Bisphenol A Adsorption on Silica Particles Modified with Beta-Cyclodextrins
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Diamino Butane Monosubstituted BCD (BCD-NH2)
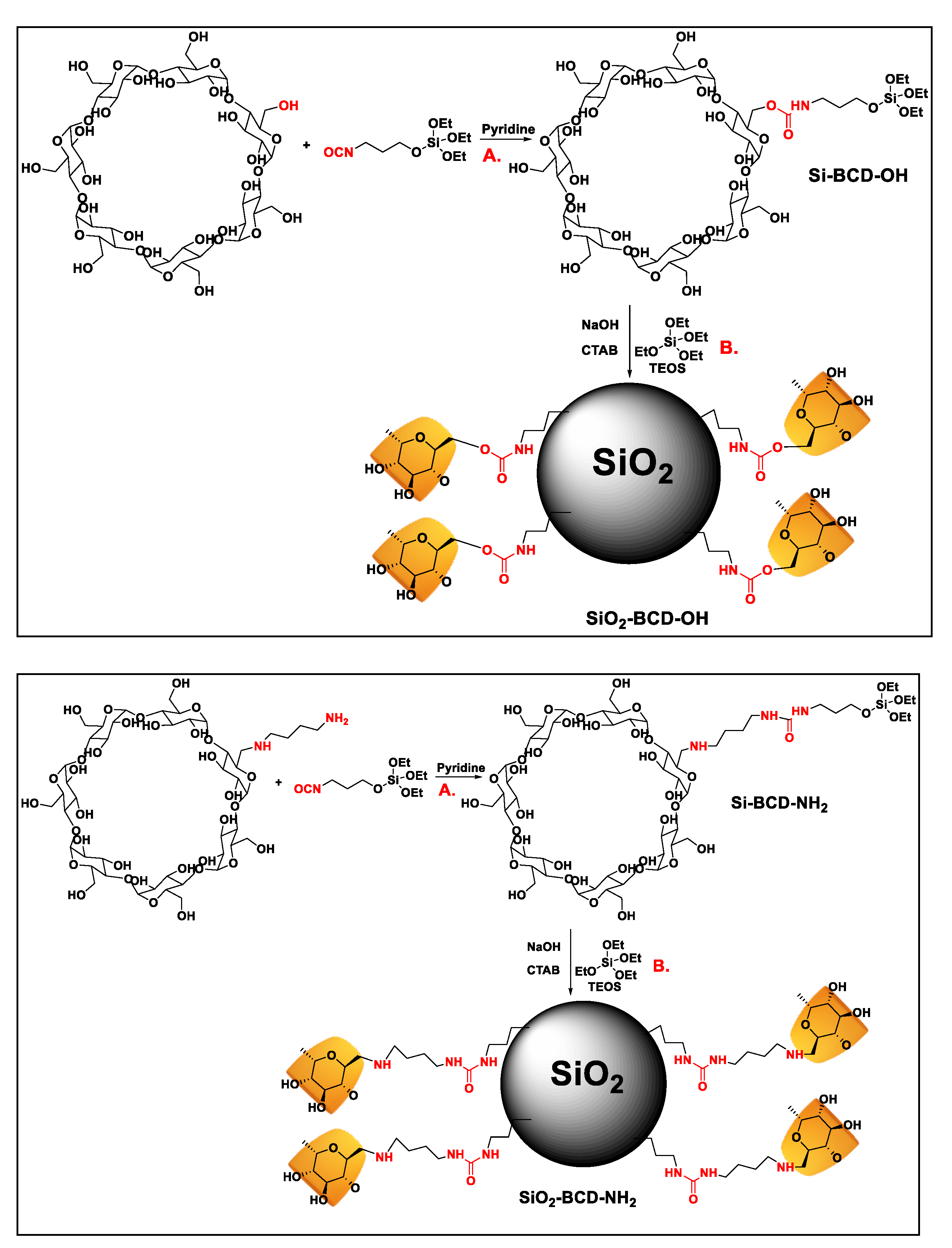
2.2. Synthesis of Si-BCD-OH and Si-BCD-NH2
2.3. Synthesis of SiO2-BCD-OH and SiO2-BCD-NH2
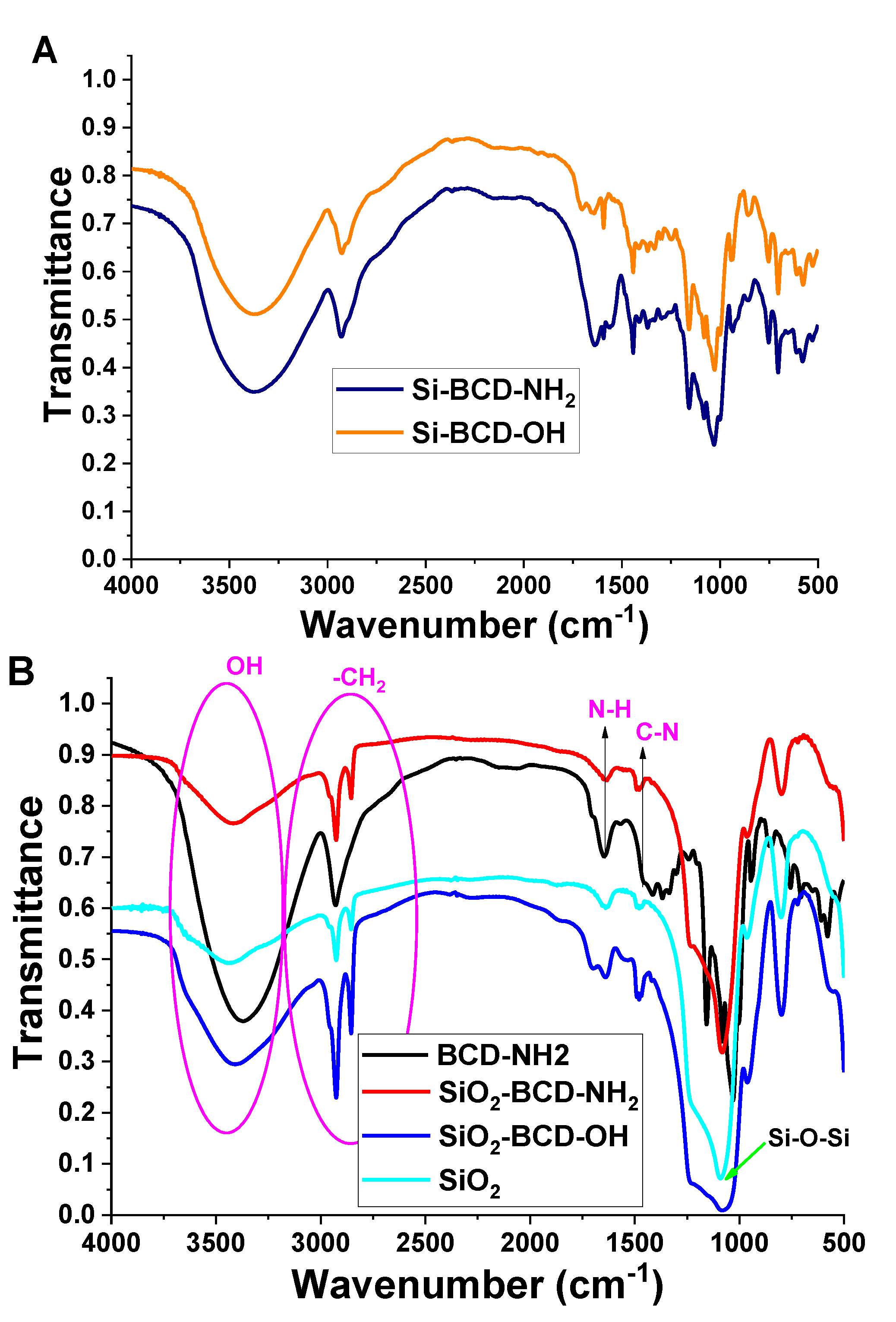
2.4. Methods
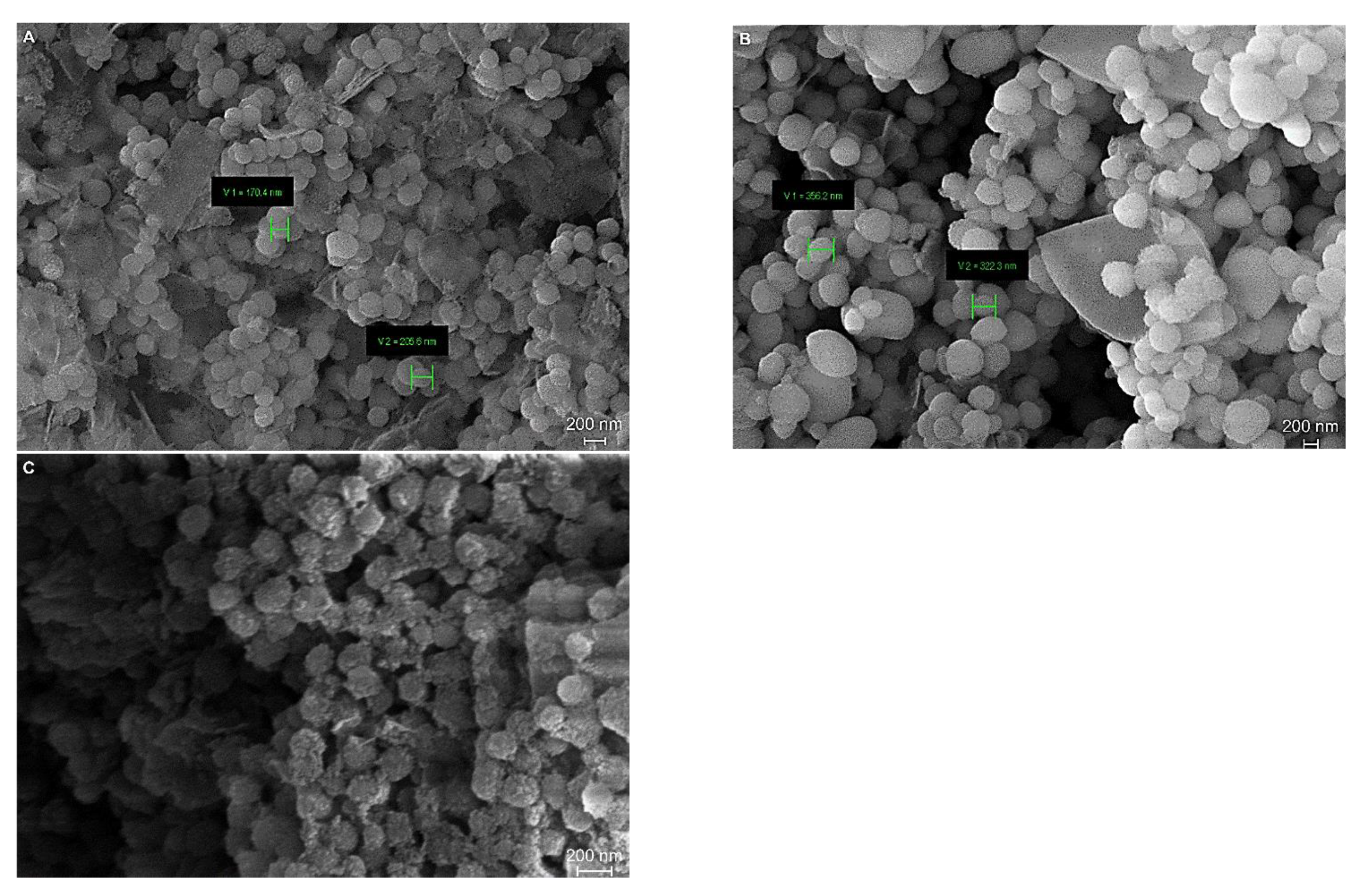
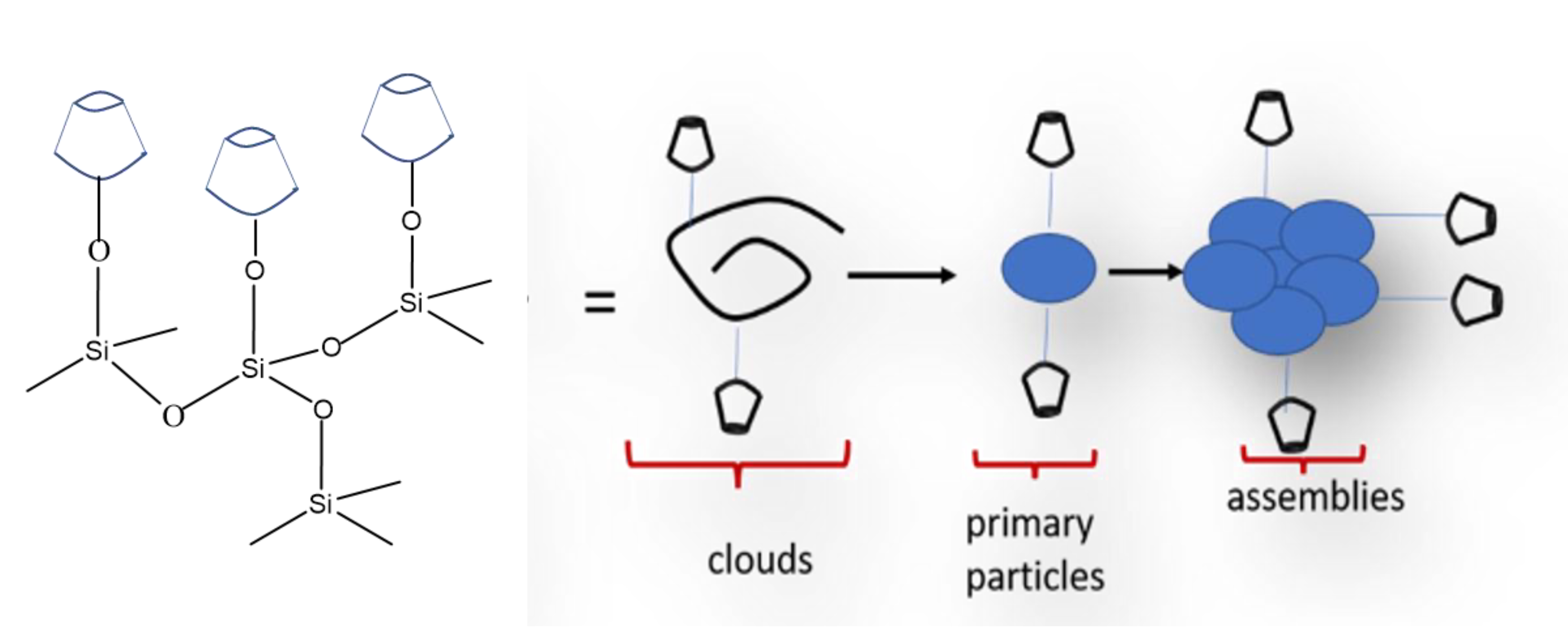
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Gogolashvili, A.; Lomsadze, K.; Chankvetadze, L.; Takaishvili, N.; Peluso, P.; Dallocchio, R.; Salgado, A.; Chankvetadze, B. Separation of tetrahydrozoline enantiomers in capillary electrophoresis with cyclodextrin-type chiral selectors and investigation of chiral recognition mechanisms. J. Chromatogr. A 2021, 1643, 462084. [Google Scholar] [CrossRef]
- Hapiot, F.; Menuel, S.; Ferreira, M.; Léger, B.; Bricout, H.; Tilloy, S.; Monflier, E. Catalysis in Cyclodextrin-Based Unconventional Reaction Media: Recent Developments and Future Opportunities. ACS Sustain. Chem. Eng. 2017, 5, 3598–3606. [Google Scholar] [CrossRef]
- Schneiderman, E.; Stalcup, A.M. Cyclodextrins: A versatile tool in separation science. J. Chromatogr. B Biomed. Sci. Appl. 2000, 745, 83–102. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; García-González, C.A.; Concheiro, A. Cyclodextrins as versatile building blocks for regenerative medicine. J. Control. Release 2017, 268, 269–281. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, L.; Xi, J.; Wang, J.; Feng, Z.-g. Cyclodextrin polymers: Structure, synthesis, and use as drug carriers. Prog. Polym. Sci. 2021, 118, 101408. [Google Scholar] [CrossRef]
- Fenyvesi, É.; Vikmon, M.; Szente, L. Cyclodextrins in Food Technology and Human Nutrition: Benefits and Limitations. Crit. Rev. Food Sci. Nutr. 2016, 56, 1981–2004. [Google Scholar] [CrossRef]
- Roy, M.N.; Ekka, D.; Saha, S.; Chandra Roy, M. Host–guest inclusion complexes of α and β-cyclodextrins with α-amino acids. RSC Adv. 2014, 4, 42383–42390. [Google Scholar] [CrossRef]
- Gong, T.; Zhou, Y.; Sun, L.; Liang, W.; Yang, J.; Shuang, S.; Dong, C. Effective adsorption of phenolic pollutants from water using β-cyclodextrin polymer functionalized Fe3O4 magnetic nanoparticles. RSC Adv. 2016, 6, 80955–80963. [Google Scholar] [CrossRef]
- Bi, J.; Tao, Q.; Huang, X.; Wang, J.; Wang, T.; Hao, H. Simultaneous decontamination of multi-pollutants: A promising approach for water remediation. Chemosphere 2021, 284, 131270. [Google Scholar] [CrossRef]
- Gentili, A. Cyclodextrin-based sorbents for solid phase extraction. J. Chromatogr. A 2020, 1609, 460654. [Google Scholar] [CrossRef]
- Zolfaghari, G. β-Cyclodextrin incorporated nanoporous carbon: Host–guest inclusion for removal of p-Nitrophenol and pesticides from aqueous solutions. Chem. Eng. J. 2016, 283, 1424–1434. [Google Scholar] [CrossRef]
- Constantin, M.; Fundueanu, G.; Bortolotti, F.; Cortesi, R.; Ascenzi, P.; Menegatti, E. Preparation and characterisation of poly(vinyl alcohol)/cyclodextrin microspheres as matrix for inclusion and separation of drugs. Int. J. Pharm. 2004, 285, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Bertini, S.; Torri, G.; Naggi, A.; Sforzini, D.; Vecchi, C.; Janus, L.; Lekchiri, Y.; Morcellet, M. Sorption of aromatic compounds in water using insoluble cyclodextrin polymers. J. Appl. Polym. Sci. 1998, 68, 1973–1978. [Google Scholar] [CrossRef]
- Mohamadhoseini, M.; Mohamadnia, Z. Supramolecular self-healing materials via host-guest strategy between cyclodextrin and specific types of guest molecules. Coord. Chem. Rev. 2021, 432, 213711. [Google Scholar] [CrossRef]
- Gómez-Graña, S.; Pérez-Juste, J.; Hervés, P. Cyclodextrins and inorganic nanoparticles: Another tale of synergy. Adv. Colloid Interface Sci. 2021, 288, 102338. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zou, C.; Liang, H.; Peng, H.; Liao, Y. The effective removal of nickel ions from aqueous solution onto magnetic multi-walled carbon nanotubes modified by β-cyclodextrin. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126544. [Google Scholar] [CrossRef]
- Alothman, Z.A.; AlMasoud, N.; Mbianda, X.Y.; Ali, I. Synthesis and characterization of γ-cyclodextrin-graphene oxide nanocomposite: Sorption, kinetics, thermodynamics and simulation studies of tetracycline and chlortetracycline antibiotics removal in water. J. Mol. Liq. 2021, 116993. [Google Scholar] [CrossRef]
- Salipira, K.L.; Mamba, B.B.; Krause, R.W.; Malefetse, T.J.; Durbach, S.H. Carbon nanotubes and cyclodextrin polymers for removing organic pollutants from water. Environ. Chem. Lett. 2007, 5, 13–17. [Google Scholar] [CrossRef]
- Prochowicz, D.; Kornowicz, A.; Lewiński, J. Interactions of Native Cyclodextrins with Metal Ions and Inorganic Nanoparticles: Fertile Landscape for Chemistry and Materials Science. Chem. Rev. 2017, 117, 13461–13501. [Google Scholar] [CrossRef]
- Cutrone, G.; Casas-Solvas, J.M.; Vargas-Berenguel, A. Cyclodextrin-Modified inorganic materials for the construction of nanocarriers. Int. J. Pharm. 2017, 531, 621–639. [Google Scholar] [CrossRef]
- Park, C.; Youn, H.; Kim, H.; Noh, T.; Kook, Y.H.; Oh, E.T.; Park, H.J.; Kim, C. Cyclodextrin-covered gold nanoparticles for targeted delivery of an anti-cancer drug. J. Mater. Chem. 2009, 19, 2310–2315. [Google Scholar] [CrossRef]
- Li, D.; Qi, L. Self-assembly of inorganic nanoparticles mediated by host-guest interactions. Curr. Opin. Colloid Interface Sci. 2018, 35, 59–67. [Google Scholar] [CrossRef]
- Theron, J.; Walker, J.A.; Cloete, T.E. Nanotechnology and Water Treatment: Applications and Emerging Opportunities. Crit. Rev. Microbiol. 2008, 34, 43–69. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Braun, J.M.; Kalkbrenner, A.E.; Calafat, A.M.; Yolton, K.; Ye, X.; Dietrich, K.N.; Lanphear, B.P. Impact of Early-Life Bisphenol A Exposure on Behavior and Executive Function in Children. Pediatrics 2011, 128, 873–882. [Google Scholar] [CrossRef]
- Wetherill, Y.B.; Akingbemi, B.T.; Kanno, J.; McLachlan, J.A.; Nadal, A.; Sonnenschein, C.; Watson, C.S.; Zoeller, R.T.; Belcher, S.M. In vitro molecular mechanisms of bisphenol A action. Reprod. Toxicol. 2007, 24, 178–198. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yasuhara, A.; Shiraishi, H.; Nakasugi, O. Bisphenol A in hazardous waste landfill leachates. Chemosphere 2001, 42, 415–418. [Google Scholar] [CrossRef]
- Available online: https://echa.europa.eu/hot-topics/bisphenol-a (accessed on 10 September 2021).
- Available online: https://reachonline.eu/reach/en/annex-xvii.html (accessed on 10 September 2021).
- Eladak, S.; Grisin, T.; Moison, D.; Guerquin, M.-J.; N’Tumba-Byn, T.; Pozzi-Gaudin, S.; Benachi, A.; Livera, G.; Rouiller-Fabre, V.; Habert, R. A new chapter in the bisphenol A story: Bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil. Steril. 2015, 103, 11–21. [Google Scholar] [CrossRef]
- Ohore, O.E.; Zhang, S. Endocrine disrupting effects of bisphenol A exposure and recent advances on its removal by water treatment systems. A review. Sci. Afr. 2019, 5, e00135. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Anastopoulos, I. Adsorptive removal of bisphenol A (BPA) from aqueous solution: A review. Chemosphere 2017, 168, 885–902. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Lee, D. Bisphenol A removal by a membrane bioreactor. Process Biochem. 2008, 43, 451–456. [Google Scholar] [CrossRef]
- Chin, S.S.; Lim, T.M.; Chiang, K.; Fane, A.G. Hybrid low-pressure submerged membrane photoreactor for the removal of bisphenol A. Desalination 2007, 202, 253–261. [Google Scholar] [CrossRef]
- Zhou, Y.; Gu, X.; Zhang, R.; Lu, J. Influences of Various Cyclodextrins on the Photodegradation of Phenol and Bisphenol A under UV Light. Ind. Eng. Chem. Res. 2015, 54, 426–433. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Liu, Z.; Hu, J.-Q.; Cai, Q.-W.; Li, X.-Y.; Wang, W.; Faraj, Y.; Ju, X.-J.; Xie, R.; Chu, L.-Y. β-Cyclodextrin-modified graphene oxide membranes with large adsorption capacity and high flux for efficient removal of bisphenol A from water. J. Membr. Sci. 2020, 595, 117510. [Google Scholar] [CrossRef]
- Bucur, S.; Mangalagiu, I.; Diacon, A.; Mocanu, A.; Rizea, F.; Somoghi, R.; Ghebaur, A.; Boscornea, A.C.; Rusen, E. Novel Chemical Architectures Based on Beta-Cyclodextrin Derivatives Covalently Attached on Polymer Spheres. Polymers 2021, 13, 2338. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, J.; Liu, Q.; Chen, H.; Liu, Y.; Zhou, Y. A novel hollow-sphere cyclodextrin nanoreactor for the enhanced removal of bisphenol A under visible irradiation. J. Hazard. Mater. 2020, 384, 121267. [Google Scholar] [CrossRef] [PubMed]
- Karnati, S.R.; Agbo, P.; Zhang, L. Applications of silica nanoparticles in glass/carbon fiber-reinforced epoxy nanocomposite. Compos. Commun. 2020, 17, 32–41. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous Materials for Drug Delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef]
- Videira-Quintela, D.; Martin, O.; Montalvo, G. Emerging opportunities of silica-based materials within the food industry. Microchem. J. 2021, 167, 106318. [Google Scholar] [CrossRef]
- Liu, T.; Ma, L.; Wang, X.; Wang, J.; Qian, H.; Zhang, D.; Li, X. Self-healing corrosion protective coatings based on micro/nanocarriers: A review. Corros. Commun. 2021, 1, 18–25. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Fourmentin, M.; Fourmentin, S.; Torri, G.; Crini, G. Silica Materials Containing Cyclodextrin for Pollutant Removal. In Cyclodextrin Applications in Medicine, Food, Environment and Liquid Crystals; Fourmentin, S., Crini, G., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 149–182. [Google Scholar]
- Topuz, F.; Uyar, T. Cyclodextrin-functionalized mesostructured silica nanoparticles for removal of polycyclic aromatic hydrocarbons. J. Colloid Interface Sci. 2017, 497, 233–241. [Google Scholar] [CrossRef]
- Carvalho, L.B.; Chagas, P.M.B.; Marques, T.R.; Razafitianamaharavo, A.; Pelletier, M.; Nolis, P.; Jaime, C.; Thomasi, S.S.; Pinto, L.d.M.A. Removal of the synthetic hormone methyltestosterone from aqueous solution using a β-cyclodextrin/silica composite. J. Environ. Chem. Eng. 2019, 7, 103492. [Google Scholar] [CrossRef]
- Phan, T.N.T.; Bacquet, M.; Morcellet, M. The removal of organic pollutants from water using new silica-supported β-cyclodextrin derivatives. React. Funct. Polym. 2002, 52, 117–125. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Mahdavi, M.; Lijan, H.; Bahadorikhalili, S.; Ma’mani, L.; Rashidi Ranjbar, P.; Shafiee, A. Copper supported β-cyclodextrin grafted magnetic nanoparticles as an efficient recyclable catalyst for one-pot synthesis of 1-benzyl-1H-1,2,3-triazoldibenzodiazepinone derivatives via click reaction. RSC Adv. 2016, 6, 28838–28843. [Google Scholar] [CrossRef]
- Knight, A.W.; Kalugin, N.G.; Coker, E.; Ilgen, A.G. Water properties under nano-scale confinement. Sci. Rep. 2019, 9, 8246. [Google Scholar] [CrossRef] [PubMed]
- Zhuravlev, L.T. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf. A Physicochem. Eng. Asp. 2000, 173, 1–38. [Google Scholar] [CrossRef]
- Carcouët, C.C.M.C.; van de Put, M.W.P.; Mezari, B.; Magusin, P.C.M.M.; Laven, J.; Bomans, P.H.H.; Friedrich, H.; Esteves, A.C.C.; Sommerdijk, N.A.J.M.; van Benthem, R.A.T.M.; et al. Nucleation and Growth of Monodisperse Silica Nanoparticles. Nano Lett. 2014, 14, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Palamà, I.E.; D’Amone, S.; Biasiucci, M.; Gigli, G.; Cortese, B. Bioinspired design of a photoresponsive superhydrophobic/oleophilic surface with underwater superoleophobic efficacy. J. Mater. Chem. A 2014, 2, 17666–17675. [Google Scholar] [CrossRef]
- Swenson, H.; Stadie, N.P. Langmuir’s Theory of Adsorption: A Centennial Review. Langmuir 2019, 35, 5409–5426. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Poulopoulos, S.G.; Kazemian, H. Insights into the S-shaped sorption isotherms and their dimensionless forms. Microporous Mesoporous Mater. 2018, 272, 166–176. [Google Scholar] [CrossRef]
- Li, X.; Zhou, M.; Jia, J.; Ma, J.; Jia, Q. Design of a hyper-crosslinked β-cyclodextrin porous polymer for highly efficient removal toward bisphenol a from water. Sep. Purif. Technol. 2018, 195, 130–137. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Sadegh, H.; Ali, G.A.M.; Bharti, A.K.; Hamdy Makhlouf, A.S. Facile route synthesis of novel graphene oxide-β-cyclodextrin nanocomposite and its application as adsorbent for removal of toxic bisphenol A from the aqueous phase. J. Mol. Liq. 2017, 237, 466–472. [Google Scholar] [CrossRef]
- Lin, Q.; Wu, Y.; Jiang, X.; Lin, F.; Liu, X.; Lu, B. Removal of bisphenol A from aqueous solution via host-guest interactions based on beta-cyclodextrin grafted cellulose bead. Int. J. Biol. Macromol. 2019, 140, 1–9. [Google Scholar] [CrossRef]
- Kitaoka, M.; Hayashi, K. Adsorption of Bisphenol A by Cross-Linked β-Cyclodextrin Polymer. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 429–431. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwak, S.-Y. Rapid adsorption of bisphenol A from wastewater by β-cyclodextrin-functionalized mesoporous magnetic clusters. Appl. Surf. Sci. 2019, 467-468, 178–184. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, L.; Guo, J.; Ye, Q.; Lin, J.-M.; Yuan, J. Adsorption of environmental pollutants using magnetic hybrid nanoparticles modified with β-cyclodextrin. Appl. Surf. Sci. 2014, 305, 267–273. [Google Scholar] [CrossRef]
- Shi, S.; Ocampo-Pérez, R.; Lv, J.; Liu, Q.; Nan, F.; Liu, X.; Xie, S.; Feng, J. Diatomite cross-linked β-Cyclodextrin polymers: A novel vision of diatomite adsorbent for the removal of bisphenol A. Environ. Technol. Innov. 2021, 23, 101602. [Google Scholar] [CrossRef]
- Bandura, L.; Białoszewska, M.; Malinowski, S.; Franus, W. Adsorptive performance of fly ash-derived zeolite modified by β-cyclodextrin for ibuprofen, bisphenol A and caffeine removal from aqueous solutions—Equilibrium and kinetic study. Appl. Surf. Sci. 2021, 562, 150160. [Google Scholar] [CrossRef]
- Rovani, S.; Santos, J.J.; Guilhen, S.N.; Corio, P.; Fungaro, D.A. Fast, efficient and clean adsorption of bisphenol-A using renewable mesoporous silica nanoparticles from sugarcane waste ash. RSC Adv. 2020, 10, 27706–27712. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Luo, L.; Shi, Y.; Chen, Y.; Wang, S.; Bian, L.; Jiang, F. One-step hydrothermal synthesis of CTAB-modified SiO2 for removal of bisphenol A. Water Sci. Technol. 2017, 76, 928–938. [Google Scholar] [CrossRef] [PubMed]

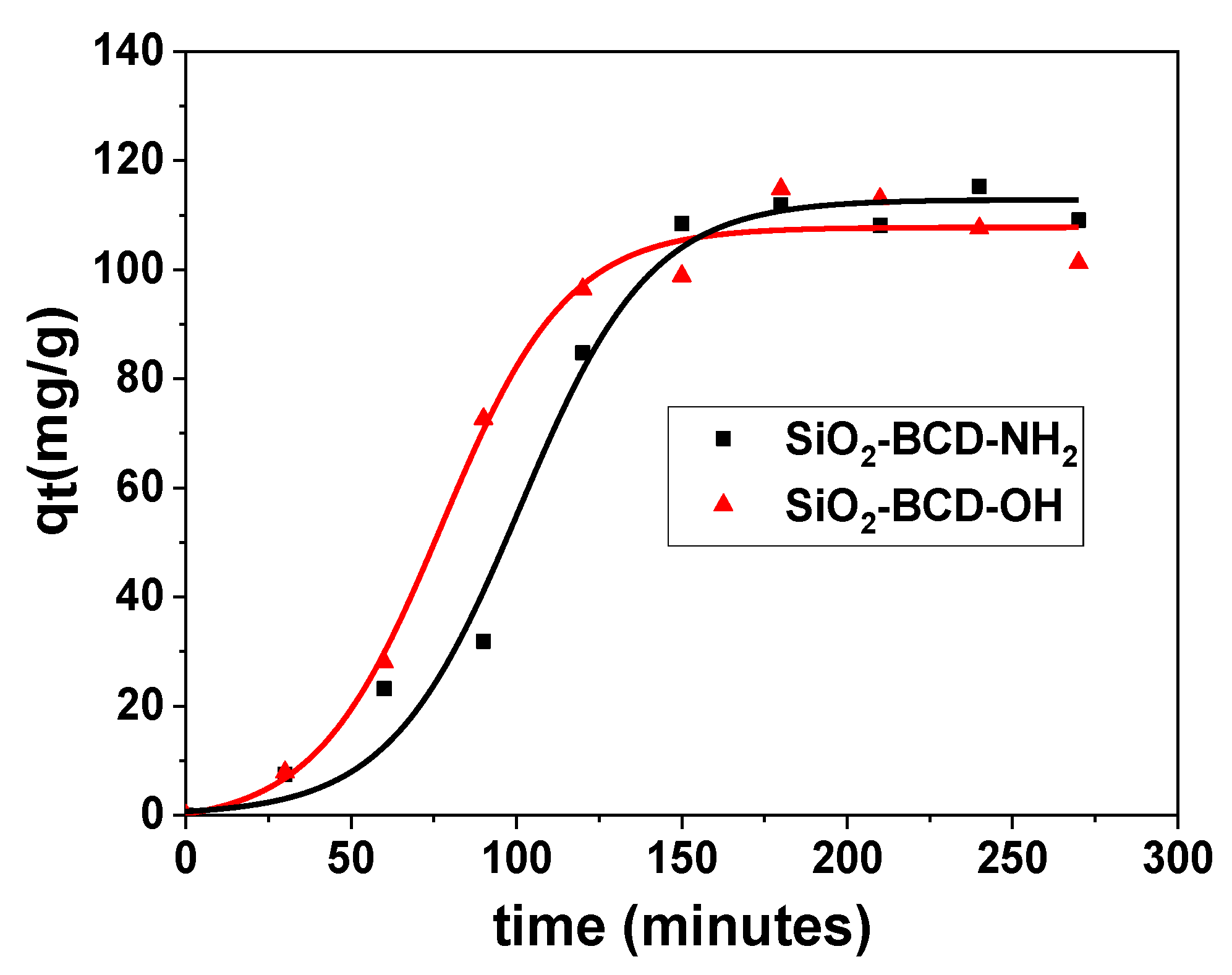
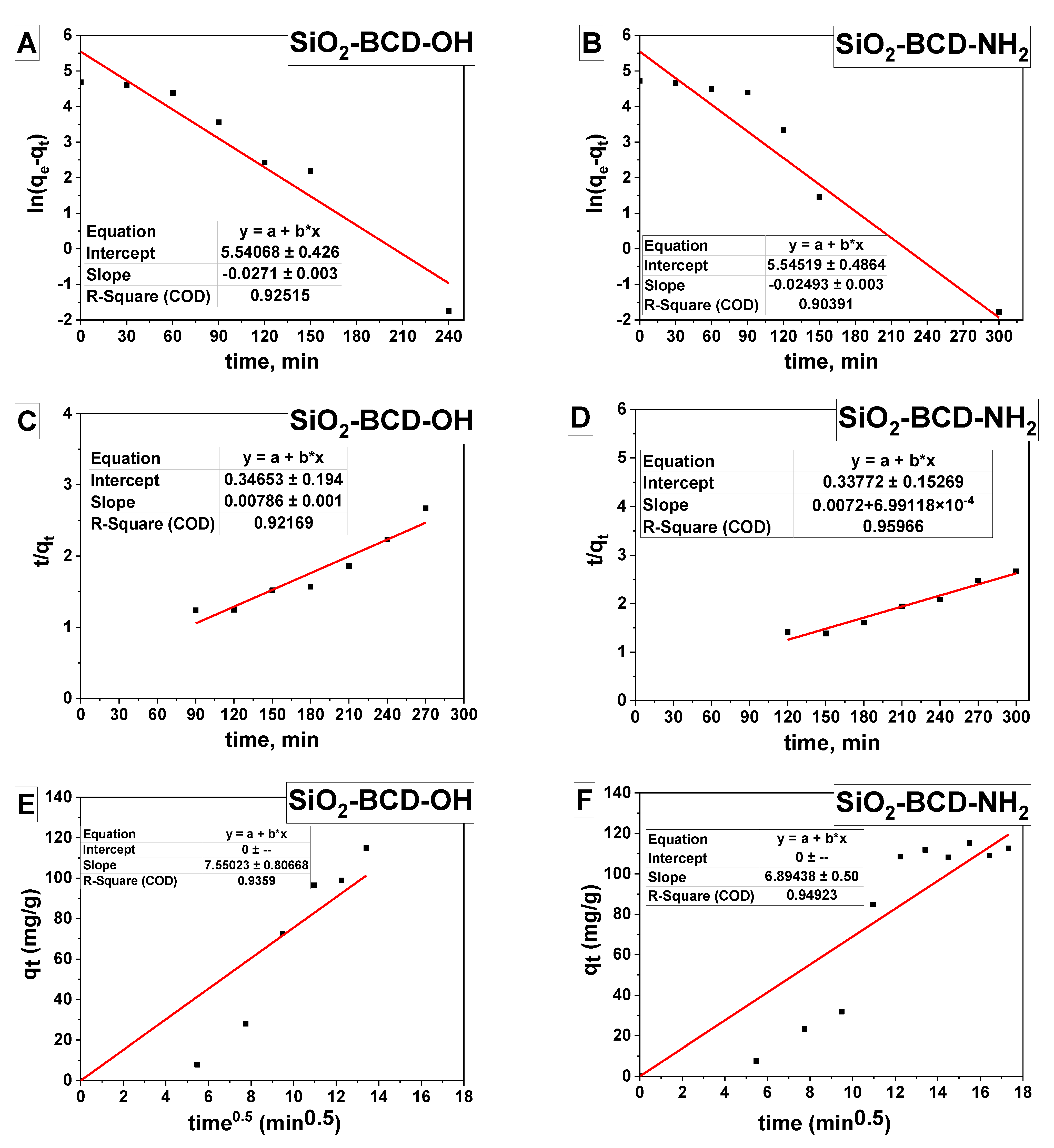
| Lagergren’s Pseudo-First-Order | Ho’s Pseudo-Second-Order Model | Weber’s Intraparticle Diffusion Model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| qe, exp (mg g−1) | k1 × 103 (min−1) | qe, calc (mg g−1) | R2 | k2 × 103 (g mg−1 min−1) | qe, calc (mg g−1) | R2 | Kp (mg g−1 min−0.5) | R2 | |
| SiO2-BCD-OH | 107.74 | 27.1 | 254.85 | 0.925 | 0.172 | 127.22 | 0.921 | 7.55 | 0.935 |
| SiO2-BCD-NH2 | 112.77 | 24.93 | 256.00 | 0.903 | 0.178 | 131.23 | 0.959 | 6.89 | 0.949 |
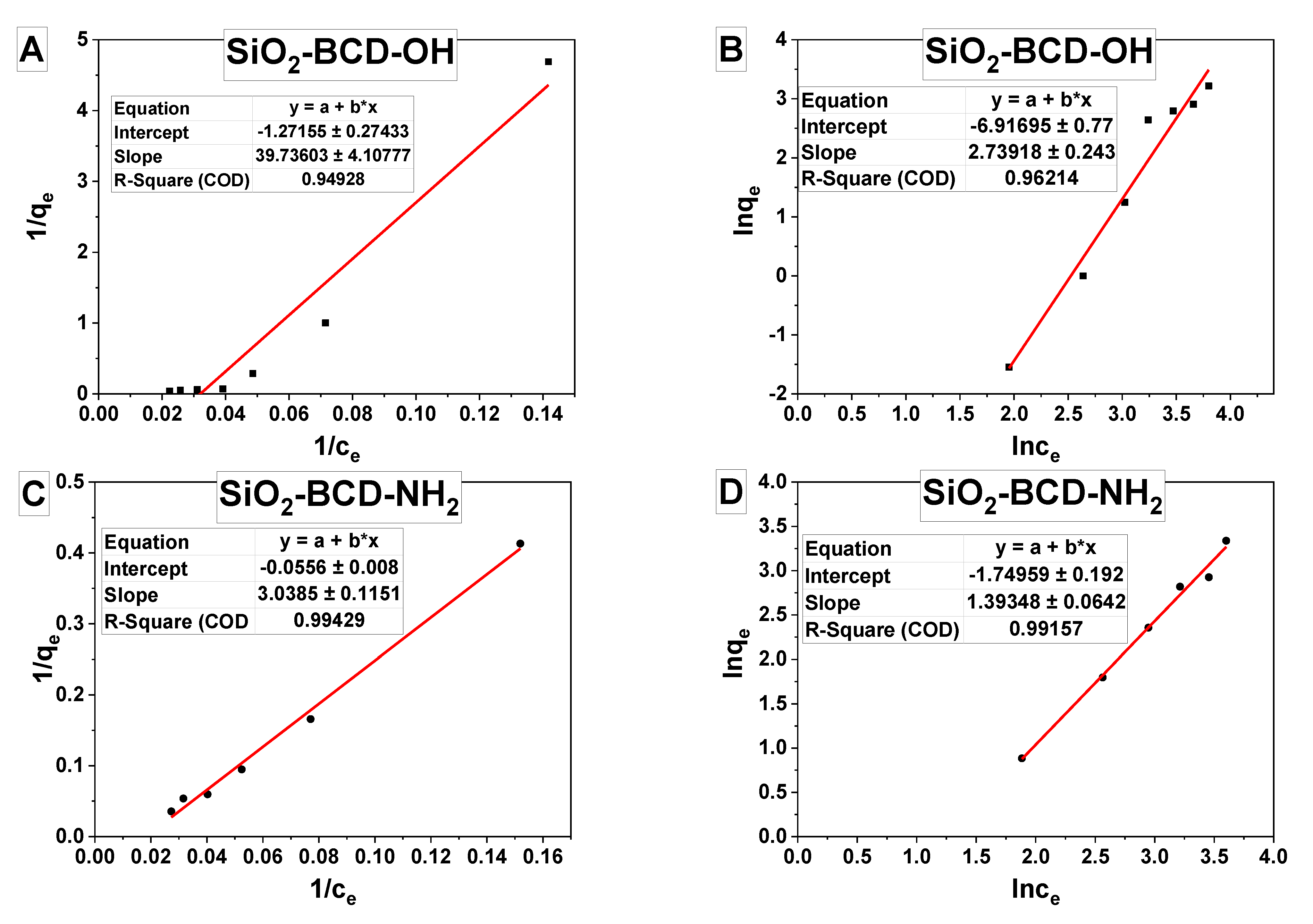
| Absorbent | Adsorbate | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| KL/ (L mg)−1 | qmax/ (mg g−1) | R2 | KF/ (L mg−1) | n | R2 | ||
| SiO2- BCD-OH | Bisphenol A | −0.032 | −0.786 | 0.949 | 0.00099 | 0.365 | 0.962 |
| SiO2- BCD-NH2 | Bisphenol A | −0.0183 | −17.9856 | 0.994 | 0.17384 | 0.717 | 0.991 |
| Adsorbent | Phenolic Pollutants | qmax (mg g−1) | References |
|---|---|---|---|
| Hyper-crosslinked β-CD porous polymer | Bisphenol A | 278 | [58] |
| Graphene oxide-β-CD nanocomposites | Bisphenol A | 373.4 | [59] |
| BCD polymer-functionalized Fe3O4 magnetic nanoparticles | Bisphenol A | 74.63 | [9] |
| BCD-grafted cellulose beads | Bisphenol A | 30.77 | [60] |
| (BCD+epichlorohydrin) polyBCD | Bisphenol A | 84 | [61] |
| BCD-Functionalized Mesoporous Magnetic Clusters | Bisphenol A | 52.7 | [62] |
| BCD-poly(glycidyl methacrylate)-SiO2- nanoparticles | Bisphenol A | 22.48 | [63] |
| Diatomite cross-linked BCD polymers | Bisphenol A | 83.57 | [64] |
| Fly ash-derived zeolite modified by BCD | Bisphenol A | 33.784 | [65] |
| BCD-modified graphene oxide membranes | Bisphenol A | 25.5 | [38] |
| Mesoporous silica nanoparticles-CTAB | Bisphenol A | 155.78 | [66] |
| CTAB-SiO2 | Bisphenol A | 198.8 | [67] |
| Polymer colloids modified with BCD-NH2 | Bisphenol A | 148.37 | [39] |
| Polymer colloids modified with BCD-OH | Bisphenol A | 37.09 | [39] |
| SiO2-BCD-NH2 | Bisphenol A | 107.7 | this study |
| SiO2-BCD-BCD-OH | Bisphenol A | 112.7 | this study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bucur, S.; Diacon, A.; Mangalagiu, I.; Mocanu, A.; Rizea, F.; Dinescu, A.; Ghebaur, A.; Boscornea, A.C.; Voicu, G.; Rusen, E. Bisphenol A Adsorption on Silica Particles Modified with Beta-Cyclodextrins. Nanomaterials 2022, 12, 39. https://doi.org/10.3390/nano12010039
Bucur S, Diacon A, Mangalagiu I, Mocanu A, Rizea F, Dinescu A, Ghebaur A, Boscornea AC, Voicu G, Rusen E. Bisphenol A Adsorption on Silica Particles Modified with Beta-Cyclodextrins. Nanomaterials. 2022; 12(1):39. https://doi.org/10.3390/nano12010039
Chicago/Turabian StyleBucur, Stefan, Aurel Diacon, Ionel Mangalagiu, Alexandra Mocanu, Florica Rizea, Adrian Dinescu, Adi Ghebaur, Aurelian Cristian Boscornea, Georgeta Voicu, and Edina Rusen. 2022. "Bisphenol A Adsorption on Silica Particles Modified with Beta-Cyclodextrins" Nanomaterials 12, no. 1: 39. https://doi.org/10.3390/nano12010039
APA StyleBucur, S., Diacon, A., Mangalagiu, I., Mocanu, A., Rizea, F., Dinescu, A., Ghebaur, A., Boscornea, A. C., Voicu, G., & Rusen, E. (2022). Bisphenol A Adsorption on Silica Particles Modified with Beta-Cyclodextrins. Nanomaterials, 12(1), 39. https://doi.org/10.3390/nano12010039











