Facile In Situ Synthesis of Co(OH)2–Ni3S2 Nanowires on Ni Foam for Use in High-Energy-Density Supercapacitors
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Ni3S2 and Co(OH)2–Ni3S2 Positive Electrode
2.3. Cell Assembly
2.4. Materials Characterization and Electrochemical Measurements
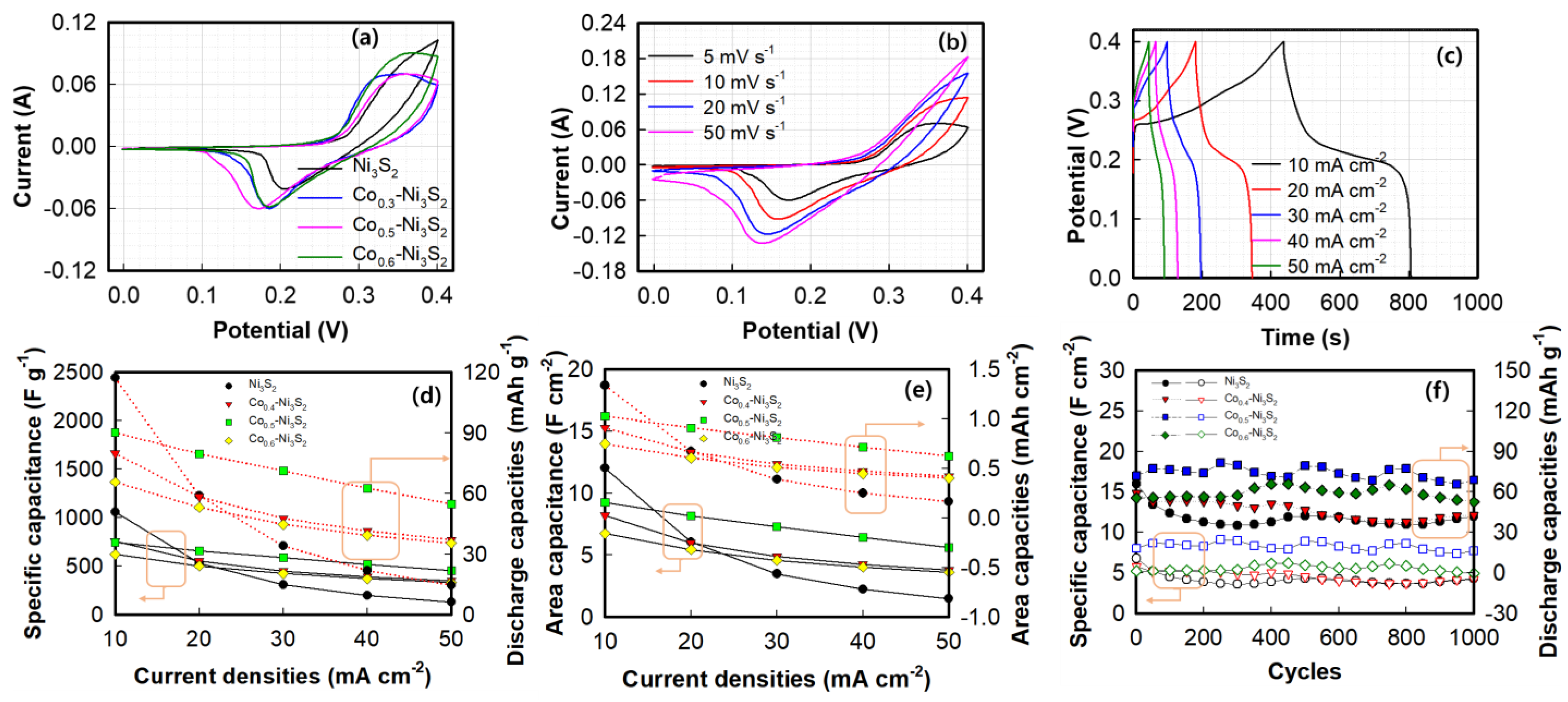
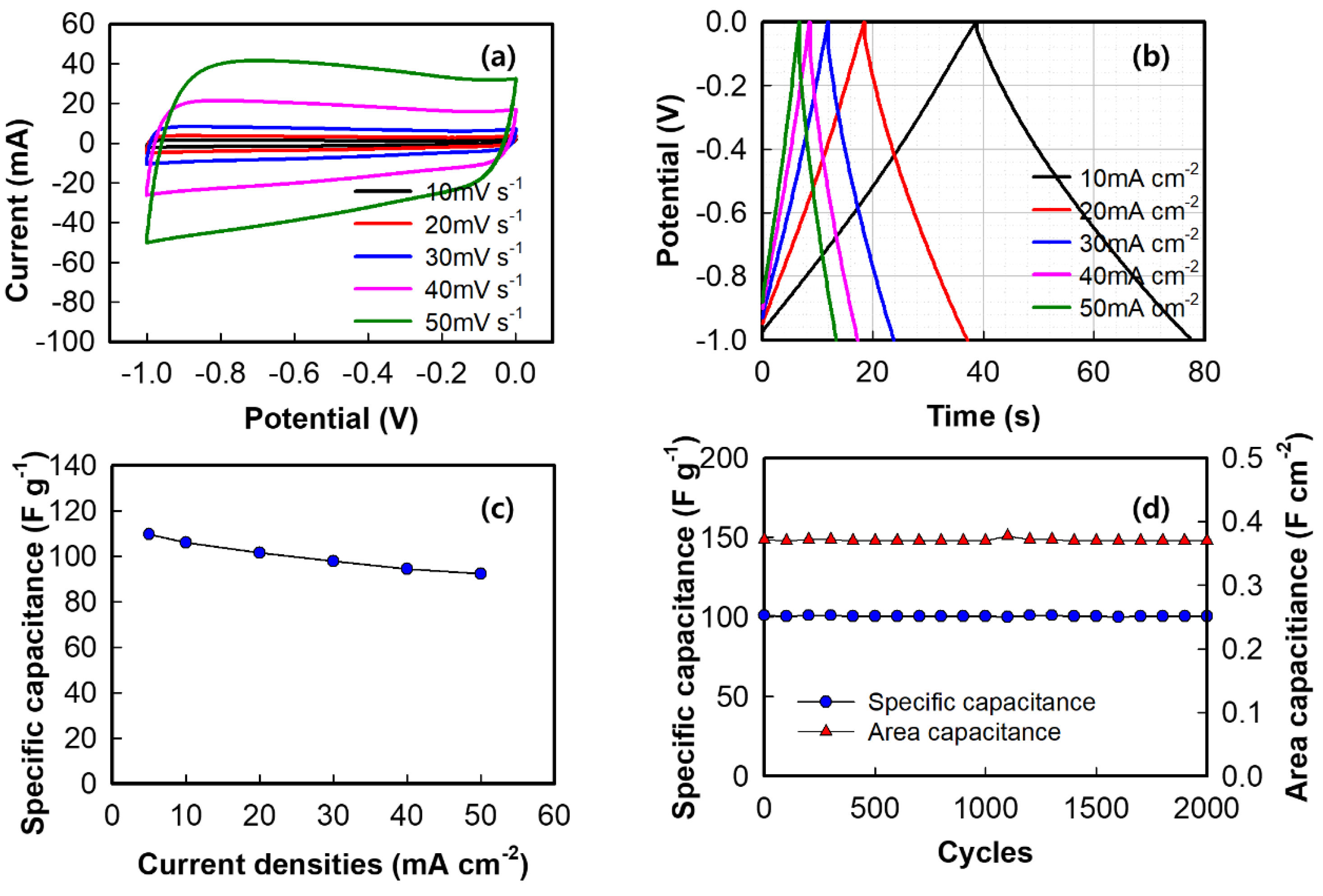
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, S.; Zhu, X.; Sarkar, S.; Zhao, Y. Challenges and opportunities for supercapacitors. APL Mater. 2019, 7, 100901. [Google Scholar] [CrossRef]
- Abdel Maksoud, M.I.A.; Fahim, R.A.; Shalan, A.E.; Abd Elkodous, M.; Olojede, S.O.; Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.a.H.; Awed, A.S.; Ashour, A.H.; et al. Advanced materials and technologies for supercapacitors used in energy conversion and storage: A review. Environ. Chem. Lett. 2020, 19, 375–439. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, K.-Y.; Yoon, J.-R. Binder- and conductive additive-free laser-induced graphene/LiNi1/3Mn1/3Co1/3O2 for advanced hybrid supercapacitors. NPG Asia Mater. 2020, 12, 28. [Google Scholar] [CrossRef]
- Lamb, J.J.; Burheim, O.S. Lithium-ion capacitors: A review of design and active materials. Energies 2021, 14, 979. [Google Scholar] [CrossRef]
- Sung, J.; Shin, C. Recent studies on supercapacitors with next-generation structures. Micromachines 2020, 11, 1125. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Obreja, V.V.N. Supercapacitors specialities-Materials review. AIP Conf. Proc. 2014, 98, 1597. [Google Scholar] [CrossRef] [Green Version]
- Danilovic, N.; Subbaraman, R.; Chang, K.C.; Chang, S.H.; Kang, Y.J.; Snyder, J.; Paulikas, A.P.; Strmcnik, D.; Kim, Y.T.; Myers, D.; et al. Activity-stability trends for the oxygen evolution reaction on monometallic oxides in acidic environments. J. Phys. Chem. Lett. 2014, 5, 2474–2478. [Google Scholar] [CrossRef]
- Branco, J.B.; Ferreira, A.C.; Vieira, F.; Martinho, J.F. Cerium-based bimetallic oxides as catalysts for the methanation of CO2: Influence of the preparation method. Energy Fuels 2021, 35, 6725–6737. [Google Scholar] [CrossRef]
- Anantharaj, S.; Karthick, K.; Kundu, S. Evolution of layered double hydroxides (LDH) as high performance water oxidation electrocatalysts: A review with insights on structure, activity and mechanism. Mater. Today Energy 2017, 6, 1–26. [Google Scholar] [CrossRef]
- Wang, A.; Wang, H.; Zhang, S.; Mao, C.; Song, J.; Niu, H.; Jin, B.; Tian, Y. Controlled synthesis of nickel sulfide/graphene oxide nanocomposite for high-performance supercapacitor. Appl. Surf. Sci. 2013, 282, 704–708. [Google Scholar] [CrossRef]
- Santhosh, N.M.; Upadhyay, K.K.; Strazar, P.; Filipic, G.; Zavasnik, J.; Mao de Ferro, A.; Silva, R.P.; Tatarova, E.; Montemor, M.F.; Cvelbar, U. Advanced carbon-nickel sulfide hybrid nanostructures: Extending the limits of battery-type electrodes for redox-based supercapacitor applications. ACS Appl Mater Interfaces 2021, 13, 20559–20572. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Liang, M.; He, Z.; Li, K.; Song, W.; Tian, S.; Duan, W.; Zhao, Y.; Miao, Z. Novel dealloying-fabricated NiS/NiO nanoparticles with superior cycling stability for supercapacitors. ACS Omega 2021, 6, 17999–18007. [Google Scholar] [CrossRef]
- Huang, H.; Deng, X.; Yan, L.; Wei, G.; Zhou, W.; Liang, X.; Guo, J. One-step synthesis of self-supported Ni3S2/NiS composite film on Ni foam by electrodeposition for high-performance supercapacitors. Nanomaterials 2019, 9, 1718. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.-Y.; Tai, S.-Y.; Chou, S.-W. Bifunctional one-dimensional hierarchical nanostructures composed of cobalt sulfide nanoclusters on carbon nanotubes backbone for dye-sensitized solar cells and supercapacitors. J. Phys. Chem. C 2013, 118, 823–830. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, C.; Tian, Y.; Tang, Y.; Yin, X.; Que, W. A long cycle life asymmetric supercapacitor based on advanced nickel-sulfide/titanium carbide (MXene) nanohybrid and MXene electrodes. J. Power Sources 2020, 450, 227694. [Google Scholar] [CrossRef]
- Shombe, G.B.; Khan, M.D.; Zequine, C.; Zhao, C.; Gupta, R.K.; Revaprasadu, N. Direct solvent free synthesis of bare alpha-NiS, beta-NiS and alpha-beta-NiS composite as excellent electrocatalysts: Effect of self-capping on supercapacitance and overall water splitting activity. Sci Rep. 2020, 10, 3260. [Google Scholar] [CrossRef]
- Jiang, N.; Tang, Q.; Sheng, M.; You, B.; Jiang, D.-E.; Sun, Y. Nickel sulfides for electrocatalytic hydrogen evolution under alkaline conditions: A case study of crystalline NiS, NiS2, and Ni3S2 nanoparticles. Catal. Sci. Technol. 2016, 6, 1077–1084. [Google Scholar] [CrossRef]
- Tang, J.; Ni, S.; Chao, D.; Liu, J.; Yang, X.; Zhao, J. High-rate and ultra-stable Na-ion storage for Ni3S2 nanoarrays via self-adaptive pseudocapacitance. Electrochim. Acta 2018, 265, 709–716. [Google Scholar] [CrossRef]
- Darezereshki, E.; Vakylabad, A.B.; Hassanzadeh, A.; Niedoba, T.; Surowiak, A.; Koohestani, B. Hydrometallurgical synthesis of nickel nano-sulfides from spent lithium-ion batteries. Minerals 2021, 11, 419. [Google Scholar] [CrossRef]
- Lai, C.-H.; Huang, K.-W.; Cheng, J.-H.; Lee, C.-Y.; Lee, W.-F.; Huang, C.-T.; Hwang, B.-J.; Chen, L.-J. Oriented growth of large-scale nickel sulfide nanowire arrays via a general solution route for lithium-ion battery cathode applications. J. Mater. Chem. 2009, 19, 7277–7283. [Google Scholar] [CrossRef]
- Zhu, X.; Wen, Z.; Gu, Z.; Huang, S. Room-temperature mechanosynthesis of Ni3S2 as cathode material for rechargeable lithium polymer batteries. J. Electrochem. Soc. 2006, 153, A504–A507. [Google Scholar] [CrossRef]
- Takeuchi, T.; Sakaebe, H.; Kageyama, H.; Sakai, T.; Tatsumi, K. Preparation of NiS2 using spark-plasma-sintering process and its electrochemical properties. J. Electrochem. Soc. 2008, 155, A679–A684. [Google Scholar] [CrossRef]
- Chou, S.-W.; Lin, J.-Y. Cathodic Deposition of flaky nickel sulfide nanostructure as an electroactive material for high-performance supercapacitors. J. Electrochem. Soc. 2013, 160, D178–D182. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Gk, V.; Radhakrishnan, S.; Kim, S. Corrigendum to “One pot hydrothermal growth of hierarchical nanostructured Ni3S2 on Ni foam for supercapacitor application” [Chem. Eng. J. 251 (2014) 116–122]. Chem. Eng. J. 2015, 266, 386. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Ding, D.; Gao, Y. Controllable synthesis of three-dimensional beta-NiS nanostructured assembly for hybrid-type asymmetric supercapacitors. Nanomaterials 2020, 10, 487. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Zhang, S.; Zou, X.; Hao, J.; Bai, Y.; Yan, L.; Li, W. Coordination agent-dominated phase control of nickel sulfide for high-performance hybrid supercapacitor. J. Colloid. Interface Sci. 2021, 607, 45–52. [Google Scholar] [CrossRef]
- Sajjad, M.; Khan, Y. Rational design of self-supported Ni3S2 nanoparticles as a battery type electrode material for high-voltage (1.8 V) symmetric supercapacitor applications. Cryst. Eng. Commun. 2021, 23, 2869–2879. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, H.; Xiong, W.; Yang, S.; Huang, H.; Zou, Y.; Zhou, W.; Cheng, Z.; Wang, J.; Luo, G. One-pot synthesis of glucose-derived carbon coated Ni3S2 nanowires as a battery-type electrode for high performance supercapacitors. Nanomaterials 2021, 11, 678. [Google Scholar] [CrossRef]
- Phonsuksawang, P.; Khajondetchairit, P.; Ngamchuea, K.; Butburee, T.; Sattayaporn, S.; Chanlek, N.; Suthirakun, S.; Siritanon, T. Enhancing performance of NiCo2S4/Ni3S2 supercapacitor electrode by Mn doping. Electrochim. Acta 2021, 368, 137634. [Google Scholar] [CrossRef]
- Shinde, N.M.; Xia, Q.X.; Shinde, P.V.; Yun, J.M.; Mane, R.S.; Kim, K.H. Sulphur source-inspired self-grown 3D NixSy nanostructures and their electrochemical supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 4551–4559. [Google Scholar] [CrossRef] [PubMed]
- Vidhya, M.S.; Ravi, G.; Yuvakkumar, R.; Velauthapillai, D.; Thambidurai, M.; Dang, C.; Saravanakumar, B. Nickel–cobalt hydroxide: A positive electrode for supercapacitor applications. RSC Adv. 2020, 10, 19410–19418. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Savu, R.; Dubey, P.K.; Kumar, P.; Moshkalev, S.A. Microwave-assisted synthesis of void-induced graphene-wrapped nickel oxide hybrids for supercapacitor applications. RSC Adv. 2016, 6, 26612–26620. [Google Scholar] [CrossRef] [Green Version]
- Vishnukumar, P.; Saravanakumar, B.; Ravi, G.; Ganesh, V.; Guduru, R.K.; Yuvakkumar, R. Synthesis and characterization of NiO/Ni3V2O8 nanocomposite for supercapacitor applications. Mater. Lett. 2018, 219, 114–118. [Google Scholar] [CrossRef]
- Anil Kumar, Y.; Dasha Kumar, K.; Kim, H.J. A novel electrode for supercapacitors: Efficient PVP-assisted synthesis of Ni3S2 nanostructures grown on Ni foam for energy storage. Dalton Trans. 2020, 49, 4050–4059. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Qian, H.; Nie, Z.; Luo, Z.; Wu, Z.; Liu, P.; He, H.; Wu, J.; Chen, S.; Zhang, F. Ni3S2 nanorods growing directly on Ni foam for all-solid-state asymmetric supercapacitor and efficient overall water splitting. J. Energy Chem. 2020, 46, 178–186. [Google Scholar] [CrossRef]
- Chen, F.; Wang, H.; Ji, S.; Linkov, V.; Wang, R. Core-shell structured Ni3S2@Co(OH)2 nano-wires grown on Ni foam as binder-free electrode for asymmetric supercapacitors. Chem. Eng. J. 2018, 345, 48–57. [Google Scholar] [CrossRef]
- Wang, X.; Shi, B.; Fang, Y.; Rong, F.; Huang, F.; Que, R.; Shao, M. High capacitance and rate capability of a Ni3S2@CdS core–shell nanostructure supercapacitor. J. Mater. Chem. A 2017, 5, 7165–7172. [Google Scholar] [CrossRef]
- Wang, J.; Chao, D.; Liu, J.; Li, L.; Lai, L.; Lin, J.; Shen, Z. Ni3S2@MoS2 Core/shell nanorod arrays on Ni foam for high-performance electrochemical energy storage. Nano Energy 2014, 7. [Google Scholar] [CrossRef]
- Liu, X.X.; Wu, R.; Wang, Y.; Xiao, S.H.; He, Q.; Niu, X.B.; Blackwood, D.J.; Chen, J.S. Self-supported core/shell Co3O4@Ni3S2 nanowires for high-performance supercapacitors. Electrochim. Acta 2019, 311, 221–229. [Google Scholar] [CrossRef]
- Tronganh, N.; Gao, Y.; Jiang, W.; Tao, H.; Wang, S.; Zhao, B.; Jiang, Y.; Chen, Z.; Jiao, Z. Hierarchically assembled 3D nanoflowers and 0D nanoparticles of nickel sulfides on reduced graphene oxide with excellent lithium storage performances. Appl. Surf. Sci. 2018, 439, 386–393. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Q.; Peng, Z.; Guan, S.; Fu, X. Ni Foam-supported tin oxide nanowall array: An integrated supercapacitor anode. Molecules 2021, 26, 4517. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Jiang, M.; Gao, S.; Wang, Z.; Wang, H.; Boczkaj, G.; Liu, Z.; Ma, J.; Li, Z. 3D mesoporous α-Co(OH)2 nanosheets electrodeposited on nickel foam: A new generation of macroscopic cobalt-based hybrid for peroxymonosulfate activation. Chem. Eng. J. 2020, 380, 122447. [Google Scholar] [CrossRef]
- Yang, H.; Long, Y.; Zhu, Y.; Zhao, Z.; Ma, P.; Jin, J.; Ma, J. Crystal lattice distortion in ultrathin Co(OH)2nanosheets inducing elongated Co–OOH bonds for highly efficient oxygen evolution reaction. Green Chem. 2017, 19, 5809–5817. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, J.; Wu, J.; Xu, R.; Lai, M.; Gong, C.; Chen, X.; Zhou, P. Excellent electrochemical performance hierarchical Co3O4@Ni3S2 core/shell nanowire arrays for asymmetric supercapacitors. Electrochim. Acta 2016, 207, 87–96. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Li, J.; Wang, E. One-step synthesis of well-structured NiS-Ni2P2S6 nanosheets on nickel foam for efficient overall water splitting. J. Mater. Chem. A 2017, 5, 22131–22136. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, T.; Xi, S.; He, C.; Yang, X.; Wu, H. One-step synthesis of self-standing Ni3S2/Ni2P heteronanorods on nickel foam for efficient electrocatalytic hydrogen evolution over a wide pH range. Chem. Cat. Chem. 2018, 10, 5487–5495. [Google Scholar] [CrossRef]
- Jing, F.; Lv, Q.; Xiao, J.; Wang, Q.; Wang, S. Highly active and dual-function self-supported multiphase NiS–NiS2–Ni3S2/NF electrodes for overall water splitting. J. Mater. Chem. A 2018, 6, 14207–14214. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, K.; Xie, D.; Zhang, Y.; Zhang, Y.; Wang, Y.; Wu, J.; Wang, X.; Fan, H.J.; Xia, X.; et al. Correction to: High-index-faceted Ni3S2 branch arrays as bifunctional electrocatalysts for efficient water splitting. Nanomicro Lett. 2020, 13, 16. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, Y.; Zhang, Y.; Qin, Q. Electrochemical reaction of nanocrystalline Co3O4 thin film with Lithium. Solid State Ion. 2004, 170, 105–109. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, J.; Li, W.; Zuo, X.; Xiang, B.; Qiang, Y.; Zou, X.; Tan, B.; Hu, Q.; Chen, F. Mn3O4/Co(OH)2 cactus-type nanoarrays for high-energy-density asymmetric supercapacitors. J. Mater. Sci. 2019, 55, 724–737. [Google Scholar] [CrossRef]
- Petitto, S.C.; Marsh, E.M.; Carson, G.A.; Langell, M.A. Cobalt oxide surface chemistry: The interaction of CoO(100), Co3O4(110) and Co3O4(111) with oxygen and water. J. Mol. Catal. A Chem. 2008, 281, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Sayeed, M.A.; Herd, T.; O’Mullane, A.P. Direct electrochemical formation of nanostructured amorphous Co(OH)2 on gold electrodes with enhanced activity for the oxygen evolution reaction. J. Mater. Chem. A 2016, 4, 991–999. [Google Scholar] [CrossRef] [Green Version]
- Shackery, I.; Patil, U.; Pezeshki, A.; Shinde, N.M.; Im, S.; Jun, S.C. Enhanced Non-enzymatic amperometric sensing of glucose using Co(OH)2 nanorods deposited on a three dimensional graphene network as an electrode material. Microchim. Acta 2016, 183, 2473–2479. [Google Scholar] [CrossRef]
- Wang, J.; Xie, T.; Deng, Q.; Wang, Y.; Zhu, Q.; Liu, S. Three-dimensional interconnected Co(OH)2 nanosheets on Ti mesh as a highly sensitive electrochemical sensor for hydrazine detection. New J. Chem. 2019, 43, 3218–3225. [Google Scholar] [CrossRef]
- Feng, S.; Liu, C.; Chai, Z.; Li, Q.; Xu, D. Cobalt-based hydroxide nanoparticles @ N-doping carbonic frameworks core–shell structures as highly efficient bifunctional electrocatalysts for oxygen evolution and oxygen reduction reactions. Nano Res. 2018, 11, 1482–1489. [Google Scholar] [CrossRef]
- Li, H.B.; Yu, M.H.; Lu, X.H.; Liu, P.; Liang, Y.; Xiao, J.; Tong, Y.X.; Yang, G.W. Amorphous cobalt hydroxide with superior pseudocapacitive performance. ACS Appl. Mater Interfaces 2014, 6, 745–749. [Google Scholar] [CrossRef]
- Kandula, S.; Shrestha, K.R.; Kim, N.H.; Lee, J.H. Fabrication of a 3D hierarchical sandwich Co9S8 /alpha-MnS@N-C@MoS2 nanowire architectures as advanced electrode material for high performance hybrid supercapacitors. Small 2018, 14, e1800291. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, L.; Zhang, H.; Zan, P.; Gen, S.; Shi, W.; Han, B.; Sun, H.; Yang, X.; Xu, T. Serpent-cactus-like Co-doped Ni(OH)2/Ni3S2 hierarchical structure composed of ultrathin nanosheets for use in efficient asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 1603–1613. [Google Scholar] [CrossRef]
- Ali Akbari, M.S.; Bagheri, R.; Song, Z.; Najafpour, M.M. Oxygen-evolution reaction by nickel/nickel oxide interface in the presence of ferrate(VI). Sci Rep. 2020, 10, 8757. [Google Scholar] [CrossRef]
- Yadav, N.; Yadav, N.; Singh, M.K.; Hashmi, S.A. Nonaqueous, redox-active gel polymer electrolyte for high-performance supercapacitor. Energy Technol. 2019, 7, 1900132. [Google Scholar] [CrossRef]
- He, L.; Wang, Y.; Xu, Y.; Cai, W.; Zhu, M.; Wang, H. Facile synthesis of core-shell NiSe@α-Ni(OH)2 as battery-type electrode for high-performance hybrid supercapacitor. J. Alloys Compd. 2021, 876, 160164. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Lu, W. Synergistic effect: Hierarchical Ni3S2@Co(OH)2 heterostructure as efficient bifunctional electrocatalyst for overall water splitting. Appl. Surf. Sci. 2018, 457, 156–163. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Xin, L.; Wu, M.; Lou, X. Single-crystal β-NiS nanorod arrays with a hollow-structured Ni3S2 framework for supercapacitor applications. J. Mater. Chem. A 2016, 4, 7700–7709. [Google Scholar] [CrossRef]
- Yilmaz, G.; Lu, X. Direct growth of 3D hierarchical porous Ni3S2 nanostructures on nickel foam for high-Performance supercapacitors. ChemNanoMat 2016, 2, 719–725. [Google Scholar] [CrossRef]
- Lin, H.; Liu, F.; Wang, X.; Ai, Y.; Yao, Z.; Chu, L.; Han, S.; Zhuang, X. Graphene-coupled flower-like Ni3S2 for a free-standing 3D aerogel with an ultra-high electrochemical capacity. Electrochim. Acta 2016, 191, 705–715. [Google Scholar] [CrossRef]
- Zhuo, M.; Zhang, P.; Chen, Y.; Li, Q. Facile construction of graphene-like Ni3S2 nanosheets through the hydrothermally assisted sulfurization of nickel foam and their application as self-supported electrodes for supercapacitors. RSC Adv. 2015, 5, 25446–25449. [Google Scholar] [CrossRef]
- Yang, J.; Guo, W.; Li, D.; Wei, C.; Fan, H.; Wu, L.; Zheng, W. Synthesis and electrochemical performances of novel hierarchical flower-like nickel sulfide with tunable number of composed nanoplates. J. Power Sources 2014, 268, 113–120. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, X.; Su, R.; Hao, Z.; Jia, B.; Li, S.; Dong, L. Highly porous graphitic activated carbons from lignite via microwave pretreatment and iron-catalyzed graphitization at low-temperature for supercapacitor electrode materials. Processes 2019, 7, 300. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef]
- Zhuoqing, C.; Li, T.; Li, G.; Wang, K. One-pot in-situ synthesis of Ni(OH)2–NiFe2O4 nanosheet arrays on nickel foam as binder-free electrodes for supercapacitors. J. Mater. Sci. Mater. Electron. 2019, 30, 600–608. [Google Scholar] [CrossRef]
- Li, R.; Wang, S.; Huang, Z.; Lu, F.; He, T. NiCo2S4@Co(OH)2 core-shell nanotube arrays in situ grown on Ni foam for high performances asymmetric supercapacitors. J. Power Sources 2016, 312, 156–164. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, T.; Yang, X.; Zhang, L.; Leng, K.; Huang, Y.; Chen, Y. A high-performance supercapacitor-battery hybrid energy storage device based on graphene-enhanced electrode materials with ultrahigh energy density. Energy Environ. Sci. 2013, 6, 1623. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, M.; Li, X.; Gao, H. NiMoO4@Ni(OH)2 core/shell nanorods supported on Ni foam for high-performance supercapacitors. RSC Adv. 2015, 5, 69365–69370. [Google Scholar] [CrossRef]
- Tian, W.; Wang, X.; Zhi, C.; Zhai, T.; Liu, D.; Zhang, C.; Golberg, D.; Bando, Y. Ni(OH)2 nanosheet @ Fe2O3 nanowire hybrid composite arrays for high-performance supercapacitor electrodes. Nano Energy 2013, 2, 754–763. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Z.; Wang, G.; Lu, L.; Liu, S.; Gao, S.; Xu, H.; Yu, Z. One-pot synthesis of a CoS-AC electrode in a redox electrolyte for high-performance supercapacitors. J. Appl. Electrochem. 2019, 49, 1069–1077. [Google Scholar] [CrossRef]
- Cevik, E.; Bozkurt, A. Design of high-performance flexible symmetric supercapacitors energized by redox-mediated hydrogels including metal-doped acidic polyelectrolyte. Int. J. Energy Res. 2020, 44, 4309–4320. [Google Scholar] [CrossRef]
- Baasanjav, E.; Bandyopadhyay, P.; Cho, J.S.; Jeong, S.M. Hierarchical MCo2O4@Ni(OH)2 (M = Zn or Mn) core@shell architectures as electrode materials for asymmetric solid-state supercapacitors. J. Energy Storage. 2021, 44, 103345. [Google Scholar] [CrossRef]
- Li, J.-J.; Hu, Y.-X.; Liu, M.-C.; Kong, L.-B.; Hu, Y.-M.; Han, W.; Luo, Y.-C.; Kang, L. Mechanical alloying synthesis of Ni3S2 nanoparticles as electrode material for pseudocapacitor with excellent performances. J. Alloys Compd. 2016, 656, 138–145. [Google Scholar] [CrossRef]
- Li, R.; Wang, S.; Wang, J.; Huang, Z. Ni3S2@CoS core-shell nano-triangular pyramid arrays on Ni foam for high-performance supercapacitors. Phys. Chem. Chem. Phys. 2015, 17, 16434–16442. [Google Scholar] [CrossRef]
- Dai, C.S.; Chien, P.Y.; Lin, J.Y.; Chou, S.W.; Wu, W.K.; Li, P.H.; Wu, K.Y.; Lin, T.W. Hierarchically structured Ni3S2/carbon nanotube composites as high performance cathode materials for asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 12168–12174. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Sui, Y.; Wei, F.; Qi, J.; Meng, Q.; Ren, Y.; He, Y. Construction of layered C@MnNiCo–OH/Ni3S2 core–shell heterostructure with enhanced electrochemical performance for asymmetric supercapacitor. J. Mater. Sci.: Mater. Electron. 2021, 32, 11145–11157. [Google Scholar] [CrossRef]
- Long, L.; Yao, Y.; Yan, M.; Wang, H.; Zhang, G.; Kong, M.; Yang, L.; Liao, X.; Yin, G.; Huang, Z. Ni3S2@polypyrrole composite supported on nickel foam with improved rate capability and cycling durability for asymmetric supercapacitor device applications. J. Mater. Sci. 2016, 52, 3642–3656. [Google Scholar] [CrossRef]
- Ye, B.; Gong, C.; Huang, M.; Ge, J.; Fan, L.; Lin, J.; Wu, J. A high-performance asymmetric supercapacitor based on Ni3S2-coated NiSe arrays as positive electrode. New J. Chem. 2019, 43, 2389–2399. [Google Scholar] [CrossRef]
- Qian, H.; Wu, B.; Nie, Z.; Liu, T.; Liu, P.; He, H.; Wu, J.; Chen, Z.; Chen, S. A flexible Ni3S2/Ni@CC electrode for high-performance battery-like supercapacitor and efficient oxygen evolution reaction. Chem. Eng. J. 2021, 420, 127646. [Google Scholar] [CrossRef]

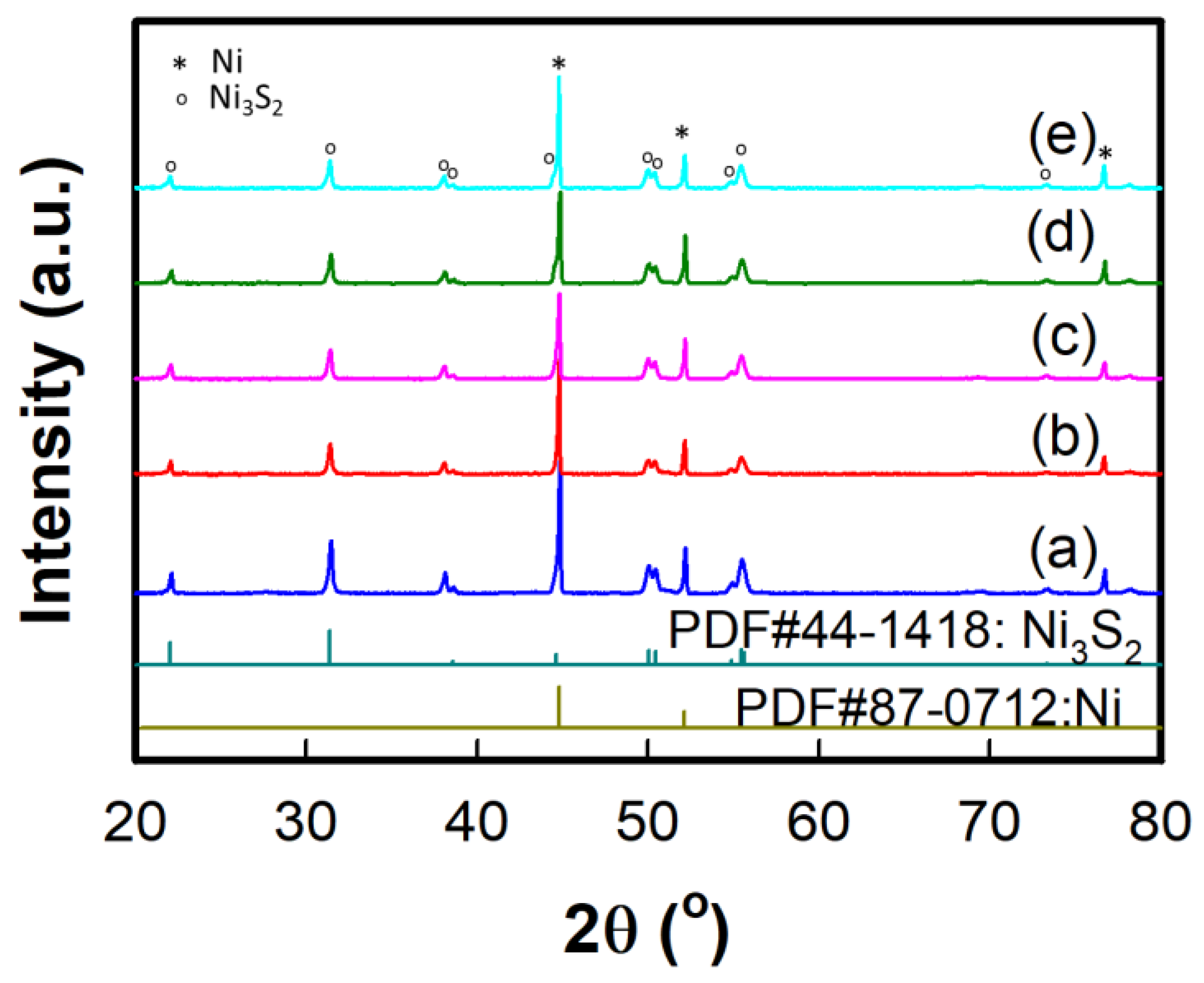
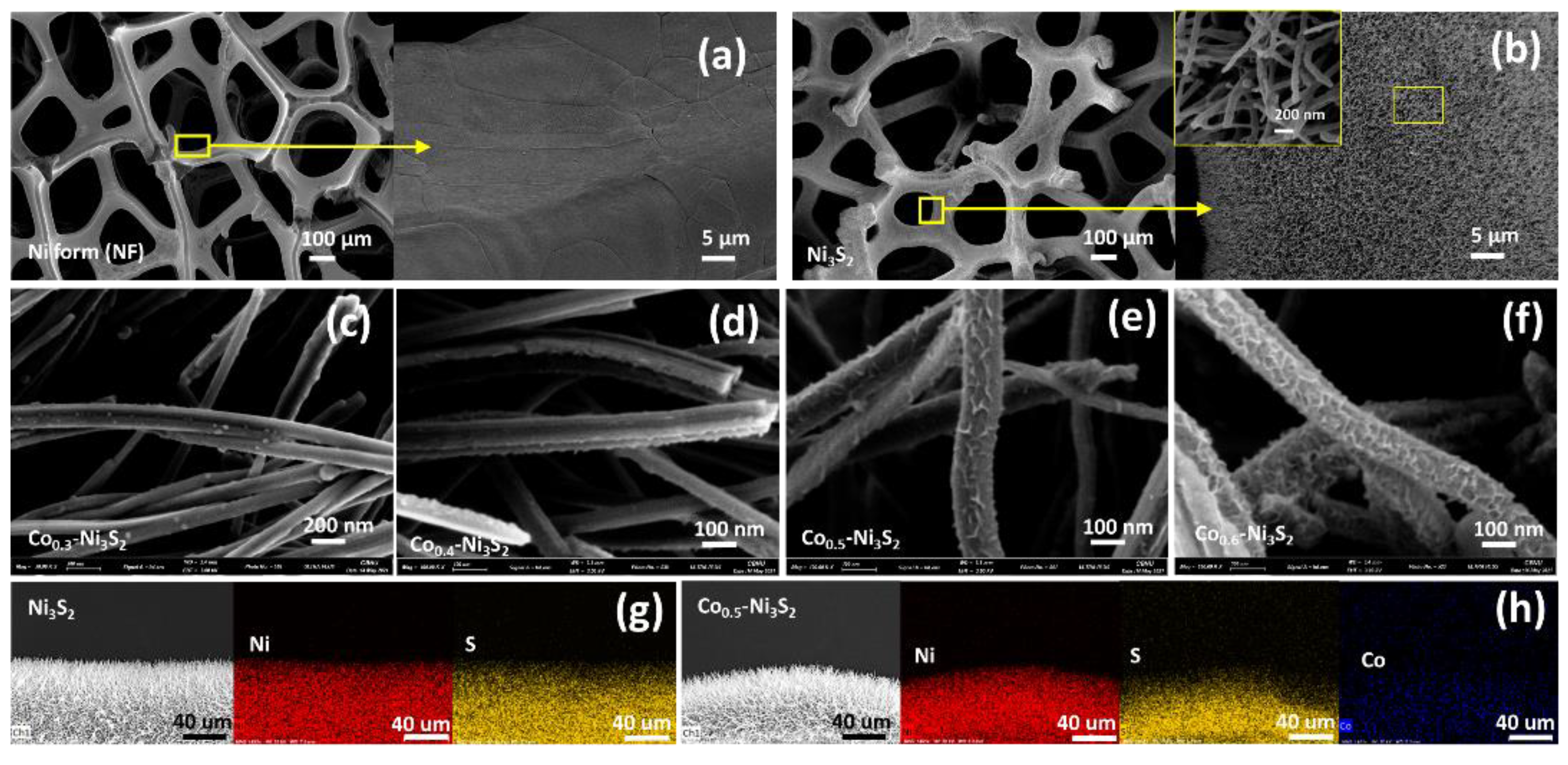
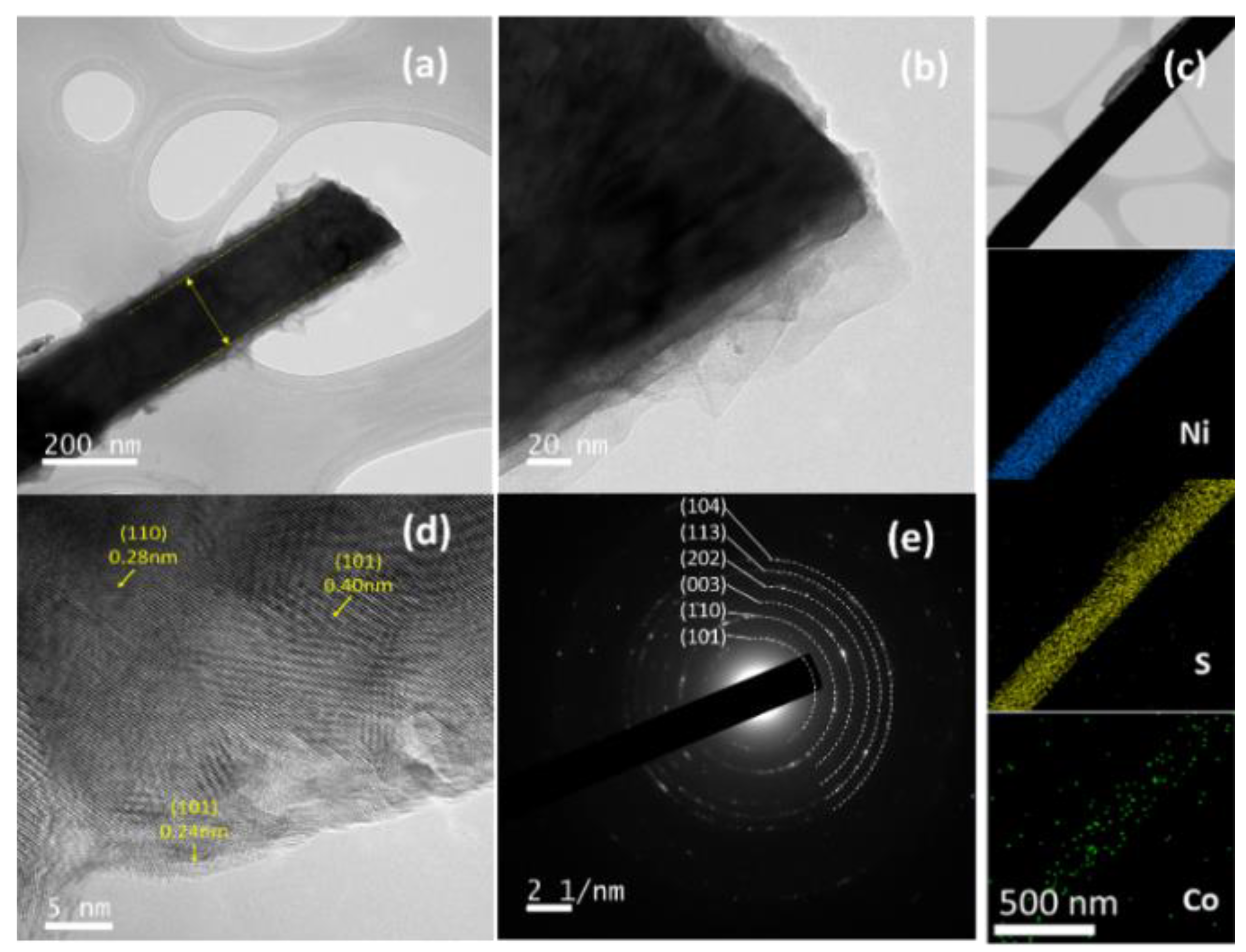
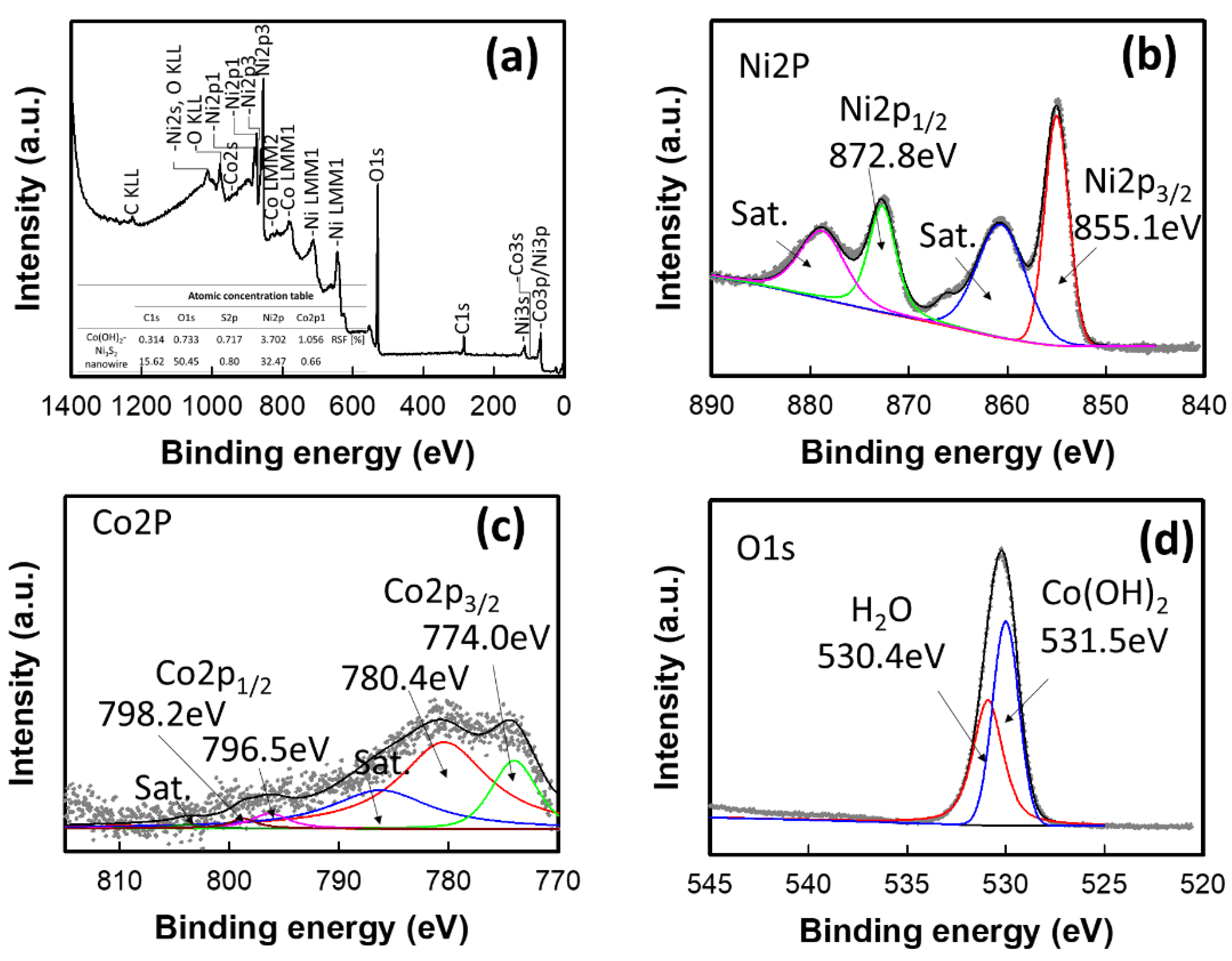

| Electrodes | Current Density | Specific or Areal Capacitance | Cycle Stability | Ref. |
|---|---|---|---|---|
| Ni3S2 | 15 A g−1 | 670 F g−1 | 97.4% (2000) | [50] |
| Ni3S2 nanosheet @ Ni3S2 nanorods | 20.6 A g−1 | 489 F g−1 | 89.3% (5000) | [51] |
| Ni3S2 nanoflake | 50 A cm−2 | 2.28 F cm−2 | 77% (5000) | [52] |
| Ni3S2 nanoporous | 1 mA cm−2 | 3.42 F cm−2 | 102% (4250) | [53] |
| Ni3S2 nanosheet | 15 mA cm−2 | 1.342 F cm−2 | 93.6% (3000) | [54] |
| Ni3S2 nest | 25 mA cm−2 | 682.9 F g−1 | 69% (1000) | [55] |
| Ni3S2 nanowire | 20 mA cm−2 | 530.3 F g−1 (6.05 F cm−2) | 83.7% (1000) | This study |
| Co(OH)2–Ni3S2 nanowire | 20 mA cm−2 | 656.2 F g−1 (8.17 F cm−2) | 95.4% (1000) |
| Electrodes | Energy Density (W h kg−1) | Power Density (W kg−1) | Ref. |
|---|---|---|---|
| rGO–Ni3S2//AC | 37.19 | 399.9 | [66] |
| Ni3S2//AC | 36 | 400 | [79] |
| Ni3S2@CoS//AC | 23.69 | 268.95 | [80] |
| Ni3S2//AC | 10.01 | 150.12 | [80] |
| Ni3S2/MWCNT–NC//AC | 19.8 | 798 | [81] |
| C@MnNiCo–OH/Ni3S2/NF//AC | 36,000 | 799.95 | [82] |
| Ni3S2@PPy//AC | 17.54 | 179.33 | [83] |
| Ni3S2//AC | 17.73 | 179.32 | [83] |
| NiSe/Ni3S2//AC | 38.7 | 192 | [84] |
| (Ni3S2/Ni@CC//AC/CC | 0.27 W h cm−2 | 4.90 W cm−2 | [85] |
| Co(OH)2–Ni3S2 nanowire | 60.3 | 1944.3 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.L.; Jin, E.M.; Chen, J.; Bandyopadhyay, P.; Jin, B.; Jeong, S.M. Facile In Situ Synthesis of Co(OH)2–Ni3S2 Nanowires on Ni Foam for Use in High-Energy-Density Supercapacitors. Nanomaterials 2022, 12, 34. https://doi.org/10.3390/nano12010034
Wang XL, Jin EM, Chen J, Bandyopadhyay P, Jin B, Jeong SM. Facile In Situ Synthesis of Co(OH)2–Ni3S2 Nanowires on Ni Foam for Use in High-Energy-Density Supercapacitors. Nanomaterials. 2022; 12(1):34. https://doi.org/10.3390/nano12010034
Chicago/Turabian StyleWang, Xuan Liang, En Mei Jin, Jiasheng Chen, Parthasarathi Bandyopadhyay, Bo Jin, and Sang Mun Jeong. 2022. "Facile In Situ Synthesis of Co(OH)2–Ni3S2 Nanowires on Ni Foam for Use in High-Energy-Density Supercapacitors" Nanomaterials 12, no. 1: 34. https://doi.org/10.3390/nano12010034
APA StyleWang, X. L., Jin, E. M., Chen, J., Bandyopadhyay, P., Jin, B., & Jeong, S. M. (2022). Facile In Situ Synthesis of Co(OH)2–Ni3S2 Nanowires on Ni Foam for Use in High-Energy-Density Supercapacitors. Nanomaterials, 12(1), 34. https://doi.org/10.3390/nano12010034









