L-Tryptophan Aqueous Systems at Low Concentrations: Interconnection between Self-Organization, Fluorescent and Physicochemical Properties, and Action on Hydrobionts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Design
2.3. Physicochemical Methods
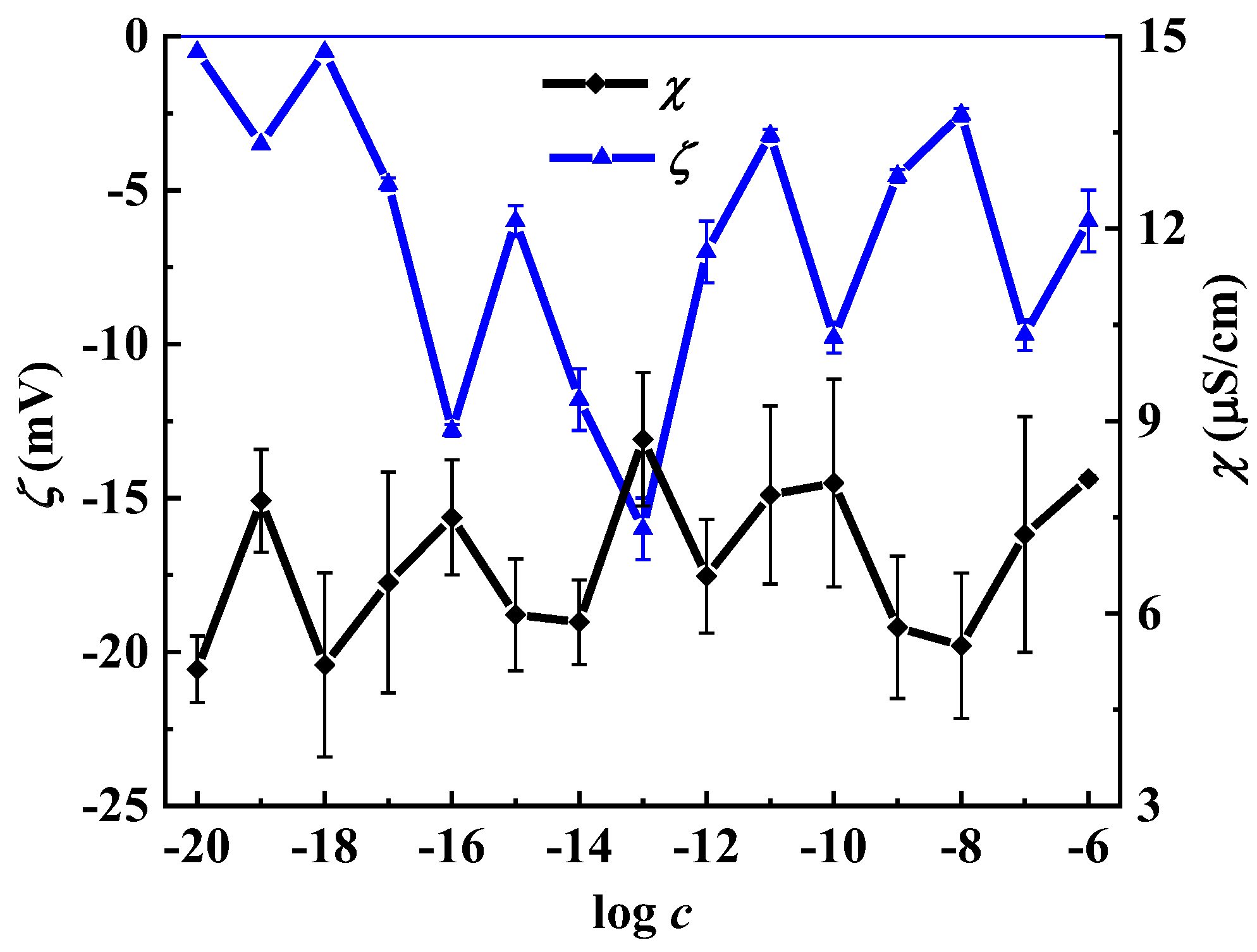
2.3.1. Conductometry
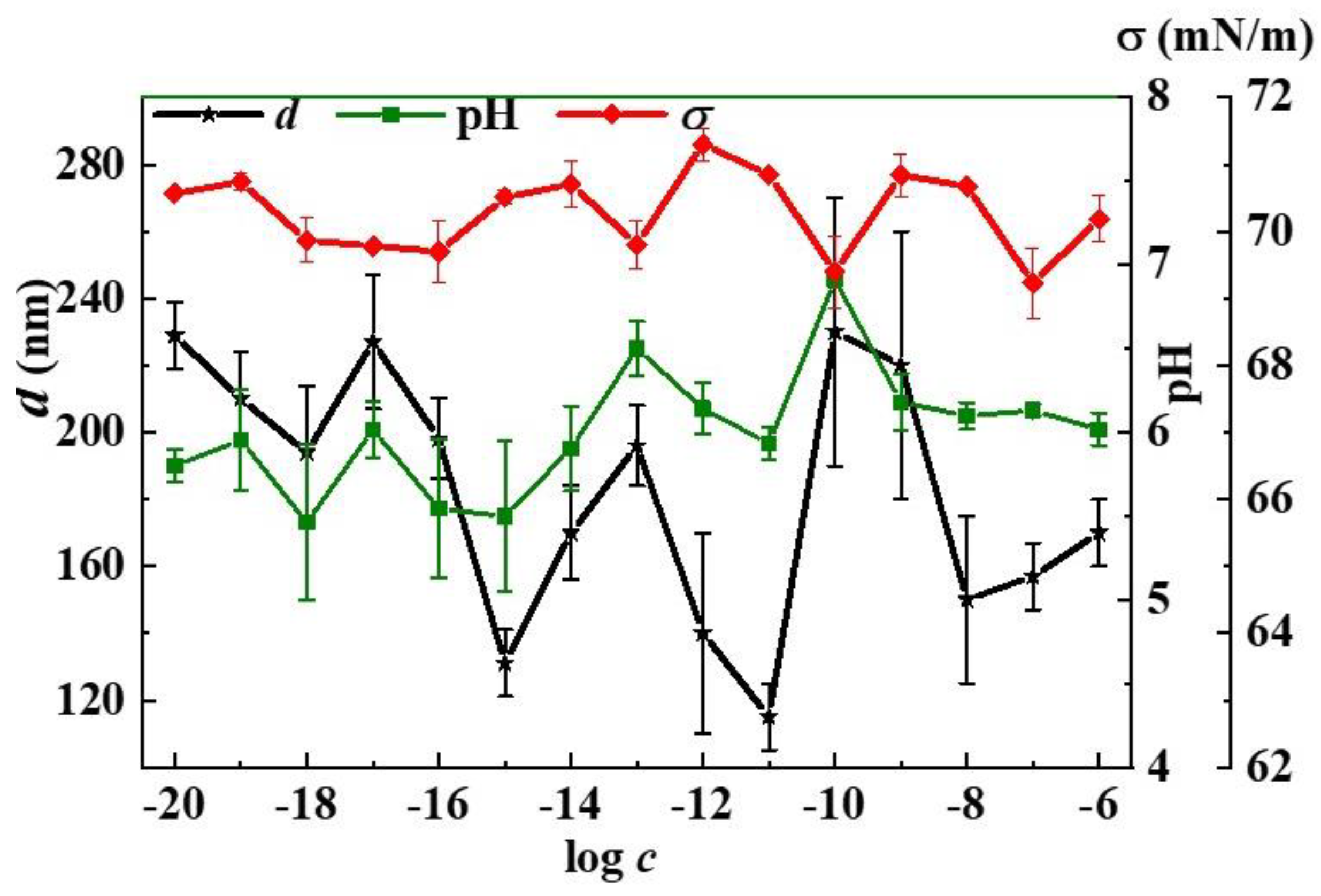
2.3.2. pH
2.3.3. Tensiometry
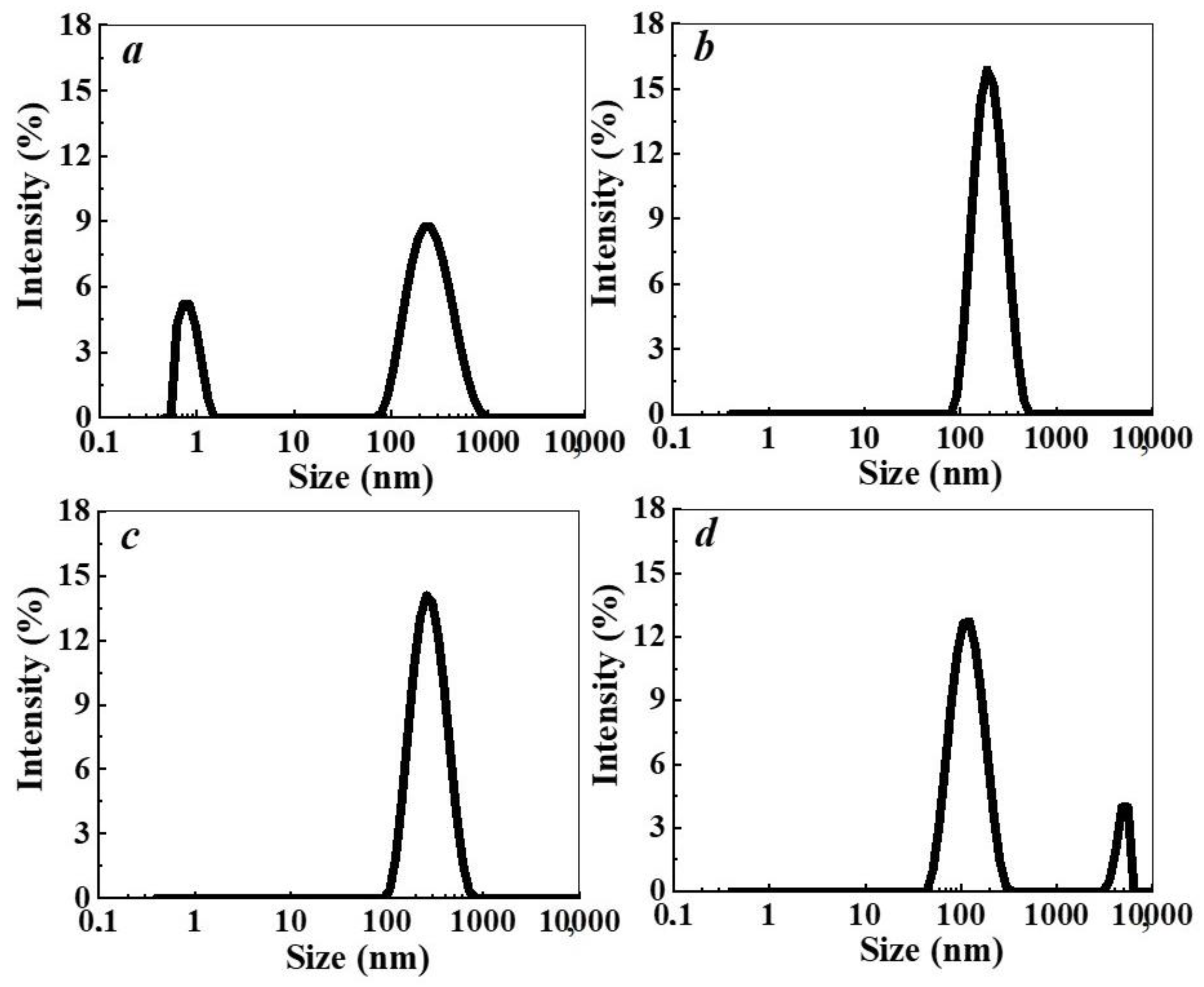
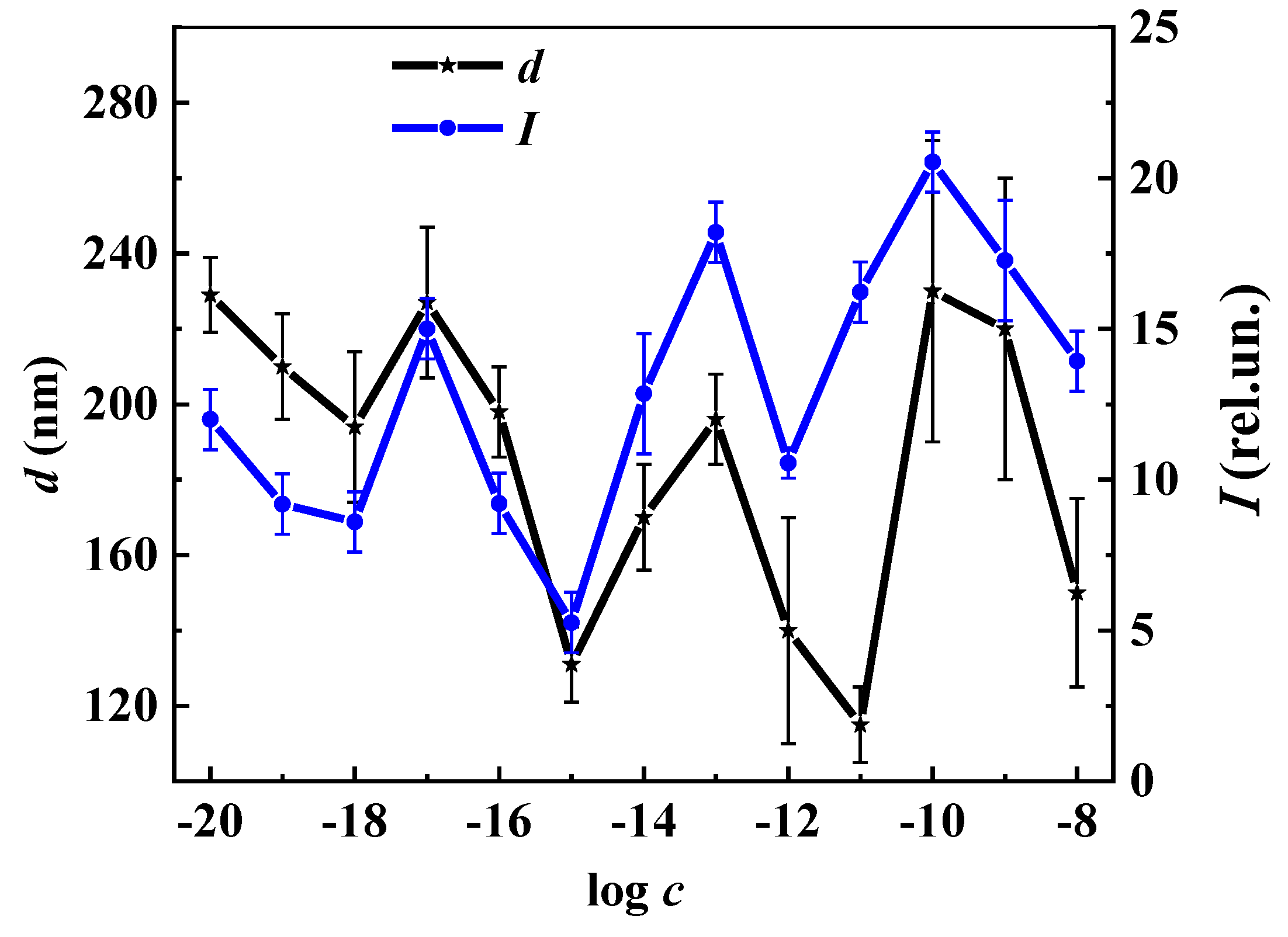
2.3.4. Dynamic Light Scattering (DLS)
2.3.5. Electrophoretic Light Scattering (ELS)
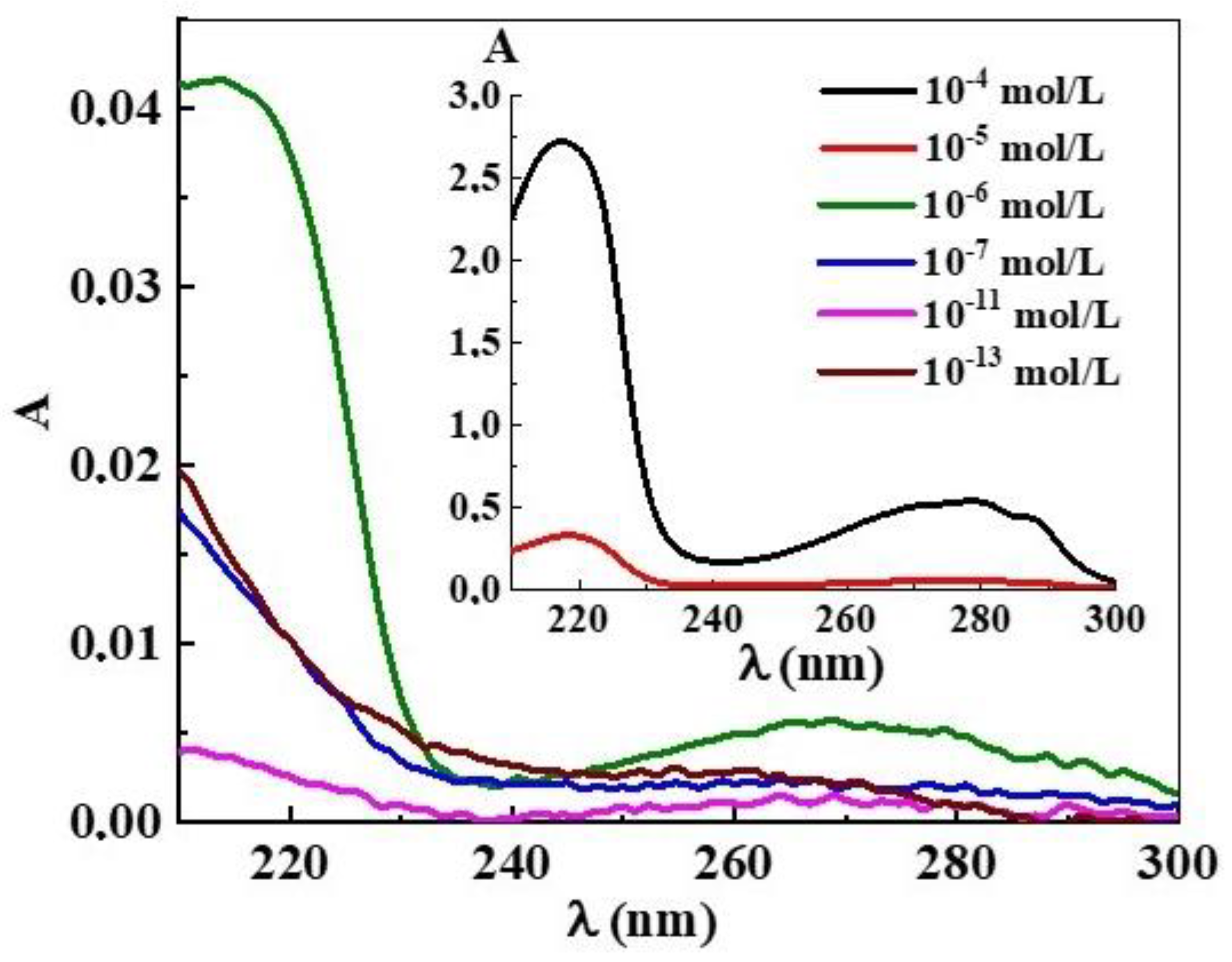
2.3.6. UV–Vis Spectroscopy
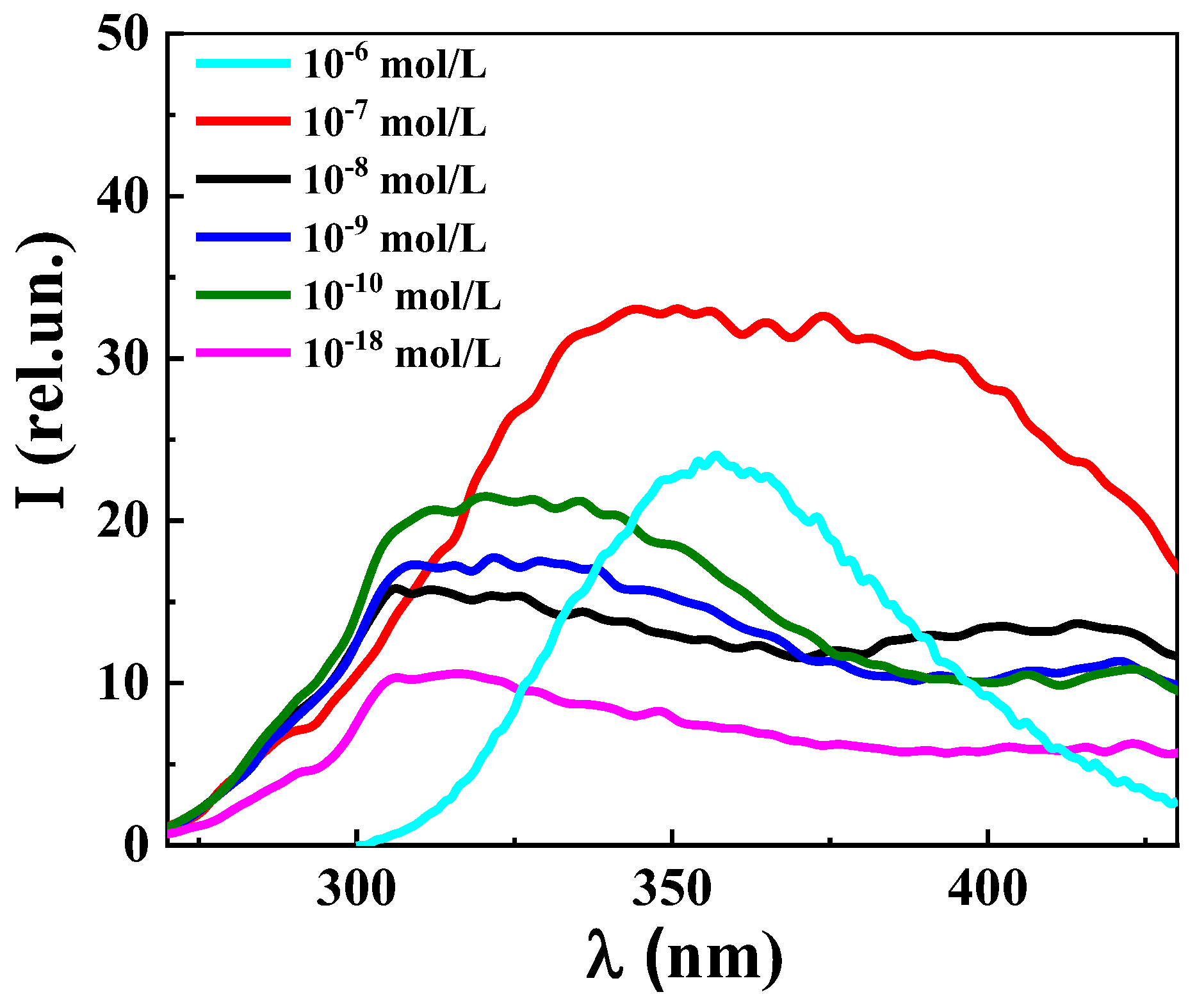
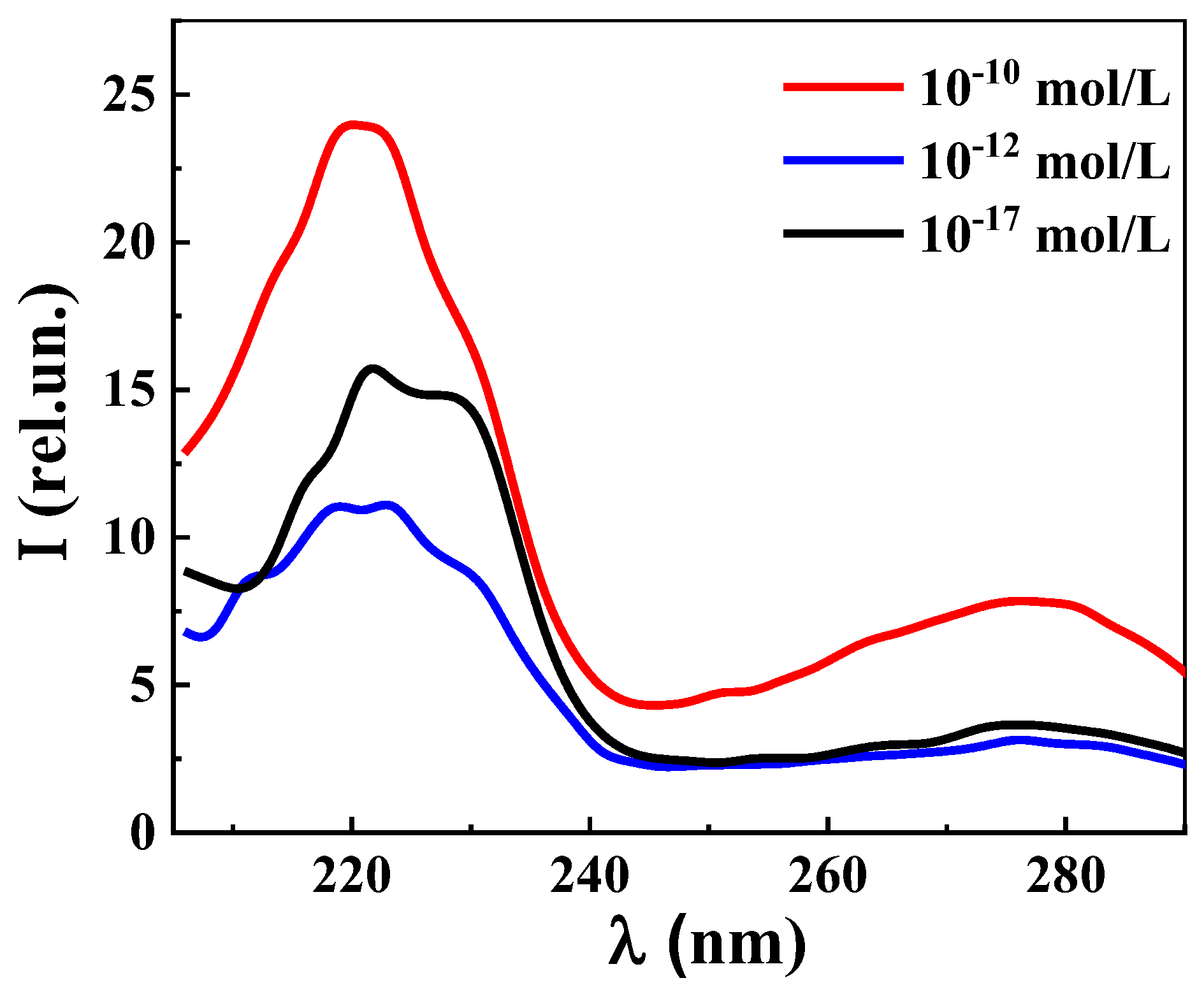
2.3.7. Fluorescence Spectroscopy
2.4. Toxicological Methods
2.4.1. Biotesting on Infusoria Paramecium caudatum
2.4.2. Biotesting on Chlorella vulgaris Green Algae
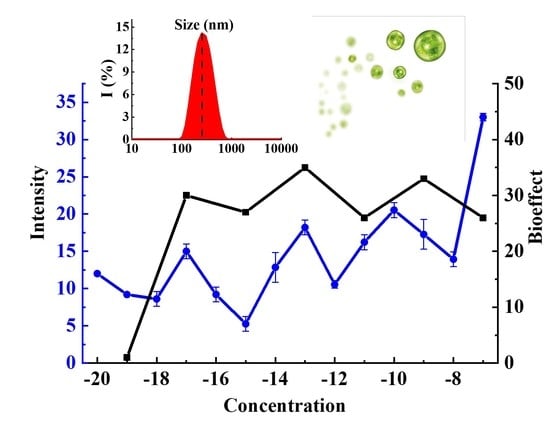
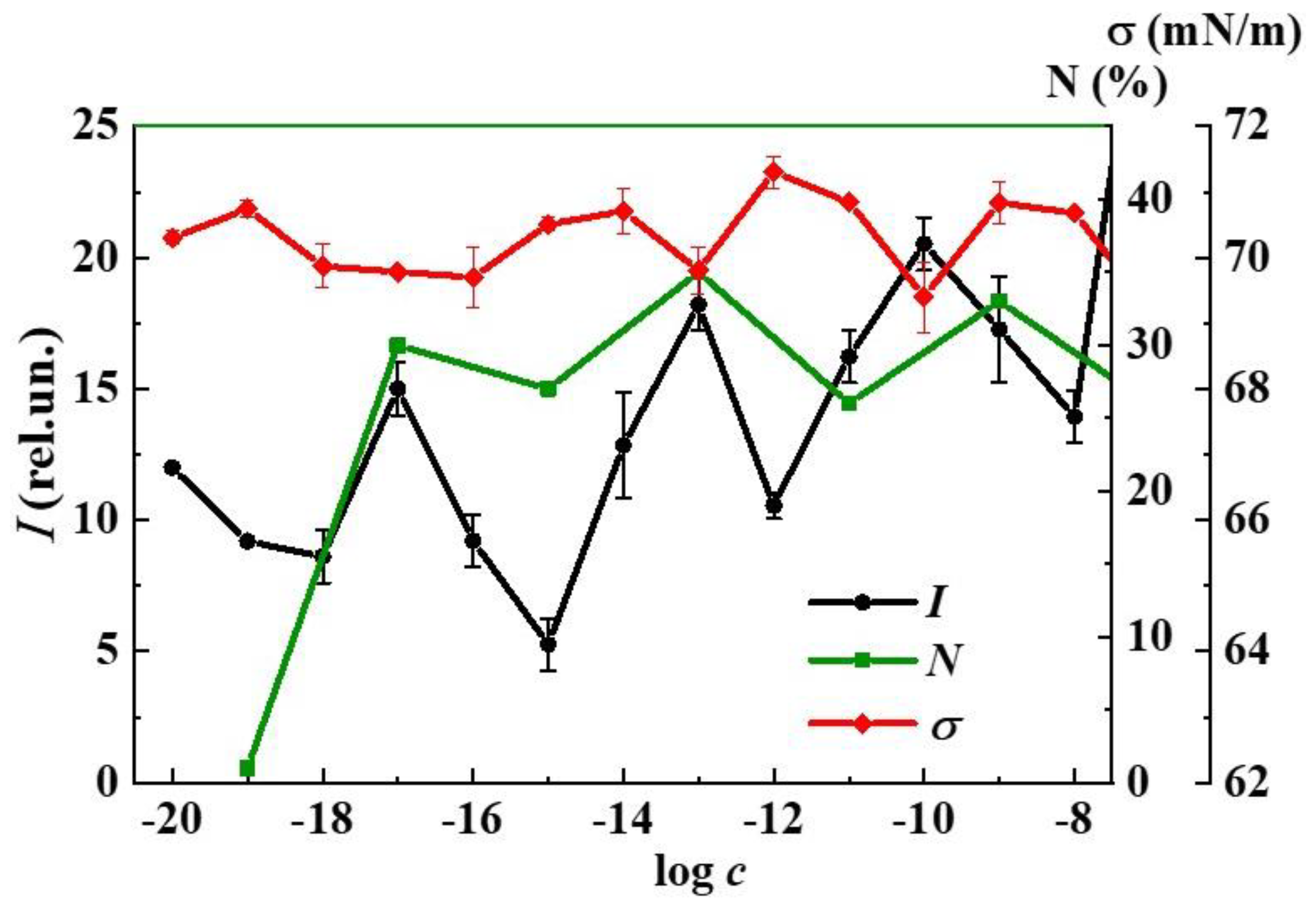
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koolman, J.; Rohm, K.H. Taschenatlas der Biochemie, Georg Thieme Verlag; Stuttgart: New York, NY, USA, 2003; 469p. [Google Scholar]
- Chernitsky, E.A.; Slobozhanina, E.I. Spectral Luminescence Analysis in Medicine; Science and Technology: Minsk, Russian, 1989; p. 141. [Google Scholar]
- Jameson, D.M. Introduction to Fluorescence; Taylor & Francis Group: Boca Raton, FL, USA, 2014; 313p. [Google Scholar]
- Akbar, S.M.; Sreeramulu, K.; Sharma, H.C. Tryptophan fluorescence quenching as a binding assay to monitor protein conformation changes in the membrane of intact mitochondria. J. Bioenerg. Biomembr. 2016, 48, 241–247. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Lei, Y.; Lei, X.; Gan, W.; Shu, L.; Yang, X. Exploration of reaction rates of chlorine dioxide with tryptophan residue in oligopeptides and proteins. J. Environ. Sci. 2020, 93, 129–136. [Google Scholar] [CrossRef]
- Carstea, E.M. Fluorescence spectroscopy as a potential tool for In-Situ monitoring of dissolved organic matter in surface water systems. Water Pollution. 2012. Available online: https://www.intechopen.com/chapters/28946 (accessed on 2 May 2022).
- Carstea, E.M.; Bridgeman, J.; Baker, A.; Reynolds, D.M. Fluorescence spectroscopy for wastewater monitoring: A review. Water Res. 2016, 95, 205–219. [Google Scholar] [CrossRef]
- Curi, R.; Newsholme, P.; Procopio, J.; Lagranha, C.; Gorjão, R.; Pithon-Curi, T.C. Glutamine, gene expression, and cell function. Front. Biosci. 2007, 12, 344–357. [Google Scholar] [CrossRef] [Green Version]
- Chalisova, N.I.; Ivanova, P.N.; Zalomaeva, E.S.; Nikitina, E.A.; Kozina, L.S. Effect of tryptophan and kynurenine on the cellular proliferation in the organotypic tissue culture of brain cortex in young and old rats. Adv. Geront. 2018, 31, 732–736. (In Russian) [Google Scholar]
- Chalisova, N.I.; Korovin, A.E.; Zalomaeva, E.S.; Menshikov, N.O. The influence of encoded amino acids and their combinations on the development of organotypic liver culture. Clinic. Pathophys. 2019, 25, 50–55. (In Russian) [Google Scholar]
- Kondakov, S.E.; Mel’nikov, M.Y.; Prokoptseva, O.S. Application of nonspecific biosensors for the determination of effective concentrations of active substances in a new pharmaceutical composition. Doklady Phys. Chem. 2014, 455, 45–48. [Google Scholar] [CrossRef]
- Konovalov, A.I.; Ryzhkina, I.S.; Pal’mina, N.P.; Mal’tseva, E.L.; Sergeeva, S.Y.; Murtazina, L.I. The relationship between self-organization and membrane effects of aqueous dispersion systems of the thyroliberin oligopeptide. Doklady Phys. Chem. 2017, 474, 80–84. [Google Scholar] [CrossRef]
- Pearce, G.; Strydom, D.; Johnson, S.; Ryan, C.A. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science 1991, 253, 895–897. [Google Scholar] [CrossRef]
- Terekhova, S.F.; Grechenko, T.N. Ultra-Low Doses of Different Biologically Active Substances Regulate Neuronal Functional States. Nonspecific Effect. Radiat. Biol. Radioecol. 2003, 3, 315–319. (In Russian) [Google Scholar]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [Green Version]
- Shimanovsky, N.L.; Epinetov, M.A.; Mel’nikov, M.Y. Molecular and Nanofarmacology; Fizmatlit: Moscow, Russia, 2010. [Google Scholar]
- Mattson, M.P.; Calabrese, E.J. (Eds.) Hormesis: A Revolution in Biology, Toxicology and Medicine, 1st ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Tushmalova, N.A.; Lebedeva, N.E.; Igolkina, Y.V.; Sarapul’tseva, E.I. Spirostomum ambiguum as a bioindicator of aquatic environment pollution. Moscow Univ. Biol. Sci. Bull. 2014, 69, 67–70. [Google Scholar] [CrossRef]
- Pikula, K.; Chaika, V.; Zakharenko, A.; Markina, Z.; Vedyagin, A.; Kuznetsov, V.; Golokhvast, K. Comparison of the level and mechanisms of toxicity of carbon nanotubes, carbon nanofibers, and silicon nanotubes in bioassay with four marine microalgae. Nanomaterials 2020, 10, 485. [Google Scholar] [CrossRef] [Green Version]
- Ryzhkina, I.S.; Sergeeva, S.Y.; Kiseleva, Y.V.; Timosheva, A.P.; Salakhutdinova, O.A.; Shevelev, M.D.; Konovalov, A.I. Self-organization and properties of dispersed systems based on dilute aqueous solutions of (S)- and (R)-lysine. Mendeleev Commun. 2018, 28, 66–69. [Google Scholar] [CrossRef]
- Ryzhkina, I.S.; Kiseleva, Y.V.; Murtazina, L.I.; Kuznetsova, T.V.; Zainulgabidinov, E.R.; Knyazev, I.V.; Petrov, A.M.; Kondakov, S.E.; Konovalov, A.I. Diclofenac sodium aqueous systems at low concentrations: Interconnection between physicochemical properties and action on hydrobionts. J. Environ. Sci. 2020, 88, 177–186. [Google Scholar] [CrossRef]
- Santos, L.H.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C.B.S.M. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef] [Green Version]
- Hearon, S.E.; Wang, M.; McDonald, T.J.; Phillips, T.D. Decreased bioavailability of aminomethylphosphonic acid (AMPA) in genetically modified corn with activated carbon or calcium montmorillonite clay inclusion in soil. J. Environ. Sci. 2021, 100, 131–143. [Google Scholar] [CrossRef]
- Mathias, F.T.; Fockink, D.H.; Disner, G.R.; Prodocimo, V.; Ribas, J.L.C.; Ramos, L.P.; Cestari, M.M.; Silva de Assis, H.C.S. Effects of low concentrations of ibuprofen on freshwater fish Rhamdiaquelen. Environ. Toxicol. Pharmacol. 2018, 59, 105–113. [Google Scholar] [CrossRef]
- Chow, L.K.; Ghaly, T.M.; Gillings, M.R. A survey of sub-inhibitory concentrations of antibiotics in the environment. J. Environ. Sci. 2021, 99, 21–27. [Google Scholar] [CrossRef]
- Li, Y.; Taggart, M.A.; McKenzie, C.; Zhang, Z.; Lu, Y.; Pap, S.; Gibb, S.W. A SPE-HPLC-MS/MS method for the simultaneous determination of prioritised pharmaceuticals and EDCs with high environmental risk potential in freshwater. J. Environ. Sci. 2021, 100, 18–27. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, S.; Huang, W.; He, L.; Li, J.; Zhou, J.; Zhou, J. Influence of eugenol on algal growth, cell physiology of cyanobacteria Microcystis aeruginosa and its interaction with signaling molecules. Chemosphere 2020, 255, 126935. [Google Scholar] [CrossRef]
- Patsaeva, S.V.; Khundzhua, D.A.; Trubetskoj, O.A.; Trubetskaya, O.E. Excitation-Dependent Fluorescence Quantum Yield for Freshwater Chromophoric Dissolved Organic Matter from Northern Russian Lakes. J. Spectrosc. 2018, 2018, 3168320. [Google Scholar] [CrossRef] [Green Version]
- Konovalov, A.I.; Ryzhkina, I.S. Highly diluted aqueous solutions: Formation of nanosized molecular assemblies (nanoassociates). Geochem. Int. 2014, 52, 1192–1210. [Google Scholar] [CrossRef]
- Elia, V.; Ausanio, G.; De Ninno, A.; Germano, R.; Napoli, E.; Niccoli, M. Experimental evidences of stable water nanostructures at standard pressure and temperature obtained by iterative filtration. Water 2014, 5, 121–130. [Google Scholar]
- Elia, V.; Oliva, R.; Napoli, E.; Germano, R.; Pinto, G.; Lista, L.; Niccoli, M.; Toso, D.; Vitiello, G.; Trifuoggi, M.; et al. Experimental study of physicochemical changes in water by iterative contact with hydrophilic polymers: A comparison between Cellulose and Nafion. J. Mol. Liq. 2018, 268, 598–609. [Google Scholar] [CrossRef]
- Elia, V.; Napoli, E.; Germano, R.; Oliv, R.; Roviello, V.; Niccoli, M.; Amoresano, A.; Naviglio, D.; Ciaravolo, M.; Trifuoggi, M.; et al. New chemical-physical properties of water after iterative procedure using hydrophilic polymers: The case of paper filter. J. Mol. Liq. 2019, 296, 111808. [Google Scholar] [CrossRef]
- Chai, B.H.; Zheng, J.M.; Zhao, Q.; Pollack, G.H. Spectroscopic Studies of Solutes in Aqueous Solution. J. Phys. Chem. A 2008, 112, 2242–2247. [Google Scholar] [CrossRef]
- Lobyshev, V.I.; Shikhlinskaya, R.E.; Ryzhikov, B.D. Experimental evidence for intrinsic luminescence of water. J. Mol. Liq. 1999, 82, 73–81. [Google Scholar] [CrossRef]
- Pollack, G.H. The Fourth Phase of Water; Ebner and Sons Publishers: Seattle, WA, USA, 2013. [Google Scholar]
- Ryzhkina, I.; Murtazina, L.; Gainutdinov, K.; Konovalov, A. Diluted Aqueous Dispersed Systems of 4-Aminopyridine: The Relationship of Self-Organization, Physicochemical Properties, and Influence on the Electrical Characteristics of Neurons. Front. Chem. 2021, 9, 81. [Google Scholar] [CrossRef]
- Ryzhkina, I.S.; Murtazina, L.I.; Sergeeva, S.Y.; Kostina, L.A.; Sharapova, D.A.; Shevelev, M.D.; Konovalov, A.I. Fluorescence characteristics of aqueous dispersed systems of succinic acid as potential markers of their self-organization and bioeffects in low concentration range. Environ. Technol. Innov. 2021, 21, 101215. [Google Scholar] [CrossRef]
- Ryzhkina, I.S.; Murtazina, L.I.; Kostina, L.A.; Sharapova, D.A.; Shevelev, M.D.; Zainulgabidinov, E.R.; Petrov, A.M.; Konovalov, A.I. Interrelation of physicochemical, spectral, and biological properties of self-organized multi-component aqueous systems based on N-(phosphonomethyl) glycine in the low concentration range. Russ. Chem. Bull. 2021, 70, 81–90. [Google Scholar] [CrossRef]
- Ryzhkina, I.S.; Murtazina, L.I.; Kostina, L.A.; Dokuchaeva, I.S.; Kuznetsova, T.V.; Petrov, A.M.; Konovalov, A.I. Physicochemical and biological properties of aqueous herbicide compositions based on N-(phosphonomethyl) glycine and succinic acid in a range of low concentrations. Russ. Chem. Bull. 2021, 70, 1499–1508. [Google Scholar] [CrossRef]
- Available online: https://kr-analytical.ru/uploadedFiles/files/documens/zetasizre_nano/Seriya_Zetasizer.pdf (accessed on 2 May 2022).
- Kononov, L.O. Chemical Reactivity and Solution Structure: On the Way to Paradigm Shift? RSC Adv. 2015, 5, 46718. [Google Scholar] [CrossRef]
- Sedl’ak, M. Large-Scale Supramolecular Structure in Solutions of Low Molar Mass Compounds und Mixtures of Liquids: 1. Light Scattering Characterization. J. Phys. Chem. B 2006, 110, 4329–4338. [Google Scholar] [CrossRef]
- Sed’lak, M.; Rak, D. Large-scale inhomogeneities in solutions of low molar mass compounds and mixtures of liquids: Supramolecular structures or nanobubbles? J. Phys. Chem. B 2013, 117, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Kaszuba, M.; McKnight, D.; Connah, M.T.; McNeil-Watson, F.K.; Nobbmann, U. Measuring sub nanometre sizes using dynamic light scattering. J Nanopart Res. 2008, 10, 823–829. [Google Scholar] [CrossRef] [Green Version]
- Andrievsky, G.V.; Bruskov, V.I.; Tykhomyrov, A.A.; Gudkov, S.V. Peculiarities of the antioxidant and radioprotective effects of hydrated C60 fullerene nanostuctures in vitro and in vivo. Free Radic. Biol. Med. 2009, 47, 786–793. [Google Scholar] [CrossRef]
- Yinnon, T.A. Liquids Prepared by Serially Diluting and Vigorously Shaking of Aqueous Solutions: Unveiling Effects of the Solute on their Properties. Water 2020, 10, 115–134. [Google Scholar]
- Kazakov, V.P.; Ostakhov, S.S.; Osina, I.O. Effect of Supramolecular Self-Organization in Water–Ethanol Mixtures on the S2→ S0 Fluorescence of Tryptophan. High Energy Chem. 2010, 44, 185–188. [Google Scholar] [CrossRef]
- Chalisova, N.I.; Ryzhak, G.A.; Ivko, O.M.; Zalomaeva, E.S.; Ivanova, P.N. Protective effect of coded L-amino acids in cytostatic presence on the development of organotypic culture of tissues of different genesis. Mol. Med. 2021, 19, 49–53. (In Russian) [Google Scholar] [CrossRef]
- Lobyshev, V.I.; Tomkevich, M.S.; Petrushanko, I.Y. Experimental study of potentiated aqueous solutions. Biophys 2005, 50, 416–420. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryzhkina, I.S.; Murtazina, L.I.; Kostina, L.A.; Sharapova, D.A.; Dokuchaeva, I.S.; Sergeeva, S.Y.; Meleshenko, K.A.; Petrov, A.M. L-Tryptophan Aqueous Systems at Low Concentrations: Interconnection between Self-Organization, Fluorescent and Physicochemical Properties, and Action on Hydrobionts. Nanomaterials 2022, 12, 1792. https://doi.org/10.3390/nano12111792
Ryzhkina IS, Murtazina LI, Kostina LA, Sharapova DA, Dokuchaeva IS, Sergeeva SY, Meleshenko KA, Petrov AM. L-Tryptophan Aqueous Systems at Low Concentrations: Interconnection between Self-Organization, Fluorescent and Physicochemical Properties, and Action on Hydrobionts. Nanomaterials. 2022; 12(11):1792. https://doi.org/10.3390/nano12111792
Chicago/Turabian StyleRyzhkina, Irina S., Lyaisan I. Murtazina, Larisa A. Kostina, Diana A. Sharapova, Irina S. Dokuchaeva, Svetlana Yu. Sergeeva, Kristina A. Meleshenko, and Andrew M. Petrov. 2022. "L-Tryptophan Aqueous Systems at Low Concentrations: Interconnection between Self-Organization, Fluorescent and Physicochemical Properties, and Action on Hydrobionts" Nanomaterials 12, no. 11: 1792. https://doi.org/10.3390/nano12111792
APA StyleRyzhkina, I. S., Murtazina, L. I., Kostina, L. A., Sharapova, D. A., Dokuchaeva, I. S., Sergeeva, S. Y., Meleshenko, K. A., & Petrov, A. M. (2022). L-Tryptophan Aqueous Systems at Low Concentrations: Interconnection between Self-Organization, Fluorescent and Physicochemical Properties, and Action on Hydrobionts. Nanomaterials, 12(11), 1792. https://doi.org/10.3390/nano12111792