Polarization of Femtosecond Laser for Titanium Alloy Nanopatterning Influences Osteoblastic Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Titanium Alloy Samples
2.2. Laser Surface Texturing
- IR linear LIPSS: They were generated on titanium alloy surfaces using the Tangor and Tangerine lasers and the 100-mm f-theta lens with a fluence of between 0.3–0.47 J/cm2. The fluence peak is defined as: . Spacing between pulses and a hatch distance of 4–5 µm were chosen with a 16-µm measured 1/e² beam diameter. This configuration makes a number of effective pulses per spot diameter of Neff_1D = 4 and a line separation distance of Δ = 4–5 µm.
- IR radial LIPSS: A 56-mm f-theta lens was deployed, leading to a 11-µm measured 1/e² beam diameter. In order to create an exotic LIPSS with ripples pointing to the outside of the center of the beam, an s-wave plate was implemented in the beam path of the laser to create a donut-shaped beam that converted the entering linear polarization into an azimuthal polarization. This decision was based on previous experiments on the behavior of cell adhesion with this type of structure. A laser pulse energy E of 0.7 µJ was applied to obtain the radial LIPSS. Impact positions were defined in order to keep the isotropic effect provided by such nanostructures. As a consequence, a 13-µm spacing between pulses led to tangency with a slight overlay on the edges of the impacts. An accumulation of 5 pulses per impact was chosen to compensate for a lack of energy accumulation due to this low recovery rate. It is worth mentioning that the work field of this f-theta lens is about 50 × 50 mm2 in area. In the current study, we produced our radially aligned LIPSS by using azimuthal polarization in a relatively small area of 10 × 10 mm2. No marked influence of mirror positions upon LIPSS quality was wittenessed in such a case. The same nominated laser conditions proceduced similar impacts at the extremity.
2.3. Surface Morphology
2.4. Surface Topography
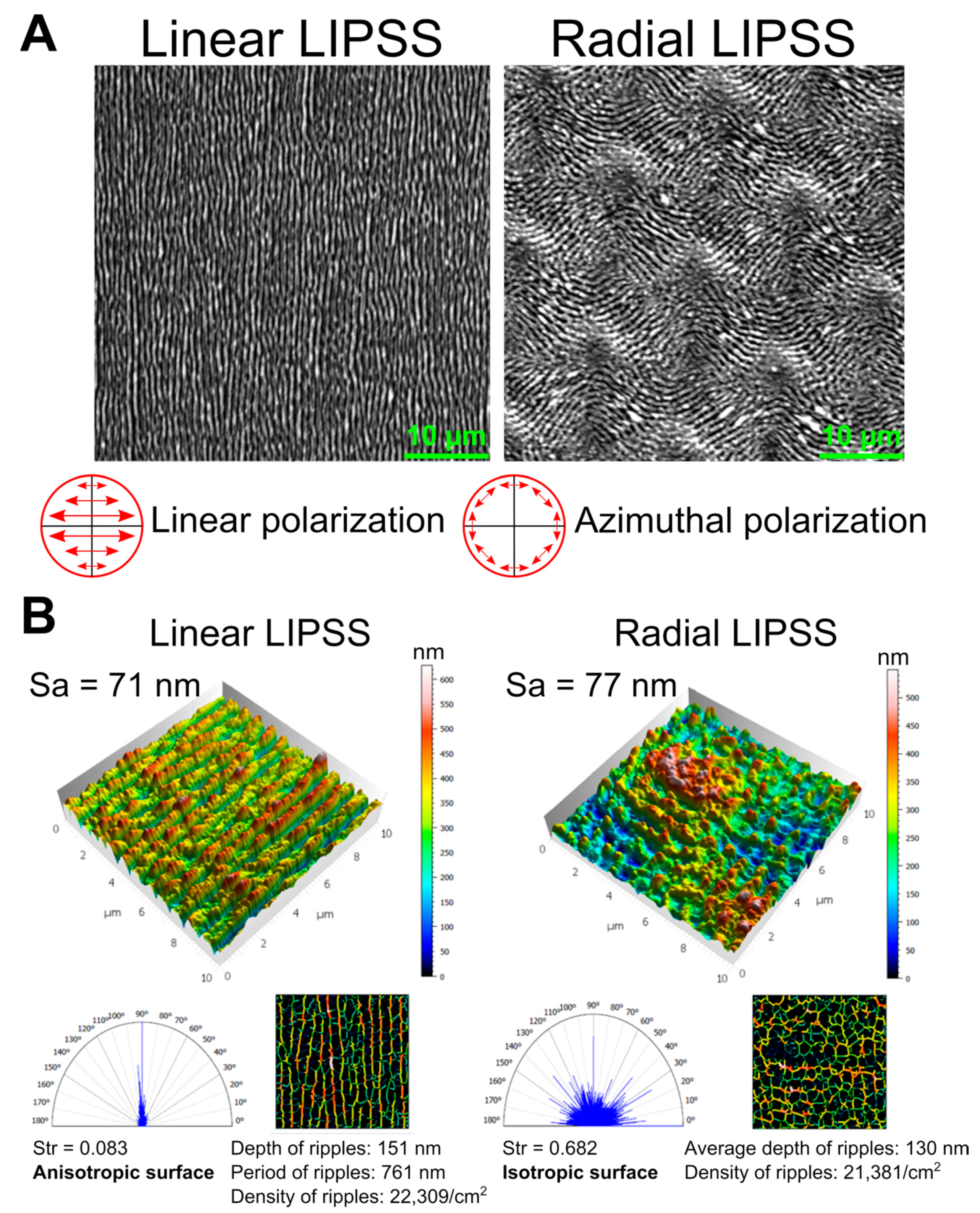
- Areal arithmetic mean height Sa (nm), which expresses the difference in height of each point compared to the arithmetical mean of the surface. This parameter is used to evaluate surface roughness.
- Texture aspect ratio Str, which expresses the isotropy and anisotropy of the topography. Str is a value ranging from 0 to 1. A value close to 0 indicates directionality (anisotropy), whereas a value close to 1 indicates that the surface does not exhibit preferred directions (isotropy).
- Polar spectrum, which shows the privileged texture directions.
- Average period and depth of the ripples.
- Ripple density per cm2.
2.5. Cell Culture
2.6. Fluorescent Cell Labeling at 24 h and 7 d Post Seeding
2.7. Quantitative Real Time PCR (qRT-qPCR) at 14 d Post Seeding
2.8. Assessment of Mineralization at 21 d Post Seeding
2.9. Image Acquisition and Analysis
2.10. Statistics
3. Results
3.1. Directionality of LIPSS Was the Main Difference between the Two Designed Textures
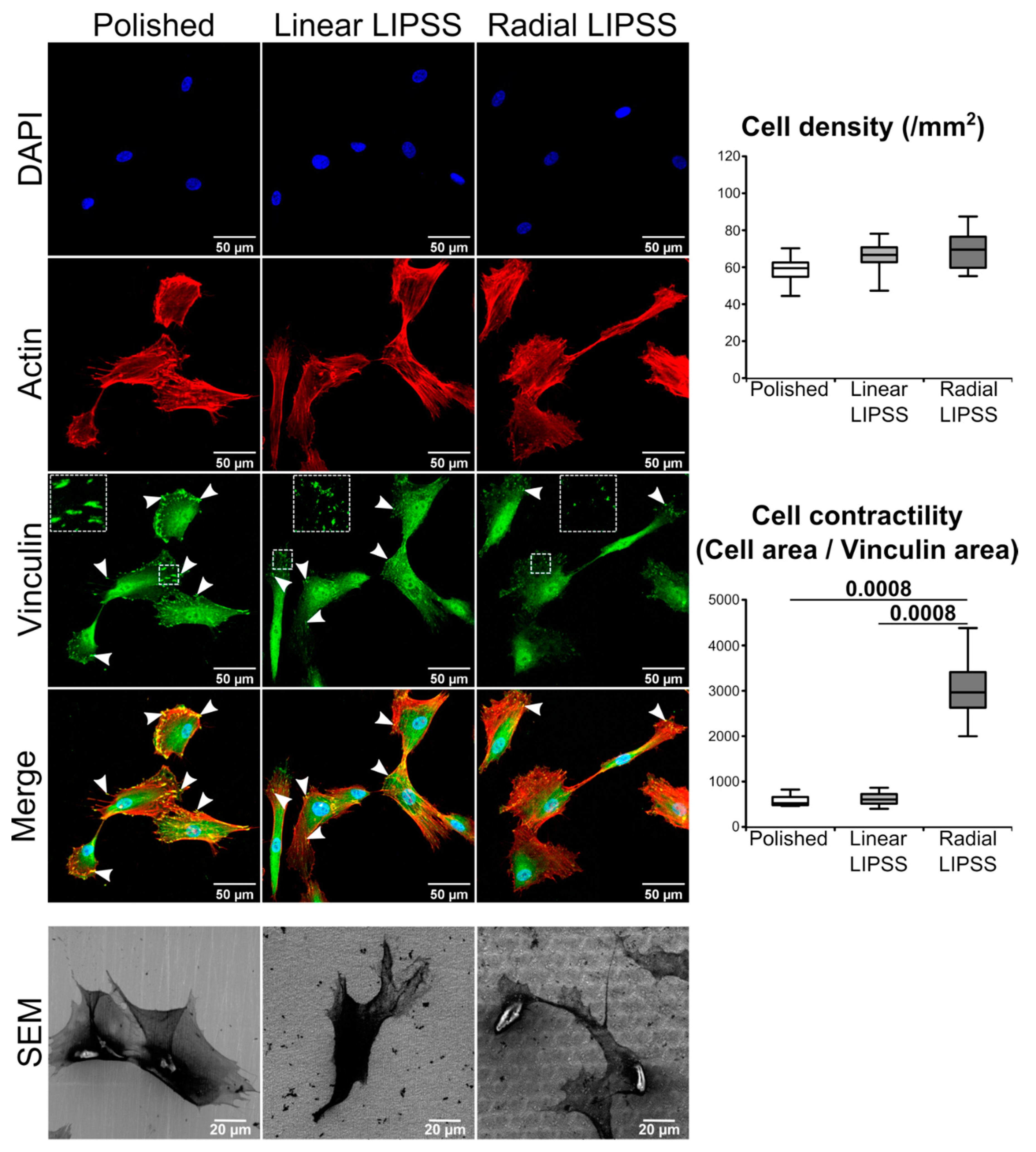
3.2. Radial LIPSS Increased Cell Contractility in the Early Stages
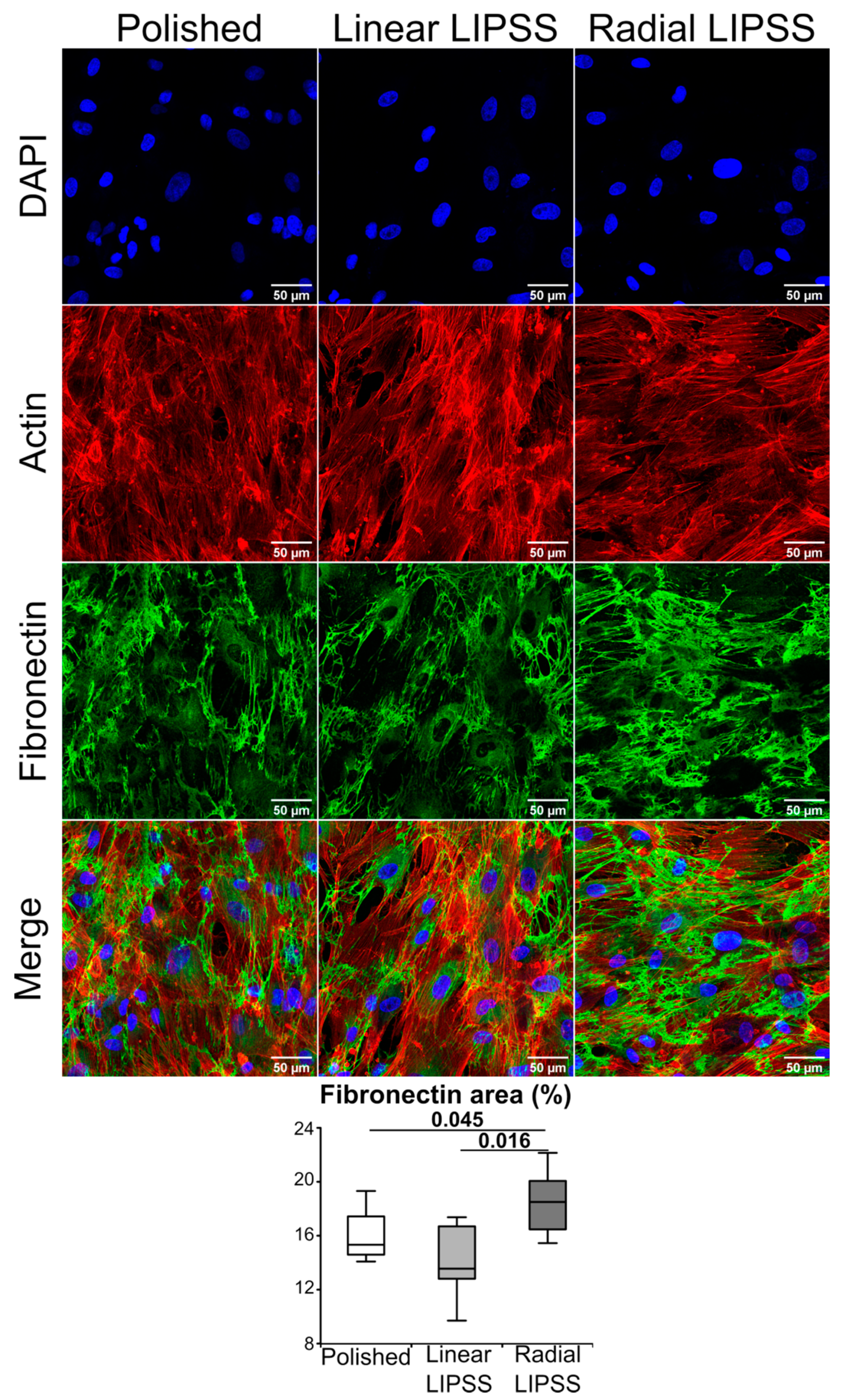
3.3. Radial LIPSS Improved Fibronectin Matrix Production
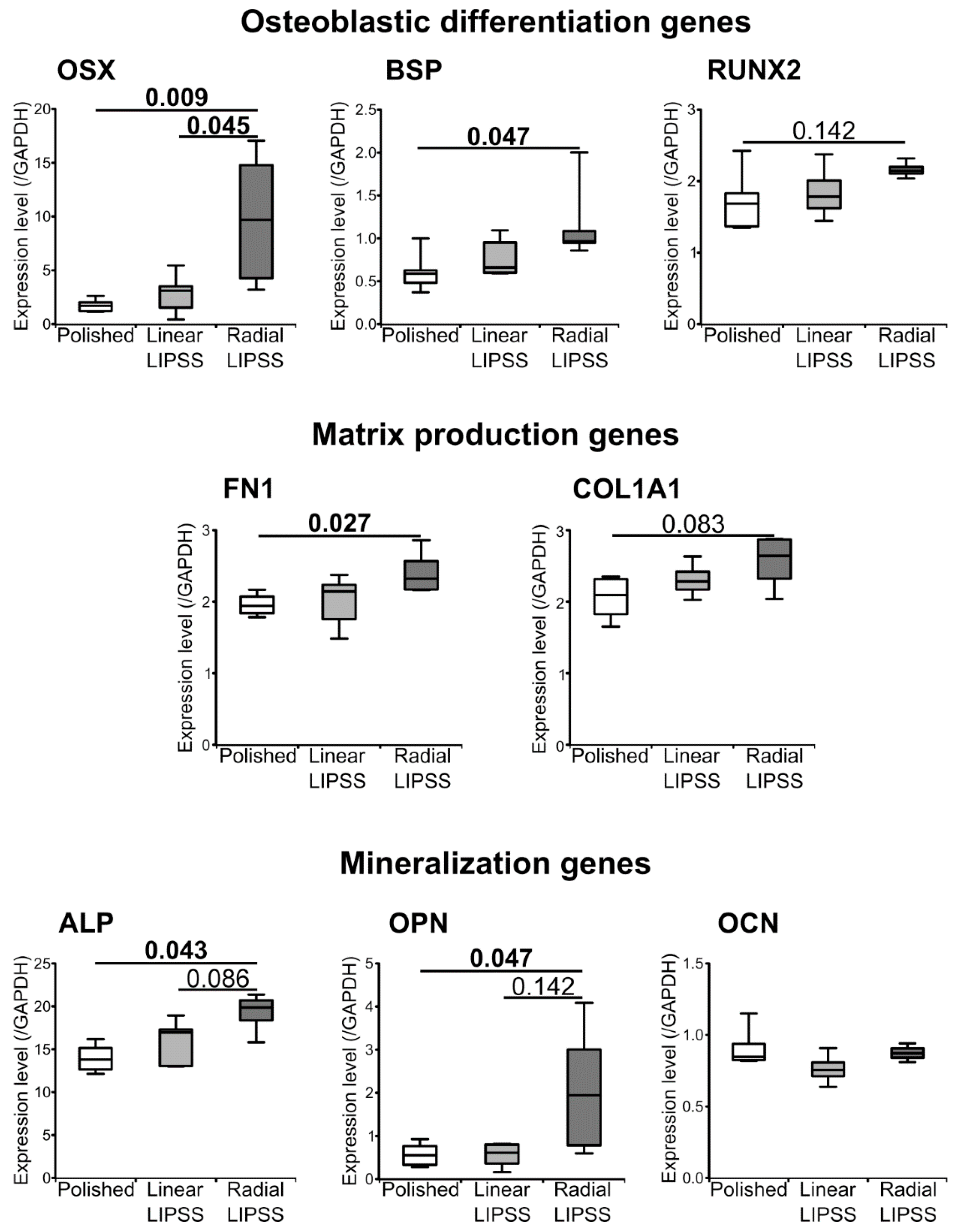
3.4. Radial LIPSS Induced Overexpression of Osteogenic Related Genes
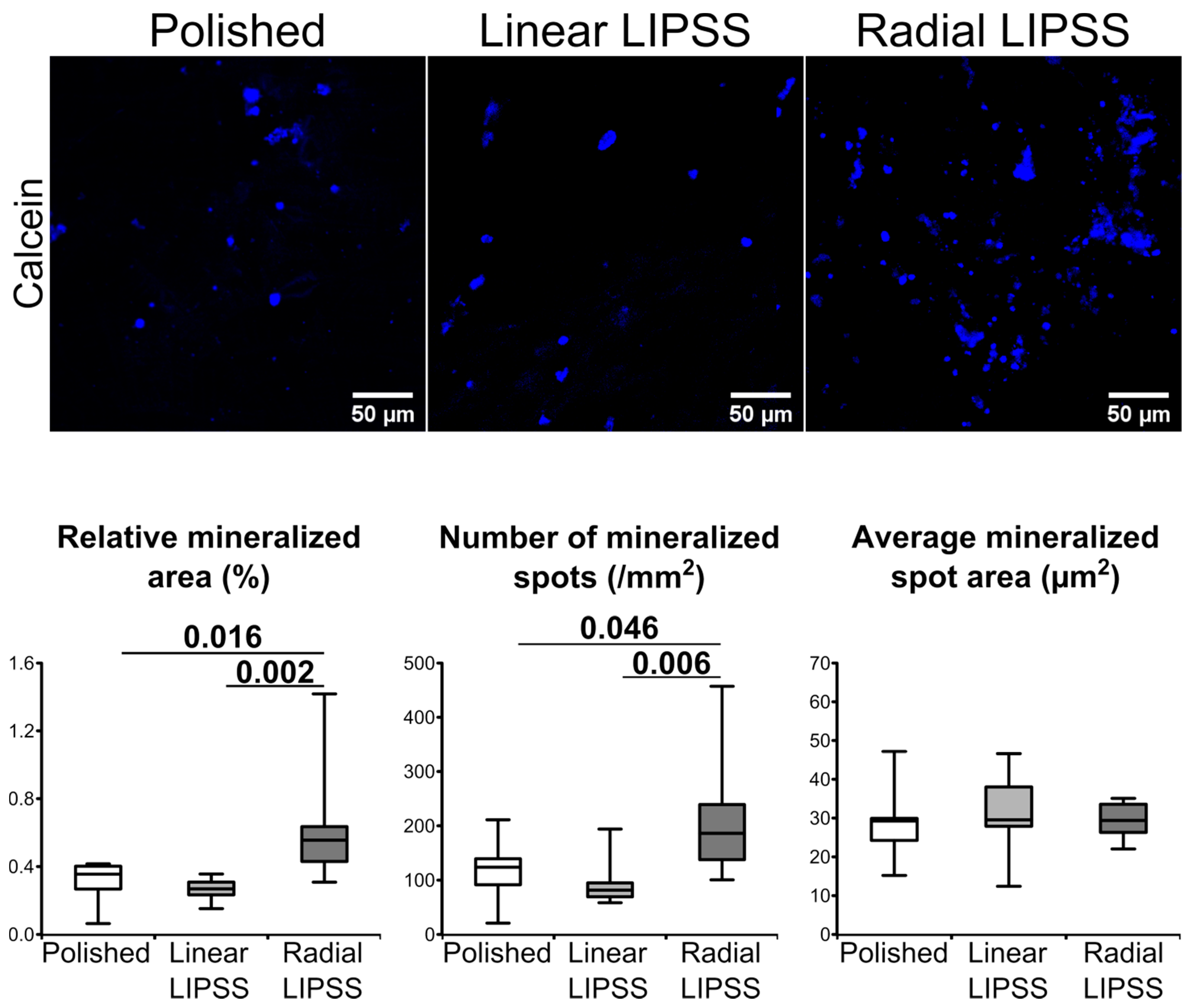
3.5. Radial LIPSS Increased the Mineralized Surfaces
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ottria, L.; Lauritano, D.; Andreasi Bassi, M.; Palmieri, A.; Candotto, V.; Tagliabue, A.; Tettamanti, L. Mechanical, chemical and biological aspects of titanium and titanium alloys in implant dentistry. J. Biol. Regul. Homeost. Agents 2018, 32 (Suppl. S1), 81–90. [Google Scholar] [PubMed]
- Shah, F.A.; Trobos, M.; Thomsen, P.; Palmquist, A. Commercially pure titanium (cp-Ti) versus titanium alloy (Ti6Al4V) materials as bone anchored implants—Is one truly better than the other? Mater. Sci. Eng. C Biol. Appl. 2016, 62, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Ermis, M.; Antmen, E.; Hasirci, V. Micro and Nanofabrication methods to control cell-substrate interactions and cell behavior: A review from the tissue engineering perspective. Bioact. Mater. 2018, 3, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Stich, T.; Alagboso, F.; Křenek, T.; Kovářík, T.; Alt, V.; Docheva, D. Implant-bone-interface: Reviewing the impact of titanium surface modifications on osteogenic processes in vitro and in vivo. Bioeng. Transl. Med. 2022, 7, e10239. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef] [Green Version]
- Brunette, D.M.; Tengvall, P.; Textor, M.; Thomsen, P. Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications; Softcover Reprint of the Original 1st ed. 2001 Édition; Springer-Verlag Berlin and Heidelberg GmbH & Co. K: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Zwahr, C.; Welle, A.; Weingärtner, T.; Heinemann, C.; Kruppke, B.; Gulow, N.; Holthaus, M.G.; Lasagni, A.F. Ultrashort Pulsed Laser Surface Patterning of Titanium to Improve Osseointegration of Dental Implants. Adv. Eng. Mater. 2019, 21, 1900639. [Google Scholar] [CrossRef] [Green Version]
- Martinez, M.A.F.; Balderrama, .D.F.; Karam, P.S.B.H.; De Oliveira, R.C.; De Oliveira, F.A.; Grandini, C.R.; Vicente, F.B.; Stavropoulos, A.; Zangrando, M.S.R.; Sant’Ana, A.C.P. Surface roughness of titanium disks influences the adhesion, proliferation and differentiation of osteogenic properties derived from human. Int. J. Implant Dent. 2020, 6, 46. [Google Scholar] [CrossRef]
- Lüthen, F.; Lange, R.; Becker, P.; Rychly, J.; Beck, U.; Nebe, J.G.B. The influence of surface roughness of titanium on beta1- and beta3-integrin adhesion and the organization of fibronectin in human osteoblastic cells. Biomaterials 2005, 26, 2423–2440. [Google Scholar] [CrossRef]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef]
- Lee, S.W.; Phillips, K.S.; Gu, H.; Kazemzadeh-Narbat, M.; Ren, D. How microbes read the map: Effects of implant topography on bacterial adhesion and biofilm formation. Biomaterials 2021, 268, 120595. [Google Scholar] [CrossRef] [PubMed]
- Linklater, D.P.; Baulin, V.A.; Juodkazis, S.; Crawford, R.J.; Stoodley, P.; Ivanova, E.P. Mechano-bactericidal actions of nanostructured surfaces. Nat. Rev. Genet. 2021, 19, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.M.; Buchberger, G.; Stifter, D.; Duchoslav, J.; Hertwig, A.; Bonse, J.; Heitz, J.; Schwibbert, K. Spatial Period of Laser-Induced Surface Nanoripples on PET Determines Escherichia coli Repellence. Nanomaterials 2021, 11, 3000. [Google Scholar] [CrossRef] [PubMed]
- Klos, A.; Sedao, X.; Itina, T.E.; Helfenstein-Didier, C.; Donnet, C.; Peyroche, S.; Vico, L.; Guignandon, A.; Dumas, V. Ultrafast Laser Processing of Nanostructured Patterns for the Control of Cell Adhesion and Migration on Titanium Alloy. Nanomaterials 2020, 10, 864. [Google Scholar] [CrossRef]
- Dumas, V.; Rattner, A.; Vico, L.; Audouard, E.; Dumas, J.C.; Naisson, P.; Bertrand, P. Multiscale grooved titanium processed with femtosecond laser influences mesenchymal stem cell morphology, adhesion, and matrix organization. J. Biomed. Mater. Res. Part A 2012, 100, 3108–3116. [Google Scholar] [CrossRef]
- Dumas, V.; Guignandon, A.; Vico, L.; Mauclair, C.; Zapata, X.; Linossier, M.T.; Bouleftour, W.; Granier, J.; Peyroche, S.; Dumas, J.-C.; et al. Femtosecond laser nano/micro patterning of titanium influences mesenchymal stem cell adhesion and commitment. Biomed. Mater. 2015, 10, 055002. [Google Scholar] [CrossRef]
- Epperlein, N.; Menzel, F.; Schwibbert, K.; Koter, R.; Bonse, J.; Sameith, J.; Krüger, J.; Toepel, J. Influence of femtosecond laser produced nanostructures on biofilm growth on steel. Appl. Surf. Sci. 2017, 418, 420–424. [Google Scholar] [CrossRef]
- Luo, X.; Yao, S.; Zhang, H.; Cai, M.; Liu, W.; Pan, R.; Chen, C.; Wang, X.; Wang, L.; Zhong, M. Biocompatible nano-ripples structured surfaces induced by femtosecond laser to rebel bacterial colonization and biofilm formation. Opt. Laser Technol. 2020, 124, 105973. [Google Scholar] [CrossRef]
- Cunha, A.; Zouani, O.F.; Plawinski, L.; Rego, A.M.B.D.; Almeida, A.; Vilar, R.; Durrieu, M.-C. Human mesenchymal stem cell behavior on femtosecond laser-textured Ti-6Al-4V surfaces. Nanomedicine 2015, 10, 725–739. [Google Scholar] [CrossRef]
- San-Blas, A.; Calderon, M.M.; Buencuerpo, J.; Sanchez-Brea, L.; del Hoyo, J.; Gomez-Aranzadi, M.; Rodríguez, A.; Olaizola, S. Femtosecond laser fabrication of LIPSS-based waveplates on metallic surfaces. Appl. Surf. Sci. 2020, 520, 146328. [Google Scholar] [CrossRef]
- Garrelie, F.; Colombier, J.-P.; Pigeon, F.; Tonchev, S.; Faure, N.; Bounhalli, M.; Reynaud, S.; Parriaux, O. Evidence of surface plasmon resonance in ultrafast laser-induced ripples. Opt. Express 2011, 19, 9035–9043. [Google Scholar] [CrossRef] [PubMed]
- Bonse, J.; Hohm, S.; Kirner, S.V.; Rosenfeld, A.; Kruger, J. Laser-Induced Periodic Surface Structures—A Scientific Evergreen. IEEE J. Sel. Top. Quantum Electron. 2016, 23. [Google Scholar] [CrossRef]
- Skoulas, E.; Manousaki, A.; Fotakis, C.; Stratakis, E. Biomimetic surface structuring using cylindrical vector femtosecond laser beams. Sci. Rep. 2017, 7, 45114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anselme, K.; Bigerelle, M. Role of materials surface topography on mammalian cell response. Int. Mater. Rev. 2011, 56, 243–266. [Google Scholar] [CrossRef]
- Théry, M.; Pépin, A.; Dressaire, E.; Chen, Y.; Bornens, M. Cell distribution of stress fibres in response to the geometry of the adhesive environment. Cell Motil. Cytoskelet. 2006, 63, 341–355. [Google Scholar] [CrossRef]
- Kilian, K.A.; Bugarija, B.; Lahn, B.T.; Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877. [Google Scholar] [CrossRef] [Green Version]
- Peng, R.; Yao, X.; Ding, J. Effect of cell anisotropy on differentiation of stem cells on micropatterned surfaces through the controlled single cell adhesion. Biomaterials 2011, 32, 8048–8057. [Google Scholar] [CrossRef]
- Yao, X.; Peng, R.; Ding, J. Effects of aspect ratios of stem cells on lineage commitments with and without induction media. Biomaterials 2013, 34, 930–939. [Google Scholar] [CrossRef]
- Han, P.; Frith, J.E.; Gomez, G.A.; Yap, A.S.; O’Neill, G.M.; Cooper-White, J.J. Five Piconewtons: The Difference between Osteogenic and Adipogenic Fate Choice in Human Mesenchymal Stem Cells. ACS Nano 2019, 13, 11129–11143. [Google Scholar] [CrossRef]
- Linsley, C.; Wu, B.; Tawil, B. The Effect of Fibrinogen, Collagen Type I, and Fibronectin on Mesenchymal Stem Cell Growth and Differentiation into Osteoblasts. Tissue Eng. Part A 2013, 19, 1416–1423. [Google Scholar] [CrossRef]
- Li, B.; Moshfegh, C.; Lin, Z.; Albuschies, J.; Vogel, V. Mesenchymal Stem Cells Exploit Extracellular Matrix as Mechanotransducer. Sci. Rep. 2013, 3, 2425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO. ISO 25178-2:2021. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/07/45/74591.html (accessed on 30 March 2022).
- Anderson, H.J.; Sahoo, J.K.; Ulijn, R.V.; Dalby, M.J. Mesenchymal Stem Cell Fate: Applying Biomaterials for Control of Stem Cell Behavior. Front. Bioeng. Biotechnol. 2016, 4, 38. Available online: https://www.frontiersin.org/article/10.3389/fbioe.2016.00038 (accessed on 3 May 2022). [CrossRef] [PubMed] [Green Version]
- Krishna, L.; Dhamodaran, K.; Jayadev, C.; Chatterjee, K.; Shetty, R.; Khora, S.S.; Das, D. Nanostructured scaffold as a determinant of stem cell fate. Stem Cell Res. Ther. 2016, 7, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamir, E.; Katz, M.; Posen, Y.; Erez, N.; Yamada, K.; Katz, B.-Z.; Lin, S.; Lin, D.C.; Bershadsky, A.; Kam, Z.; et al. Dynamics and segregation of cell–matrix adhesions in cultured fibroblasts. Nat. Cell Biol. 2000, 2, 191–196. [Google Scholar] [CrossRef]
- Yuan, H.; Zhou, Y.; Lee, M.-S.; Zhang, Y.; Li, W.-J. A newly identified mechanism involved in regulation of human mesenchymal stem cells by fibrous substrate stiffness. Acta Biomater. 2016, 42, 247–257. [Google Scholar] [CrossRef] [Green Version]
- Wennerberg, A.; Albrektsson, T. Suggested guidelines for the topographic evaluation of implant surfaces. Int. J. Oral Maxillofac. Implant. 2000, 15, 331–344. [Google Scholar]
- Faia-Torres, A.B.; Charnley, M.; Goren, T.; Guimond-Lischer, S.; Rottmar, M.; Maniura, K.; Spencer, N.D.; Reis, R.L.; Textor, M.; Neves, N.M. Osteogenic differentiation of human mesenchymal stem cells in the absence of osteogenic supplements: A surface-roughness gradient study. Acta Biomater. 2015, 28, 64–75. [Google Scholar] [CrossRef] [Green Version]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20 (Suppl. S4), 172–184. [Google Scholar] [CrossRef]
- Melo, M.A. Bacterial Interactions with Dental and Medical Materials. J. Funct. Biomater. 2020, 11, 83. [Google Scholar] [CrossRef]
- Bollenl, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Diaz, C.; Cortizo, M.C.; Schilardi, P.L.; De Saravia, S.G.G.; De Mele, M.A.F.L. Influence of the nano-micro structure of the surface on bacterial adhesion. Mater. Res. 2007, 10, 11–14. [Google Scholar] [CrossRef]
- Fiedler, J.; Özdemir, B.; Bartholomä, J.; Plettl, A.; Brenner, R.E.; Ziemann, P. The effect of substrate surface nanotopography on the behavior of multipotnent mesenchymal stromal cells and osteoblasts. Biomaterials 2013, 34, 8851–8859. [Google Scholar] [CrossRef] [PubMed]
- You, M.-H.; Kwak, M.K.; Kim, D.-H.; Kim, K.; Levchenko, A.; Kim, D.-Y.; Suh, K.-Y. Synergistically Enhanced Osteogenic Differentiation of Human Mesenchymal Stem Cells by Culture on Nanostructured Surfaces with Induction Media. Biomacromolecules 2010, 11, 1856–1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.W.; Oreffo, R.O.C. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Metavarayuth, K.; Sitasuwan, P.; Zhao, X.; Lin, Y.; Wang, Q. Influence of surface topographical cues on the differentiation of mesenchymal stem cells in vitro. ACS Biomater. Sci. Eng. 2016, 2, 142–151. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Lim, J.; Ho, Y.S.; Zhang, Q.-Y.; Chong, M.S.K.; Tang, M.; Hong, M.-H.; Chan, J.K.Y.; Teoh, S.H.; Thian, E.S. Biomimetic three-dimensional anisotropic geometries by uniaxial stretching of poly(ε-caprolactone) films: Degradation and mesenchymal stem cell responses. J. Biomed. Mater. Res. Part A 2014, 102, 2197–2207. [Google Scholar] [CrossRef]
- Wang, P.-Y.; Li, W.-T.; Yu, J.; Tsai, W.-B. Modulation of osteogenic, adipogenic and myogenic differentiation of mesenchymal stem cells by submicron grooved topography. J. Mater. Sci. Mater. Med. 2012, 23, 3015–3028. [Google Scholar] [CrossRef]
- Biggs, M.J.P.; Richards, R.G.; McFarlane, S.; Wilkinson, C.D.W.; Oreffo, R.O.C.; Dalby, M. Adhesion formation of primary human osteoblasts and the functional response of mesenchymal stem cells to 330 nm deep microgrooves. J. R. Soc. Interface 2008, 5, 1231–1242. [Google Scholar] [CrossRef] [Green Version]
- Sjöström, T.; Dalby, M.J.; Hart, A.; Tare, R.; Oreffo, R.O.C.; Su, B. Fabrication of pillar-like titania nanostructures on titanium and their interactions with human skeletal stem cells. Acta Biomater. 2009, 5, 1433–1441. [Google Scholar] [CrossRef]
- McNamara, L.E.; Sjöström, T.; Burgess, K.E.; Kim, J.J.; Liu, E.; Gordonov, S.; Moghe, P.V.; Meek, R.D.; Oreffo, R.O.; Su, B.; et al. Skeletal stem cell physiology on functionally distinct titania nanotopographies. Biomaterials 2011, 32, 7403–7410. [Google Scholar] [CrossRef]
- Sjöström, T.; McNamara, L.E.; Meek, R.M.D.; Dalby, M.J.; Su, B. 2D and 3D Nanopatterning of Titanium for Enhancing Osteoinduction of Stem Cells at Implant Surfaces. Adv. Healthc. Mater. 2013, 2, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Florian, C.; Skoulas, E.; Puerto, D.; Mimidis, A.; Stratakis, E.; Solis, J.; Siegel, J. Controlling the Wettability of Steel Surfaces Processed with Femtosecond Laser Pulses. ACS Appl. Mater. Interfaces 2018, 10, 36564–36571. [Google Scholar] [CrossRef] [PubMed]
- Florian, C.; Wonneberger, R.; Undisz, A.; Kirner, S.V.; Wasmuth, K.; Spaltmann, D.; Krüger, J.; Bonse, J. Chemical effects during the formation of various types of femtosecond laser-generated surface structures on titanium alloy. Appl. Phys. A 2020, 126, 266. [Google Scholar] [CrossRef] [Green Version]
- Kirner, S.V.; Wirth, T.; Sturm, H.; Krüger, J.; Bonse, J. Nanometer-resolved chemical analyses of femtosecond laser-induced periodic surface structures on titanium. J. Appl. Phys. 2017, 122, 104901. [Google Scholar] [CrossRef]
- Yang, G.-L.; He, F.-M.; Yang, X.-F.; Wang, X.-X.; Zhao, S.-F. Bone responses to titanium implants surface-roughened by sandblasted and double etched treatments in a rabbit model. Oral Sur. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, 516–524. [Google Scholar] [CrossRef]
- de Oliveira, P.T.; Zalzal, S.F.; Beloti, M.M.; Rosa, A.L.; Nanci, A. Enhancement of in vitro osteogenesis on titanium by chemically produced nanotopography. J. Biomed. Mater. Res. Part A 2007, 80, 554–564. [Google Scholar] [CrossRef]
- Carbone, R.; Marangi, I.; Zanardi, A.; Giorgetti, L.; Chierici, E.; Berlanda, G.; Podestà, A.; Fiorentini, F.; Bongiorno, G.; Piseri, P. Biocompatibility of cluster-assembled nanostructured TiO2 with primary and cancer cells. Biomaterials 2006, 27, 3221–3229. [Google Scholar] [CrossRef]
- Huang, H.-H.; Pan, S.-J.; Lai, Y.-L.; Lee, T.-H.; Chen, C.-C.; Lu, F.-H. Osteoblast-like cell initial adhesion onto a network-structured titanium oxide layer. Scr. Mater. 2004, 51, 1017–1021. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Oreffo, R.O.C. Harnessing nanotopography and integrin–matrix interactions to influence stem cell fate. Nat. Mater. 2014, 13, 558–569. [Google Scholar] [CrossRef]
- Heitz, J.; Plamadeala, C.; Muck, M.; Armbruster, O.; Baumgartner, W.; Weth, A.; Steinwender, C.; Blessberger, H.; Kellermair, J.; Kirner, S.V.; et al. Femtosecond laser-induced microstructures on Ti substrates for reduced cell adhesion. Appl. Phys. A 2017, 123, 734. [Google Scholar] [CrossRef] [Green Version]
| Topographies | Laser Pulse Condition | Distance between Pulses | Hatching Distance | F-Theta |
|---|---|---|---|---|
| Linear LIPSS | = 0.3–0.47 J/cm2 | 4–5 µm | 4–5 µm | 100 mm |
| Radial LIPSS | E = 0.7 µJ/pulse 5 pulses/impact | 13 µm | 13 µm | 56 mm |
| Protein | Forward | Reverse | Gene Bank ID |
|---|---|---|---|
| ALP | tgtaaggacatcgcctacca | gaagctcttccaggtgtcaa | NM_000478.5 |
| BSP | gaagactctgaggctgagaa | cctctgtgctgttggtactg | NM_004967.3 |
| COL1A1 | tccggctcctgctcctctta | gttgtcgcagacgcagatcc | NM_000088 |
| FN1 | ggctggatgatggtagattg | tgcctctcacacttccactc | NM_212482.4 |
| GAPDH | catcaccatcttccaggagcga | gtggtcatgagtccttccacga | NM_001289745.1 |
| OCN | agcggtgcagagtccagcaa | agccgatgtggtcagccaac | NM_199173.5 |
| OPN | tgatggccgaggtgatagtg | atcagaaggcgcgttcaggt | NM_001251830.1 |
| OSX | ctggctgcggcaaggtgtat | ccagctcatccgaacgagtg | NM_001300837.1 |
| RUNX2 | ccttgaccataaccgtcttc | aaggacttggtgcagagttc | NM_001024630.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maalouf, M.; Abou Khalil, A.; Di Maio, Y.; Papa, S.; Sedao, X.; Dalix, E.; Peyroche, S.; Guignandon, A.; Dumas, V. Polarization of Femtosecond Laser for Titanium Alloy Nanopatterning Influences Osteoblastic Differentiation. Nanomaterials 2022, 12, 1619. https://doi.org/10.3390/nano12101619
Maalouf M, Abou Khalil A, Di Maio Y, Papa S, Sedao X, Dalix E, Peyroche S, Guignandon A, Dumas V. Polarization of Femtosecond Laser for Titanium Alloy Nanopatterning Influences Osteoblastic Differentiation. Nanomaterials. 2022; 12(10):1619. https://doi.org/10.3390/nano12101619
Chicago/Turabian StyleMaalouf, Mathieu, Alain Abou Khalil, Yoan Di Maio, Steve Papa, Xxx Sedao, Elisa Dalix, Sylvie Peyroche, Alain Guignandon, and Virginie Dumas. 2022. "Polarization of Femtosecond Laser for Titanium Alloy Nanopatterning Influences Osteoblastic Differentiation" Nanomaterials 12, no. 10: 1619. https://doi.org/10.3390/nano12101619
APA StyleMaalouf, M., Abou Khalil, A., Di Maio, Y., Papa, S., Sedao, X., Dalix, E., Peyroche, S., Guignandon, A., & Dumas, V. (2022). Polarization of Femtosecond Laser for Titanium Alloy Nanopatterning Influences Osteoblastic Differentiation. Nanomaterials, 12(10), 1619. https://doi.org/10.3390/nano12101619








