRef]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. C60: A new form of carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- Namin, H.S.; Rahimpour, E.; Ozkan, S.A.; Jouyban, A. An overview on nanostructure-modified supported liquid membranes for the electro membrane extraction method. Anal. Methods-UK 2022, 14, 212–221. [Google Scholar] [CrossRef]
- Vallant, R.M.; Szabo, Z.; Bachmann, S.; Bakry, R.; Najam-ul-Haq, M.; Rainer, M.; Heigl, N.; Petter, C.; Huck, C.W.; Bonn, G.K. Development and application of C60-fullerene bound silica for solid-phase extraction of biomolecules. Anal. Chem. 2007, 79, 8144–8153. [Google Scholar] [CrossRef]
- Alheety, M.A.; Raoof, A.; Al-Jibori, S.A.; Karadağ, A.; Khaleel, A.I.; Akbaş, H.; Uzun, O. Eco-friendly C60-SESMP-Fe3O4 inorganic magnetizable nanocomposite as high-performance adsorbent for magnetic removal of arsenic from crude oil and water samples. Mater. Chem. Phys. 2019, 231, 292–300. [Google Scholar] [CrossRef]
- Ghorbani, M.; Seyedin, O.; Aghamohammadhassan, M. Adsorptive removal of lead (II) ion from water and wastewater media using carbon-based nanomaterials as unique sorbents: A review. J. Environ. Manag. 2020, 254, 109814. [Google Scholar] [CrossRef]
- Journet, C.W.; Maser, W.K.; Bernier, P.; Loiseau, A.; Fischer, J.E. Large-scale production of single-walled carbon nanotubes by the electric-arc technique. Nature 1997, 388, 756–758. [Google Scholar] [CrossRef]
- Ishigami, M.; Cumings, J.; Zettl, A.; Chen, S. A simple method for the continuous production of carbon nanotubes. Chem Phys. Lett. 2000, 319, 457–459. [Google Scholar] [CrossRef]
- Hsin, Y.L.; Hwang, K.C.; Chen, F.R.; Kai, J.J. Production and in-situ metal filling of carbon nanotubes in water. Adv. Mater. 2001, 12, 830–833. [Google Scholar] [CrossRef]
- Zhu, H.W.; Li, X.S.; Jiang, B.; Xu, C.L.; Zhu, Y.F.; Wu, D.H.; Chen, X.H. Formation of carbon nanotubes in water by the electric-arc technique. Chem. Phys. Lett. 2002, 366, 664–669. [Google Scholar] [CrossRef]
- Vittori Antisari, M.; Marazzi, R.; Krsmanovic, R. Synthesis of multiwall carbon nanotubes by electric arc discharge in liquid environments. Carbon 2003, 41, 2393–2401. [Google Scholar] [CrossRef]
- Guo, T.; Nikolaev, P.; Thess, A.; Colbert, D.T.; Smalley, R.E. Catalytic growth of single-walled manotubes by laser vaporization. Chem. Phys. Lett. 1996, 243, 49–54. [Google Scholar] [CrossRef]
- Rinzler, G.A.; Liu, J.; Dai, H.; Nikolaev, P.; Huffman, B.C. Large-scale purification of single-wall carbon nanotubes: Process, product, and characterization. Appl. Phys. A 1998, 67, 29–37. [Google Scholar] [CrossRef]
- Nikolaev, P.; Bronikowski, M.J.; Bradley, R.K.; Rohmund, F.; Colbert, D.T.; Smith, K.A.; Smalley, R.E. Gas-phase catalytic growth of single-walled carbon nanotubes from carbon monoxide. Chem. Phys. Lett. 1999, 313, 91–97. [Google Scholar] [CrossRef]
- Cheng, H.M.; Li, F.; Su, G.; Pan, H.Y.; He, L.L.; Sun, X.; Dresselhaus, G. Large-scale and low-cost synthesis of single-walled carbon nanotubes by the catalytic pyrolysis of hydrocarbons. Appl. Phys. Lett. 1998, 72, 3282–3284. [Google Scholar] [CrossRef]
- Andrews, R.; Jacques, D.; Rao, A.M.; Derbyshire, F.; Qian, D.; Fan, X.; Dickey, E.C.; Chen, J. Continuous production of aligned carbon nanotubes: A step closer to commercial realization. Chem. Phys. Lett. 1999, 303, 467–474. [Google Scholar] [CrossRef]
- Feist, B.; Sitko, R. Method for the determination of Pb, Cd, Zn, Mn and Fe in rice samples using carbon nanotubes and cationic complexes of batophenanthroline. Food Chem. 2018, 249, 38–44. [Google Scholar] [CrossRef]
- Khamirchi, R.; Hosseini-Bandegharaei, A.; Alahabadi, A.; Sivamani, S.; Rahmani-Sani, A.; Shahryari, T.; Anastopoulos, I.; Miri, M.; Tran, H.N. Adsorption property of Br-PADAP-impregnated multiwall carbon nanotubes towards uranium and its performance in the selective separation and determination of uranium in different environmental samples. Ecotox. Environ. Saf. 2018, 150, 136–143. [Google Scholar] [CrossRef]
- Kedziora, K.; Wasiak, W. Extraction media used in needle trap devices-Progress in development and applications. J. Chromatogr. A 2017, 1505, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Shi, J.; Jiang, G. Application of graphene in analytical sample preparation. TrAC Trend Anal. Chem. 2012, 37, 1–11. [Google Scholar]
- Sitko, R.; Zawisza, B.; Malicka, E. Graphene as a new sorbent in analytical chemistry. TrAC Trend Anal. Chem. 2013, 51, 33–43. [Google Scholar] [CrossRef]
- Wang, J.; Mei, X.; Huang, L.; Zheng, Q.; Qiao, Y.; Zang, K.; Mao, S.; Yang, R.; Zhang, Z.; Gao, Y. Synthesis of layered double hydroxides/graphene oxide nanocomposite as a novel high-temperature CO2 adsorbent. J. Energy Chem. 2015, 24, 127–137. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheng, B.; You, W.; Yu, J.; Ho, W.K. 3D hierarchical graphene oxide-NiFe LDH composite with enhanced adsorption affinity to Congo red, methyl orange and Cr (VI) ions. J. Hazard. Mater. 2019, 369, 214–225. [Google Scholar] [CrossRef]
- Somani, P.R.; Somani, S.P.; Umeno, M. Planer nano-graphenes from camphor by CVD. Chem. Phys. Lett. 2006, 430, 56–59. [Google Scholar] [CrossRef]
- Arco, L.D.; Yi, Z.; Kumar, A.; Zhou, C. Synthesis, transfer, and devices of single- and few-layer graphene by chemical vapor deposition. IEEE T Nanotechnol. 2009, 8, 135–138. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.T.; Ho, J.; Nezich, D.; Son, H.B.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical Vapor deposition. Nano Lett. 2008, 9, 30–35. [Google Scholar] [CrossRef]
- Silvestro, I.; Ciarlantini, C.; Francolini, I.; Tomai, P.; Gentili, A.; Dal Bosco, C.; Piozzi, A. Chitosan-graphene oxide composite membranes for solid-phase extraction of pesticides. Int. J. Mol. Sci. 2021, 22, 8374. [Google Scholar] [CrossRef]
- Sun, W.J.; Hu, X.Y.; Meng, X.Y.; Xiang, Y.H.; Ye, N.S. Molybdenum disulfide-graphene oxide composites as dispersive solid-phase extraction adsorbents for the enrichment of four paraben preservatives in cosmetics. Microchim. Acta 2021, 188, 256. [Google Scholar] [CrossRef]
- Yan, W.F.; Qiu, S.L. Research progress of porous materials. Chemistry 2014, 77, 703–708. [Google Scholar]
- Zhang, C.H.; Li, G.J.; Wang, J.H. Application of nanopore and porous materials for heavy metal ion detection. Chin. J. Anal. Chem. 2014, 42, 607–615. [Google Scholar] [CrossRef]
- Zhang, H.F.; Cooper, A.I. Synthesis and applications of emulsion-templated porous materials. Soft Matter. 2005, 1, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Llewellyn, P.; Maurin, G. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Academic Press: Cambridge, MA, USA, 1999; pp. 163–165. [Google Scholar]
- Peng, G.L.; Sharshir, S.W.; Wang, Y.P.; An, M.; Ma, D.K.; Zang, J.F.; Kabeel, A.E.; Yang, N.U. Potential and challenges of improving solar still by micro/nano-particles and porous materials-A review. J. Clean. Prod. 2021, 311, 127432. [Google Scholar] [CrossRef]
- Suga, M.; Asahina, S.; Sakuda, Y.; Kazumori, H.; Nishiyama, H.; Nokuo, T.; Alfredsson, V.; Kjellman, T.; Stevens, S.M.; Cho, H.S.; et al. Recent progress in scanning electron microscopy for the characterization of fine structural details of nano materials. Prog. Solid. State. Chem. 2014, 42, 1–21. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, G.M.; Li, H.L. Selective binding and removal of guests in a microporous metal-organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Li, X.J.; Ma, W.; Li, H.M.; Zhang, Q.H.; Liu, H.W. Sulfur-functionalized metal-organic frameworks: Synthesis and applications as advanced adsorbents. Coordin. Chem. Rev. 2020, 408, 213191. [Google Scholar] [CrossRef]
- Wu, Y.L.; Chen, H.L.; Chen, Y.J.; Sun, N.A.R.; Deng, C.H. Metal organic frameworks as advanced extraction adsorbents for separation and analysis in proteomics and environmental research. Sci. China Chem. 2022, 65, 650–677. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Fourmentin, M.; Ribeiro, A.R.L.; Noutsopoulos, C.; Mapelli, F.; Fenyvesi, E.; Vieira, M.G.A.; Picos-Corrales, L.A.; Moreno-Pirajan, J.C.; et al. Removal of emerging contaminants from wastewater using advanced treatments. Rev. Environ. Chem. Lett. 2022, 20, 1333–1375. [Google Scholar] [CrossRef]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): A review. J. Hazard. Mater. 2013, 244, 444–456. [Google Scholar] [CrossRef]
- Barea, E.; Montoro, C.; Navarro, J.A.R. Toxic gas removal--metal-organic frameworks for the capture and degradation of toxic gases and vapours. Chem. Soc. Rev. 2014, 43, 5419–5430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, F.; Qiu, L.G.; Yuan, Y.P.; Peng, F.M.; Jiang, X.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. Thiol-functionalization of metal-organic framework by a facile coordination-based postsynthetic strategy and enhanced removal of Hg2+ from water. J. Hazard. Mater. 2011, 196, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Shekhah, O.; Liu, J.; Fischer, R.A.; Woll, C. MOF thin films: Existing and future applications. Chem. Soc. Rev. 2011, 40, 1081–1106. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Masel, R.I. Rapid production of metal-organic frameworks via microwave-assisted solvothermal synthesis. J. Am. Chem. Soc. 2006, 128, 12394–12395. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ma, J.P.; Wang, S.S.; Chai, H.N.; Guo, L.; Li, J.H.; Ostovan, A.; Guan, Y.F.; Chen, L.X. Cationic metal-organic framework based mixed-matrix membrane for extraction of phenoxy carboxylic acid (PCA) herbicides from water samples followed by UHPLC-MS/MS determination. J. Hazard. Mater. 2020, 394, 122556. [Google Scholar] [CrossRef] [PubMed]
- Daliran, S.; Miri, M.G.; Oveisi, A.R.; Khajeh, M.; Navalon, S.; Alvaro, M.; Ghaffari-Moghaddam, M.; Delarami, H.S.; Garcia, H. A pyridyltriazol functionalized zirconium metal-organic framework for selective and highly efficient adsorption of palladium. ACS Appl. Mater. Interfaces 2020, 12, 25221–25232. [Google Scholar] [CrossRef]
- Amini, S.; Ebrahimzadeh, H.; Seidi, S.; Jalilian, N. Preparation of polyacrylonitrile/Ni-MOF electrospun nanofiber as an efficient fiber coating material for headspace solid-phase microextraction of diazinon and chlorpyrifos followed by CD-IMS analysis. Food. Chem. 2021, 350, 129242. [Google Scholar] [CrossRef]
- Cote, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crustalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [Green Version]
- El-Kaderi, H.M.; Hunt, J.R.; Mendoza-Cortes, J.L.; Cote, A.P.; Taylor, R.E.; O’Keeffe, M.; Yaghi, O.M. Designed synthesis of 3D covalent organic frameworks. Science 2007, 316, 268–272. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.M.; Gan, L.; Zhang, Y. Matrix effect-free on-line pass-through cleanup procedure for the fast determination of local anesthetic drug by LC-MS/MS. J. Chromatogr. B 2019, 1130–1131, 121831. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.L.; Li, C.R.; Li, Q.L.; Zhang, S.X.; Lv, F.; Yan, Z.M. Electrospinning fabrication of covalent organic framework composite nanofibers for pipette tip solid phase extraction of tetracycline antibiotics in grass carp and duck. J. Chromatogr. A 2020, 1622, 461098. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.B.; Wu, H.W.; Liao, Y.F.; Rui, Q.H.; Zhu, Y.; Zhang, Y. Application of petal-shaped ionic liquids modified covalent organic frameworks for one step cleanup and extraction of general anesthetics in human plasma samples. Talanta 2020, 210, 120652. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, M.V.; Khim, Z.F. Adsorption properties and structure of silica gel. J. Phys. Chem. 1931, 2, 799–805. [Google Scholar]
- Fischer, L.; Müller, R.; Ekberg, B.; Mosbach, K. Direct enantioseparation of β–adrenergic blocker using a chiral stationary phase prepared by molecular imprinting. J. Am. Chem. Soc. 1991, 113, 9358–9360. [Google Scholar] [CrossRef]
- Belbruno, J.J. Molecularly imprinted polymers. Chem. Rev. 2019, 199, 94–119. [Google Scholar] [CrossRef]
- Kumar, V.; Kim, K.H. Use of molecular imprinted polymers as sensitive/selective luminescent sensing probes for pesticides/herbicides in water and food samples. Environ. Pollut. 2022, 299, 118824. [Google Scholar] [CrossRef]
- Aylaz, G.; Kuhn, J.; Lau, E.C.H.T.; Yeung, C.C.; Roy, V.A.L.; Duman, M.; Yiu, H.H.P. Recent developments on magnetic molecular imprinted polymers (MMIPs) for sensing, capturing, and monitoring pharmaceutical and agricultural pollutants. J. Chem. Technol. Biot. 2021, 96, 1151–1160. [Google Scholar] [CrossRef]
- Beyazit, S.; Bui, B.T.S.; Haupt, K.; Gonzato, C. Molecularly imprinted polymer nanomaterials and nanocomposites by controlled/living radical polymerization. Prog. Polym. Sci. 2016, 62, 1–21. [Google Scholar] [CrossRef]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerda, V. Solid-phase extraction of organic compounds: A critical review (Part I). TrAC Trends. Anal. Chem. 2016, 80, 641–654. [Google Scholar] [CrossRef]
- Hu, T.L.; Chen, R.; Wang, Q.; He, C.Y.; Liu, S.R. Recent advances and applications of molecularly imprinted polymers in solid-phase extraction for real sample analysis. J. Sep. Sci. 2021, 44, 274–309. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.; Wang, X.Y.; Lu, W.H.; Wu, X.Q.; Li, J.H. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.; Xu, S.F.; Li, J.H. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef] [PubMed]
- Saad, E.M.; El Gohary, N.A.; Abdel-Halim, M.; Handoussa, H.; El Nashar, R.M.; Mizaikoff, B. Molecularly imprinted polymers for selective extraction of rosmarinic acid from Rosmarinus officinalis L. Food Chem. 2021, 335, 127644. [Google Scholar] [CrossRef]
- Li, Z.Z.; Chen, X.J.; Zhang, X.W.; Wang, Y.; Li, D.M.; Gao, H.L.; Duan, X. Selective solid-phase extraction of four phenylarsonic compounds from feeds, edible chicken and pork with tailoring imprinted polymer. Food Chem. 2021, 347, 129054. [Google Scholar] [CrossRef]
- Abdar, A.; Amiri, A.; Mirzaei, M. Semi-automated solid-phase extraction of polycyclic aromatic hydrocarbons based on stainless steel meshes coated with metal–organic framework/graphene oxide. Microchem. J. 2020, 17, 107269. [Google Scholar] [CrossRef]
- Feng, J.B.; Li, Y.Y.; Zhang, Y.; Xu, Y.Y.; Cheng, X.W. Adsorptive removal of indomethacin and diclofenac from water by polypyrrole doped-GO/COF-300 nanocomposites. Chem. Eng. J. 2022, 429, 132499. [Google Scholar] [CrossRef]
- Wu, F.F.; Chen, Q.Y.; Ma, X.J.; Li, T.T.; Wang, L.F.; Hong, J.; Sheng, Y.H.; Ye, M.L.; Zhu, Y. N-doped magnetic covalent organic frameworks for preconcentration of allergenic disperse dyes in textiles of fall protection equipment. Anal. Methods-UK 2019, 11, 3381–3387. [Google Scholar] [CrossRef]
- Lu, D.K.; Qin, M.H.; Liu, C.; Deng, J.J.; Shi, G.Y.; Zhou, T.S. Ionic liquid-functionalized magnetic metal-organic framework nanocomposites for efficient extraction and sensitive detection of fluoroquinolone antibiotics in environmental water. ACS. Appl. Mater. Interfaces 2021, 13, 5357–5367. [Google Scholar] [CrossRef]
- Li, F.; Li, X.X.; Su, J.; Li, Y.J.; He, X.W.; Chen, L.X.; Zhang, Y.K. Hydrophilic molecularly imprinted polymers functionalized magnetic carbon nanotubes for selective extraction of cyclic adenosine monophosphate from winter jujube. J. Sep. Sci. 2021, 44, 2131–2142. [Google Scholar] [CrossRef]
- Cheng, H.L.; Wang, F.L.; Zhao, Y.G.; Zhang, Y.; Jin, M.C.; Zhu, Y. Simultaneous determination of fifteen toxic alkaloids in meat dishes and vegetable dishes using double layer pipette tip magnetic dispersive solid phase extraction followed by UFLC-MS/MS. Anal. Methods-UK 2018, 10, 1151–1162. [Google Scholar] [CrossRef]
- Lu, Q.; Guo, H.; Zhang, Y.Y.; Tang, X.D.; Lei, W.B.; Qi, R.J.; Chu, J.H.; Li, D.Z.; Zhao, Q.B. Graphene oxide-Fe3O4 nanocomposite magnetic solid phase extraction followed by UHPLC-MS/MS for highly sensitive determination of eight psychoactive drugs in urine samples. Talanta 2020, 206, 120212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.G.; Li, X.P.; Yao, S.S.; Zhao, P.P.; Liu, J.C.; Xu, C.P.; Lu, Y.Y.; Chen, X.H.; Jin, M.C. Fast throughput determination of 21 allergenic disperse dyes from river water using reusable three-dimensional interconnected magnetic chemically modified graphene oxide followed by liquid chromatography–tandem quadrupole mass spectrometry. J. Chromatogr. A 2016, 1431, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.G.; Zhang, Y.; Wang, F.L.; Zhou, J.; Zhao, Q.M.; Zeng, X.Q.; Hu, M.Q.; Jin, M.C.; Zhu, Y. Determination of perchlorate from tea leaves using quaternary ammonium modified magnetic carboxyl-carbon nanotubes followed by liquid chromatography-tandem quadrupole mass spectrometry. Talanta 2018, 185, 411–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhao, Y.G.; Chen, W.S.; Cheng, H.L.; Zeng, X.Q.; Zhu, Y. Three-dimensional ionic liquid-ferrite functionalized graphene oxide nanocomposite for pipette-tip solid phase extraction of 16 polycyclic aromatic hydrocarbons in human blood sample. J. Chromatogr. A 2018, 1552, 1–9. [Google Scholar] [CrossRef] [PubMed]




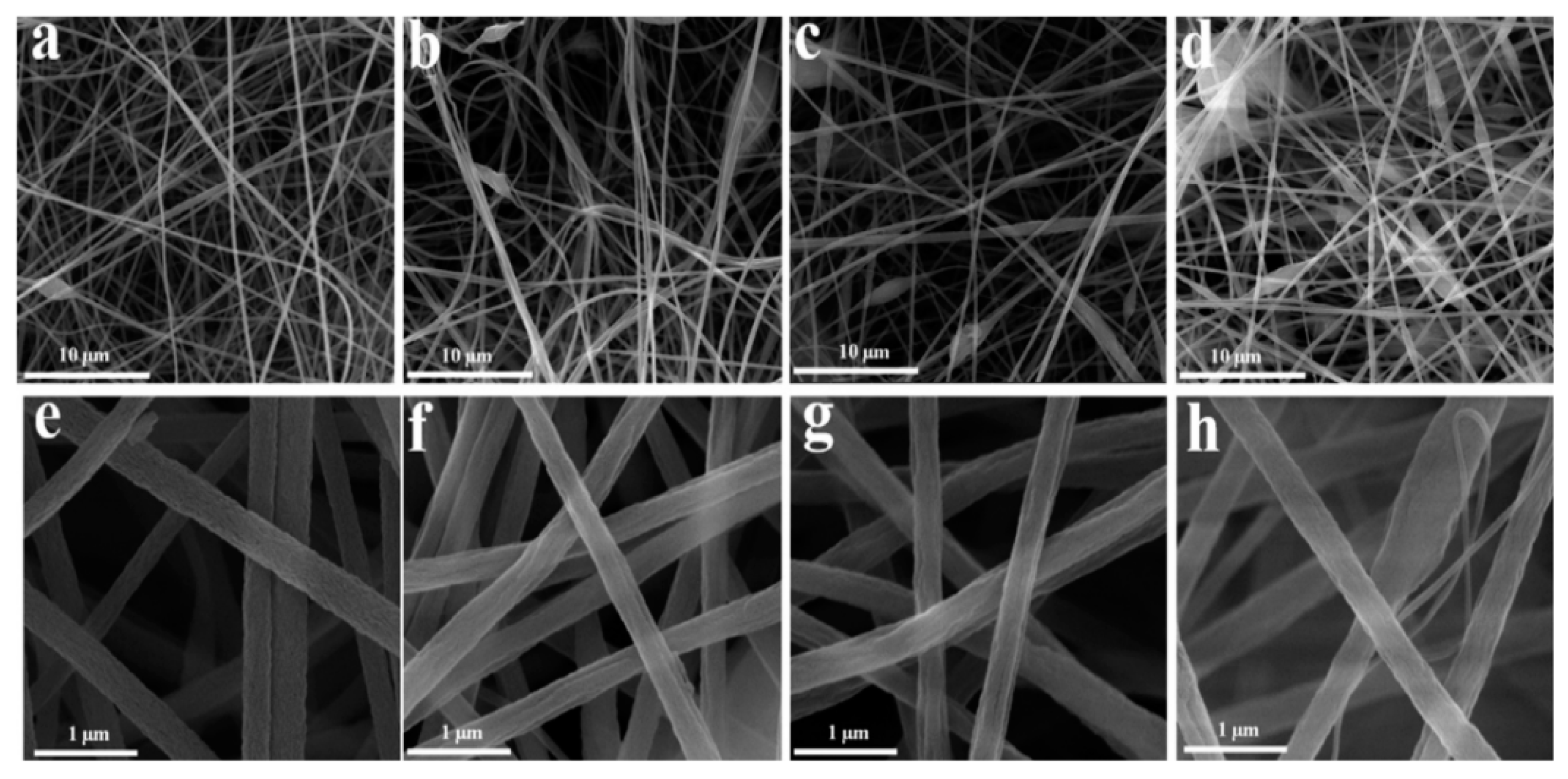
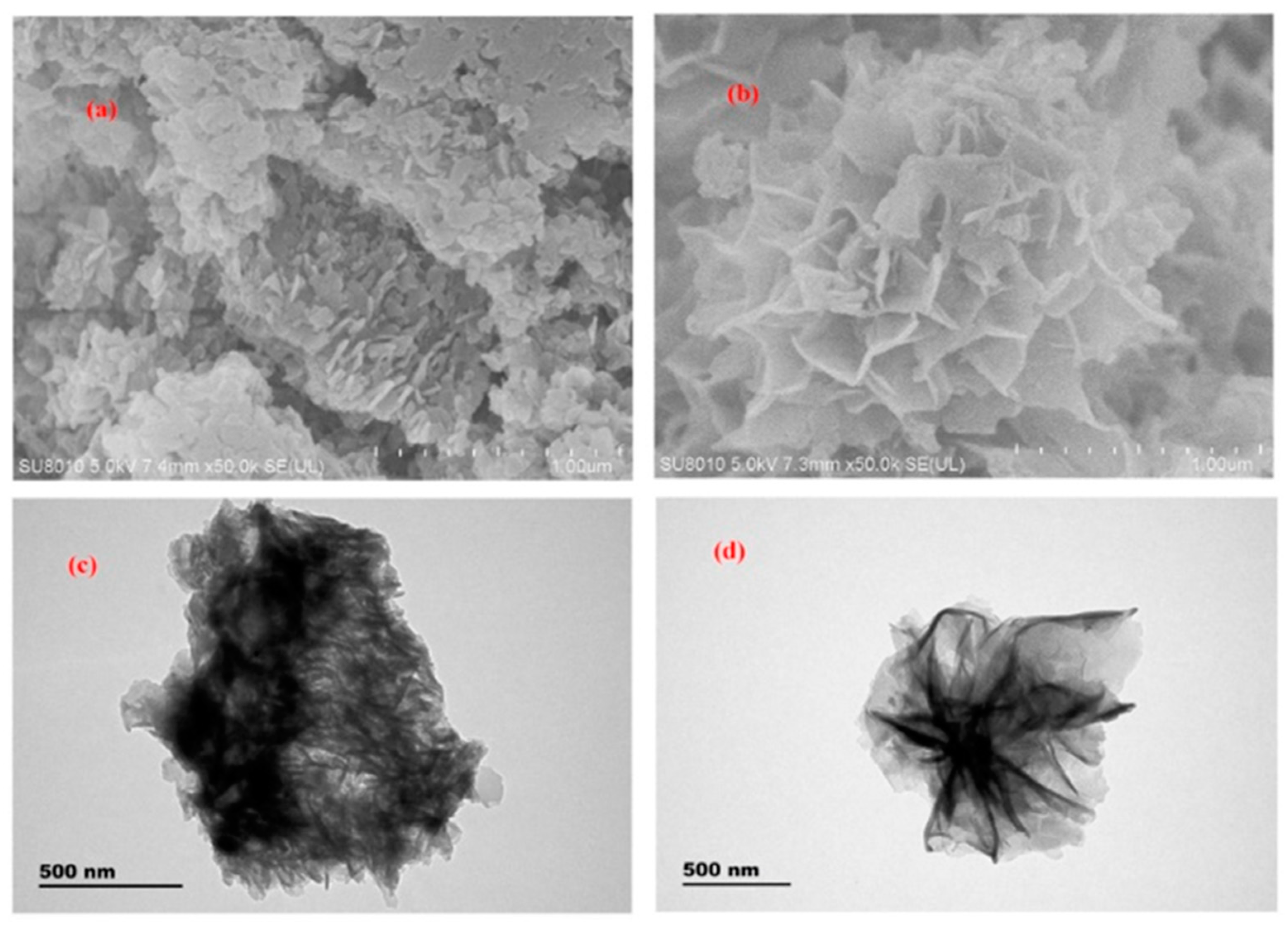
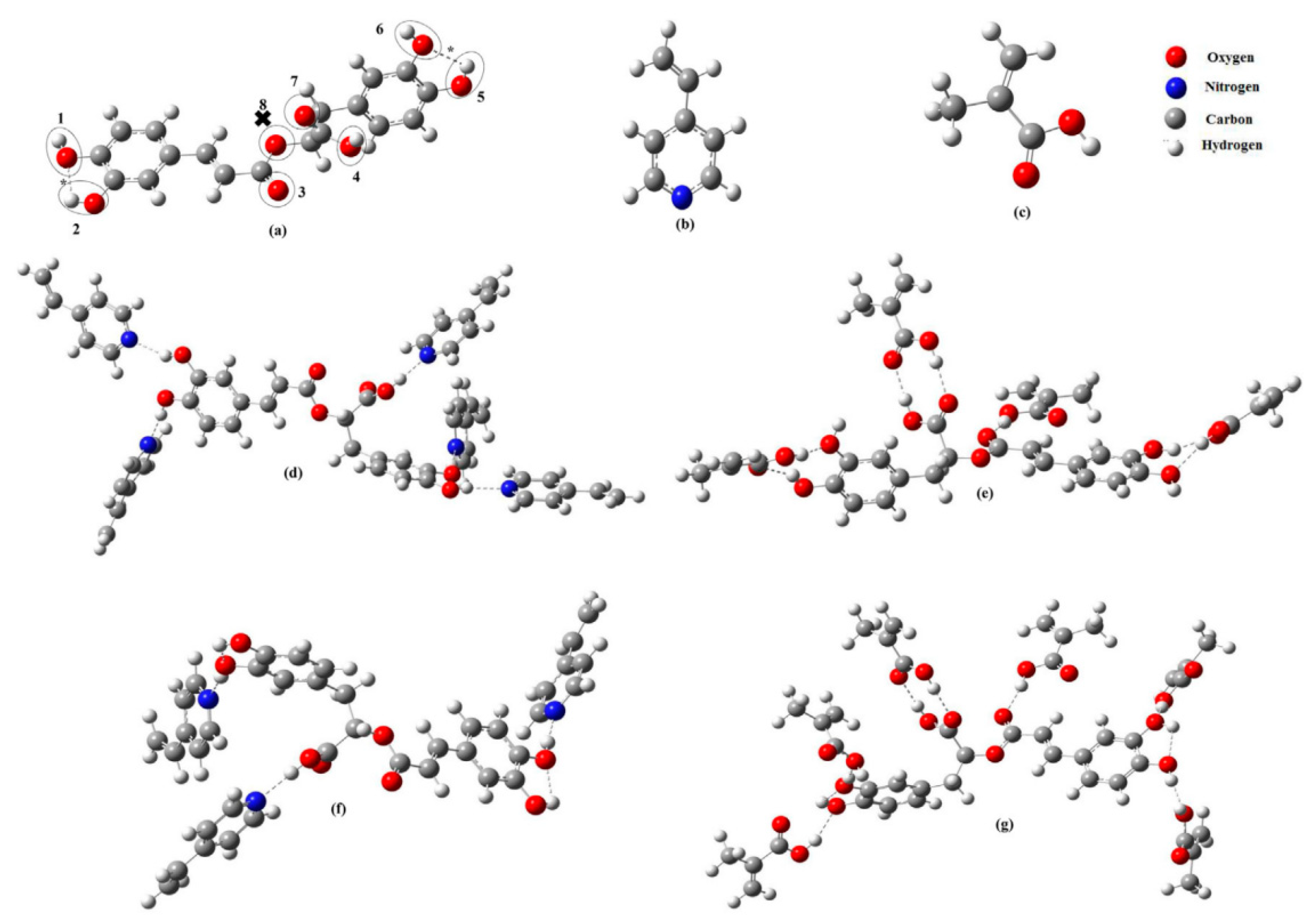
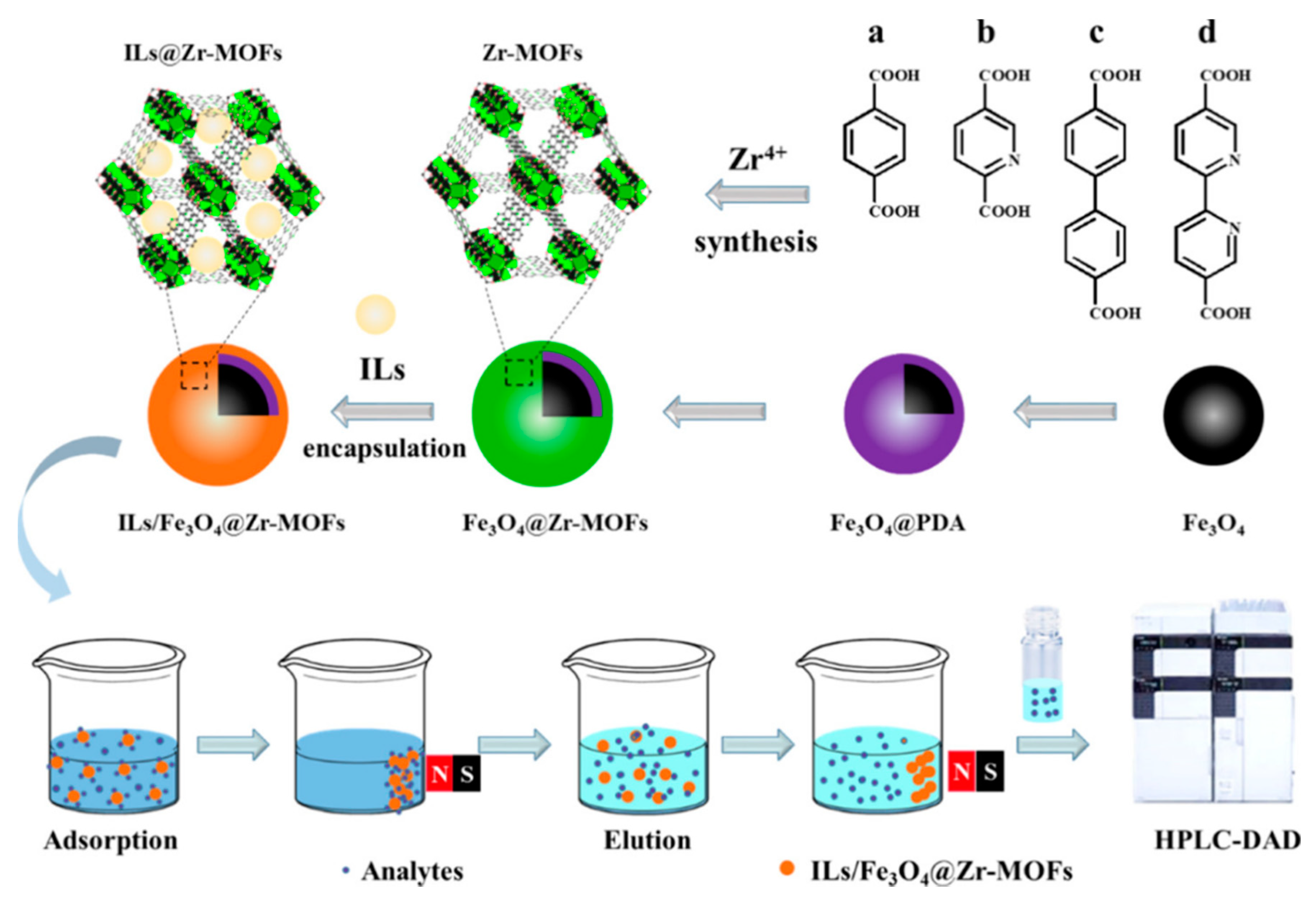
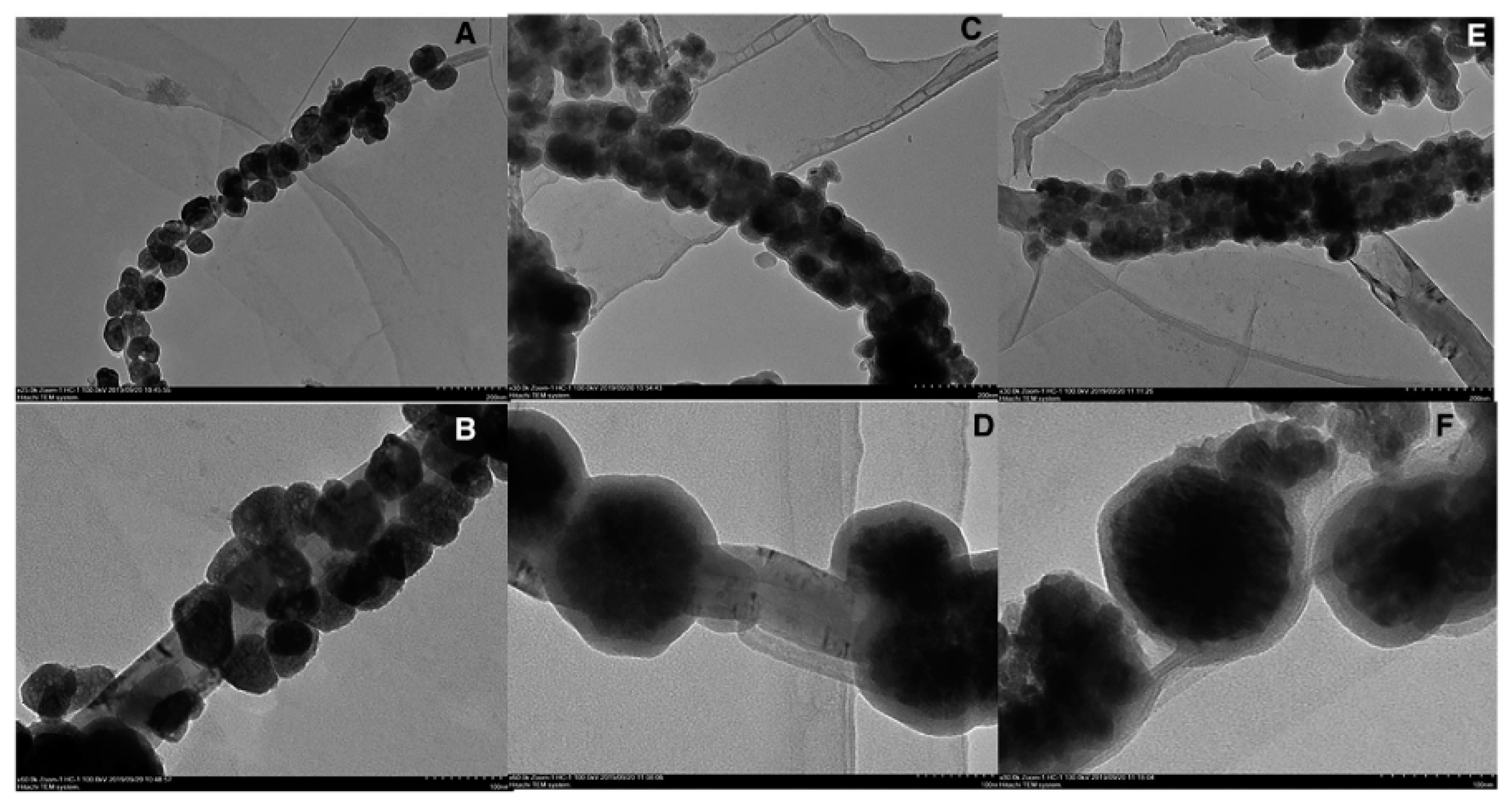
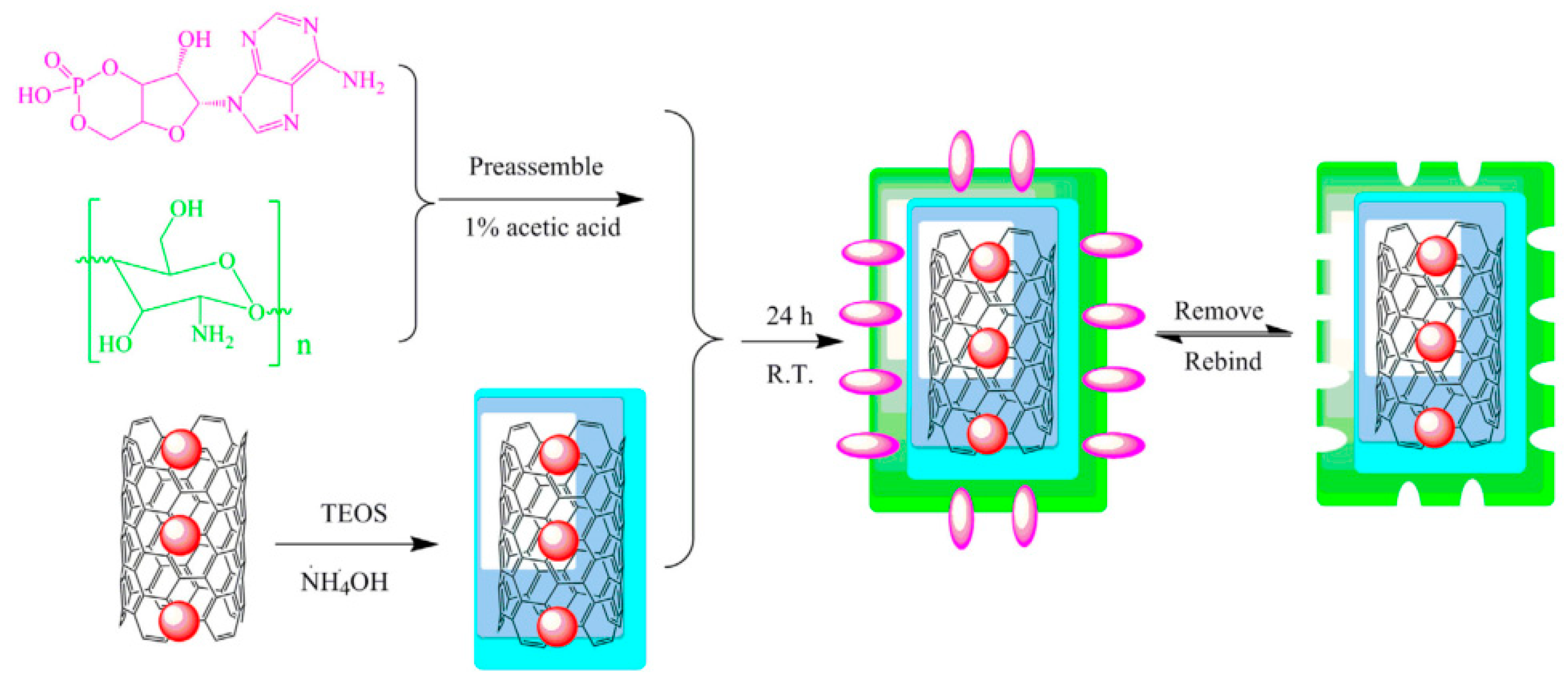
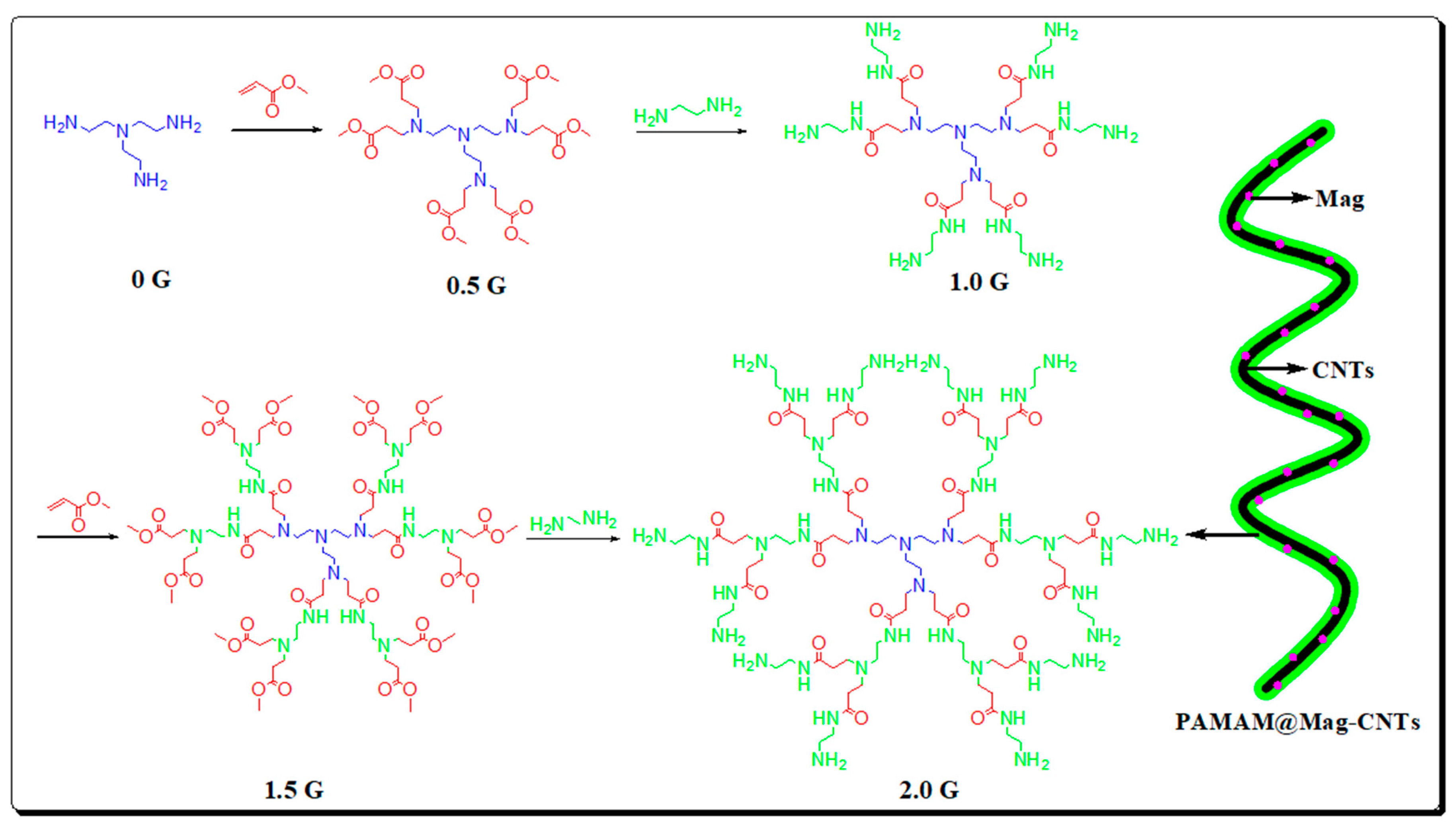


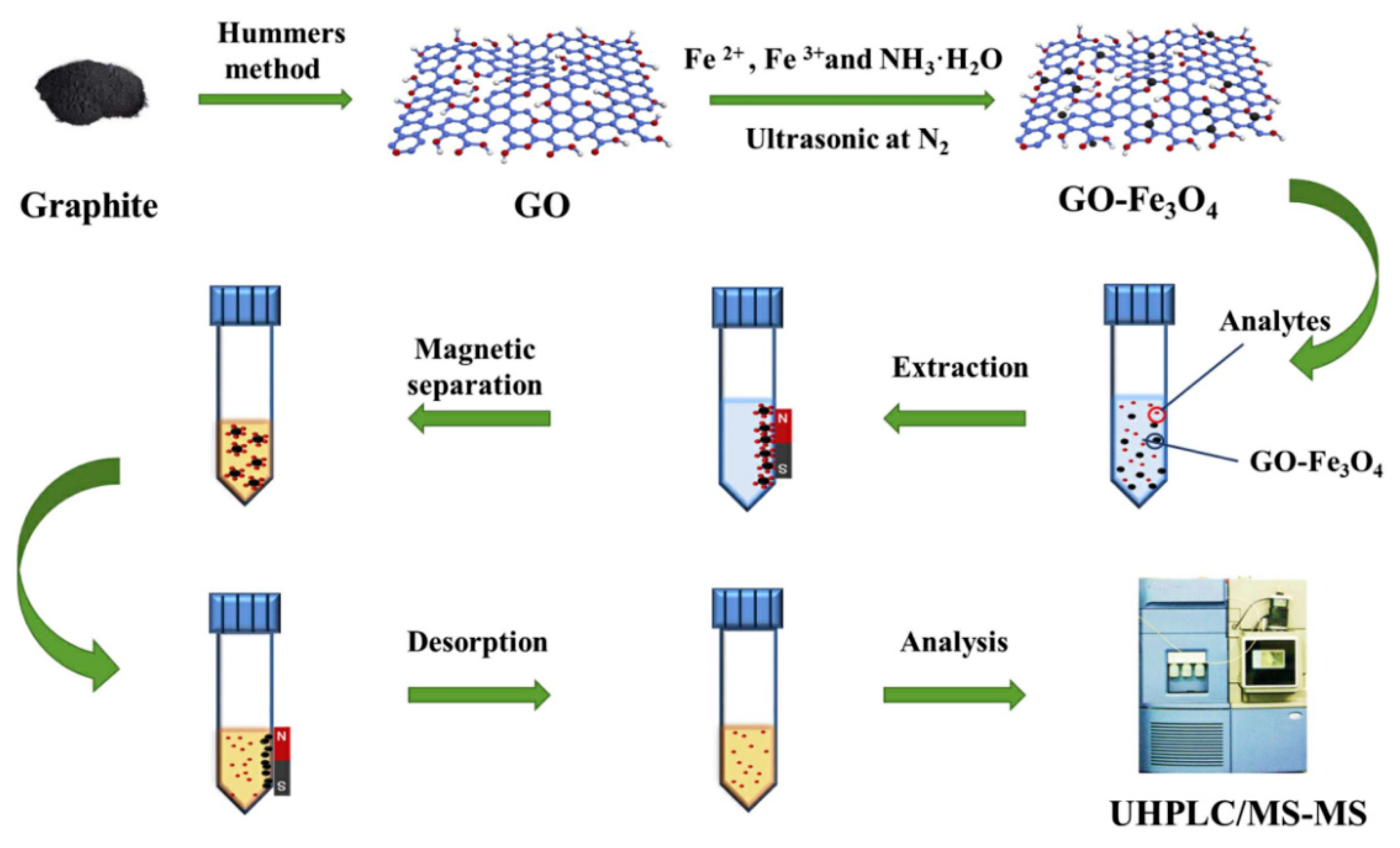
| Sample | Target Analyte | Adsorbent | Pretreatment Method | Detection | LOD (µg/mL) | Recovery (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Crude oil | As(III) As(V) | C60-SESMP-Fe3O4 | MSPE | ICP-OES | 0.047–0.032 | 96.5–98.0 | [48] |
| Wild rice | Pb (II) | ox-MWCNTs/ batophenanth | DMSPE | FAAS | 0.25 | 93.8 | [60] |
| Sewage water | U(VI) | Br-PADAP/MWCNT | MSPE | ICP-AES | 0.14 | 99.7 | [61] |
| Cosmetics | Parabens | MoS2/GO | DSPE | UPLC-PDA | 0.40~2.3 | 91.3–124 | [71] |
| Sewage water | PCAs | UIO-66-NMe3+MMM | DME | UHPLC-MS/MS | 0.030–0.59 | 80.1–117 | [88] |
| Tap water | Pd(II) | UIO-66-Pyta | SPE | ICP-AES | 1.9 | 99.0 | [89] |
| Duck, Grass Carp | TCs | PAN@COF-SCU1 | PT-SPE | HPLC | 0.60–3.0 | 84.0–117 | [94] |
| Human Plasma prior | Anesthetics | PS-IL-COFS | OSCE | LC-MS/MS | 0.016–0.18 | 82.5–115 | [95] |
| Rice, apples, green groceries | PCAs | TAPT-DHTA-COF | SPE | LC-MS/MS | 0.0070–0.030 | 81.2–107 | [96] |
| River water | OPPS | PAN/Ni-MOF | HS-SPME | CD-IMS | 0.20–0.30 | 87.0–98.0 | [90] |
| River water | FQS | IL-COOH/Fe3O4@Zr-MOF | MSPE | HPLC-DAD | <0.020 | 90.0–110 | [111] |
| Meat, vegetables | Toxic alkaloids | PAMAM@Mag-CNTs | DPT-MSPE | UFLC-MS/MS | 0.011~0.329 | 83.4–125 | [113] |
| Urine | Psychoactive drugs | GO-Fe3O4 | MSPE | UHPLC-MS/MS | 0.020–0.20 | 80.4–105 | [114] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.-X.; Song, S.; Ye, M.-L.; Zhu, Y.; Zhao, Y.-G.; Lu, Y. Nanomaterials with Excellent Adsorption Characteristics for Sample Pretreatment: A Review. Nanomaterials 2022, 12, 1845. https://doi.org/10.3390/nano12111845
Liu W-X, Song S, Ye M-L, Zhu Y, Zhao Y-G, Lu Y. Nanomaterials with Excellent Adsorption Characteristics for Sample Pretreatment: A Review. Nanomaterials. 2022; 12(11):1845. https://doi.org/10.3390/nano12111845
Chicago/Turabian StyleLiu, Wen-Xin, Shuang Song, Ming-Li Ye, Yan Zhu, Yong-Gang Zhao, and Yin Lu. 2022. "Nanomaterials with Excellent Adsorption Characteristics for Sample Pretreatment: A Review" Nanomaterials 12, no. 11: 1845. https://doi.org/10.3390/nano12111845
APA StyleLiu, W.-X., Song, S., Ye, M.-L., Zhu, Y., Zhao, Y.-G., & Lu, Y. (2022). Nanomaterials with Excellent Adsorption Characteristics for Sample Pretreatment: A Review. Nanomaterials, 12(11), 1845. https://doi.org/10.3390/nano12111845








