Gas-Phase Fluorination of g-C3N4 for Enhanced Photocatalytic Hydrogen Evolution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Samples
2.2. Characterization
2.3. Photoelectrochemical Measurement
2.4. Photocatalytic Measurement
2.5. Theoretical Calculations
3. Results and Discussion
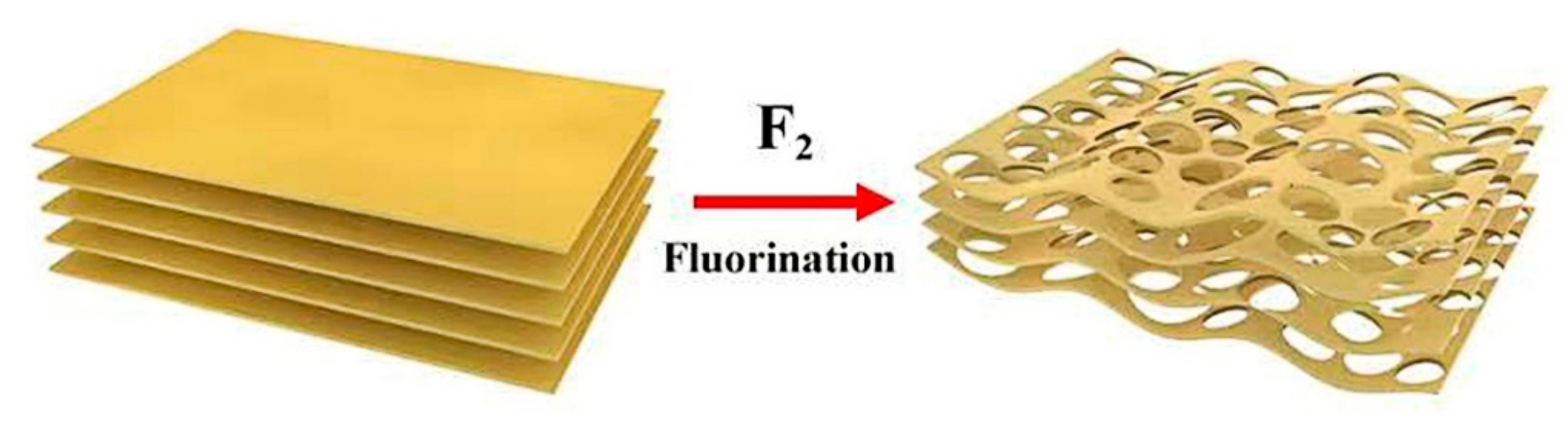
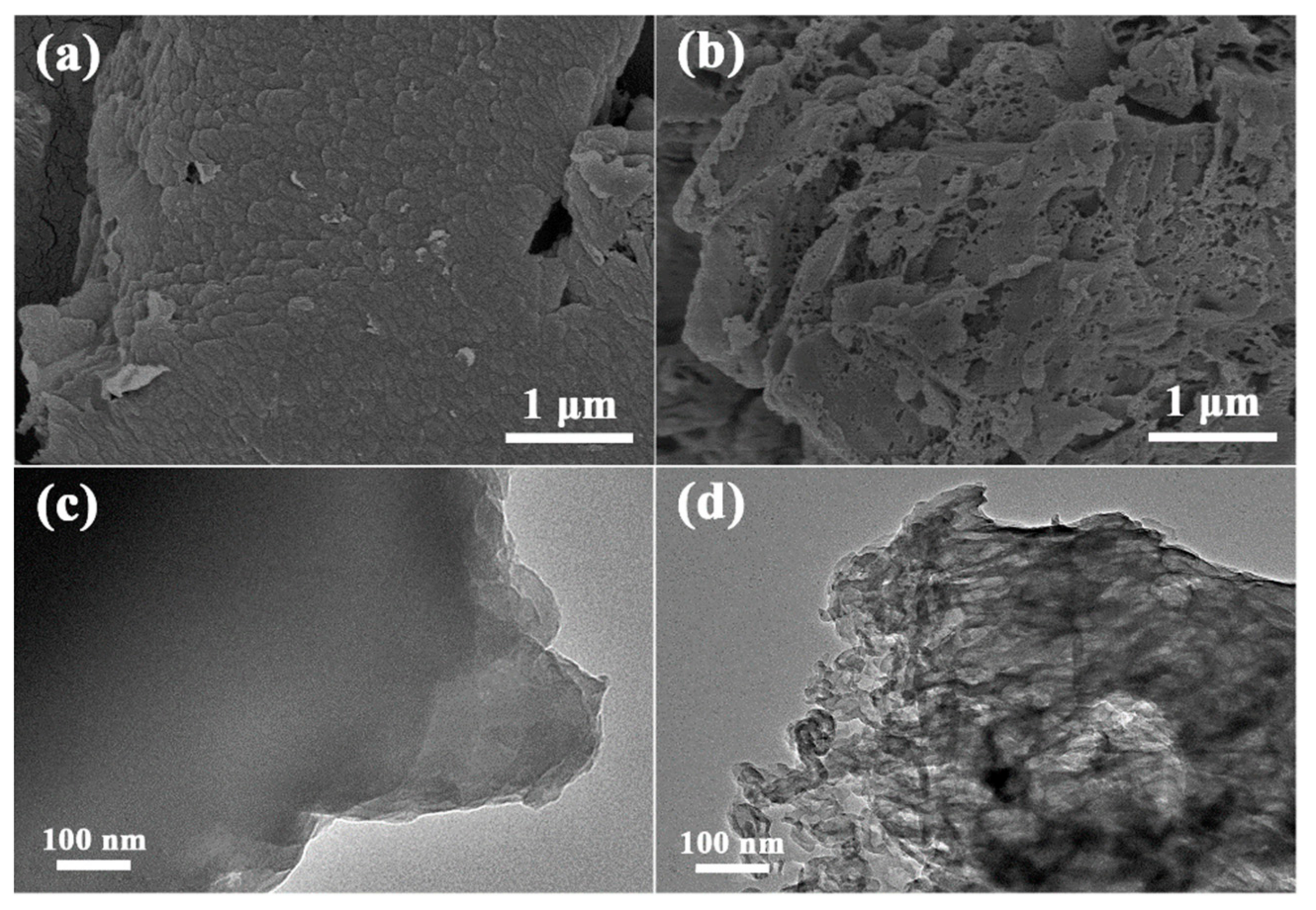
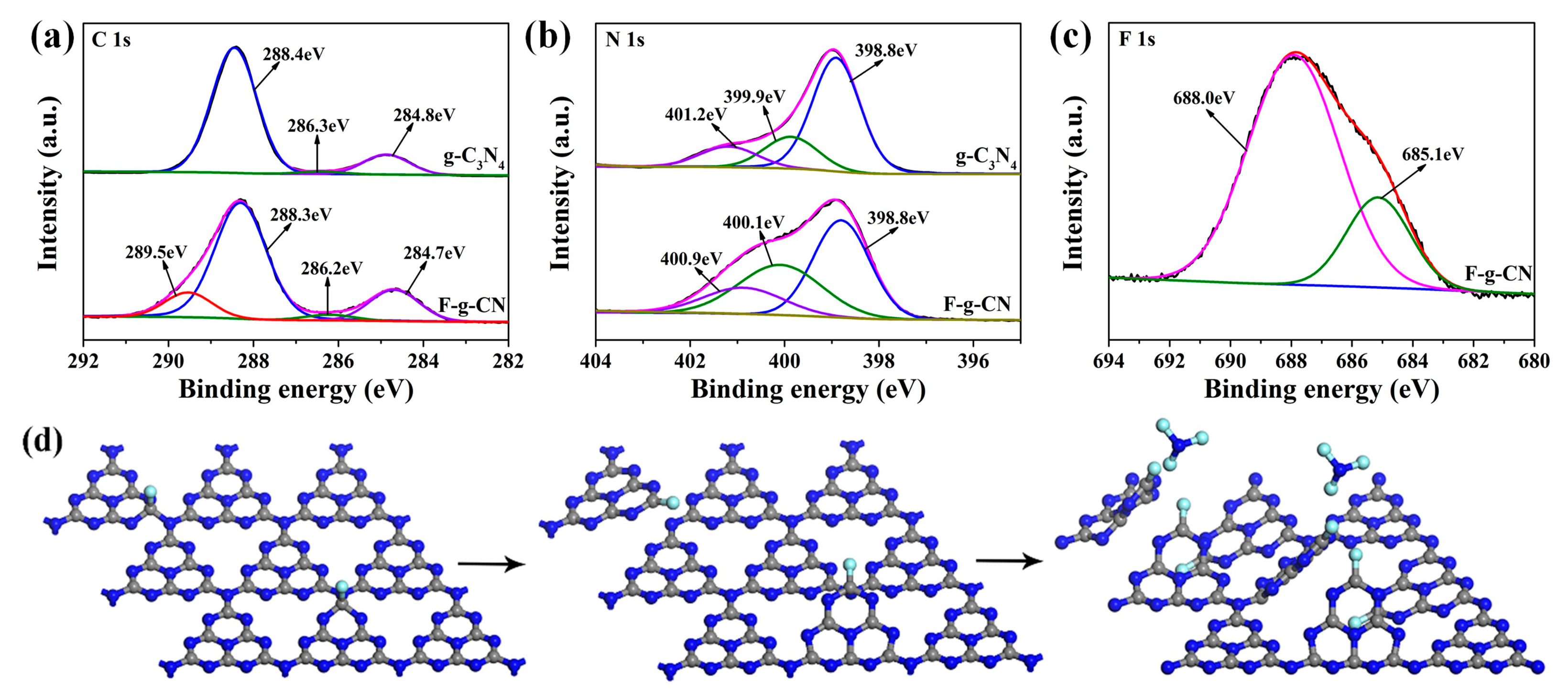
3.1. Morphology and Structural Analysis
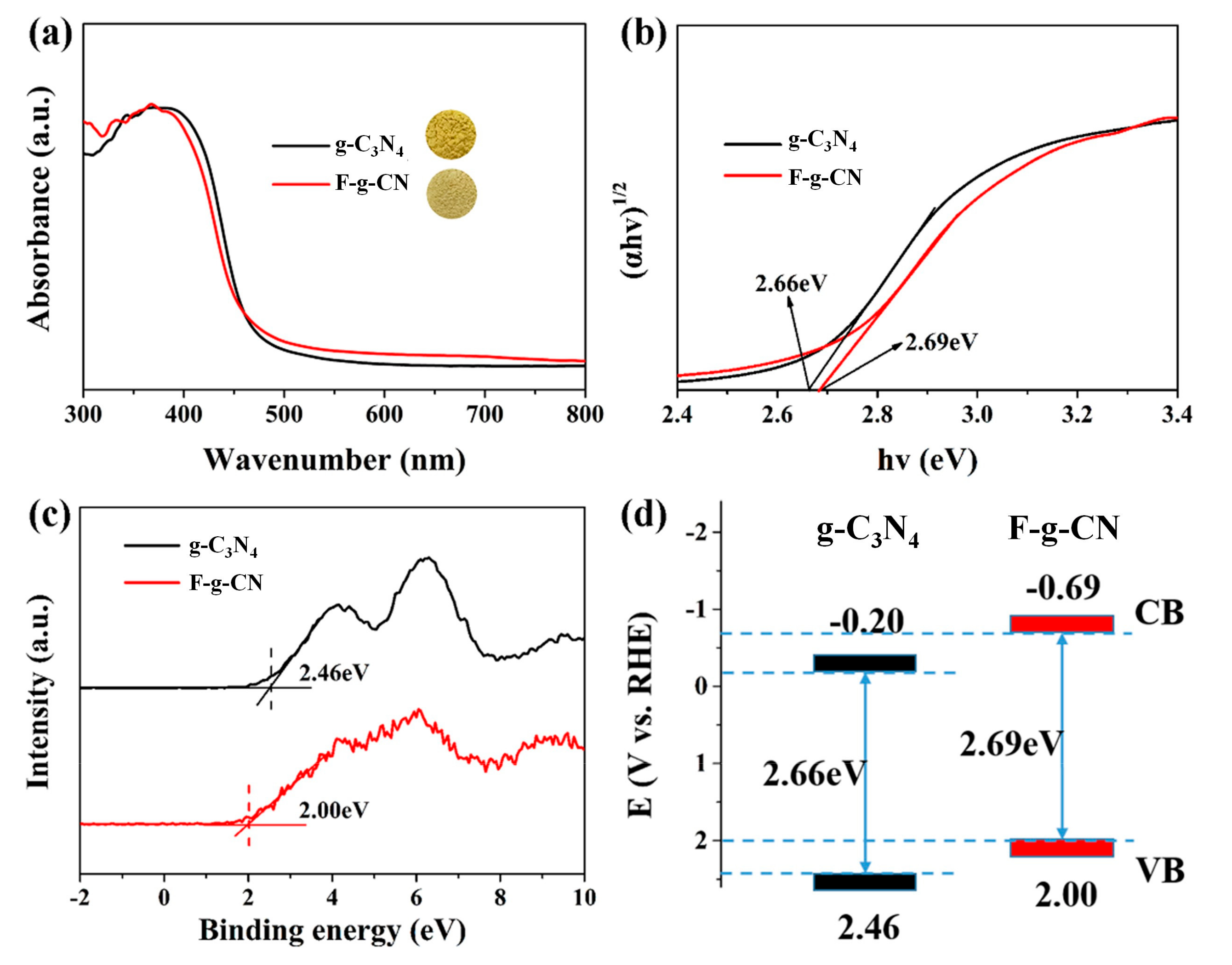
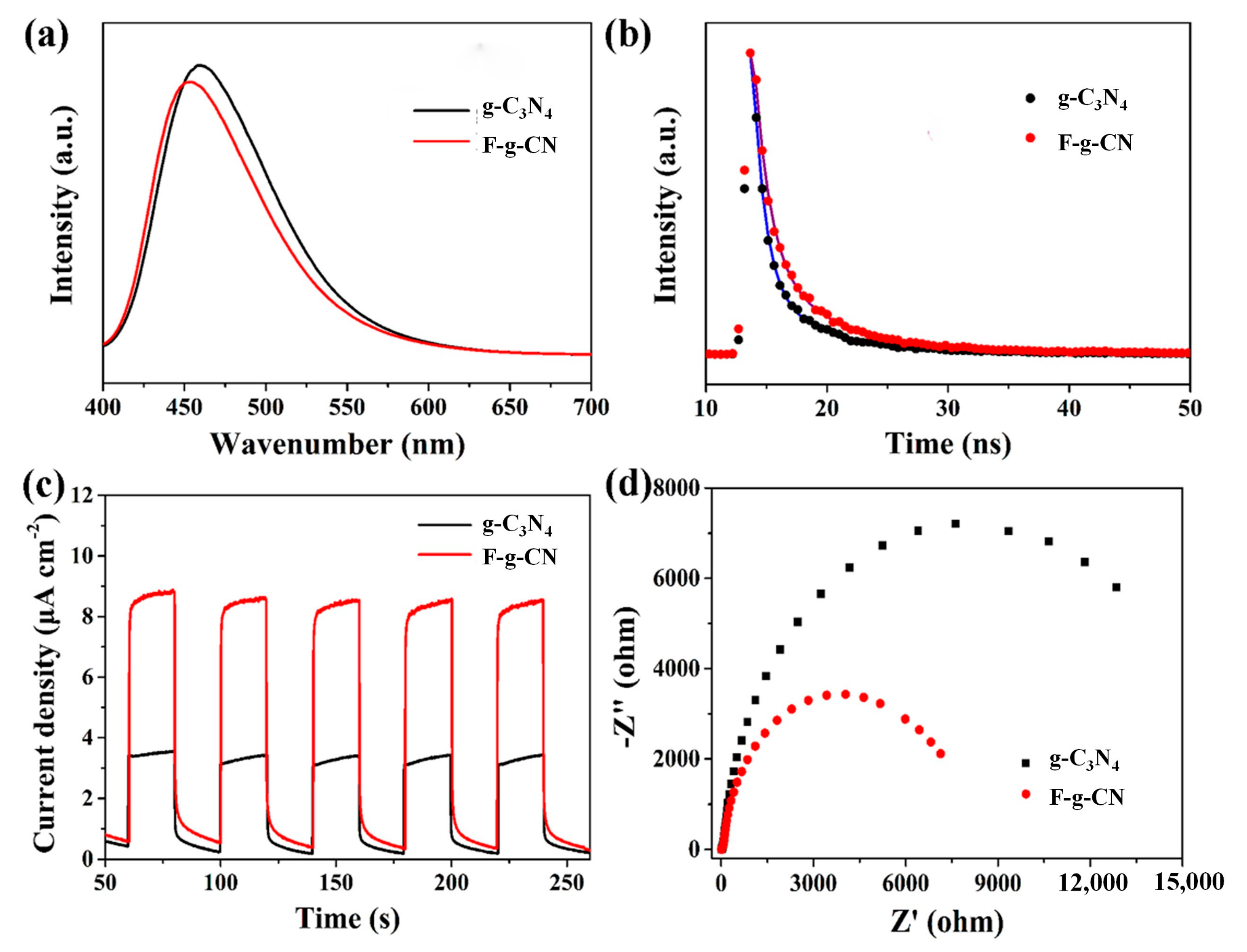
3.2. Optical and Electronic Properties
3.3. Photocatalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yan, H.J.; Yang, J.H.; Ma, G.J.; Wu, G.P.; Zong, X.; Lei, Z.B.; Shi, J.Y.; Li, C. Visible-light-driven hydrogen production with extremely high quantum efficiency on Pt-PdS/CdS photocatalyst. J. Catal. 2009, 266, 165–168. [Google Scholar] [CrossRef]
- Chen, X.B.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhao, Y.; Gao, Y.Y.; Li, D.; Li, Z.; Zhang, F.X.; Li, C. Redox-based visible-light-driven Z-scheme overall water splitting with apparent quantum efficiency exceeding 10%. Joule 2018, 2, 2393–2402. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.C.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.X.; Zhang, Y.Y.; Shen, Y.F.; Liu, S.Q.; Zhang, Y.J. Molecular engineering of polymeric carbon nitride: Advancing applications from photocatalysis to biosensing and more. Chem. Soc. Rev. 2018, 47, 2298–2321. [Google Scholar] [CrossRef]
- Fu, J.W.; Zhu, B.C.; Jiang, C.J.; Cheng, B.; You, W.; Yu, J.G. Hierarchical porous O-doped g-C3N4 with enhanced photocatalytic CO2 deduction activity. Small 2017, 13, 1603938. [Google Scholar] [CrossRef] [PubMed]
- Jorge, A.B.; Dedigama, I.; Miller, T.S.; Shearing, P.; Brett, D.J.L.; McMilan, P.F. Carbon nitride materials as efficient catalyst supports for proton exchange membrane water electrolyzers. Nanomaterials 2018, 6, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.W.; Low, J.X.; Yu, J.G.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.Y.; Han, Y.Z.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.T.; Zhong, J.; Kang, Z.H. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.T.; Wang, Z.Q.; Zhang, S.C.; Ye, H.N.; Kong, K.Y.; Gong, X.Q.; Hua, J.L.; Tian, H. Molecular engineering of donor-acceptor conjugated polymer/g-C3N4 heterostructures for significantly enhanced hydrogen evolution under visible-light irradiation. Adv. Funct. Mater. 2018, 28, 1804512. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Niu, P.; Sun, C.H.; Smith, S.C.; Chen, Z.G.; Lu, G.Q.; Cheng, H.M. Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4. J. Am. Chem. Soc. 2010, 132, 11642–11648. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.R.; Ma, T.Y.; Gao, G.P.; Du, X.W.; Qiao, S.Z. Porous P-doped graphitic carbon nitride nanosheets for synergistically enhanced visible-light photocatalytic H2 production. Energy Environ. Sci. 2015, 8, 3708–3717. [Google Scholar] [CrossRef]
- Zhang, G.G.; Zhang, M.W.; Ye, X.X.; Qiu, X.Q.; Sen, L.; Wang, X.C. Iodine modified carbon nitride semiconductors as visible light photocatalysts for hydrogen evolution. Adv. Mater. 2014, 26, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Praus, P.; Smykalova, S.; Foniok, K.; Velisek, P.; Cvejn, D.; Zadny, J.; Storch, J. Post-synthetic derivatization of graphitic carbon nitride with methanesulfonyl chloride: Synthesis, characterization and photocatalysis. Nanomaterials 2020, 10, 193. [Google Scholar] [CrossRef] [Green Version]
- Jeon, I.Y.; Ju, M.J.; Xu, J.T.; Choi, H.J.; Seo, J.M.; Kim, M.J.; Choi, I.T.; Kim, H.M.; Kim, J.C.; Lee, J.J.; et al. Edge-fluorinated graphene nanoplatelets as high performance electrodes for dye-sensitized solar cells and lithium ion batteries. Adv. Funct. Mater. 2015, 25, 1170–1179. [Google Scholar] [CrossRef]
- Karlicky, F.; Datta, K.K.R.; Otyepka, M.; Zboril, R. Halogenated graphenes: Rapidly growing family of graphene derivatives. ACS Nano 2013, 7, 6434–6464. [Google Scholar] [CrossRef]
- Feng, W.; Long, P.; Feng, Y.Y.; Li, Y. Two-dimensional fluorinated graphene: Synthesis, structures, properties and applications. Adv. Sci. 2016, 3, 1500413. [Google Scholar] [CrossRef]
- Chen, X.Y.; Fan, K.; Liu, Y.; Li, Y.; Liu, X.Y.; Feng, W.; Wang, X. Recent advances in fluorinated graphene from synthesis to applications: Critical review on functional chemistry and structure engineering. Adv. Mater. 2021, 2101665. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.; Yan, D.; Antonietti, M.; Lic, H.R.; Chen, X.F.; Wang, X.C. Excellent visible-light photocatalysis of fluorinated polymeric carbon nitride solids. Chem. Mater. 2010, 22, 5119–5121. [Google Scholar]
- Gao, D.; Liu, Y.; Song, M.; Shi, S.; Si, M.; Xue, D. Manifestation of high-temperature ferromagnetism in fluorinated graphitic carbon nitride nanosheets. J. Mater. Chem. C 2015, 3, 12230–12235. [Google Scholar] [CrossRef]
- Xu, M.Q.; Chai, B.; Yan, J.T.; Wang, H.B.; Ren, Z.D.; Paik, K.W. Facile synthesis of fluorine doped graphitic carbon nitride with enhanced visible light photocatalytic activity. Nano 2016, 11, 1650137. [Google Scholar] [CrossRef]
- Palanivel, B.; Hu, C.; Shkir, M.; AlFaify, S.; Ibrahim, F.A.; Hamdy, M.S.; Mani, A. Fluorine doped g-C3N4 coupled NiFe2O4 heterojunction: Consumption of H2O2 for production of hydroxyl radicals towards paracetamol degradation. Colloid Interface Sci. Commun. 2021, 42, 100410. [Google Scholar] [CrossRef]
- Zeng, L.; Ding, X.; Sun, Z.Z.; Hua, W.M.; Song, W.L.; Liu, S.Y.; Huang, L.M. Enhancement of photocatalytic hydrogen evolution activity of g-C3N4 induced by structural distortion via post-fluorination treatment. Appl. Catal. B Environ. 2018, 227, 276–284. [Google Scholar] [CrossRef]
- Lin, W.; Lu, K.C.; Zhou, S.J.; Wang, J.; Mu, F.H.; Wang, Y.; Wu, Y.; Kong, Y. Defects remodeling of g-C3N4 nanosheets by fluorine-containing solvothermal treatment to enhance their photocatalytic activities. Appl. Surf. Sci. 2019, 474, 194–202. [Google Scholar] [CrossRef]
- Ma, F.K.; Sun, C.L.; Shao, Y.L.; Wu, Y.Z.; Huang, B.B.; Hao, X.P. One-step exfoliation and fluorination of g-C3N4 nanosheets with enhanced photocatalytic activities. New. J. Chem. 2017, 41, 3061–3067. [Google Scholar] [CrossRef]
- Xue, M.T.; Tan, G.Q.; Liu, T.; Lv, L.; Li, B.; Zhang, D.; Dang, M.Y.; Ren, H.J.; Xia, A. Insights into the improved photocatalytic performance of fluorine surface modified mpg-C3N4 at room temperature under aqueous conditions. Appl. Catal. A Gen. 2019, 578, 89–97. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Magnetic fluorinated mesoporous g-C3N4 for photocatalytic degradation of amoxicillin: Transformation mechanism and toxicity assessment. Appl. Catal. B Environ. 2019, 242, 337–348. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.D.; Xie, J.F.; Zhang, J.J.; Ma, P.; Pan, B.C.; Xie, Y. Structural distortion in graphitic-C3N4 realizing an efficient photoreactivity. Nanoscale 2015, 7, 5152–5156. [Google Scholar] [CrossRef] [PubMed]
- Guerin, K.; Pinheiro, J.P.; Dubois, M.; Fawal, Z.; Masin, F.; Yazami, R.; Hamwi, A. Synthesis and characterization of highly fluorinated graphite containing sp2 and sp3 carbon. Chem. Mater. 2004, 16, 1786–1792. [Google Scholar] [CrossRef]
- Peng, C.; Li, Y.; Yao, F.N.; Fu, H.Y.; Zhou, R.X.; Feng, Y.Y.; Feng, W. Ultrahigh-energy-density fluorinated calcinated macadamia nut shell cathodes for lithium/fluorinated carbon batteries. Carbon 2019, 153, 783–791. [Google Scholar] [CrossRef]
- Fulvio, P.F.; Brown, S.S.; Adcock, J.; Mayes, R.T.; Guo, B.; Sun, X.G.; Dai, S. Low-temperature fluorination of soft-templated mesoporous carbons for a high-power lithium/carbon fluoride battery. Chem. Mater. 2011, 23, 4420–4427. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.L.; Liu, G.; Cheng, H.M. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.Y.; Wang, S.; Zhou, P.F.; Zhao, Z.D.; Li, X.W.; Zhuo, S.P. Fluorinated nanographite as a cathode material for lithium primary batteries. ChemElectroChem 2019, 6, 2201–2207. [Google Scholar] [CrossRef]
- Lotsch, B.V.; Doeblinger, M.; Sehnert, J.; Seyfarth, L.; Senker, J.; Oeckler, O.; Schnick, W. Unmasking melon by a complementary approach employing electron diffraction, solid-state NMR spectroscopy, and theoretical calculations-structural characterization of a carbon nitride polymer. Chem. Eur. J. 2007, 13, 4969–4980. [Google Scholar] [CrossRef]
- Liang, Q.H.; Li, Z.; Huang, Z.H.; Kang, F.Y.; Yang, Q.H. Holey graphitic carbon nitride nanosheets with carbon vacancies for highly improved photocatalytic hydrogen production. Adv. Funct. Mater. 2015, 25, 6885–6892. [Google Scholar] [CrossRef]
- Chetia, T.R.; Ansari, M.S.; Qureshi, M. Graphitic carbon nitride as a photovoltaic booster in quantum dot sensitized solar cells: A synergistic approach for enhanced charge separation and injection. J. Mater. Chem. A 2016, 4, 5528–5541. [Google Scholar] [CrossRef]
- Yang, L.Q.; Huang, J.F.; Li, S.; Cao, L.Y.; Yu, Q.; Jie, Y.N.; Jie, F.; Ouyang, H.B.; Ye, J.H. A surface modification resultant thermally oxidized porous g-C3N4 with enhanced photocatalytic hydrogen production. Appl. Catal. B Environ. 2017, 204, 335–345. [Google Scholar] [CrossRef]
- Wang, H.; Bian, Y.R.; Hu, J.T.; Dai, L.M. Highly crystalline sulfur-doped carbon nitride as photocatalyst for efficient visible-light hydrogen generation. Appl. Catal. B Environ. 2018, 238, 592–598. [Google Scholar] [CrossRef]
- Meng, N.N.; Jian, R.; Yang, L.; Yi, H.; Petit, T.; Zhang, B. Engineering oxygen-containing and amino groups into two-dimensional atomically-thin porous polymeric carbon nitrogen for enhanced photocatalytic hydrogen production. Energy Environ. Sci. 2018, 11, 566–571. [Google Scholar] [CrossRef] [Green Version]
- She, X.J.; Wu, J.J.; Zhong, J.; Xu, H.; Yang, Y.C.; Vajtai, R.; Lou, J.; Liu, Y.; Du, D.L.; Li, H.M.; et al. Oxygenated monolayer carbon nitride for excellent photocatalytic hydrogen evolution and external quantum efficiency. Nano Energy 2016, 27, 138–146. [Google Scholar] [CrossRef]
- Jiang, Y.B.; Sun, Z.Z.; Tang, C.; Zhou, Y.X.; Zeng, L.; Huang, L.M. Enhancement of photocatalytic hydrogen evolution activity of porous oxygen doped g-C3N4 with nitrogen defects induced by changing electron transition. Appl. Catal. B Environ. 2019, 240, 30–38. [Google Scholar] [CrossRef]
- Yu, H.J.; Shi, R.; Zhao, Y.X.; Bian, T.; Zhao, Y.F.; Zhou, C.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; Zhang, T.R. Alkali-assisted synthesis of nitrogen deficient graphitic carbon nitride with tunable band structures for efficient visible-light-driven hydrogen evolution. Adv. Mater. 2017, 29, 1605148. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.N.; Chen, G.; Li, C.M.; Han, Z.H.; Hu, Y.D.; Meng, Q.Q. Doping effect of non-metal group in porous ultrathin g-C3N4 nanosheets towards synergistically improved photocatalytic hydrogen evolution. Nanoscale 2018, 10, 5239–5245. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Fan, H.; Sun, J.C.; Han, Z.W.; Dong, J.; Ai, S.Y. Fluorine-doped carbon nitride quantum dots: Ethylene glycol-assisted synthesis, fluorescent properties, and their application for bacterial imaging. Carbon 2016, 109, 141–148. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Wolf, A.K.; Glinnemann, J.; Schmidt, M.U. Packing of tetrahedral EX4 molecules with E = C, Si, Ge, Sn, Pb and X = F, Cl, Br, I. CrystEngComm 2008, 10, 1364–1371. [Google Scholar] [CrossRef]
- Wang, Y.J.; Li, L.B.; Wei, Y.Y.; Xue, J.; Chen, H.; Ding, L.; Caro, J.; Wang, H.H. Water transport with ultralow friction through partially exfoliated g-C3N4 nanosheet membranes with self-supporting spacers. Angew. Chem. Int. Ed. 2017, 56, 8974–8980. [Google Scholar] [CrossRef]
- Li, Y.F.; Yang, M.; Xing, Y.; Liu, X.C.; Yang, Y.; Wang, X.; Song, S.Y. Preparation of carbon-rich g-C3N4 nanosheets with enhanced visible light utilization for efficient photocatalytic hydrogen production. Small 2017, 13, 1701552. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Yin, L.C.; Yang, Y.Q.; Liu, G.; Cheng, H.M. Increasing the visible light absorption of graphitic carbon nitride (Melon) photocatalysts by homogeneous self-modification with nitrogen vacancies. Adv. Mater. 2014, 26, 8046–8052. [Google Scholar] [CrossRef]
- Han, Q.; Wang, B.; Gao, J.; Cheng, Z.H.; Zhao, Y.; Zhang, Z.P.; Qu, L.T. Atomically thin mesoporous nanomesh of graphitic C3N4 for high-efficiency photocatalytic hydrogen evolution. ACS Nano 2016, 10, 2745–2751. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cao, L.L.; Cheng, W.R.; Cao, Y.J.; Liu, X.K.; Zhang, W.; Mou, X.L.; Jin, L.L.; Zheng, X.S.; Che, W.; et al. Single-site active cobalt-based photocatalyst with a long carrier lifetime for spontaneous overall water splitting. Angew. Chem. Int. Ed. 2017, 129, 9440–9445. [Google Scholar] [CrossRef]
- Wen, W.; Wang, J.B.; Tong, R.F.; Liu, D.N.; Huang, H.; Yu, Y.; Zhou, Z.K.; Chu, P.K.; Yu, X.F. A low-cost metal-free photocatalyst based on black phosphorus. Adv. Sci. 2019, 6, 1801321. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Gim, S.; Jeon, T.H.; Kim, H.; Choi, W. Distorted carbon nitride structure with substituted benzene moieties for enhanced visible light photocatalytic activities. ACS Appl. Mater. Interfaces 2017, 9, 40360–40368. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Li, Y.; Feng, W. Gas-Phase Fluorination of g-C3N4 for Enhanced Photocatalytic Hydrogen Evolution. Nanomaterials 2022, 12, 37. https://doi.org/10.3390/nano12010037
Sun L, Li Y, Feng W. Gas-Phase Fluorination of g-C3N4 for Enhanced Photocatalytic Hydrogen Evolution. Nanomaterials. 2022; 12(1):37. https://doi.org/10.3390/nano12010037
Chicago/Turabian StyleSun, Lidong, Yu Li, and Wei Feng. 2022. "Gas-Phase Fluorination of g-C3N4 for Enhanced Photocatalytic Hydrogen Evolution" Nanomaterials 12, no. 1: 37. https://doi.org/10.3390/nano12010037
APA StyleSun, L., Li, Y., & Feng, W. (2022). Gas-Phase Fluorination of g-C3N4 for Enhanced Photocatalytic Hydrogen Evolution. Nanomaterials, 12(1), 37. https://doi.org/10.3390/nano12010037





