On the Use of Carbon Cables from Plastic Solvent Combinations of Polystyrene and Toluene in Carbon Nanotube Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Synthesis of Carbon Nanotubes
2.2. The Characterization and Measurement of Material Properties
2.3. Preparation of CNTs for Testing Voltage Drop
2.4. Device Preparation and Measurement
2.5. Life Cycle Assessment(LCA) Methodolgy, Assumptions, and Boundary Conditions
3. Results and Discussion
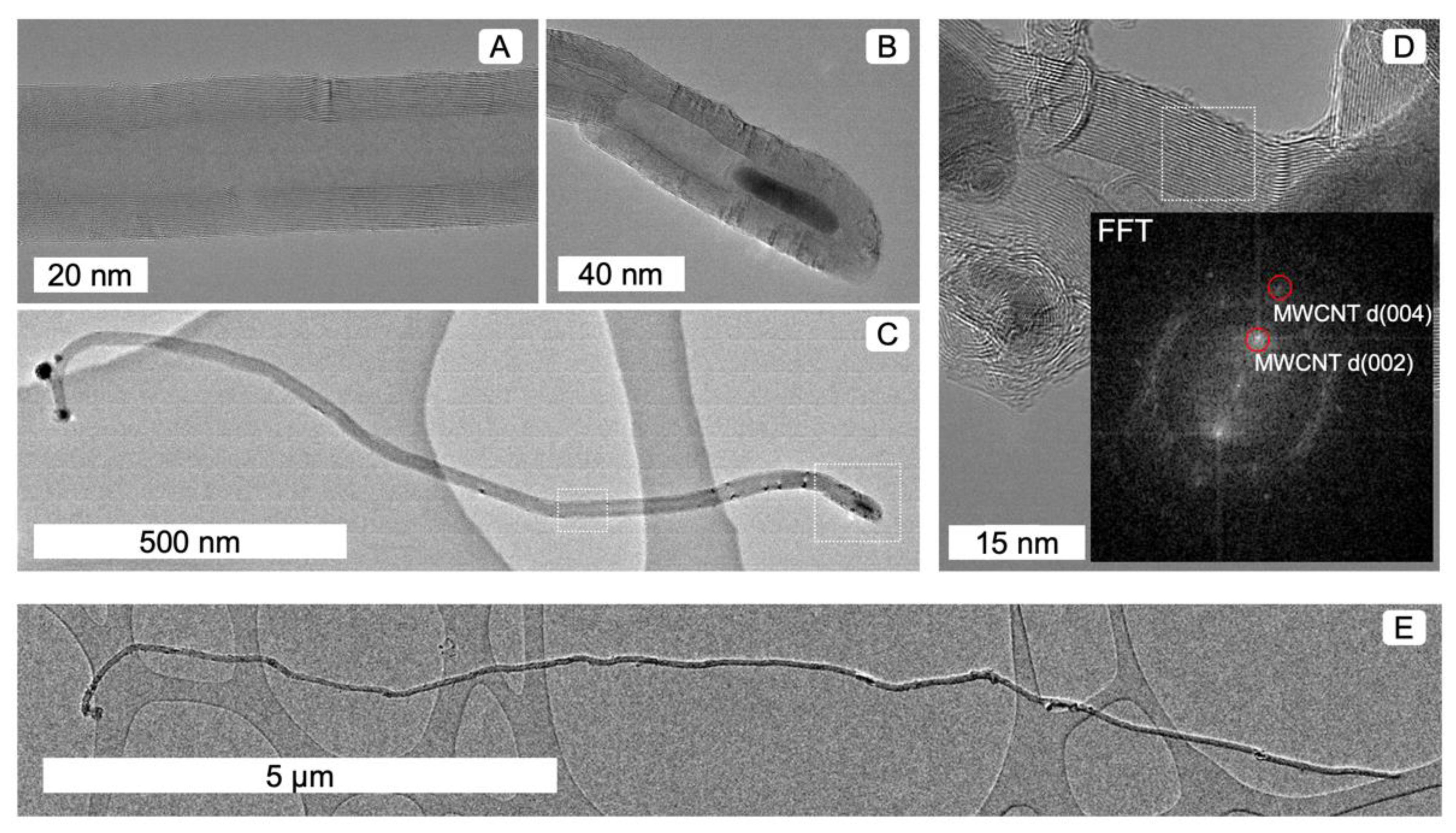
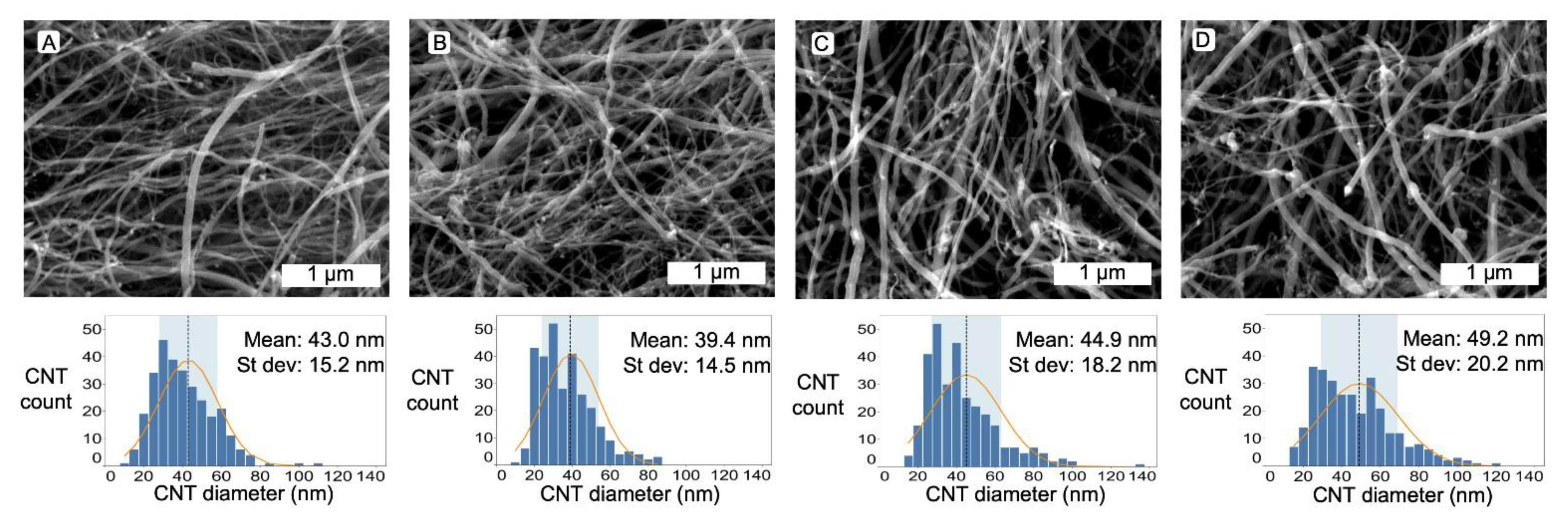
3.1. Material Characteristics Determind Using Microscopy
3.2. Material Characteristics Determined Using Spectroscopy
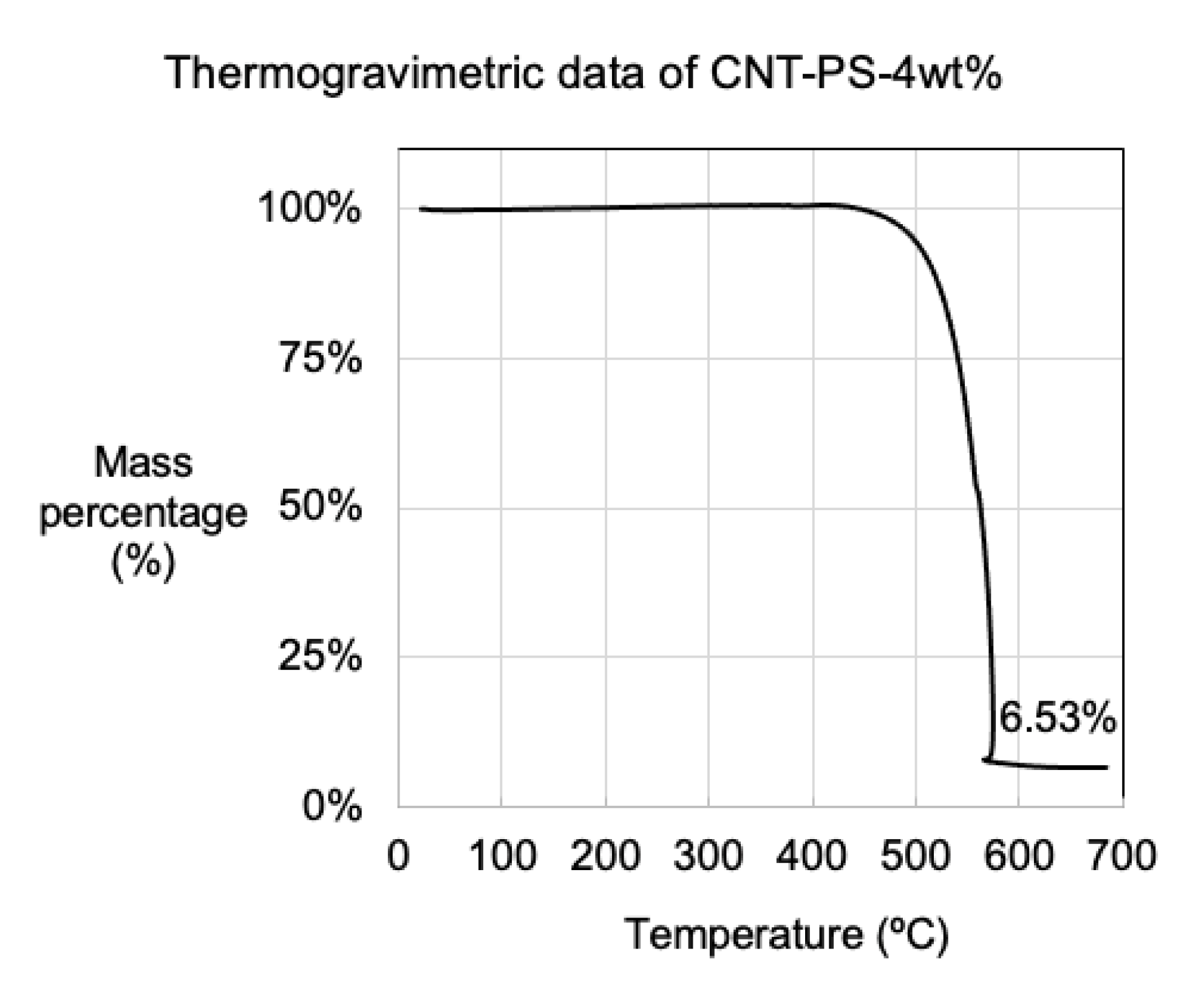
3.3. Material Properties Determined Using Mass Balance and Thermogravimetric Analysis
3.4. General Mechanism of Carbon Nanotube Growth
3.5. Contribution to CNT Growth from TOLUENE
3.6. Contribution to CNT Growth from Polystyrene
3.7. Contribution to CNT Growth from Cyclopentadienyl Rings
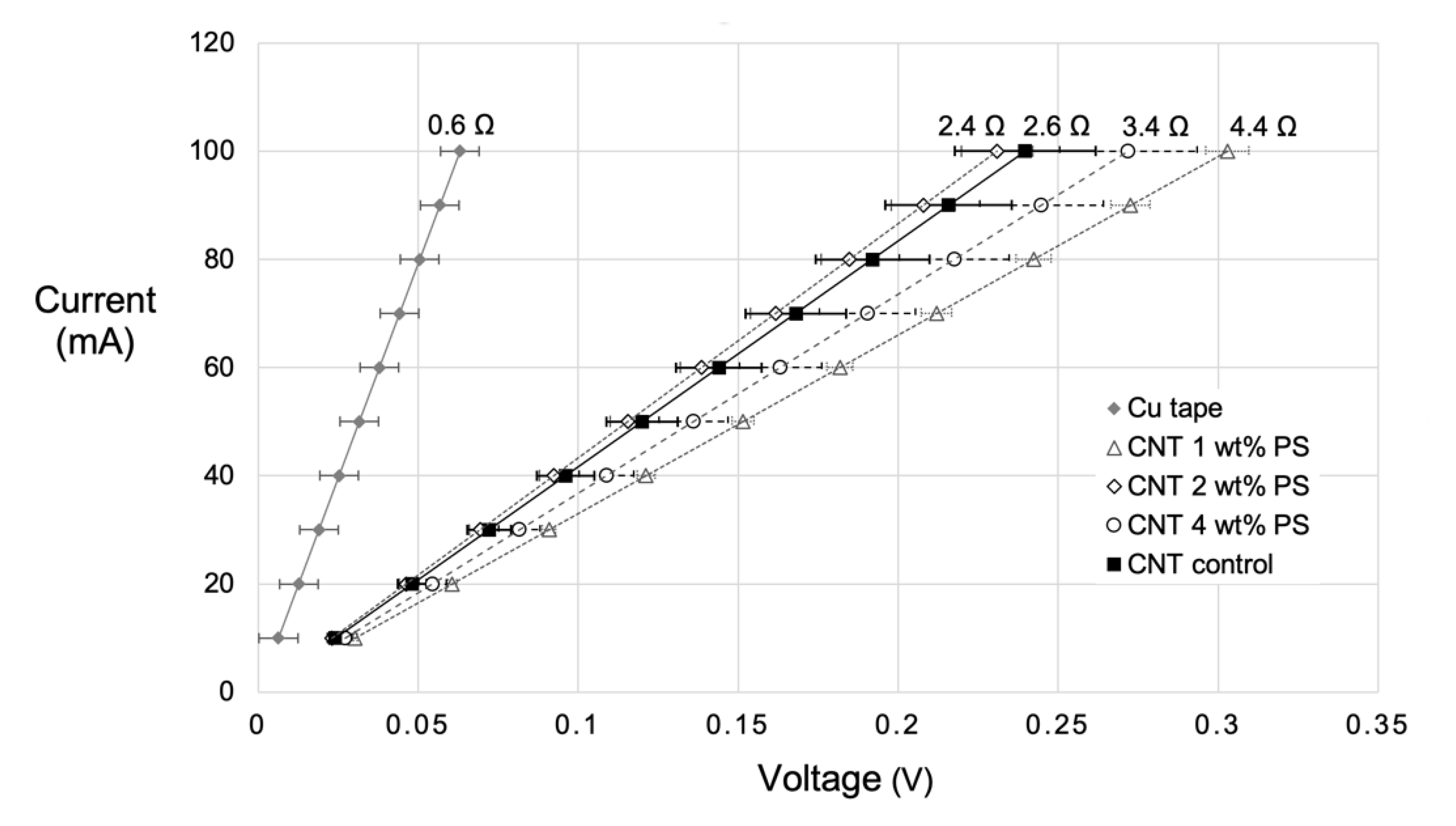
3.8. Carbon Nanotube Devices Measurement and Application
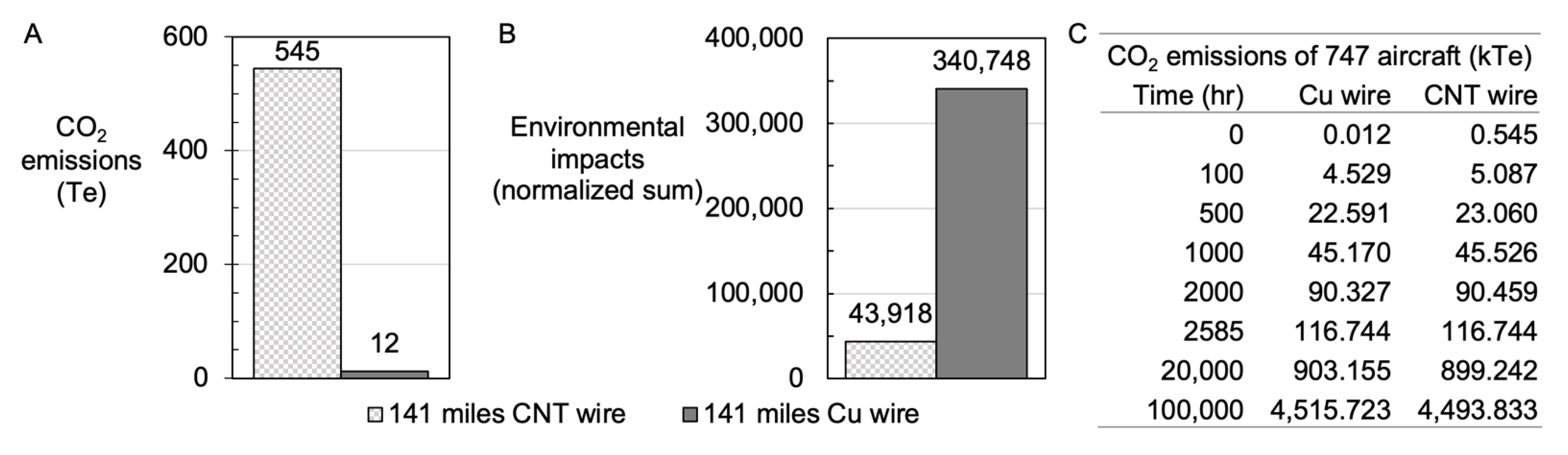
3.9. The Life Cycle Assessment of MWCNT Growth and Wire Production Versus Cu Wire Formation
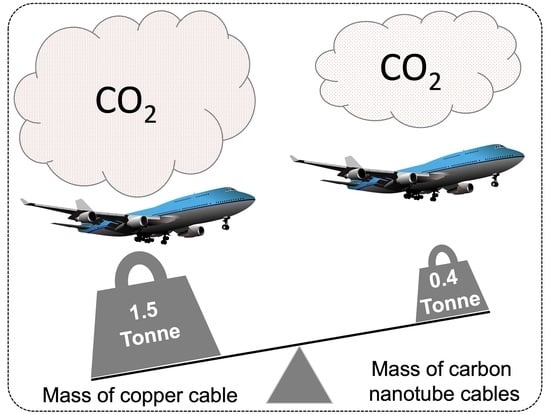
3.10. The Life Cycle Assessment of Use Case of MWCNTs in a Boeing 747-400 Aircraft
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smalley, R.E. Future Global Energy Prosperity: The Terawatt Challenge. MRS Bull. 2005, 67, 412–417. [Google Scholar] [CrossRef] [Green Version]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A Review on Pyrolysis of Plastic Wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Khodabakhshi, S.; Kiani, S.; Niu, Y.; White, A.O.; Suwaileh, W.; Palmer, R.E.; Barron, A.R.; Andreoli, E. Facile and Environmentally Friendly Synthesis of Ultramicroporous Carbon Spheres: A Significant Improvement in CVD Method. Carbon 2021, 171, 426–436. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Uses, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Bazargan, A.; McKay, G. A Review—Synthesis of Carbon Nanotubes from Plastic Wastes. Chem. Eng. J. 2012, 195–196, 377–391. [Google Scholar] [CrossRef]
- Acomb, J.C.; Wu, C.; Williams, P.T. Control of Steam Input to the Pyrolysis-Gasification of Waste Plastics for Improved Production of Hydrogen or Carbon Nanotubes. Appl. Catal. B Environ. 2014, 147, 571–584. [Google Scholar] [CrossRef]
- Aboul-Enein, A.A.; Adel-Rahman, H.; Haggar, A.M.; Awadallah, A.E. Simple Method for Synthesis of Carbon Nanotubes over Ni-Mo/Al 2 O 3 Catalyst via Pyrolysis of Polyethylene Waste Using a Two-Stage Process. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 211–222. [Google Scholar] [CrossRef]
- El Essawy, N.A.; Konsowa, A.H.; Elnouby, M.; Farag, H.A. A Novel One-Step Synthesis for Carbon-Based Nanomaterials from Polyethylene Terephthalate (PET) Bottles Waste. J. Air Waste Manag. Assoc. 2017, 67, 358–370. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Sun, H.; Wu, C.; Patel, D.; Huang, J. Thermal Chemical Conversion of High-Density Polyethylene for the Production of Valuable Carbon Nanotubes Using Ni/AAO Membrane Catalyst. Energy Fuels 2018, 32, 4511–4520. [Google Scholar] [CrossRef]
- Arena, U.; Mastellone, M.L.; Camino, G.; Boccaleri, E. An Innovative Process for Mass Production of Multi-Wall Carbon Nanotubes by Means of Low-Cost Pyrolysis of Polyolefins. Polym. Degrad. Stab. 2006, 91, 763–768. [Google Scholar] [CrossRef]
- Miller-Chou, B.A.; Koenig, J.L. A Review of Polymer Dissolution. Prog. Polym. Sci. 2003, 28, 1223–1270. [Google Scholar] [CrossRef] [Green Version]
- Puengjinda, P.; Sano, N.; Tanthapanichakoon, W.; Charinpanitkul, T. Selective Synthesis of Carbon Nanotubes and Nanocapsules Using Naphthalene Pyrolysis Assisted with Ferrocene. J. Ind. Eng. Chem. 2009, 15, 375–380. [Google Scholar] [CrossRef]
- Charinpanitkul, T.; Sano, N.; Puengjinda, P.; Klanwan, J.; Akrapattangkul, N.; Tanthapanichakoon, W. Naphthalene as an Alternative Carbon Source for Pyrolytic Synthesis of Carbon Nanostructures. J. Anal. Appl. Pyrolysis 2009, 86, 386–390. [Google Scholar] [CrossRef]
- Orbaek, A.W.; Barron, A.R. Towards a ‘Catalyst Activity Map’ Regarding the Nucleation and Growth of Single Walled Carbon Nanotubes. J. Exp. Nanosci. 2015, 10, 66–76. [Google Scholar] [CrossRef]
- Hedayati, A.; Barnett, C.; Swan, G.; Orbaek White, A. Chemical Recycling of Consumer-Grade Black Plastic into Electrically Conductive Carbon Nanotubes. C 2019, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Narasimhan, B.; Peppas, N.A. Disentanglement and Reptation during Dissolution of Rubbery Polymers. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 947–961. [Google Scholar] [CrossRef]
- Achilias, D.S.; Giannoulis, A.; Papageorgiou, G.Z. Recycling of Polymers from Plastic Packaging Materials Using the Dissolution–Reprecipitation Technique. Polym. Bull. 2009, 63, 449–465. [Google Scholar] [CrossRef]
- Bjørn, A.; Owsianiak, M.; Molin, C.; Laurent, A. Main Characteristics of LCA. In Life Cycle Assessment Theory and Practice, 1st ed.; Hauschild, M., Rosenbaum, R., Olsen, S., Eds.; Springer: Cham, Switzerland, 2018; pp. 9–16. [Google Scholar]
- Heidari, A.; Younesi, H. Synthesis, Characterization and Life Cycle Assessment of Carbon Nanospheres from Waste Tires Pyrolysis over Ferrocene Catalyst. J. Environ. Chem. Eng. 2020, 8, 103669. [Google Scholar] [CrossRef]
- Rybicka, J.; Tiwari, A.; Leeke, G.A. Technology Readiness Level Assessment of Composites Recycling Technologies. J. Clean. Prod. 2016, 112, 1001–1012. [Google Scholar] [CrossRef] [Green Version]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and Recovery Routes of Plastic Solid Waste (PSW): A Review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Kim, J.-W.; Pham, T.D.; Tarafdar, A.; Hong, S.; Chun, S.-H.; Lee, S.-H.; Kang, D.-Y.; Kim, J.-Y.; Kim, S.-B.; et al. Microplastics in Food: A Review on Analytical Methods and Challenges. Int. J. Environ. Res. Public Health 2020, 17, 6710. [Google Scholar] [CrossRef]
- Orbaek, A.W.; Aggarwal, N.; Barron, A.R. The Development of a ‘Process Map’ for the Growth of Carbon Nanomaterials from Ferrocene by Injection CVD. J. Mater. Chem. A 2013, 1, 14122. [Google Scholar] [CrossRef]
- Gangoli, V.S.; Raja, P.M.V.; Esquenazi, G.L.; Barron, A.R. The Safe Handling of Bulk Low-Density Nanomaterials. SN Appl. Sci. 2019, 1, 644. [Google Scholar] [CrossRef] [Green Version]
- Dee, N.T.; Li, J.; White, A.O.; Jacob, C.; Shi, W.; Kidambi, P.R.; Cui, K.; Zakharov, D.N.; Janković, N.Z.; Bedewy, M.; et al. Carbon-Assisted Catalyst Pretreatment Enables Straightforward Synthesis of High-Density Carbon Nanotube Forests. Carbon N. Y. 2019, 153, 196–205. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next Generation of Scientific Image Data. BMC Bioinform. 2017, 18, 1–26. [Google Scholar] [CrossRef]
- King, S.G.; McCafferty, L.; Stolojan, V.; Silva, S.R.P. Highly Aligned Arrays of Super Resilient Carbon Nanotubes by Steam Purification. Carbon N. Y. 2015, 84, 130–137. [Google Scholar] [CrossRef]
- Chiang, I.W.; Brinson, B.E.; Smalley, R.E.; Margrave, J.L.; Hauge, R.H. Purification and Characterization of Single-Wall Carbon Nanotubes. J. Phys. Chem. B 2001, 105, 1157–1161. [Google Scholar] [CrossRef]
- Mansfield, E.; Kar, A.; Hooker, S.A. Applications of TGA in Quality Control of SWCNTs. Anal. Bioanal. Chem. 2010, 396, 1071–1077. [Google Scholar] [CrossRef]
- Khodabakhhshi, S.; Fulvio, P.F.; Sousaraei, A.; Kiani, S.; Niu, Y.; Palmer, R.E.; Kuo, W.C.H.; Rudd, J.; Barron, A.R.; Andreoli, E. Oxidative Synthesis of Yellow Photoluminescent Carbon Nanoribbons from Carbon Black. Carbon N. Y. 2021, 183, 495–503. [Google Scholar] [CrossRef]
- Andreoli, E.; Suzuki, R.; Orbaek, A.W.; Bhutani, M.S.; Hauge, R.H.; Adams, W.; Fleming, J.B.; Barron, A.R. Preparation and Evaluation of Polyethyleneimine-Single Walled Carbon Nanotube Conjugates as Vectors for Pancreatic Cancer Treatment. J. Mater. Chem. B 2014, 2, 4740. [Google Scholar] [CrossRef]
- Chiang, I.W.; Brinson, B.E.; Huang, A.Y.; Willis, P.A.; Bronikowski, M.J.; Margrave, J.L.; Smalley, R.E.; Hauge, R.H. Purification and Characterization of Single-Wall Carbon Nanotubes (SWNTs) Obtained from the Gas-Phase Decomposition of CO (HiPco Process). J. Phys. Chem. B 2001, 105, 8297–8301. [Google Scholar] [CrossRef] [Green Version]
- López-Lorente, A.I.; Simonet, B.M.; Valcárcel, M. The Potential of Carbon Nanotube Membranes for Analytical Separations. Anal. Chem. 2010, 82, 5399–5407. [Google Scholar] [CrossRef]
- Aircraft Electrical Wire. Available online: https://www.mitrecaasd.org/atsrac/FAA_PI-Engineer_Workshop/2001/aircraft_electrical_wire.pdf (accessed on 21 December 2021).
- Lu, Q.; Keskar, G.; Ciocan, R.; Rao, R.; Mathur, R.B.; Rao, A.M.; Larcom, L.L. Determination of Carbon Nanotube Density by Gradient Sedimentation. J. Phys. Chem. B 2006, 110, 24371–24376. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Jorio, A. Raman Spectroscopy of Carbon Nanotubes in 1997 and 2007. J. Phys. Chem. C 2007, 111, 17887–17893. [Google Scholar] [CrossRef]
- Schwan, J.; Ulrich, S.; Batori, V.; Ehrhardt, H.; Silva, S.R.P. Raman Spectroscopy on Amorphous Carbon Films. J. Appl. Phys. 1996, 80, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Box Plot. Available online: https://www.itl.nist.gov/div898/handbook/eda/section3/boxplot.htm (accessed on 21 October 2021).
- Meam Plot. Available online: https://www.itl.nist.gov/div898/handbook/eda/section3/meanplot.htm (accessed on 21 October 2021).
- Baker, R.T.K. Catalytic Growth of Carbon Filaments. Carbon N. Y. 1989, 27, 315–323. [Google Scholar] [CrossRef]
- Wood, R.F.; Pannala, S.; Wells, J.C.; Puretzky, A.A.; Geohegan, D.B. Simple Model of the Interrelation between Single- and Multiwall Carbon Nanotube Growth Rates for the CVD Process. Phys. Rev. B 2007, 75, 235446. [Google Scholar] [CrossRef]
- Somorjai, G.A.; Carrazza, J. Structure Sensitivity of Catalytic Reactions. Ind. Eng. Chem. Fundam. 1986, 25, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Rao, R.; Liptak, D.; Cherukuri, T.; Yakobson, B.I.; Maruyama, B. In Situ Evidence for Chirality-Dependent Growth Rates of Individual Carbon Nanotubes. Nat. Mater. 2012, 11, 213–216. [Google Scholar] [CrossRef]
- Marchand, M.; Journet, C.; Guillot, D.; Benoit, J.-M.; Yakobson, B.I.; Purcell, S.T. Growing a Carbon Nanotube Atom by Atom: “And Yet It Does Turn”. Nano Lett. 2009, 9, 2961–2966. [Google Scholar] [CrossRef]
- Maksimova, N.I.; Krivoruchko, O.P.; Mestl, G.; Zaikovskii, V.I.; Chuvilin, A.L.; Salanov, A.N.; Burgina, E.B. Catalytic Synthesis of Carbon Nanostructures from Polymer Precursors. J. Mol. Catal. A Chem. 2000, 158, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Gogate, P.R.; Prajapat, A.L. Depolymerization using sonochemical reactors: A critical review. Ultrason. Sonochem. 2015, 27, 480–494. [Google Scholar] [CrossRef]
- Matsugi, A. Thermal Decomposition of Benzyl Radicals: Kinetics and Spectroscopy in a Shock Tube. J. Phys. Chem. A 2020, 124, 824–835. [Google Scholar] [CrossRef]
- Brouwer, L.D.; Mueller-Markgraf, W.; Troe, J. Thermal Decomposition of Toluene: A Comparison of Thermal and Laser-Photochemical Activation Experiments. J. Phys. Chem. 1988, 92, 4905–4914. [Google Scholar] [CrossRef]
- Oehlschlaeger, M.A.; Davidson, D.F.; Hanson, R.K. Thermal Decomposition of Toluene: Overall Rate and Branching Ratio. Proc. Combust. Inst. 2007, 31, 211–219. [Google Scholar] [CrossRef]
- Maafa, I.M. Pyrolysisof Polystyrene Waste: A Review. Polymers 2021, 13, 225. [Google Scholar] [CrossRef]
- Fürstner, A. Iron Catalysis in Organic Synthesis: A Critical Assessment of What It Takes to Make This Base Metal a Multitasking Champion. ACS Cent. Sci. 2016, 2, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.T. Hydrogen and Carbon Nanotubes from Pyrolysis-Catalysis of Waste Plastics: A Review. Waste Biomass Valorization 2021, 12, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Rao, C.N.R.; Sen, R. Large aligned-nanotube bundles from ferrocene pyrolysis. Chem. Commun. 1998, 15, 1525–1526. [Google Scholar] [CrossRef]
- An, J.; Choi, E.; Shim, S.; Kim, H.; Kang, G.; Yun, J. Thermal Decomposition In Situ Monitoring System of the Gas Phase Cyclopentadienyl Tris(dimethylamino) Zirconium (CpZr(NMe2)3) Based on FT-IR and QMS for Atomic Layer Deposition. Nanoscale Res. Lett. 2020, 15, 175. [Google Scholar] [CrossRef]
- Wulan, P.P.D.K.; Rivai, G.T. Synthesis of carbon nanotube using ferrocene as carbon source and catalyst in a vertically structured catalyst reactor. E3S Web Conf. 2018, 67, 03038. [Google Scholar] [CrossRef]
- Barnett, C.J.; McCormack, J.E.; Deemer, E.M.; Evans, C.R.; Evans, J.E.; White, A.O.; Dunstan, P.R.; Chianelli, R.R.; Cobley, R.J.; Barron, A.R. Enhancement of Multiwalled Carbon Nanotubes’ Electrical Conductivity Using Metal Nanoscale Copper Contacts and Its Implications for Carbon Nanotube-Enhanced Copper Conductivity. J. Phys. Chem. C 2020, 124, 18777–18783. [Google Scholar] [CrossRef]
- Cesano, F.; Uddin, M.J.; Lozano, K.; Zanetti, M.; Scarano, D. All-Carbon Conductors for Electronic and Electrical Wiring Applications. Front. Mater. 2020, 7, 219. [Google Scholar] [CrossRef]
- Jarosz, P.; Schauerman, C.; Alvarenga, J.; Moses, B.; Mastrangelo, T.; Raffaelle, R.; Ridgley, R.; Landi, B. Carbon Nanotube Wires and Cables: Near-Term Applications and Future Perspectives. Nanoscale 2011, 3, 4542. [Google Scholar] [CrossRef] [PubMed]
- House of Commons Library, Broadband. Available online: https://commonslibrary.parliament.uk/broadband-faqs/ (accessed on 27 October 2021).
- Upadhyayula, V.K.K.; Meyer, D.E.; Curran, M.A.; Gonzalez, M.A. Life Cycle Assessment as a Tool to Enhance the Environmental Performance of Carbon Nanotube Products: A Review. J. Clean. Prod. 2012, 26, 37–47. [Google Scholar] [CrossRef]
- Griffiths, O.G.; O’Byrne, J.P.; Torrente-Murciano, L.; Jones, M.D.; Mattia, D.; McManus, M.C. Identifying the Largest Environmental Life Cycle Impacts during Carbon Nanotube Synthesis via Chemical Vapour Deposition. J. Clean. Prod. 2013, 42, 180–189. [Google Scholar] [CrossRef]
- Nagy, B.; Farmer, J.D.; Bui, Q.M.; Trancik, J.E. Statistical Basis for Predicting Technological Progress. PLoS ONE 2013, 8, e52669. [Google Scholar] [CrossRef] [Green Version]
- Pawelke, R.H. Marrying Wright’s Law to Thermodynamics for an Ideal Relative Final Cost-Predicting Model of Carbon-Fuel Substitution. ChemRxiv 2021. [Google Scholar] [CrossRef]
- Gavankar, S.; Suh, S.; Keller, A.A. The Role of Scale and Technology Maturity in Life Cycle Assessment of Emerging Technologies: A Case Study on Carbon Nanotubes. J. Ind. Ecol. 2015, 19, 51–60. [Google Scholar] [CrossRef]
- Healy, M.L.; Dahlben, L.J.; Isaacs, J.A. Environmental Assessment of Single-Walled Carbon Nanotube Processes. J. Ind. Ecol. 2008, 12, 376–393. [Google Scholar] [CrossRef]
- Boeing 747-400. Available online: https://en.wikipedia.org/wiki/Boeing_747-400 (accessed on 21 December 2021).
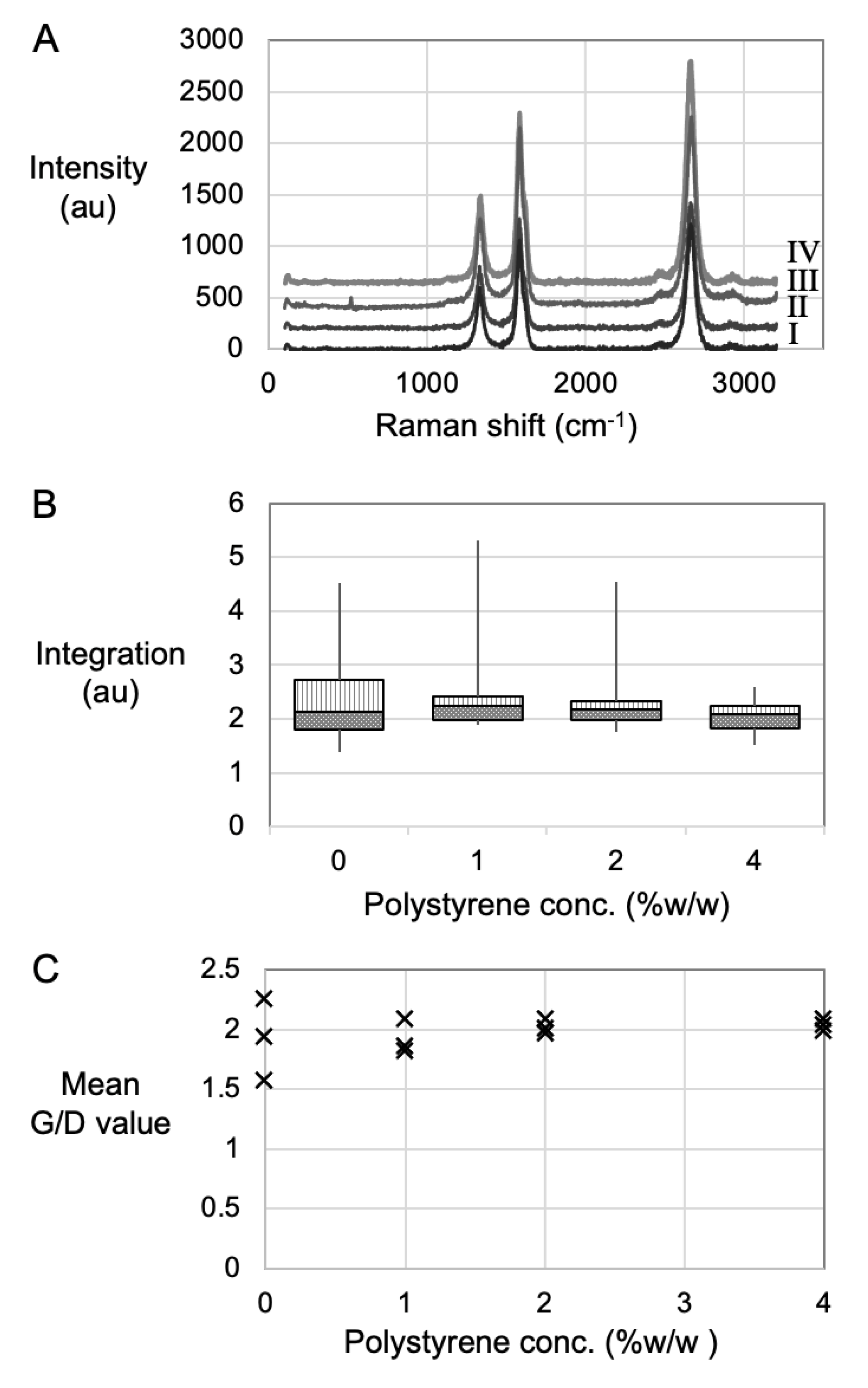

| PS Concentration (wt%) | ||||
|---|---|---|---|---|
| 0 | 1 | 2 | 4 | |
| Mean G/D value | 1.9 | 2.0 | 2.0 | 2.0 |
| Maximum G/D value | 2.3 | 2.3 | 2.3 | 2.5 |
| Sample | Mass of Carbon Reactant (mg) | Average CNT Product (mg) | Maximum CNT Product (mg) | Maximum CNT Yield (%) |
|---|---|---|---|---|
| Control | 815.87 | 89.80 | 92.80 | 11.37% |
| 1 wt% PS | 824.20 | 80.47 | 89.36 | 10.84% |
| 2 wt% PS | 832.57 | 100.36 | 107.83 | 12.95% |
| 4 wt% PS | 849.23 | 106.58 | 121.55 | 14.31% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orbaek White, A.; Hedayati, A.; Yick, T.; Gangoli, V.S.; Niu, Y.; Lethbridge, S.; Tsampanakis, I.; Swan, G.; Pointeaux, L.; Crane, A.; et al. On the Use of Carbon Cables from Plastic Solvent Combinations of Polystyrene and Toluene in Carbon Nanotube Synthesis. Nanomaterials 2022, 12, 9. https://doi.org/10.3390/nano12010009
Orbaek White A, Hedayati A, Yick T, Gangoli VS, Niu Y, Lethbridge S, Tsampanakis I, Swan G, Pointeaux L, Crane A, et al. On the Use of Carbon Cables from Plastic Solvent Combinations of Polystyrene and Toluene in Carbon Nanotube Synthesis. Nanomaterials. 2022; 12(1):9. https://doi.org/10.3390/nano12010009
Chicago/Turabian StyleOrbaek White, Alvin, Ali Hedayati, Tim Yick, Varun Shenoy Gangoli, Yubiao Niu, Sean Lethbridge, Ioannis Tsampanakis, Gemma Swan, Léo Pointeaux, Abigail Crane, and et al. 2022. "On the Use of Carbon Cables from Plastic Solvent Combinations of Polystyrene and Toluene in Carbon Nanotube Synthesis" Nanomaterials 12, no. 1: 9. https://doi.org/10.3390/nano12010009
APA StyleOrbaek White, A., Hedayati, A., Yick, T., Gangoli, V. S., Niu, Y., Lethbridge, S., Tsampanakis, I., Swan, G., Pointeaux, L., Crane, A., Charles, R., Sallah-Conteh, J., Anderson, A. O., Davies, M. L., Corr, S. J., & Palmer, R. E. (2022). On the Use of Carbon Cables from Plastic Solvent Combinations of Polystyrene and Toluene in Carbon Nanotube Synthesis. Nanomaterials, 12(1), 9. https://doi.org/10.3390/nano12010009