Facile Pretreatment of Three-Dimensional Graphene through Electrochemical Polarization for Improved Electrocatalytic Performance and Simultaneous Electrochemical Detection of Catechol and Hydroquinone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Measurements and Instrumentations
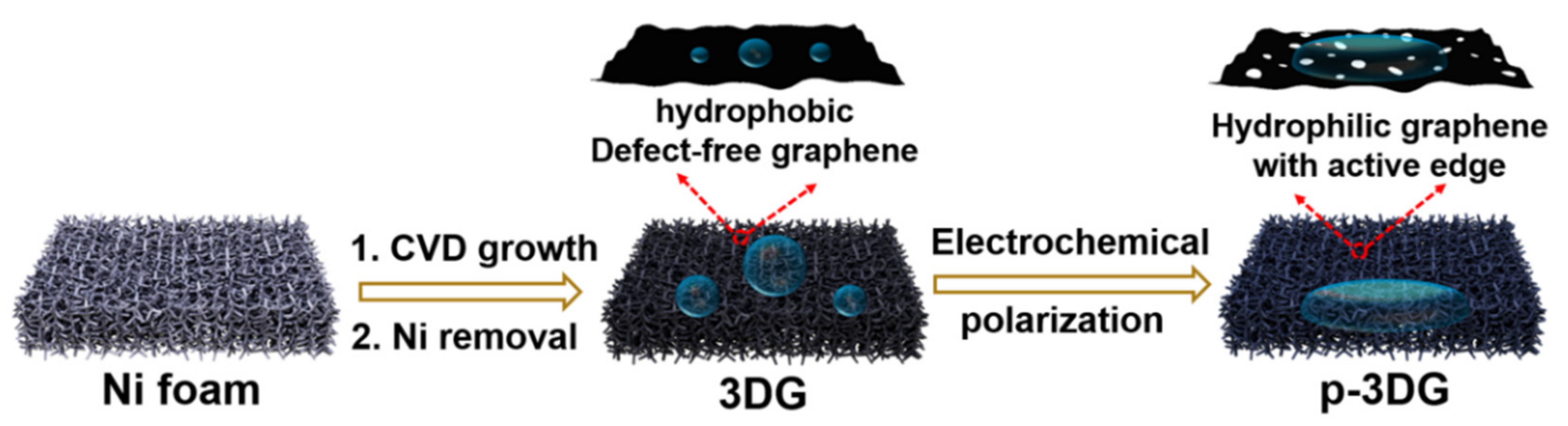
2.3. Preparation of 3DG Electrode
2.4. Electrochemical Polarization of 3DG Electrode to Prepare p-3DG
2.5. Electrochemical Detection of p-BD and o-BD
3. Results and Discussion
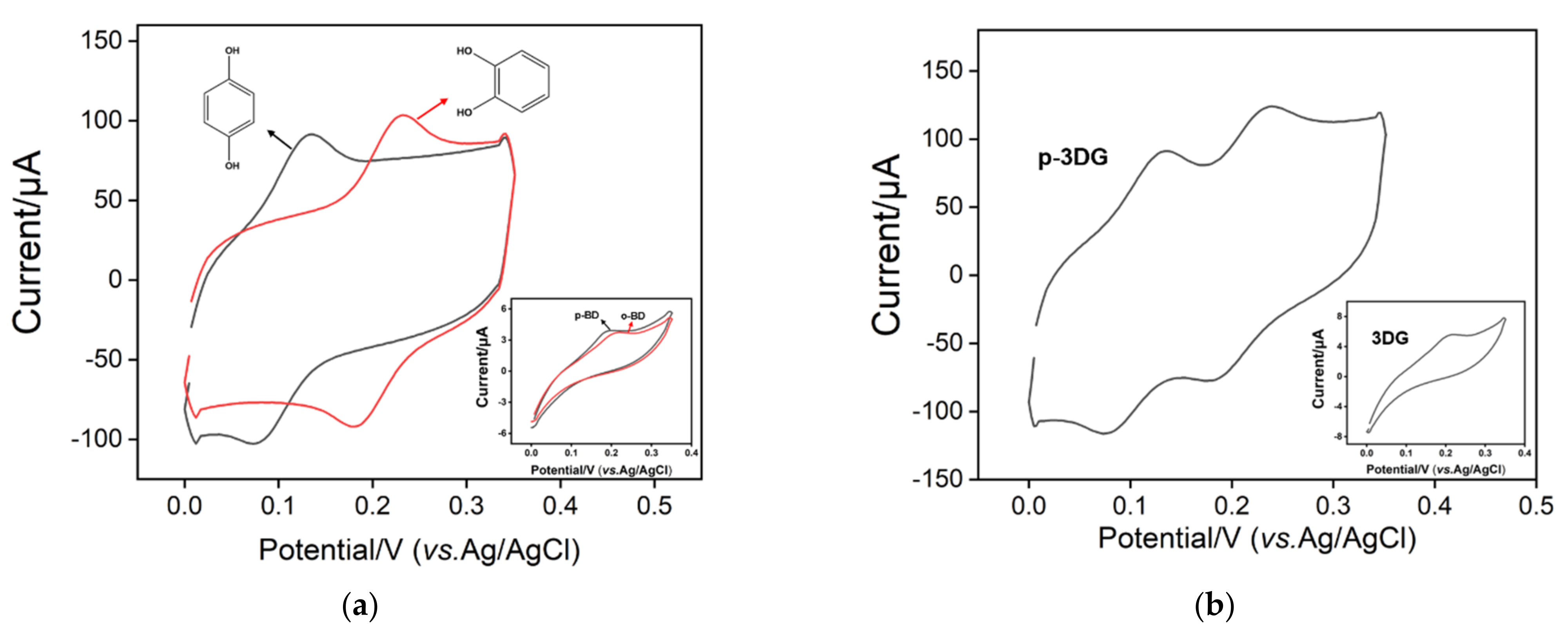
3.1. Electrochemical Polarization of Three-Dimensional Graphene
3.2. Structure and Composition Characterization of p-3DG
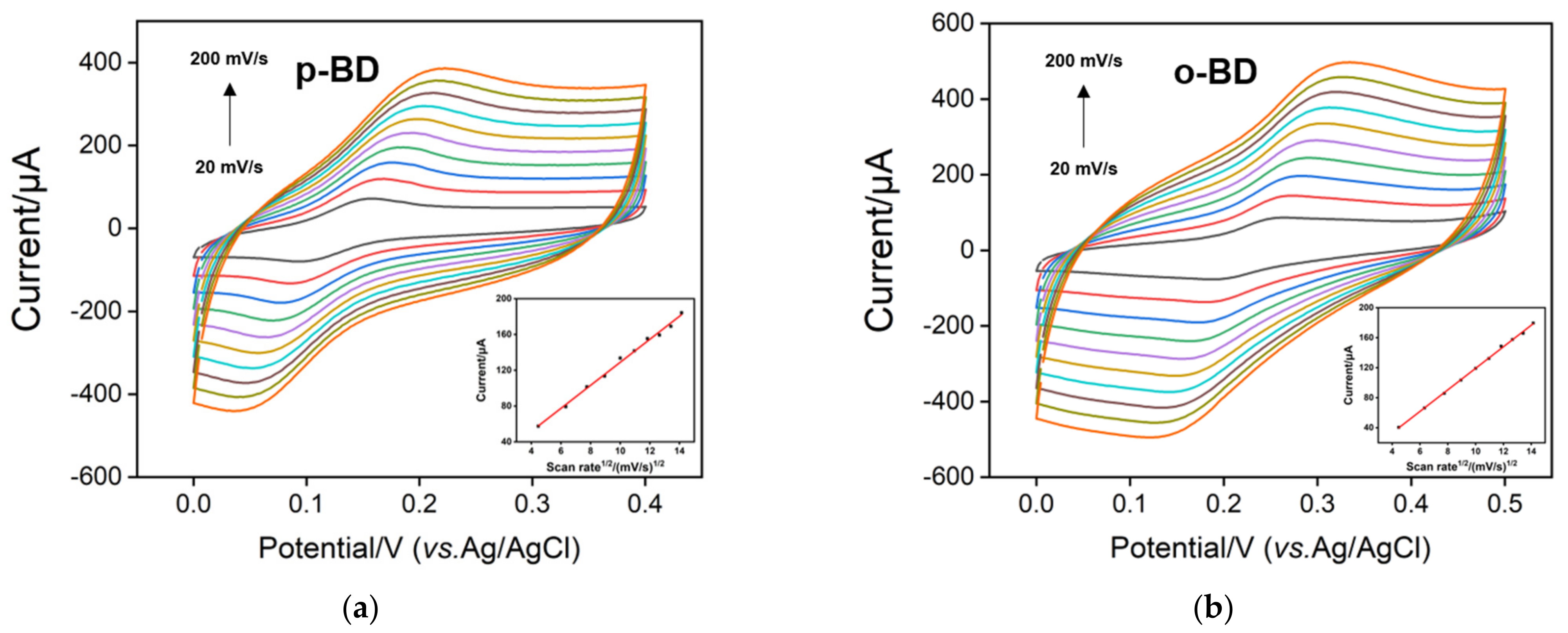
3.3. Electrocatalytic Activity of p-3DG towards Hydroquinone and Catechol
3.4. Electrocatalytic Activity of p-3DG towards Hydroquinone and Catechol
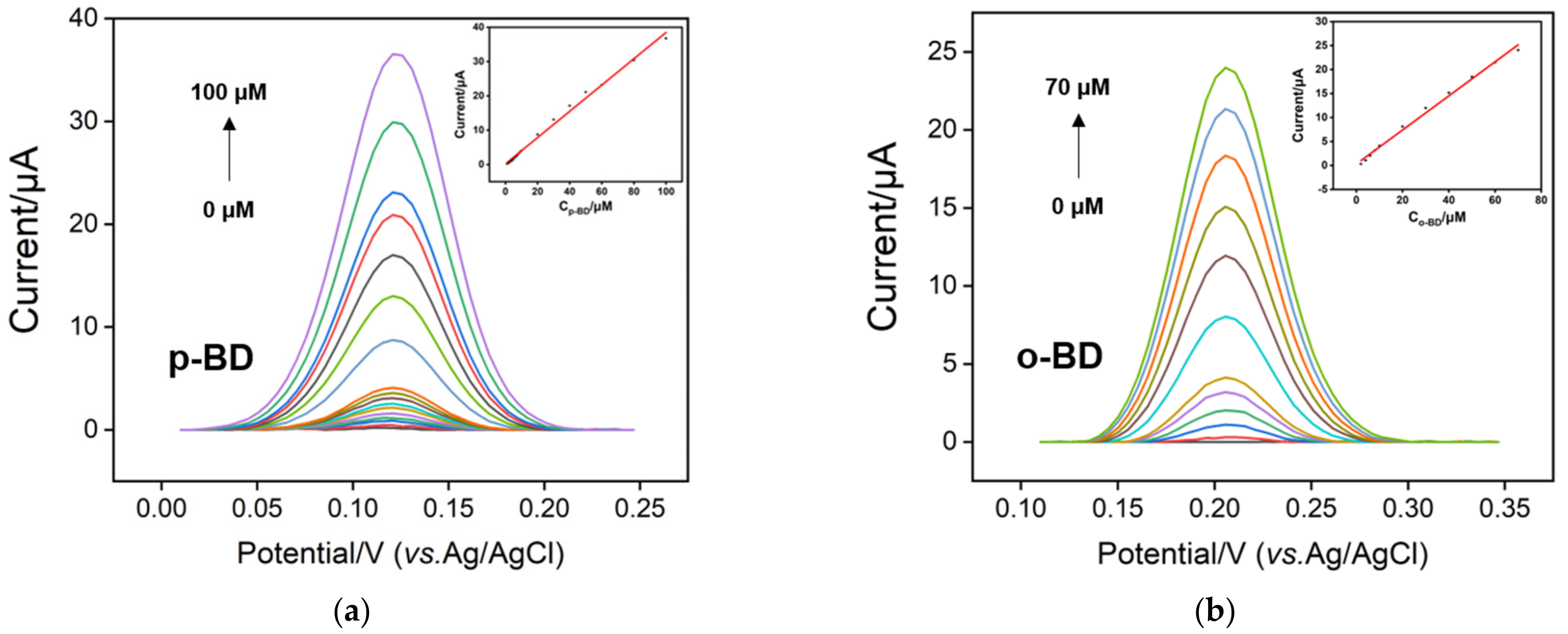
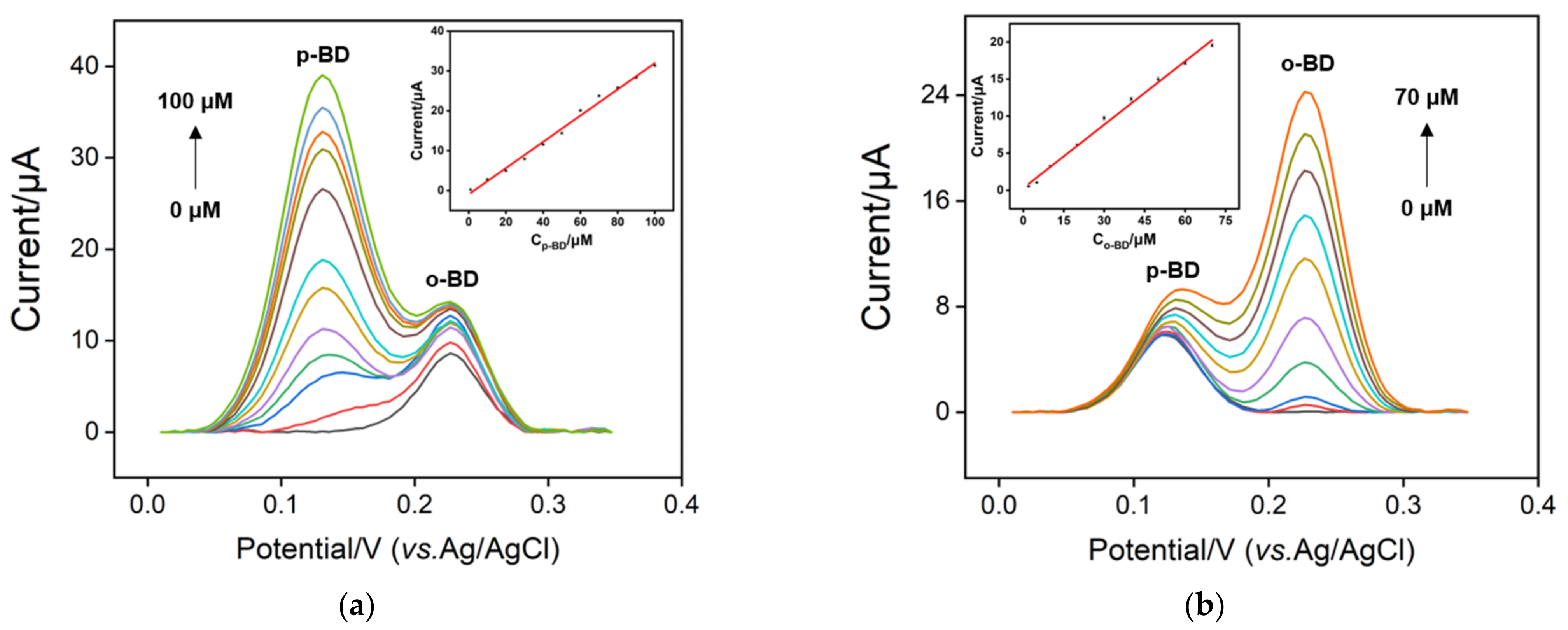
3.5. Individual and Selective Detection of p-BD and o-BD
3.6. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.Y.; Liu, M.L.; Tian, X.; Xu, P.; Fu, C.Y.; Wang, S.; Liu, Y.Q. Scalable fabrication of ultrathin free-standing graphene nanomesh films for flexible ultrafast electrochemical capacitors with AC line-filtering performance. Nano Energy 2018, 50, 182–191. [Google Scholar] [CrossRef]
- Zhu, X.; Ye, J.W.; Lu, Y.F.; Jia, X.L. 3D Graphene nanostructure composed of porous carbon sheets and interconnected nanocages for high-performance lithium-ion battery anodes and lithium−sulfur batteries. ACS Sustain. Chem. Eng. 2019, 7, 11241–11249. [Google Scholar] [CrossRef]
- Gupta, S.; Wood, R. Development of FRET biosensor based on aptamer/functionalized graphene for ultrasensitive detection of bisphenol A and discrimination from analogs. Nano-Struct. Nano-Objects 2016, 8, 2505–2510. [Google Scholar] [CrossRef]
- Chen, H.J.; Zhang, Z.H.; Cai, R.; Kong, X.Q.; Chen, X.; Liu, Y.N.; Yao, S.Z. Molecularly imprinted electrochemical sensor based on a reduced graphene modified carbon electrode for tetrabromobisphenol A detection. Analyst 2013, 138, 2769–2776. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhong, H.; Chen, M.; Zhao, C.; Liu, Y.; Xi, F.; Luo, T. Functional nanostructure-loaded three-dimensional graphene foam as a non-enzymatic electrochemical sensor for reagentless glucose detection. RSC Adv. 2020, 10, 33739–33746. [Google Scholar] [CrossRef]
- Chen, D.H.; Wang, F.; Li, Y.J.; Wang, W.W.; Huang, T.X.; Li, J.F.; Novoselov, K.S.; Tian, Z.Q.; Zhan, D.P. Programmed electrochemical exfoliation of graphite to high quality graphene. Chem. Commun. 2019, 55, 3379–3382. [Google Scholar] [CrossRef]
- Chen, K.; Shi, L.R.; Zhang, Y.F.; Liu, Z.F. Scalable chemical-vapour-deposition growth of three-dimensional graphene materials towards energy-related applications. Chem. Soc. Rev. 2018, 47, 3018–3036. [Google Scholar] [CrossRef]
- Chen, Z.P.; Ren, W.C.; Gao, L.B.; Liu, B.L.; Pei, S.F.; Cheng, H.M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef]
- Amani, H.; Mostafavi, E.; Arzaghi, H.; Davaran, S.; Akbarzadeh, A.; Akhavan, O.; Pazoki-Toroudi, H.; Webster, T.J. Three-Dimensional graphene foams: Synthesis, properties, biocompatibility, biodegradability, and applications in tissue engineering. ACS Biomater. Sci. Eng. 2019, 5, 193–214. [Google Scholar] [CrossRef]
- Qiu, B.C.; Xing, M.Y.; Zhang, J.L. Recent advances in three-dimensional graphene based materials for catalysis applications. Chem. Soc. Rev. 2018, 47, 2165–2216. [Google Scholar] [CrossRef]
- Ananthanarayanan, A.; Wang, X.W.; Routh, P.; Sana, B.; Lim, S.; Kim, D.H.; Lim, K.H.; Li, J.; Chen, P. Facile synthesis of graphene quantum dots from 3D graphene and their application for Fe3+ sensing. Adv. Funct. Mater. 2014, 24, 3021–3026. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, Y.; Pang, Y.; Chen, J.; Zhang, Z.; Xi, F.; Chen, P. Graphene quantum dots as full-color and stimulus responsive fluorescence ink for information encryption. J. Colloid Interf. Sci. 2020, 579, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Neumann, C.C.M.; Batchelor-McAuley, C.; Downing, C.; Compton, R.G. Anthraquinone monosulfonate adsorbed on graphite shows two very different rates of electron transfer: Surface heterogeneity due to basal and edge plane dites. Chem. Eur. J. 2011, 17, 7320–7326. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Duan, W.; Jin, Y.; Wo, F.; Xi, F.; Wu, J. Graphene quantum dot-decorated luminescent porous silicon dressing for theranostics of diabetic wounds. Acta Biomater. 2021, 272, 120772. [Google Scholar] [CrossRef]
- Engstrom, R.C.; Strasser, V.A. Characterization of electrochemically pretreated glassy carbon electrodes. Anal. Chem. 1984, 56, 136–141. [Google Scholar] [CrossRef]
- Nagaoka, T.; Yoshino, T. Surface properties of electrochemically pretreated glassy carbon. Anal. Chem. 1986, 58, 1037–1042. [Google Scholar] [CrossRef]
- Santhiago, M.; Maroneze, C.M.; Silva, C.C.C.; Camargo, M.N.L.; Kubota, L.T. Electrochemical oxidation of glassy carbon provides similar electrochemical response as graphene oxide prepared by tour or hummers routes. ChemElectroChem 2015, 2, 761–767. [Google Scholar] [CrossRef]
- Li, Y.W.; Zhou, J.; Song, J.; Liang, X.S.; Zhang, Z.P.; Men, D.; Wang, D.B.; Zhang, X.E. Chemical nature of electrochemical activation of carbon electrodes. Biosens. Bioelectron. 2019, 144, 111534. [Google Scholar] [CrossRef]
- Xi, F.N.; Zhao, D.J.; Wang, X.W.; Chen, P. Non-enzymatic detection of hydrogen peroxide using a functionalized three-dimensional graphene electrode. Electrochem. Commun. 2013, 26, 81–84. [Google Scholar] [CrossRef]
- Ouyang, B.; Jacob, M.V.; Rawat, R.S. Free standing 3D graphene nano-mesh synthesis by RF plasma CVD using non-synthetic precursor. Mater. Res. Bull. 2015, 71, 61–66. [Google Scholar] [CrossRef]
- Liu, J.Y.; Wang, X.H.; Wang, T.S.; Li, D.; Xi, F.N.; Wang, J.; Wang, E.K. Functionalization of monolithic and porous three-dimensional graphene by one-step chitosan electrodeposition for enzymatic biosensor. ACS Appl. Mater. Interfaces 2014, 6, 19997–20002. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Wang, J.; Wang, T.S.; Li, D.; Xi, F.N.; Wang, J.; Wang, E.K. Three-dimensional electrochemical immunosensor for sensitive detection of carcinoembryonic antigen based on monolithic and macroporous graphene foam. Biosens. Bioelectron. 2015, 65, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.L.; Wang, J.; Cheng, F.; Ali, A.; Guo, H.S.; Ying, X.; Si, L.P.; Liu, H.Y. Synergistic effect of a cobalt fluoroporphyrin and graphene oxide on the simultaneous voltammetric determination of catechol and hydroquinone. Microchim. Acta 2019, 186, 381. [Google Scholar]
- Bu, C.H.; Liu, X.H.; Zhang, Y.J.; Li, L.; Zhou, X.B.; Lu, X.Q. A sensor based on the carbon nanotubes-ionic liquid composite for simultaneous determination of hydroquinone and catechol. Colloids Surf. B. 2011, 88, 292–296. [Google Scholar] [CrossRef]
- Du, H.J.; Ye, J.S.; Zhang, J.Q.; Huang, X.D.; Yu, C.Z. A voltammetric sensor based on graphene-modified electrode for simultaneous determination of catechol and hydroquinone. J. Electroanal. Chem. 2011, 650, 209–213. [Google Scholar] [CrossRef]
- Zhang, H.S.; Bo, X.J.; Guo, L.P. Electrochemical preparation of porous graphene and its electrochemical application in the simultaneous determination of hydroquinone, catechol, and resorcinol. Sens. Actuators B Chem. 2015, 220, 919–926. [Google Scholar] [CrossRef]
- Song, K.L.; Li, R.; Li, K.; Yu, H. Simultaneous determination of dihydroxybenzene isomers using a three-dimensional over-oxidized polypyrrole–reduced graphene oxide composite film electrode prepared by an electrochemical method. New J. Chem. 2020, 44, 20294–20302. [Google Scholar] [CrossRef]
- Duan, W.; Jin, Y.; Cui, Y.; Xi, F.; Liu, X.; Wo, F.; Wu, J. A co-delivery platform for synergistic promotion of angiogenesis based on biodegradable, therapeutic and self-reporting luminescent porous silicon microparticles. Biomaterials 2021, 272, 120772. [Google Scholar] [CrossRef]
- Cui, Y.; Duan, W.; Jin, W.F.; Xi, F.; Wu, J. Ratiometric fluorescent nanohybrid for noninvasive and visual monitoring of sweat glucose. ACS Sens. 2020, 5, 2096–2105. [Google Scholar] [CrossRef]
- Guan, J.F.; Zou, J.; Liu, Y.P.; Jiang, X.Y.; Yu, J.G. Hybrid carbon nanotubes modified glassy carbon electrode for selective, sensitive and simultaneous detection of dopamine and uric acid. Ecotoxicol. Environ. Saf. 2020, 201, 110872. [Google Scholar] [CrossRef]
- Jothi, L.; Neogi, S.; Jaganathan, S.K.; Nageswaran, G. Simultaneous determination of ascorbic acid, dopamine and uric acid by a novel electrochemical sensor based on N2/Ar RF plasma assisted graphene nanosheets/graphene nanoribbons. Biosens. Bioelectron. 2018, 105, 236–242. [Google Scholar] [CrossRef] [PubMed]




| Sample | Added p-BD (μM) | Added o-BD (μM) | p-BD Found (μM) | RSD (%) | Recovery (%) |
|---|---|---|---|---|---|
| Pond water | 5 | 20 | 5.1 | 3.0 | 102.0 |
| 15 | 20 | 14.3 | 1.6 | 95.2 | |
| 50 | 20 | 49.6 | 0.3 | 99.2 |
| Sample | Added o-BD (μM) | Added p-BD (μM) | o-BD Found (μM) | RSD (%) | Recovery (%) |
|---|---|---|---|---|---|
| Pond water | 5 | 20 | 4.8 | 2.5 | 96.0 |
| 15 | 20 | 15.5 | 3.1 | 103.3 | |
| 50 | 20 | 51.2 | 1.9 | 102.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Dong, G.; Sailjoi, A.; Liu, J. Facile Pretreatment of Three-Dimensional Graphene through Electrochemical Polarization for Improved Electrocatalytic Performance and Simultaneous Electrochemical Detection of Catechol and Hydroquinone. Nanomaterials 2022, 12, 65. https://doi.org/10.3390/nano12010065
Zhou H, Dong G, Sailjoi A, Liu J. Facile Pretreatment of Three-Dimensional Graphene through Electrochemical Polarization for Improved Electrocatalytic Performance and Simultaneous Electrochemical Detection of Catechol and Hydroquinone. Nanomaterials. 2022; 12(1):65. https://doi.org/10.3390/nano12010065
Chicago/Turabian StyleZhou, Huaxu, Guotao Dong, Ajabkhan Sailjoi, and Jiyang Liu. 2022. "Facile Pretreatment of Three-Dimensional Graphene through Electrochemical Polarization for Improved Electrocatalytic Performance and Simultaneous Electrochemical Detection of Catechol and Hydroquinone" Nanomaterials 12, no. 1: 65. https://doi.org/10.3390/nano12010065
APA StyleZhou, H., Dong, G., Sailjoi, A., & Liu, J. (2022). Facile Pretreatment of Three-Dimensional Graphene through Electrochemical Polarization for Improved Electrocatalytic Performance and Simultaneous Electrochemical Detection of Catechol and Hydroquinone. Nanomaterials, 12(1), 65. https://doi.org/10.3390/nano12010065







