Fish Oil Nanoemulsion Supplementation Attenuates Bleomycin-Induced Pulmonary Fibrosis BALB/c Mice
Abstract
1. Introduction
2. Materials and Methods
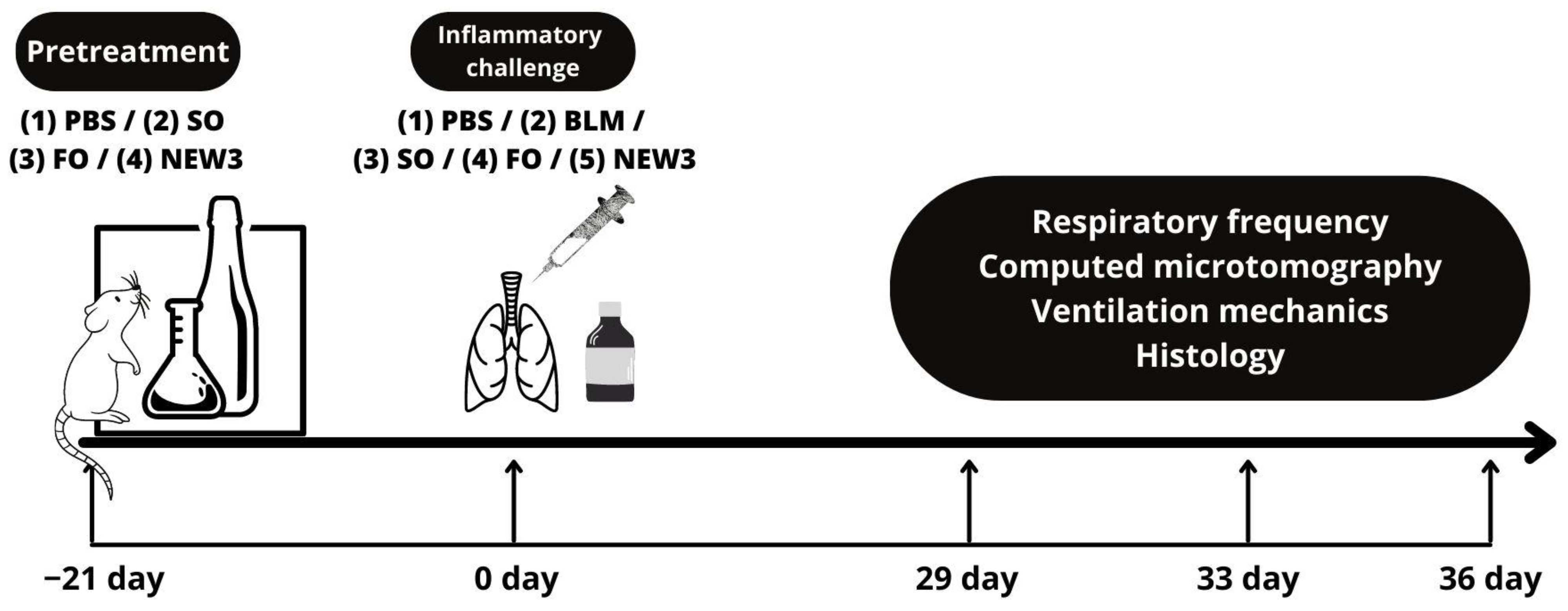
2.1. Methodological Steps
2.2. Materials and Reagents
2.3. Nanoformulation
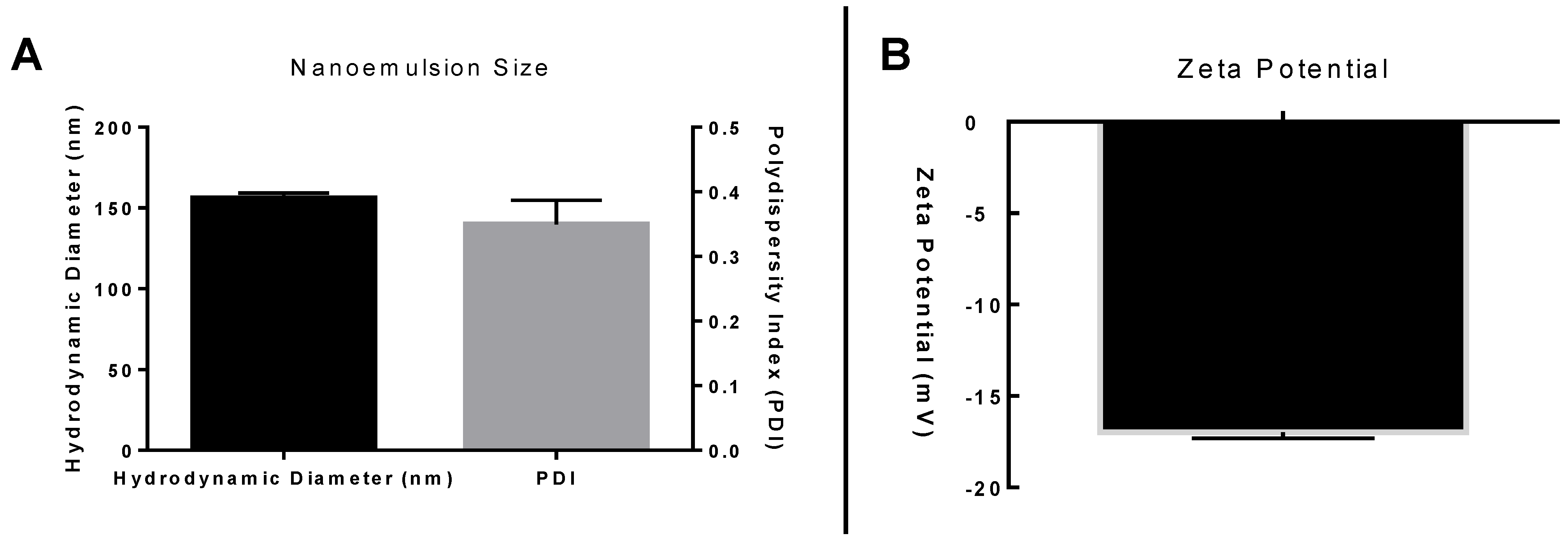
2.4. Nanoemulsion Characterization
2.5. Animal Model
2.6. Animal Treatments
2.7. Respiratory Frequency
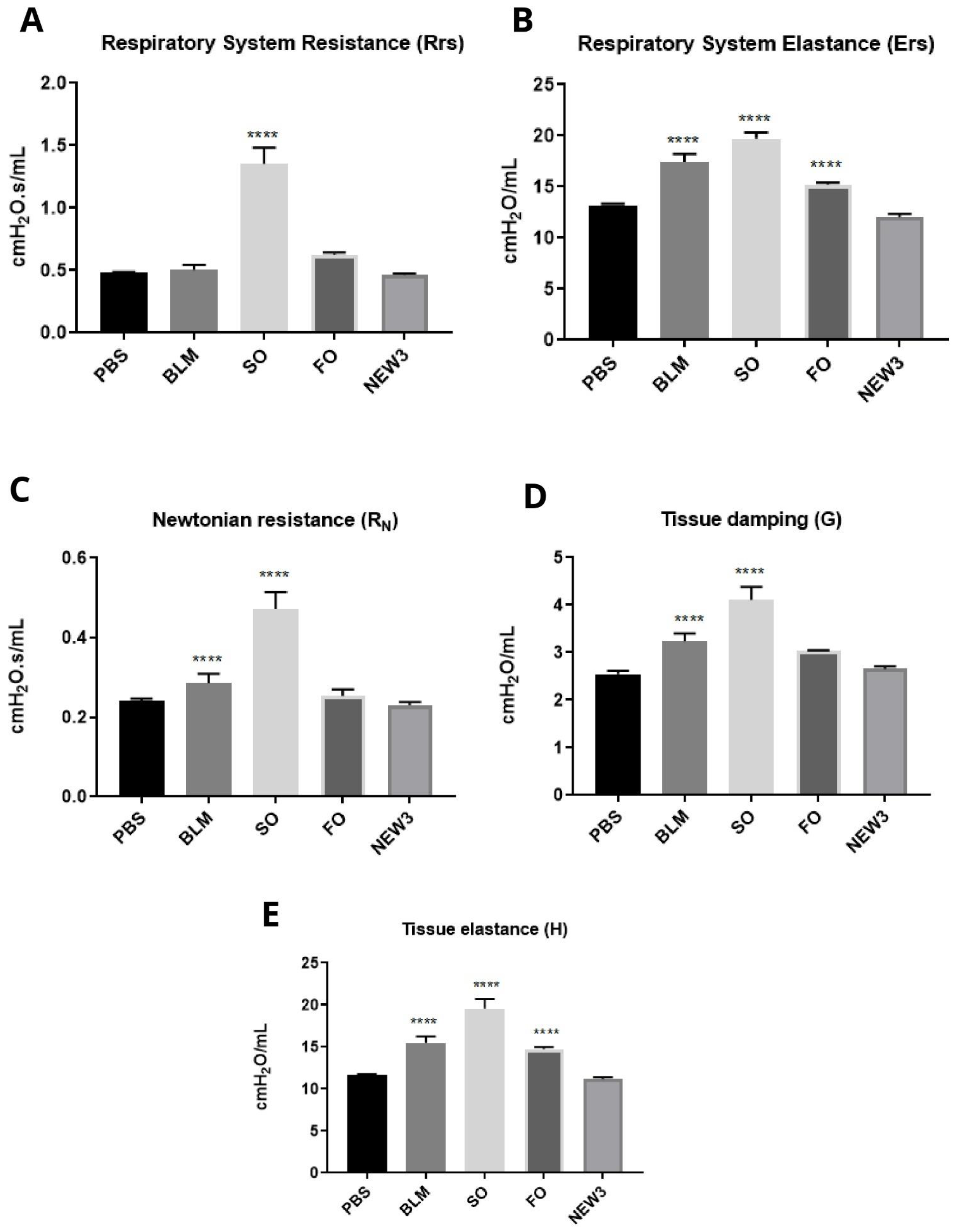
2.8. Respiratory Mechanics Measurements
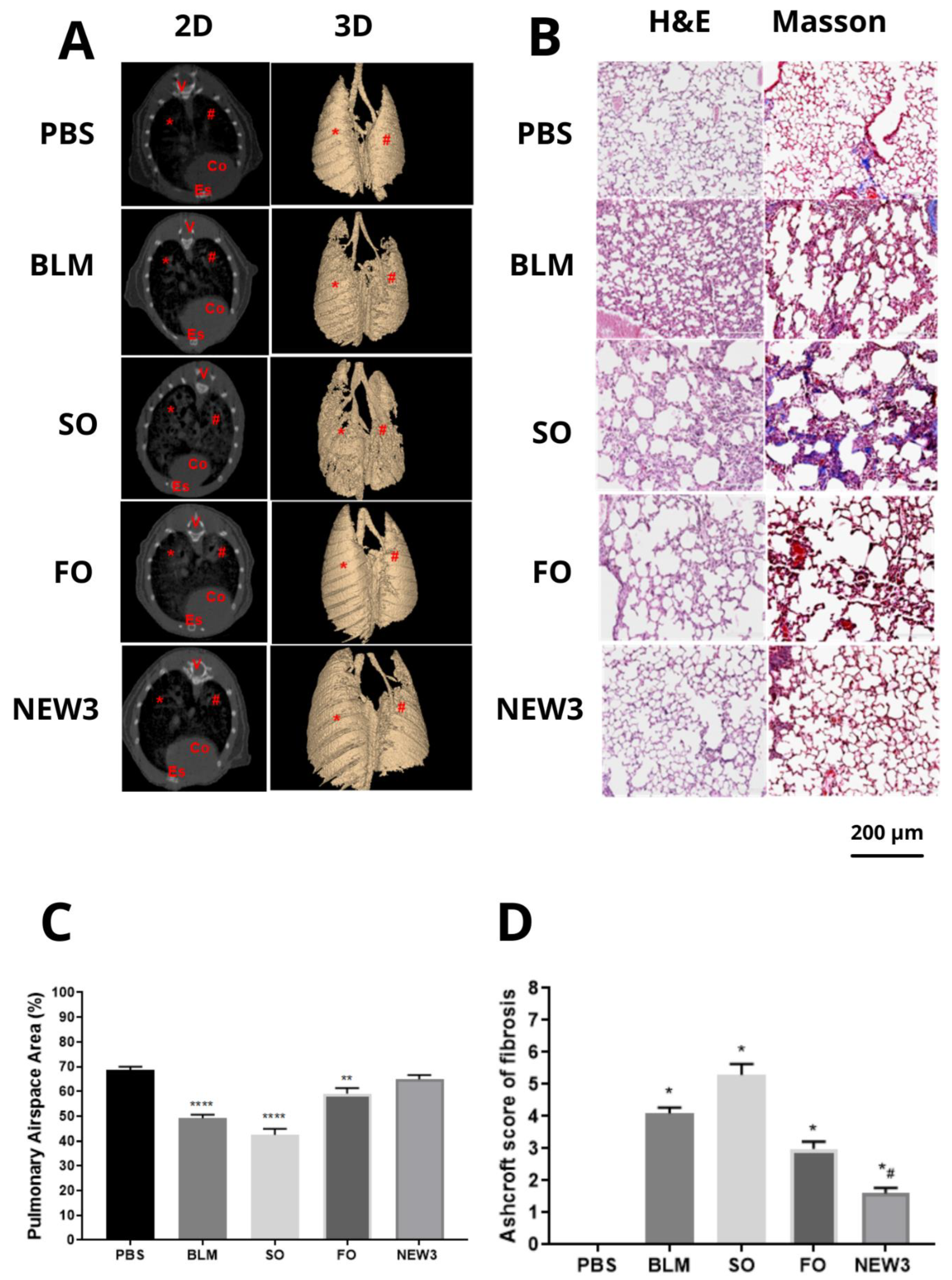
2.9. Histopathological Examination
2.10. Alveolar Air Area
2.11. Ashcroft Score
2.12. Computed Microtomography (Micro-CT)
2.13. Statistics
3. Results and Discussion
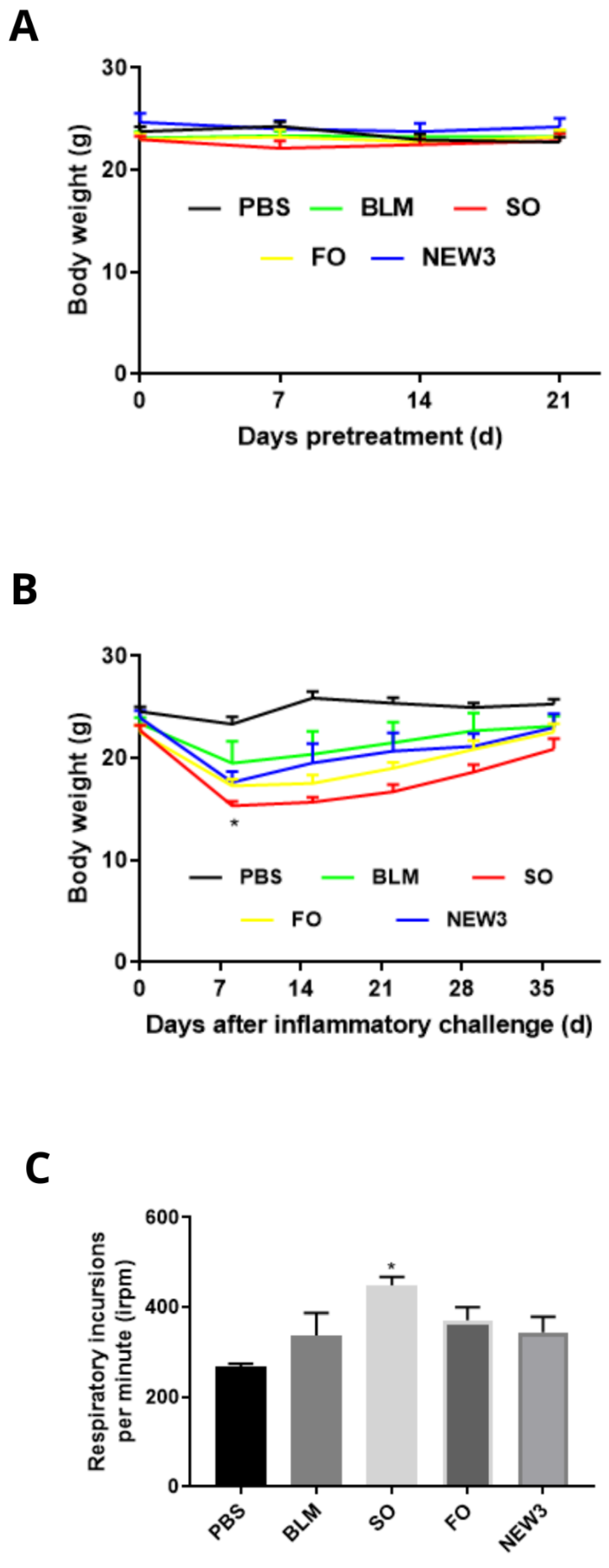
3.1. Nanoformulation Attenuates Impacts on Body Weight
3.2. Nanoformulation Attenuates Impacts on Respiratory Rate
3.3. Nanoformulation Attenuates Morphological Aspects of Pulmonary Fibrosis
3.4. Nanoformulation Attenuates Physiological Aspects of Pulmonary Fibrosis
3.5. Omega-3 Fatty Acids Promote Effects on Inflammatory Resolution
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glasser, S.W.; Hagood, J.S.; Wong, S.; Taype, C.A.; Madala, S.K.; Hardie, W.D. Mechanisms of Lung Fibrosis Resolution. Am. J. Pathol. 2016, 186, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Katzen, J.; Beers, M.F. Contributions of alveolar epithelial cell quality control to pulmonary fibrosis. J. Clin. Investig. 2020, 130, 5088–5099. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Goto, T. Molecular mechanisms of pulmonary fibrogenesis and its progression to lung cancer: A review. Int. J. Mol. Sci. 2019, 20, 1461. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Murtha, L.A.; Schuliga, M.J.; Mabotuwana, N.S.; Hardy, S.A.; Waters, D.W.; Burgess, J.K.; Knight, D.A.; Boyle, A.J. The processes and mechanisms of cardiac and pulmonary fibrosis. Front. Physiol. 2017, 8, 777. [Google Scholar] [CrossRef]
- Liu, T.; Santos, F.G.D.L.; Phan, S.H. The Bleomycin Model of Pulmonary. In Fibrosis; Humana Press: New York, NY, USA, 2017; Volume 1627. [Google Scholar] [CrossRef]
- Dei-Adomakoh, Y.A.; Afriyie-Mensah, J.S.; Gbadamosi, H. Bleomycin-induced pneumonitis in a young Ghanaian male with Hodgkin’s Lymphoma. Ghana Med. J. 2020, 54, 279–283. [Google Scholar] [CrossRef]
- Ruscitti, F.; Ravanetti, F.; Bertani, V.; Ragionieri, L.; Mecozzi, L.; Sverzellati, N.; Silva, M.; Ruffini, L.; Menozzi, V.; Civelli, M.; et al. Quantification of Lung Fibrosis in IPF-Like Mouse Model and Pharmacological Response to Treatment by Micro-Computed Tomography. Front. Pharmacol. 2020, 11, 1117. [Google Scholar] [CrossRef]
- Pinart, M.; Serrano-Mollar, A.; Llatjós, R.; Rocco, P.R.M.; Romero, P.V. Single and repeated bleomycin intratracheal instillations lead to different biomechanical changes in lung tissue. Respir. Physiol. Neurobiol. 2009, 166, 41–46. [Google Scholar] [CrossRef]
- Lamb, Y.N. Nintedanib: A Review in Fibrotic Interstitial Lung Diseases. Drugs 2021, 81, 575–586. [Google Scholar] [CrossRef]
- Galli, J.A.; Pandya, A.; Vega-Olivo, M.; Dass, C.; Zhao, H.; Criner, G.J. Pirfenidone and nintedanib for pulmonary fibrosis in clinical practice: Tolerability and adverse drug reactions. Respirology 2017, 22, 1171–1178. [Google Scholar] [CrossRef]
- Williamson, J.D.; Sadofsky, L.R.; Hart, S.P. The pathogenesis of bleomycin-induced lung injury in animals and its applicability to human idiopathic pulmonary fibrosis. Exp. Lung Res. 2015, 41, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Pietrocola, F.; Kroemer, G. Nutrition, inflammation and cancer. Nat. Immunol. 2017, 18, 843–850. [Google Scholar] [CrossRef] [PubMed]
- De Lima, L.I.; Py-Daniel, K.R.; Guimarães, M.A.; Muehlmann, L.A.; Mafud, A.C.; Mascarenhas, Y.P.; de Moraes, J.; de Souza de Almeida Leite, J.S.; Jiang, C.-S.; Azevedo, R.B.; et al. Self-nanoemulsifying drug-delivery systems improve oral absorption and antischistosomal activity of epiisopiloturine. Nanomedicine 2018, 13, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Mengarda, A.C.; Iles, B.; Longo, F.J.P.; de Moraes, J. Recent trends in praziquantel nanoformulations for helminthiasis treatment. Expert Opin. Drug Deliv. 2022, 19, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.S.; Morais, J.A.V.; Vanderlei, Í.A.C.; Santos, A.S.; Azevedo, R.B.; Muehlmann, L.A.; Osmindo, R.P., Jr.; Mortari, M.R.; da Silva, J.R.; da Silva, S.W.; et al. Oral delivery of fish oil in oil-in-water nanoemulsion: Development, colloidal stability and modulatory effect on in vivo inflammatory induction in mice. Biomed. Pharmacother. 2021, 133, 110980. [Google Scholar] [CrossRef]
- Monge-Fuentes, V.; Muehlmann, L.A.; Longo, J.P.F.; Silva, J.R.; Fascineli, M.L.; de Souza, P.; Faria, F.; Degterev, I.A.; Rodriguez, A.; Pirani Carneiro, F.; et al. Photodynamic therapy mediated by acai oil (Euterpe oleracea Martius) in nanoemulsion: A potential treatment for melanoma. J. Photochem. Photobiol. B Biol. 2017, 166, 301–310. [Google Scholar] [CrossRef]
- Smidt, K.S. Bases Moleculares da Modulação da Fibrose Pulmonar e Possíveis Aplicações Terapêuticas. Ph.D. Thesis, University of Brasília, Brasilia, Brazil, 2019. [Google Scholar]
- Melo-Silva, C.A.; Gaio, E.; Trevizoli, J.E.; Souza, C.S.; Gonçalves, A.S.; Sousa, G.C.C.; Takano, G.; Tavares, P.; Amado, V.M. Respiratory mechanics and lung tissue remodeling in a hepatopulmonary syndrome rat model. Respir. Physiol. Neurobiol. 2011, 179, 326–333. [Google Scholar] [CrossRef]
- Guttmann, J.; Eberhard, L.; Wolff, G.; Bertschmann, W.; Zeravik, J.; Adolph, M. Maneuver-free determination of compliance and resistance in ventilated ARDS patients. Chest 1992, 102, 1235–1242. [Google Scholar] [CrossRef][Green Version]
- Hantos, Z.; Daroczy, B.; Suki, B.; Nagy, S.; Fredberg, J.J. Input impedance and peripheral inhomogeneity of dog lungs. J. Appl. Physiol. 1992, 72, 168–178. [Google Scholar] [CrossRef]
- Longo, F.J.P.; Lucci, C.M.; Muehlmann, L.A.; Azevedo, R.B. Nanomedicine for cutaneous tumors—Lessons since the successful treatment of the Kaposi sarcoma. Nanomedicine 2018, 13, 2957–2959. [Google Scholar] [CrossRef]
- Ashcroft, T.; Simpson, J.M.; Timbrell, V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 1988, 41, 467–470. [Google Scholar] [CrossRef]
- Caputo, L.S.; Campos, M.I.C.; Dias, H.J.; Crotti, A.E.M.; Fajardo, J.B.; Vanelli, C.P.; Oresto, A.C.D.; Alves, M.S.; Aarestrup, F.M.; Paula, A.C.C.; et al. Copaiba oil suppresses inflammation in asthmatic lungs of BALB/c mice induced with ovalbumin. Int. Immunopharmacol. 2020, 80, 106177. [Google Scholar] [CrossRef] [PubMed]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A comprehensive review of chemistry, sources and bioavailability of omega-3 fatty acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Waitzberg, D.L.; Klek, S.; Martindale, R.G. Lipids in Parenteral Nutrition: Biological Aspects. J. Parenter. Enter. Nutr. 2020, 44, S21–S27. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez Lozada, M.I.; Rodrigues Maldonade, I.; Bobrowski Rodrigues, D.; Silva Santos, D.; Ortega Sanchez, B.A.; Narcizo de Souza, P.E.; Longo, J.P.; Bernardo Amaro, G.; de Lacerda de Oliveira, L. Physicochemical characterization and nano-emulsification of three species of pumpkin seed oils with focus on their physical stability. Food Chem. 2021, 343, 128512. [Google Scholar] [CrossRef] [PubMed]
- Laxmi, M.; Bhardwaj, A.; Mehta, S.; Mehta, A. Development and characterization of nanoemulsion as carrier for the enhancement of bioavailability of artemether. Artif. Cells Nanomed. Biotechnol. 2015, 43, 334–344. [Google Scholar] [CrossRef]
- Chircov, C.; Grumezescu, A.M. Nanoemulsion preparation, characterization, and application in the field of biomedicine. In Nanoarchitectonics in Biomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 169–188. [Google Scholar] [CrossRef]
- Manali, E.D.; Moschos, C.; Triantafillidou, C.; Kotanidou, A.; Psallidas, I.; Karabela, S.P.; Roussos, C.; Papiris, S.; Armaganidis, A.; Stathopoulos, G.T.; et al. Static and dynamic mechanics of the murine lung after intratracheal bleomycin. BMC Pulm. Med. 2011, 11, 33. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, T.; Zhao, X.; Xiea, K.; Bi, Y.; Zhong, Z.; Zhao, X. Docosahexaenoic acid (DHA) ameliorates paraquat-induced pulmonary fibrosis in rats possibly through up-regulation of Smad 7 and SnoN. Food Chem. Toxicol. 2013, 57, 330–337. [Google Scholar] [CrossRef]
- Zhao, H.; Chan-Li, Y.; Collins, S.L.; Zhang, Y.; Hallowell, R.W.; Mitzner, W.; Horton, M.R. Pulmonary delivery of docosahexaenoic acid mitigates bleomycin-induced pulmonary fibrosis. BMC Pulm. Med. 2014, 14, 64. [Google Scholar] [CrossRef]
- Ruscitti, F.; Ravanetti, F.; Essers, J.; Ridwan, Y.; Belenkov, S.; Vos, W.; Ferreira, F.; KleinJan, A.; van Heijningen, P.; Van Holsbeke, C.; et al. Longitudinal assessment of bleomycin-induced lung fibrosis by Micro-CT correlates with histological evaluation in mice. Multidiscip. Respir. Med. 2017, 12, 8. [Google Scholar] [CrossRef]
- Aburto, M.; Herráez, I.; Iturbe, D.; Jiménez-Romero, A. Diagnosis of Idiopathic Pulmonary Fibrosis: Differential Diagnosis. Med. Sci. 2018, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Elicker, B.; de Castro Pereira, C.A.; Webb, R.; Leslie, K.O. High-resolution computed tomography patterns of diffuse interstitial lung disease with clinical and pathological correlation. J. Bras. Pneumol. 2008, 34, 715–744. [Google Scholar] [CrossRef] [PubMed]
- Mecozzi, L.; Mambrini, M.; Ruscitti, F.; Ferrini, E.; Ciccimarra, R.; Ravanetti, F.; Sverzellati, N.; Silva, M.; Ruffini, L.; Belenkov, S.; et al. In-vivo lung fibrosis staging in a bleomycin-mouse model: A new micro-CT guided densitometric approach. Sci. Rep. 2020, 10, 18735. [Google Scholar] [CrossRef] [PubMed]
- Gilhodes, J.C.; Julé, Y.; Kreuz, S.; Stierstorfer, B.; Stiller, D.; Wollin, L. Quantification of pulmonary fibrosis in a bleomycin mouse model using automated histological image analysis. PLoS ONE 2017, 12, e0170561. [Google Scholar] [CrossRef] [PubMed]
- Hübner, R.H.; Gitter, W.; El Mokhtari, N.E.; Mathiak, M.; Both, M.; Bolte, H.; Freitag-Wold, S.; Bewig, B. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques 2008, 44, 507–517. [Google Scholar] [CrossRef]
- Albert, R.K.; Smith, B.; Perlman, C.E.; Schwartz, D.A. Is Progression of Pulmonary Fibrosis due to Ventilation-induced Lung Injury? Am. J. Respir. Crit. Care Med. 2019, 200, 140–151. [Google Scholar] [CrossRef]
- Tanaka, K.I.; Azuma, A.; Miyazaki, Y.; Sato, K.; Mizushima, T. Effects of lecithinized superoxide dismutase and/or pirfenidone against bleomycin-induced pulmonary fibrosis. Chest 2012, 142, 1011–1019. [Google Scholar] [CrossRef]
- Rago, F.; Melo, E.M.; Kraemer, L.; Galvão, I.; Cassali, G.D.; Santos, R.A.S.; Russo, R.C.; Teixeira, M.M. Effect of preventive or therapeutic treatment with angiotensin 1-7 in a model of bleomycin-induced lung fibrosis in mice. J. Leukoc. Biol. 2019, 106, 677–686. [Google Scholar] [CrossRef]
- Xisto, D.G.; Farias, L.L.; Ferreira, H.C.; Picanço, M.R.; Amitrano, D.; Lapa e Silva, J.R.; Negri, E.M.; Mauad, T.; Carnielli, D.; Silva, F.D.F.; et al. Lung parenchyma remodeling in a murine model of chronic allergic inflammation. Am. J. Respir Crit. Care Med. 2005, 171, 829–837. [Google Scholar] [CrossRef]
- Devos, F.C.; Maaske, A.; Robichaud, A.; Pollaris, L.; Seys, S.; Lopez, C.A.; Verbeken, E.; Tenbusch, M.; Lories, R.; Nemery, B.; et al. Forced expiration measurements in mouse models of obstructive and restrictive lung diseases. Respir. Res. 2017, 18, 123. [Google Scholar] [CrossRef]
- Nava, S.; Rubini, F. Lung and chest wall mechanics in ventilated patients with end stage idiopathic pulmonary fibrosis. Thorax 1999, 54, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The omega-6/omega-3 fatty acid ratio: Health implications. Oléagineux Corps Gras Lipides 2010, 17, 267–275. [Google Scholar] [CrossRef]
- Ishihara, T.; Yoshida, M.; Arita, M. Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. Int. Immunol. 2019, 31, 559–567. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galdino de Souza, D.; Santos, D.S.; Simon, K.S.; Morais, J.A.V.; Coelho, L.C.; Pacheco, T.J.A.; Azevedo, R.B.; Bocca, A.L.; Melo-Silva, C.A.; Longo, J.P.F. Fish Oil Nanoemulsion Supplementation Attenuates Bleomycin-Induced Pulmonary Fibrosis BALB/c Mice. Nanomaterials 2022, 12, 1683. https://doi.org/10.3390/nano12101683
Galdino de Souza D, Santos DS, Simon KS, Morais JAV, Coelho LC, Pacheco TJA, Azevedo RB, Bocca AL, Melo-Silva CA, Longo JPF. Fish Oil Nanoemulsion Supplementation Attenuates Bleomycin-Induced Pulmonary Fibrosis BALB/c Mice. Nanomaterials. 2022; 12(10):1683. https://doi.org/10.3390/nano12101683
Chicago/Turabian StyleGaldino de Souza, Danielle, Débora Silva Santos, Karina Smidt Simon, José Athayde Vasconcelos Morais, Luísa Coutinho Coelho, Thyago José Arruda Pacheco, Ricardo Bentes Azevedo, Anamélia Lorenzetti Bocca, César Augusto Melo-Silva, and João Paulo Figueiró Longo. 2022. "Fish Oil Nanoemulsion Supplementation Attenuates Bleomycin-Induced Pulmonary Fibrosis BALB/c Mice" Nanomaterials 12, no. 10: 1683. https://doi.org/10.3390/nano12101683
APA StyleGaldino de Souza, D., Santos, D. S., Simon, K. S., Morais, J. A. V., Coelho, L. C., Pacheco, T. J. A., Azevedo, R. B., Bocca, A. L., Melo-Silva, C. A., & Longo, J. P. F. (2022). Fish Oil Nanoemulsion Supplementation Attenuates Bleomycin-Induced Pulmonary Fibrosis BALB/c Mice. Nanomaterials, 12(10), 1683. https://doi.org/10.3390/nano12101683