Structural Investigation of the Synthesized Few-Layer Graphene from Coal under Microwave
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Synthesis of the FLG Materials
2.3. Structural Analysis of the FLG Materials
3. Results and Discussion
3.1. Morphological Analysis of the FLG Materials
3.2. AFM and TEM Examine the FLG Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Díaz, P.; González, Z.; Santamaría, R.; Granda, M.; Menéndez, R.; Blanco, C. Enhancing energy density of carbon-based supercapacitors using Prussian Blue modified positive electrodes. Electrochim. Acta 2016, 212, 848–855. [Google Scholar] [CrossRef]
- Dobrota, A.S.; Pašti, I.A.; Mentus, S.V.; Johansson, B.; Skorodumova, N.V. Functionalized graphene for sodium battery applications: The DFT insights. Electrochim. Acta 2017, 250, 185–195. [Google Scholar] [CrossRef]
- Xia, F.; Mueller, T.; Lin, Y.-M.; Valdes-Garcia, A.; Avouris, P. Ultrafast graphene photodetector. Nat. Nanotechnol. 2009, 4, 839–843. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Sierra, U.; Mercado, A.; Cuara, E.; Barriga-Castro, E.D.; Cortés, A.; Gallardo-Vega, C.; Fernández, S. Coke-derived few layer graphene-like materials by mild planetary milling exfoliation. Fuel 2020, 262, 116455. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 11–19. [Google Scholar]
- Tse, W.-K.; Hwang, E.; Das Sarma, S. Ballistic hot electron transport in graphene. Appl. Phys. Lett. 2008, 93, 023128. [Google Scholar] [CrossRef]
- Neto, A.C.; Guinea, F.; Peres, N.M.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109. [Google Scholar] [CrossRef]
- Castro, E.V.; Novoselov, K.; Morozov, S.; Peres, N.; Dos Santos, J.L.; Nilsson, J.; Guinea, F.; Geim, A.; Neto, A.C. Biased bilayer graphene: Semiconductor with a gap tunable by the electric field effect. Phys. Rev. Lett. 2007, 99, 216802. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.; Holland, B.; Byrne, M.; Gun’Ko, Y.K. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Song, Z.; Li, T.; Li, X.; Ogbazghi, A.Y.; Feng, R.; Dai, Z.; Marchenkov, A.N.; Conrad, E.H.; First, P.N. Ultrathin epitaxial graphite: 2D electron gas properties and a route toward graphene-based nanoelectronics. J. Phys. Chem. B 2004, 108, 19912–19916. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Muñoz, R.; Gómez-Aleixandre, C. Review of CVD synthesis of graphene. Chem. Vap. Depos. 2013, 19, 297–322. [Google Scholar] [CrossRef]
- Abdolhosseinzadeh, S.; Asgharzadeh, H.; Kim, H.S. Fast and fully-scalable synthesis of reduced graphene oxide. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Galindo, B.; Alcolea, S.G.; Gómez, J.; Navas, A.; Murguialday, A.O.; Fernandez, M.P.; Puelles, R. Effect of the number of layers of graphene on the electrical properties of TPU polymers. IOP Conf. Ser. Mater. Sci. Eng. 2014, 64, 012008. [Google Scholar] [CrossRef]
- Krane, N. Preparation of Graphene. Selected Topics in Physics: Physics of Nanoscale Carbon 2011. Available online: https://www.physik.fu-berlin.de/einrichtungen/ag/ag-reich/lehre/Archiv/ss2011/docs/Nils_Krane-Handout.pdf (accessed on 28 October 2021).
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Vijapur, S.H.; Wang, D.; Botte, G.G. Raw coal derived large area and transparent graphene films. ECS Solid State Lett. 2013, 2, M45. [Google Scholar] [CrossRef]
- Bi, H.; Wan, S.; Cao, X.; Wu, X.; Zhou, Y.; Yin, K.; Su, S.; Ma, Q.; Sindoro, M.; Zhu, J. A general and facile method for preparation of large-scale reduced graphene oxide films with controlled structures. Carbon 2019, 143, 162–171. [Google Scholar] [CrossRef]
- Menéndez, J.; Arenillas, A.; Fidalgo, B.; Fernández, Y.; Zubizarreta, L.; Calvo, E.G.; Bermúdez, J.M. Microwave heating processes involving carbon materials. Fuel Process. Technol. 2010, 91, 1–8. [Google Scholar] [CrossRef]
- Fung, D.Y.; Cunningham, F. Effect of microwaves on microorganisms in foods. J. Food Prot. 1980, 43, 641–650. [Google Scholar] [CrossRef]
- Soto-Reyes, N.; Temis-Pérez, A.L.; López-Malo, A.; Rojas-Laguna, R.; Sosa-Morales, M.E. Effects of shape and size of agar gels on heating uniformity during pulsed microwave treatment. J. Food Sci. 2015, 80, E1021–E1025. [Google Scholar] [CrossRef]
- Jiang, F.; Yu, Y.; Wang, Y.; Feng, A.; Song, L. A novel synthesis route of graphene via microwave assisted intercalation-exfoliation of graphite. Mater. Lett. 2017, 200, 39–42. [Google Scholar] [CrossRef]
- Amiri, A.; Sadri, R.; Shanbedi, M.; Ahmadi, G.; Kazi, S.; Chew, B.; Zubir, M.N.M. Synthesis of ethylene glycol-treated graphene nanoplatelets with one-pot, microwave-assisted functionalization for use as a high performance engine coolant. Energy Convers. Manag. 2015, 101, 767–777. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Velamakanni, A.; Piner, R.D.; Ruoff, R.S. Microwave assisted exfoliation and reduction of graphite oxide for ultracapacitors. Carbon 2010, 48, 2118–2122. [Google Scholar] [CrossRef]
- Liu, T.; Chai, H.; Jia, D.; Su, Y.; Wang, T.; Zhou, W. Rapid microwave-assisted synthesis of mesoporous nimoo4 nanorod/reduced graphene oxide composites for high-performance supercapacitors. Electrochim. Acta 2015, 180, 998–1006. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, J.; Liu, Y.; Su, Q.; Zhang, J.; Du, G. Microwave-assisted synthesis of hollow cuo–Cu2O nanosphere/graphene composite as anode for lithium-ion battery. J. Alloys Compd. 2014, 615, 390–394. [Google Scholar] [CrossRef]
- Kim, T.; Lee, J.; Lee, K.-H. Microwave heating of carbon-based solid materials. Carbon Lett. 2014, 15, 15–24. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-inhibiting coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Xiao, D.; Niu, Q. Valley-dependent optoelectronics from inversion symmetry breaking. Phys. Rev. B 2008, 77, 235406. [Google Scholar] [CrossRef]
- Thomsen, C.; Reich, S. Double resonant Raman scattering in graphite. Phys. Rev. Lett. 2000, 85, 5214. [Google Scholar] [CrossRef]
- Malard, L.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Govind Raj, K.; Joy, P.A. Role of localized graphitization on the electrical and magnetic properties of activated carbon. J. Am. Ceram. Soc. 2017, 100, 5151–5161. [Google Scholar] [CrossRef]
- Islam, F.; Tahmasebi, A.; Wang, R.; Yu, J. Structure of Coal-Derived Metal-Supported Few-Layer Graphene Composite Materials Synthesized Using a Microwave-Assisted Catalytic Graphitization Process. Nanomaterials 2021, 11, 1672. [Google Scholar] [CrossRef]
- Yeh, T.-S.; Wu, Y.-S.; Lee, Y.-H. Graphitization of unburned carbon from oil-fired fly ash applied for anode materials of high power lithium ion batteries. Mater. Chem. Phys. 2011, 130, 309–315. [Google Scholar] [CrossRef]
- Wang, R.; Lu, G.; Qiao, W.; Yu, J. Catalytic graphitization of coal-based carbon materials with light rare earth elements. Langmuir 2016, 32, 8583–8592. [Google Scholar] [CrossRef]
- Xiong, W.; Zhou, Y.S.; Hou, W.J.; Guillemet, T.; Silvain, J.-F.; Gao, Y.; Lahaye, M.; Lebraud, E.; Xu, S.; Wang, X. Solid-state graphene formation via a nickel carbide intermediate phase. RSC Adv. 2015, 5, 99037–99043. [Google Scholar] [CrossRef]
- Kim, T.; Lee, J.; Lee, K.-H. Full graphitization of amorphous carbon by microwave heating. RSC Adv. 2016, 6, 24667–24674. [Google Scholar] [CrossRef]
- Inagaki, M. New Carbons-Control of Structure and Functions; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Kim, Y.-J.; Yang, H.; Yoon, S.-H.; Korai, Y.; Mochida, I.; Ku, C.-H. Anthracite as a candidate for lithium ion battery anode. J. Power Sources 2003, 113, 157–165. [Google Scholar] [CrossRef]
- Islam, F.; Wang, J.; Tahmasebi, A.; Wang, R.; Moghtaderi, B.; Yu, J. Microwave-Assisted Coal-Derived Few-Layer Graphene as an Anode Material for Lithium-Ion Batteries. Materials 2021, 14, 6468. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Iqbal, M.Z.; Iqbal, M.W.; Eom, J. Improving the electrical properties of graphene layers by chemical doping. Sci. Technol. Adv. Mater. 2014, 15, 055004. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Prekodravac, J.; Marković, Z.; Jovanović, S.; Holclajtner-Antunović, I.; Pavlović, V.; Todorović-Marković, B. Raman spectroscopy study of graphene thin films synthesized from solid precursor. Opt. Quantum Electron. 2016, 48, 115. [Google Scholar] [CrossRef]
- Bîru, E.I.; Iovu, H. Graphene nanocomposites studied by Raman spectroscopy. Raman Spectrosc. 2018, 179, 179–201. [Google Scholar]
- Ni, Z.; Wang, Y.; Yu, T.; Shen, Z. Raman spectroscopy and imaging of graphene. Nano Res. 2008, 1, 273–291. [Google Scholar] [CrossRef]
- Sharma, B.; Schumann, T.; de Oliveira, M.H., Jr.; Lopes, J.M.J. Controlled synthesis and characterization of multilayer graphene films on the C-face of silicon carbide. Phys. Status Solidi 2017, 214, 1600721. [Google Scholar] [CrossRef]
- Kim, K.; Coh, S.; Tan, L.Z.; Regan, W.; Yuk, J.M.; Chatterjee, E.; Crommie, M.; Cohen, M.L.; Louie, S.G.; Zettl, A. Raman spectroscopy study of rotated double-layer graphene: Misorientation-angle dependence of electronic structure. Phys. Rev. Lett. 2012, 108, 246103. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Raman mapping of a single-layer to double-layer graphene transition. Eur. Phys. J. Spec. Top. 2007, 148, 171–176. [Google Scholar] [CrossRef][Green Version]
- Gayathri, S.; Jayabal, P.; Kottaisamy, M.; Ramakrishnan, V. Synthesis of few layer graphene by direct exfoliation of graphite and a Raman spectroscopic study. Aip Adv. 2014, 4, 027116. [Google Scholar] [CrossRef]
- Ramalingam, P.; Pusuluri, S.T.; Periasamy, S.; Veerabahu, R.; Kulandaivel, J. Role of deoxy group on the high concentration of graphene in surfactant/water media. RSC Adv. 2013, 3, 2369–2378. [Google Scholar] [CrossRef]
- Peng, K.-J.; Lin, Y.-H.; Wu, C.-L.; Lin, S.-F.; Yang, C.-Y.; Lin, S.-M.; Tsai, D.-P.; Lin, G.-R. Dissolution-and-reduction CVD synthesis of few-layer graphene on ultra-thin nickel film lifted off for mode-locking fiber lasers. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Ōya, A.; Ōtani, S. Catalytic graphitization of carbons by various metals. Carbon 1979, 17, 131–137. [Google Scholar] [CrossRef]
- Derbyshire, F.; Presland, A.; Trimm, D. Graphite formation by the dissolution—precipitation of carbon in cobalt, nickel and iron. Carbon 1975, 13, 111–113. [Google Scholar] [CrossRef]
- Bystrzejewski, M. Synthesis of carbon-encapsulated iron nanoparticles via solid state reduction of iron oxide nanoparticles. J. Solid State Chem. 2011, 184, 1492–1498. [Google Scholar] [CrossRef]
- Zan, R. Microscopy and Spectroscopy of Graphene: Atomic Scale Structure and Interaction with Foreign Atom Species; The University of Manchester: Manchester, UK, 2013. [Google Scholar]
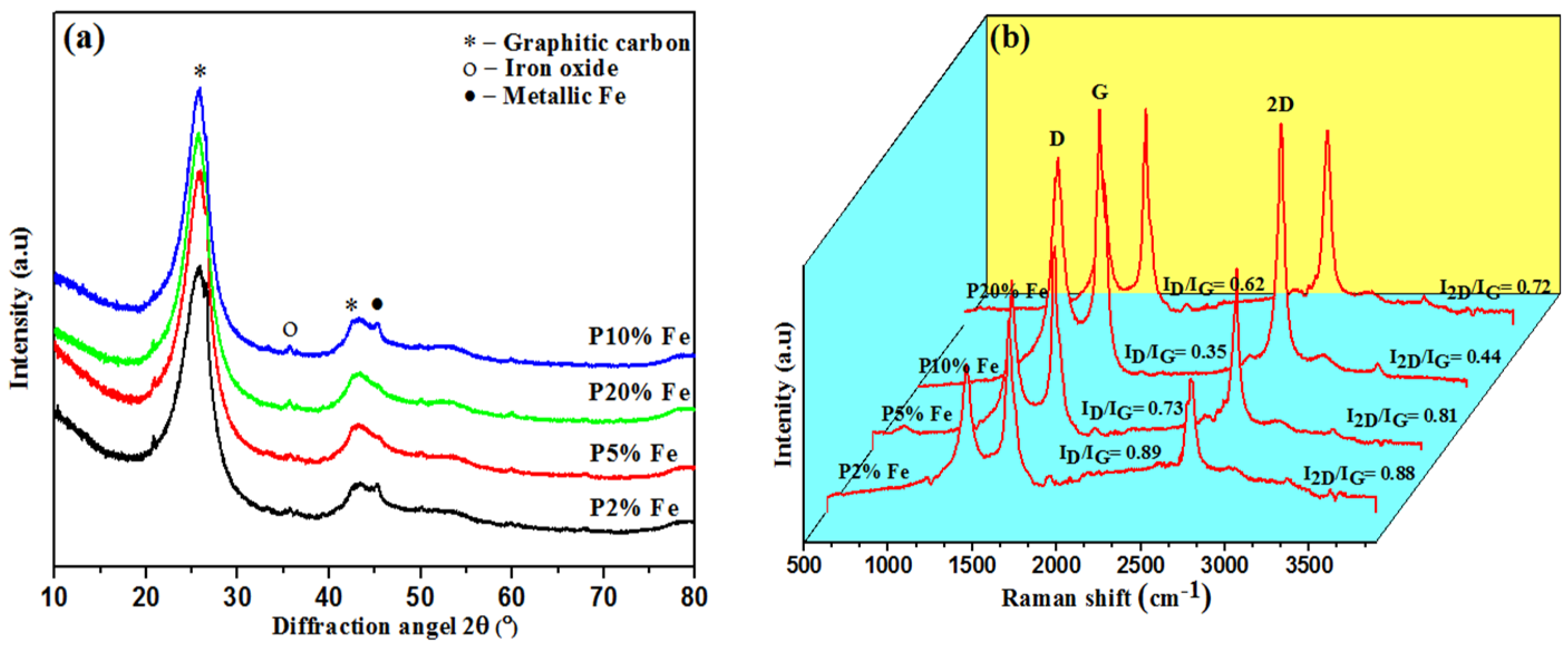

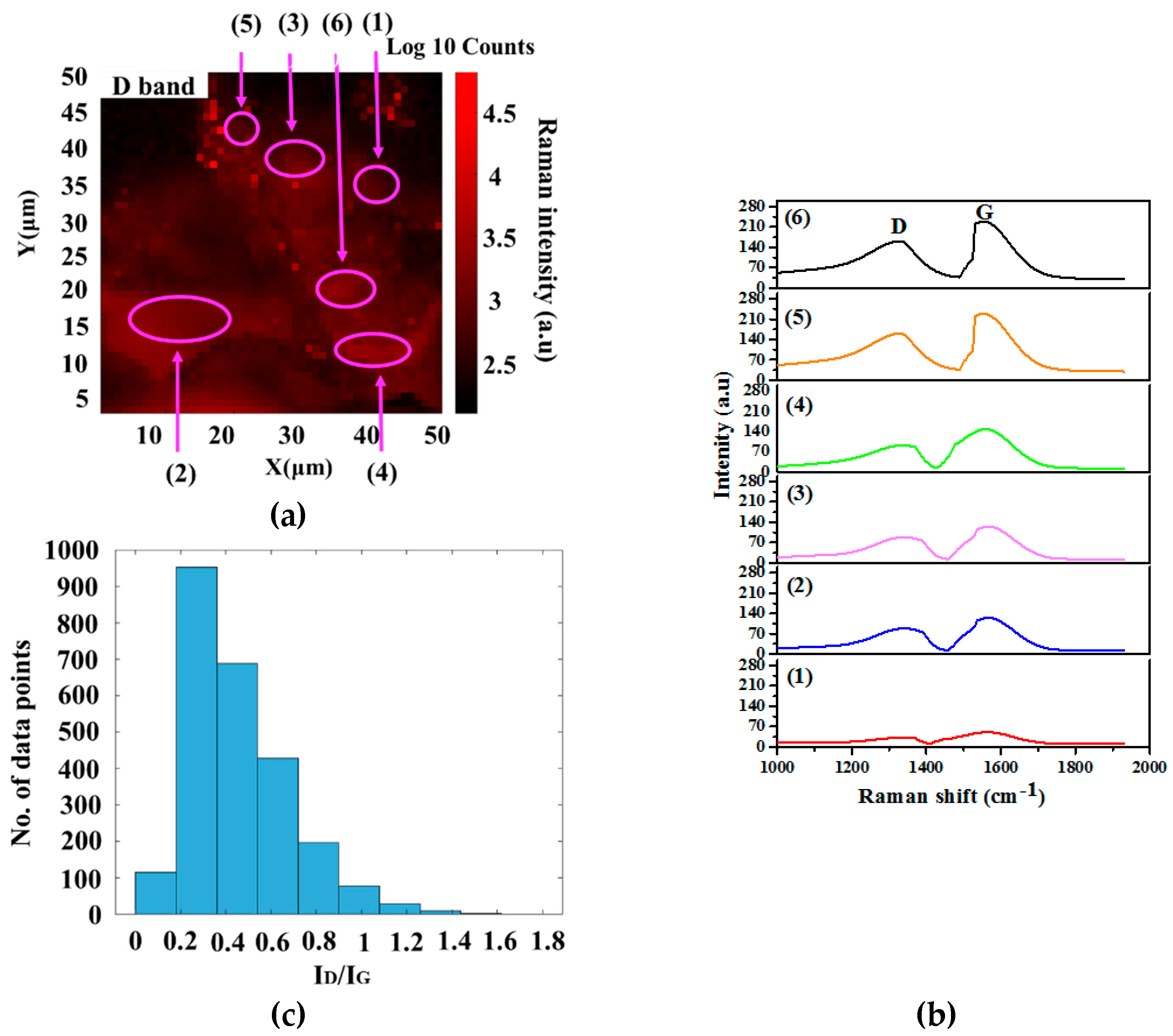
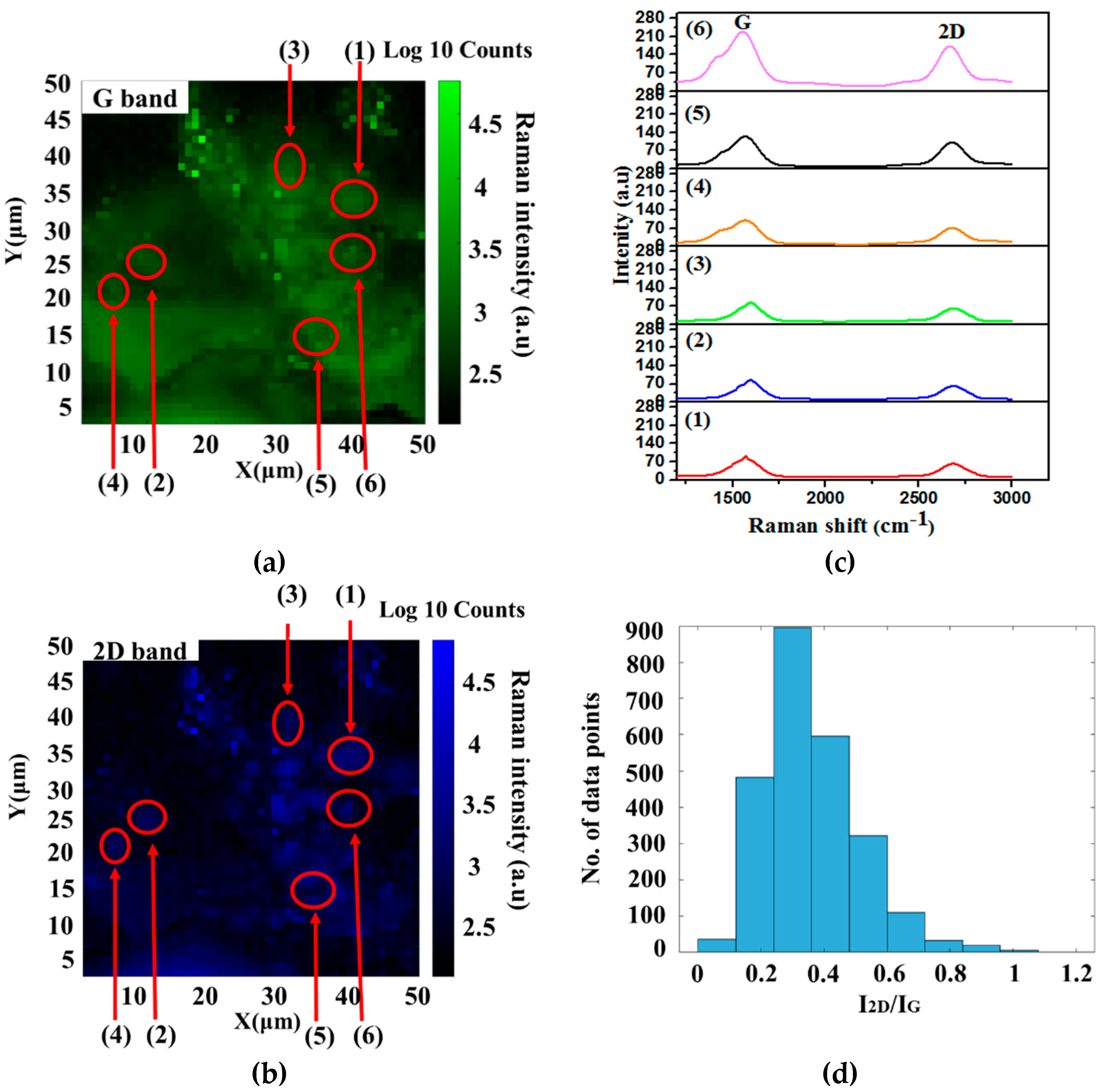


| Catalyst Loading (% of Fe)/Parameters | P2 | P5 | P10 | P20 |
|---|---|---|---|---|
| Interlayer spacing (d002) (nm) 1 | 0.3366 | 0.3362 | 0.3357 | 0.3361 |
| Crystal size (Lc) (nm) 1 | 1.42 | 1.50 | 1.98 | 1.79 |
| In-plane crystal thickness (La) (nm) | 3.07 | 3.24 | 4.27 | 3.85 |
| ID/IG | 0.89 | 0.73 | 0.35 | 0.62 |
| Surface area (m2g−1) | 221.08 | 275.74 | 315.45 | 282.36 |
| “g” factor (%) 2 | 85.9 | 90.7 | 96.8 | 92.1 |
| Fe (%) Loading/Parameters | P2 | P5 | P10 | P20 |
|---|---|---|---|---|
| Position of 2D (cm−1) | 2698 ± 0.19 | 2699 ± 0.21 | 2702 ± 0.24 | 2703 ± 0.22 |
| I2D/IG | 0.88 | 0.81 | 0.44 | 0.72 |
| I2D/ID | 0.98 | 1.11 | 1.24 | 1.17 |
| FWHM (2D) | 48 ± 0.15 | 64 ± 0.17 | 75 ± 0.26 | 74 ± 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, F.; Tahmasebi, A.; Moghtaderi, B.; Yu, J. Structural Investigation of the Synthesized Few-Layer Graphene from Coal under Microwave. Nanomaterials 2022, 12, 57. https://doi.org/10.3390/nano12010057
Islam F, Tahmasebi A, Moghtaderi B, Yu J. Structural Investigation of the Synthesized Few-Layer Graphene from Coal under Microwave. Nanomaterials. 2022; 12(1):57. https://doi.org/10.3390/nano12010057
Chicago/Turabian StyleIslam, Faridul, Arash Tahmasebi, Behdad Moghtaderi, and Jianglong Yu. 2022. "Structural Investigation of the Synthesized Few-Layer Graphene from Coal under Microwave" Nanomaterials 12, no. 1: 57. https://doi.org/10.3390/nano12010057
APA StyleIslam, F., Tahmasebi, A., Moghtaderi, B., & Yu, J. (2022). Structural Investigation of the Synthesized Few-Layer Graphene from Coal under Microwave. Nanomaterials, 12(1), 57. https://doi.org/10.3390/nano12010057






