Accelerated Formation of 2D Ruddlesden—Popper Perovskite Thin Films by Lewis Bases for High Efficiency Solar Cell Applications
Abstract
:1. Introduction
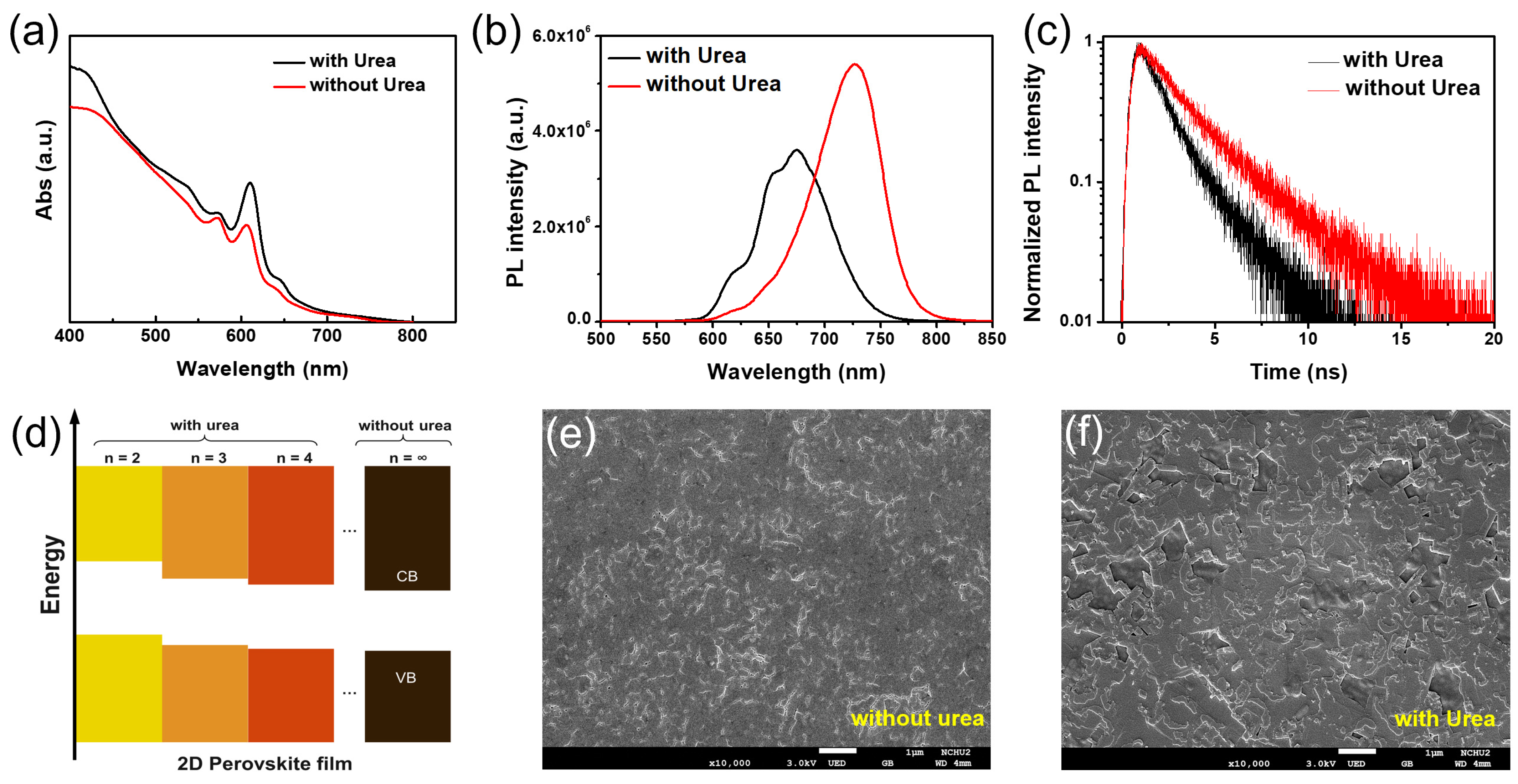
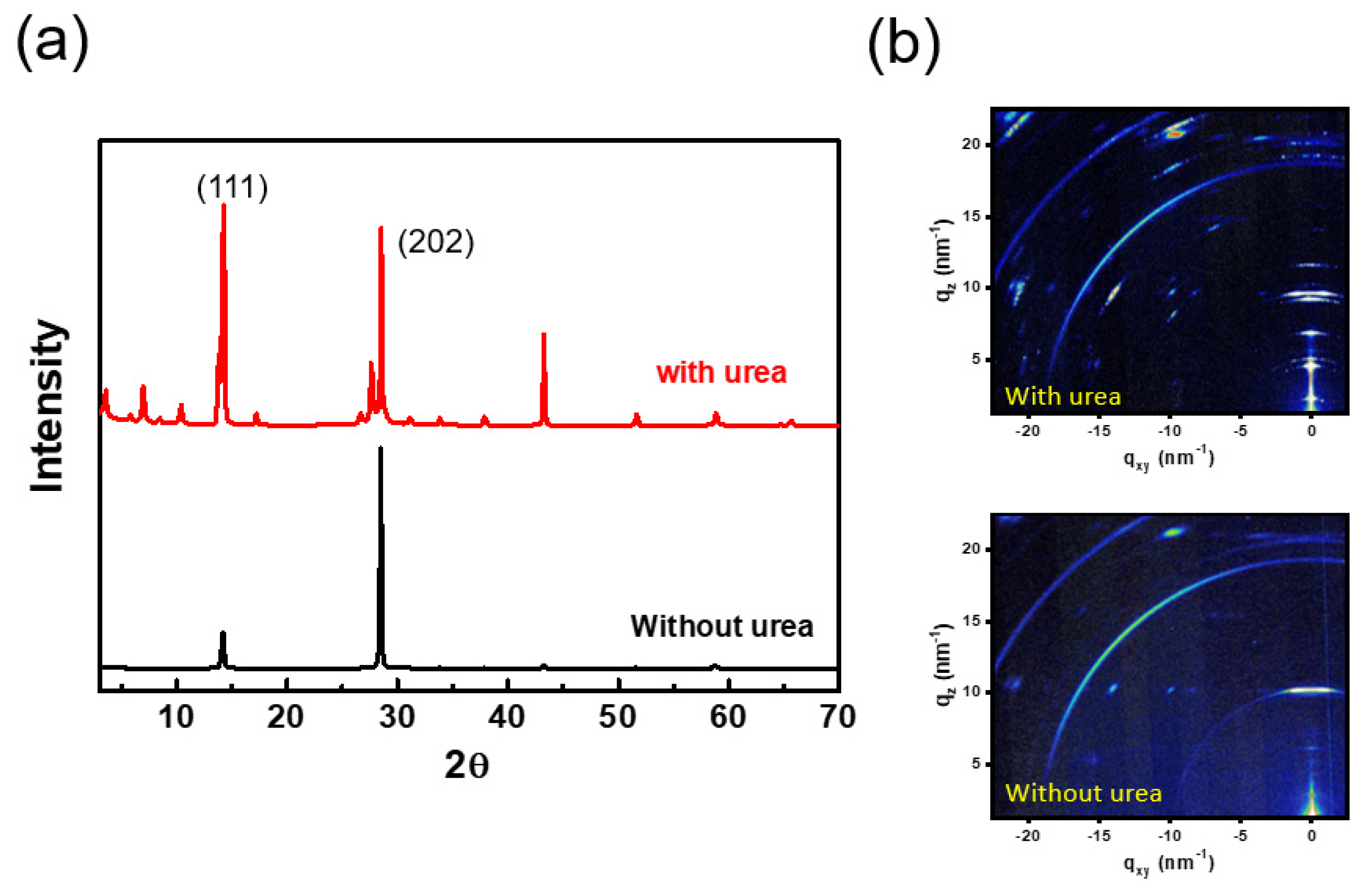
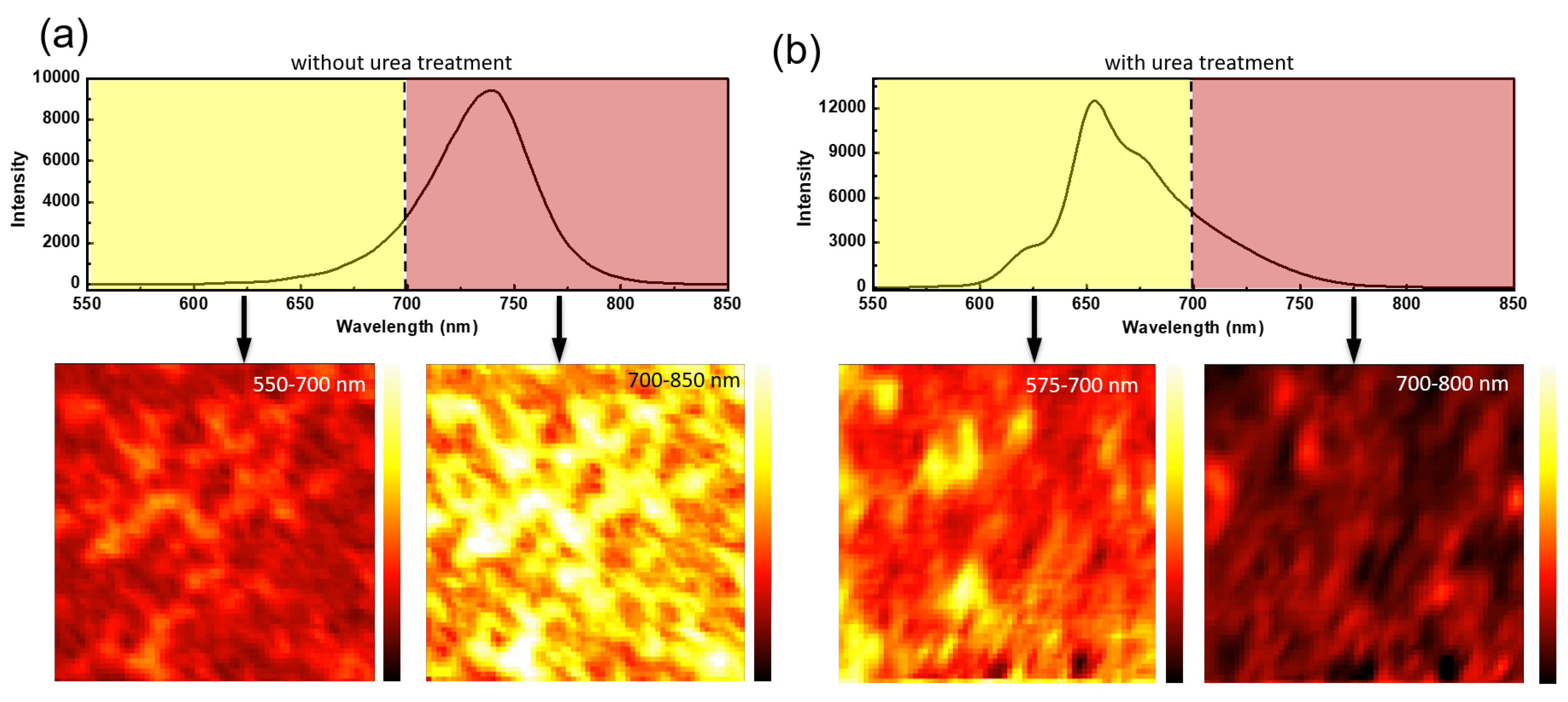
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. Chemicals
4.2. The Precursor Preparation of 2D RP Perovskite Thin Film
4.3. The Preparation of ITO Substrate
4.4. Device Fabrication
4.5. Characterization
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, P.; Xu, Z.; Li, J.; Liu, Y.; Fan, Y.; Yu, L.; Smilgies, D.-M.; Müller, C.; Zhao, K.; Liu, S.F. Highly Efficient Ruddlesden—Popper Halide Perovskite PA2MA4Pb5I16 Solar Cells. ACS Energy Lett. 2018, 3, 1975–1982. [Google Scholar] [CrossRef]
- Tsai, H.; Nie, W.; Blancon, J.C.; Stoumpos, C.C.; Soe, C.M.M.; Yoo, J.; Crochet, J.; Tretiak, S.; Even, J.; Sadhanala, A.; et al. Stable Light-Emitting Diodes Using Phase-Pure Ruddlesden-Popper Layered Perovskites. Adv. Mater. 2018, 30, 1704217. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, N.R.; Labram, J.G.; Chabinyc, M.L. Charge-carrier dynamics and crystalline texture of layered Ruddlesden—Popper hybrid lead iodide perovskite thin films. ACS Energy Lett. 2018, 3, 380–386. [Google Scholar] [CrossRef]
- Zhang, T.; Long, M.; Qin, M.; Lu, X.; Chen, S.; Xie, F.; Gong, L.; Chen, J.; Chu, M.; Miao, Q.; et al. Stable and Efficient 3D-2D Perovskite-Perovskite Planar Heterojunction Solar Cell without Organic Hole Transport Layer. Joule 2018, 2, 2706–2721. [Google Scholar] [CrossRef] [Green Version]
- Vashishtha, P.; Ng, M.; Shivarudraiah, S.B.; Halpert, J.E. High efficiency blue and green light-emitting diodes using Ruddlesden—Popper inorganic mixed halide perovskites with butylammonium interlayers. Chem. Mater. 2019, 31, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-K.; Gao, F. Organic–inorganic hybrid Ruddlesden—Popper perovskites: An emerging paradigm for high-performance light-emitting diodes. J. Phys. Chem. Lett. 2018, 9, 2251–2258. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Lu, D.; Xu, Z.; Lai, H.; Liu, Y. 2-Thiopheneformamidinium-based 2D Ruddlesden—Popper perovskite solar cells with efficiency of 16.72% and negligible hysteresis. Adv. Energy Mater. 2020, 10, 2000694. [Google Scholar] [CrossRef]
- Wei, Y.; Chu, H.; Tian, Y.; Chen, B.; Wu, K.; Wang, J.; Yang, X.; Cai, B.; Zhang, Y.; Zhao, J. Reverse-graded 2D Ruddlesden—Popper perovskites for efficient air-stable solar cells. Adv. Energy Mater. 2019, 9, 1900612. [Google Scholar] [CrossRef]
- Zhang, H.; Liao, Q.; Wu, Y.; Zhang, Z.; Gao, Q.; Liu, P.; Li, M.; Yao, J.; Fu, H. 2D Ruddlesden—Popper perovskites microring laser array. Adv. Mater. 2018, 30, 1706186. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Y.; Liao, Q.; Zhang, Z.; Liu, Y.; Gao, Q.; Liu, P.; Li, M.; Yao, J.; Fu, H. A Two-Dimensional Ruddlesden-Popper Perovskite Nanowire Laser Array based on Ultrafast Light-Harvesting Quantum Wells. Angew. Chem. 2018, 57, 7748–7752. [Google Scholar] [CrossRef]
- Tsai, H.; Nie, W.; Blancon, J.C.; Stoumpos, C.C.; Asadpour, R.; Harutyunyan, B.; Neukirch, A.J.; Verduzco, R.; Crochet, J.J.; Tretiak, S.; et al. High-efficiency two-dimensional Ruddlesden-Popper perovskite solar cells. Nature 2016, 536, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Soe, C.M.M.; Nie, W.; Stoumpos, C.C.; Tsai, H.; Blancon, J.C.; Liu, F.; Even, J.; Marks, T.J.; Mohite, A.D.; Kanatzidis, M.G. Understanding Film Formation Morphology and Orientation in High Member 2D Ruddlesden—Popper Perovskites for High-Efficiency Solar Cells. Adv. Energy Mater. 2017, 8, 1700979. [Google Scholar] [CrossRef]
- Kim, E.-B.; Akhtar, M.S.; Shin, H.-S.; Ameen, S.; Nazeeruddin, M.K. A review on two-dimensional (2D) and 2D-3D multidimensional perovskite solar cells: Perovskites structures, stability, and photovoltaic performances. J. Photochem. Photobiol. C 2021, 48, 100405. [Google Scholar] [CrossRef]
- Gharibzadeh, S.; Hossain, I.M.; Fassl, P.; Nejand, B.A.; Abzieher, T.; Schultes, M.; Ahlswede, E.; Jackson, P.; Powalla, M.; Schäfer, S.; et al. 2D/3D Heterostructure for Semitransparent Perovskite Solar Cells with Engineered Bandgap Enables Efficiencies Exceeding 25% in Four—Terminal Tandems with Silicon and CIGS. Adv. Funct. Mater. 2020, 30, 1909919. [Google Scholar] [CrossRef] [Green Version]
- Tong, Y.; Najar, A.; Wang, L.; Liu, L.; Du, M.; Yang, J.; Li, J.; Wang, K.; Liu, S. Wide-Bandgap Organic—Inorganic Lead Halide Perovskite Solar Cells. Adv. Sci. 2022, 9, 2105085. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.S.; Naidu, K.C.B. A review on perovskite solar cells (PSCs), materials and applications. J. Mater. 2021, 7, 940–956. [Google Scholar]
- Zhen, J.; Zhou, W.; Chen, M.; Li, B.; Jia, L.; Wang, M.; Yang, S. Pyridine-functionalized fullerene additive enabling coordination interactions with CH3NH3PbI3 perovskite towards highly efficient bulk heterojunction solar cells. J. Mater. Chem. A 2019, 7, 2754–2763. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, L.; Deng, L.; Ren, L.; Zhao, N.; Huang, J.; Yu, Y.; Gao, P. Fused dithienopicenocarbazole enabling high mobility dopant-free hole-transporting polymers for efficient and stable perovskite solar cells. ACS Appl. Mater. Interfaces 2021, 13, 6688–6698. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, K. Additive engineering for efficient and stable perovskite solar cells. Adv. Energy Mater. 2020, 10, 1902579. [Google Scholar] [CrossRef]
- Lin, C. Stabilizing organic–inorganic lead halide perovskite solar cells with efficiency beyond 20%. Front. Chem. 2020, 8, 592. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, Y.; Zhang, P.; Shi, J.; Zhao, Y.; Zhang, H.; Wu, J.; Luo, Y.; Li, D.; Meng, Q. Investigation on the role of Lewis bases in the ripening process of perovskite films for highly efficient perovskite solar cells. J. Mater. Chem. A 2017, 5, 20874–20881. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, A.; Zou, T.; Jung, H.; Heo, S.; Noh, Y.-Y. A Lewis base and boundary passivation bifunctional additive for high performance lead-free layered-perovskite transistors and phototransistors. Mater. Today Energy 2021, 21, 100722. [Google Scholar] [CrossRef]
- Patil, J.V.; Mali, S.S.; Hong, C.K. A thiourea additive-based quadruple cation lead halide perovskite with an ultra-large grain size for efficient perovskite solar cells. Nanoscale 2019, 11, 21824–21833. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Bae, S.-H.; Hsieh, Y.-T.; De Marco, N.; Wang, M.; Sun, P.; Yang, Y. A bifunctional lewis base additive for microscopic homogeneity in perovskite solar cells. Chem 2017, 3, 290–302. [Google Scholar] [CrossRef]
- Hsieh, C.-M.; Liao, Y.-S.; Lin, Y.-R.; Chen, C.-P.; Tsai, C.-M.; Diau, E.W.-G.; Chuang, S.-C. Low-temperature, simple and efficient preparation of perovskite solar cells using Lewis bases urea and thiourea as additives: Stimulating large grain growth and providing a PCE up to 18.8%. RSC Adv. 2018, 8, 19610–19615. [Google Scholar] [CrossRef] [Green Version]
- Stoumpos, C.C.; Cao, D.H.; Clark, D.J.; Young, J.; Rondinelli, J.M.; Jang, J.I.; Hupp, J.T.; Kanatzidis, M.G. Ruddlesden—Popper hybrid lead iodide perovskite 2D homologous semiconductors. Chem. Mater. 2016, 28, 2852–2867. [Google Scholar] [CrossRef]
- Liu, J.; Leng, J.; Wu, K.; Zhang, J.; Jin, S. Observation of internal photoinduced electron and hole separation in hybrid two-dimentional perovskite films. J. Am. Chem. Soc. 2017, 139, 1432–1435. [Google Scholar] [CrossRef]
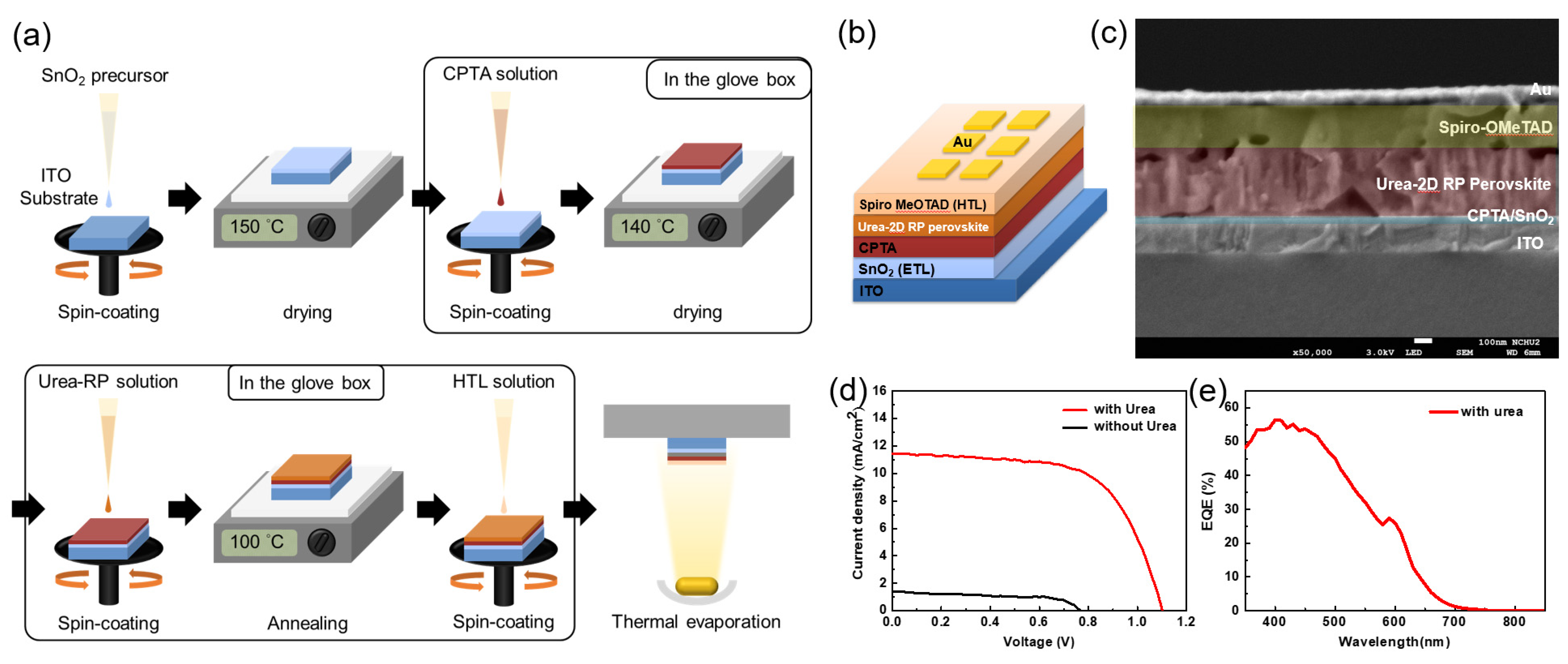

| Jsc (mA) | Voc (mV) | FF (%) | h (%) | |
|---|---|---|---|---|
| With Urea | 11.4 | 1103.6 | 62.82 | 7.9 |
| Without Urea | 1.4 | 771 | 59.08 | 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gowdru, S.M.; Lin, J.-C.; Wang, S.-T.; Chen, Y.-C.; Wu, K.-C.; Jiang, C.-N.; Chen, Y.-D.; Li, S.-S.; Chang, Y.J.; Wang, D.-Y. Accelerated Formation of 2D Ruddlesden—Popper Perovskite Thin Films by Lewis Bases for High Efficiency Solar Cell Applications. Nanomaterials 2022, 12, 1816. https://doi.org/10.3390/nano12111816
Gowdru SM, Lin J-C, Wang S-T, Chen Y-C, Wu K-C, Jiang C-N, Chen Y-D, Li S-S, Chang YJ, Wang D-Y. Accelerated Formation of 2D Ruddlesden—Popper Perovskite Thin Films by Lewis Bases for High Efficiency Solar Cell Applications. Nanomaterials. 2022; 12(11):1816. https://doi.org/10.3390/nano12111816
Chicago/Turabian StyleGowdru, Swathi M., Jou-Chun Lin, Szu-Tan Wang, Yi-Chia Chen, Kuan-Chang Wu, Cheng-Nan Jiang, Yu-Dian Chen, Shao-Sian Li, Yuan Jay Chang, and Di-Yan Wang. 2022. "Accelerated Formation of 2D Ruddlesden—Popper Perovskite Thin Films by Lewis Bases for High Efficiency Solar Cell Applications" Nanomaterials 12, no. 11: 1816. https://doi.org/10.3390/nano12111816
APA StyleGowdru, S. M., Lin, J.-C., Wang, S.-T., Chen, Y.-C., Wu, K.-C., Jiang, C.-N., Chen, Y.-D., Li, S.-S., Chang, Y. J., & Wang, D.-Y. (2022). Accelerated Formation of 2D Ruddlesden—Popper Perovskite Thin Films by Lewis Bases for High Efficiency Solar Cell Applications. Nanomaterials, 12(11), 1816. https://doi.org/10.3390/nano12111816







