Biomass-Derived Porous Carbon from Agar as an Anode Material for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
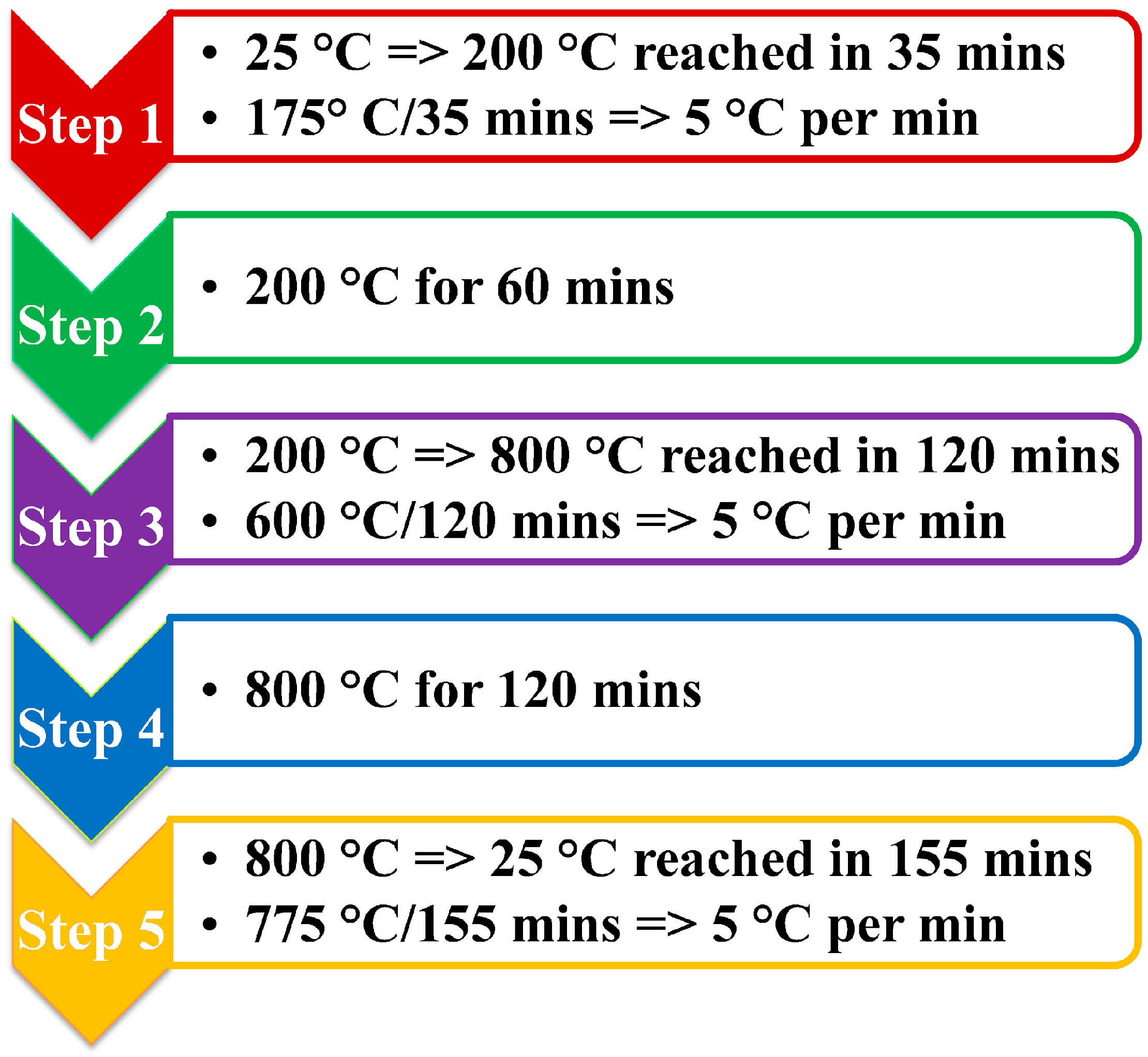
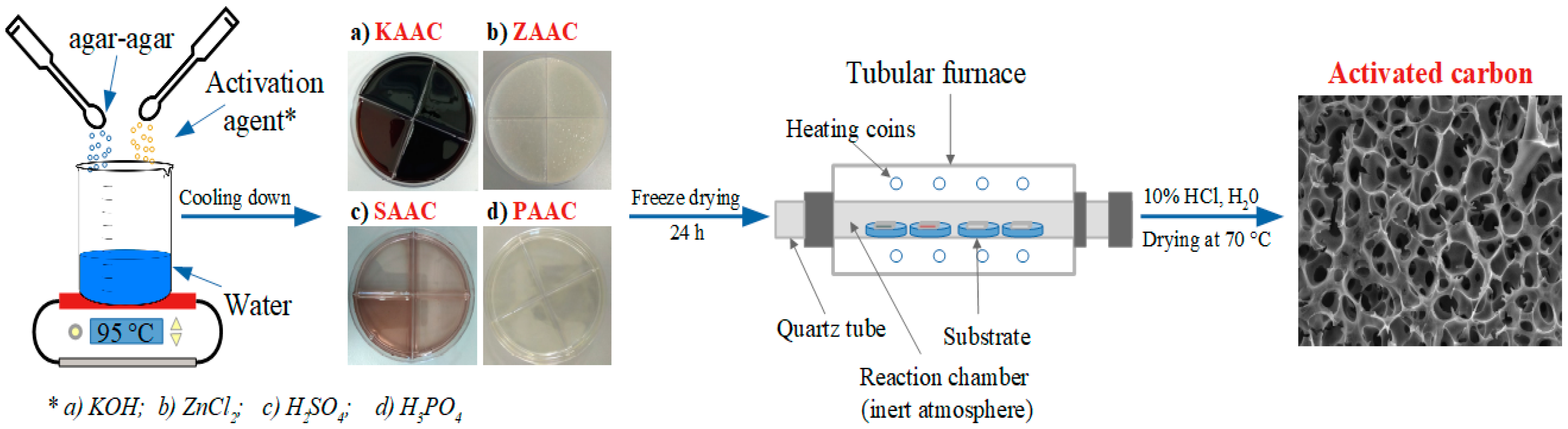
2.1. Fabrication of the Activated Carbon
2.2. Characterizations
2.3. Cell Preparation
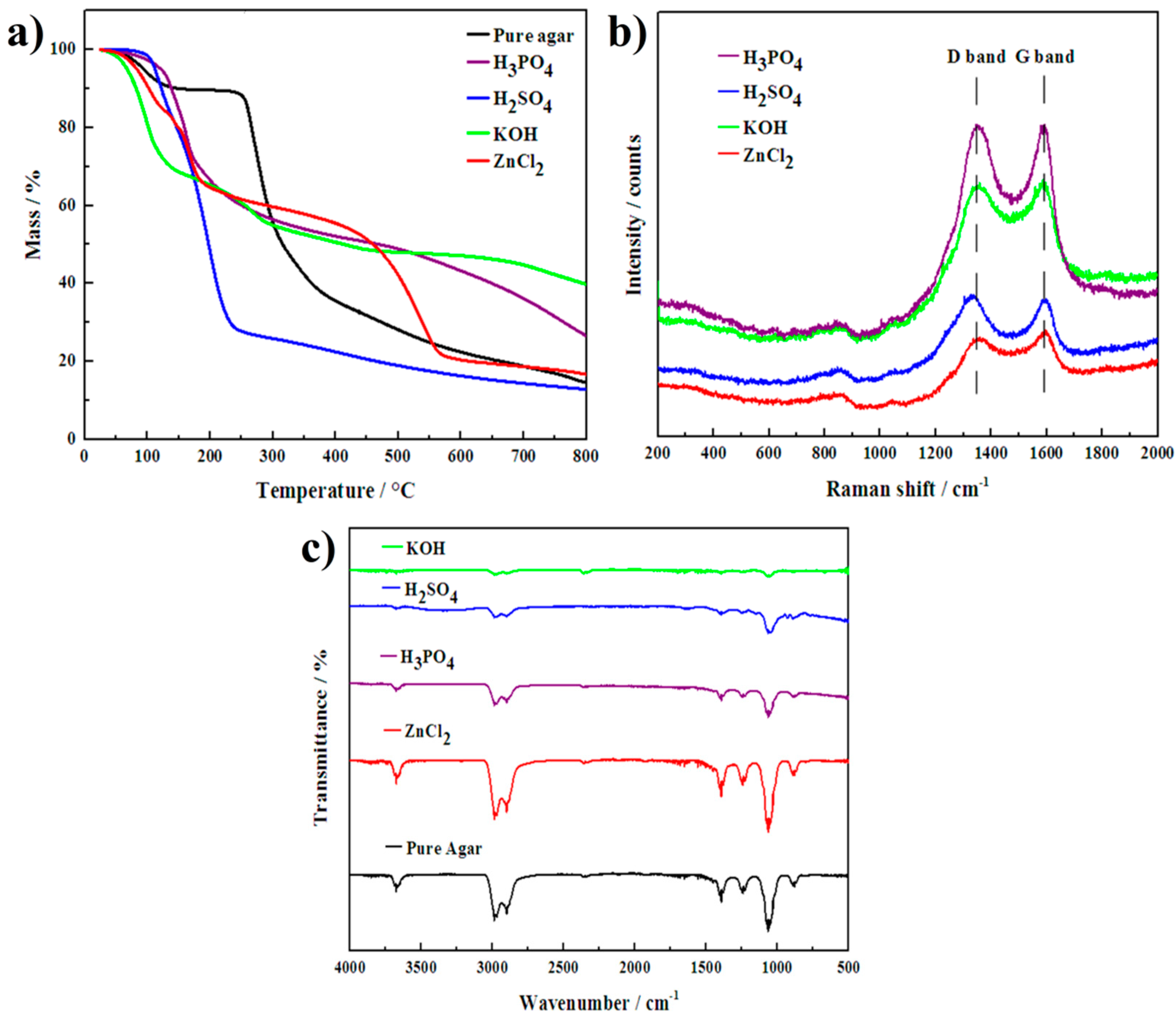
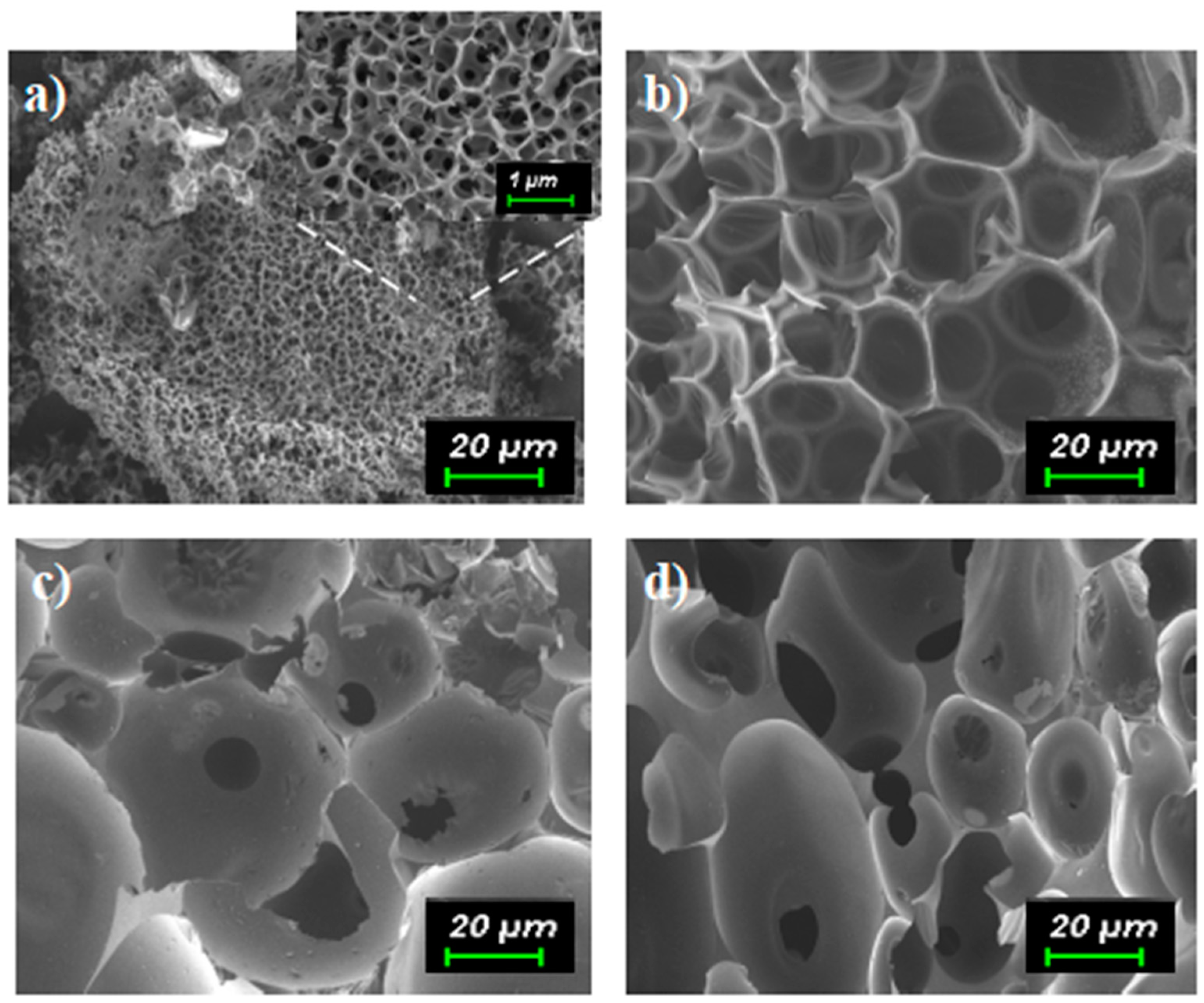
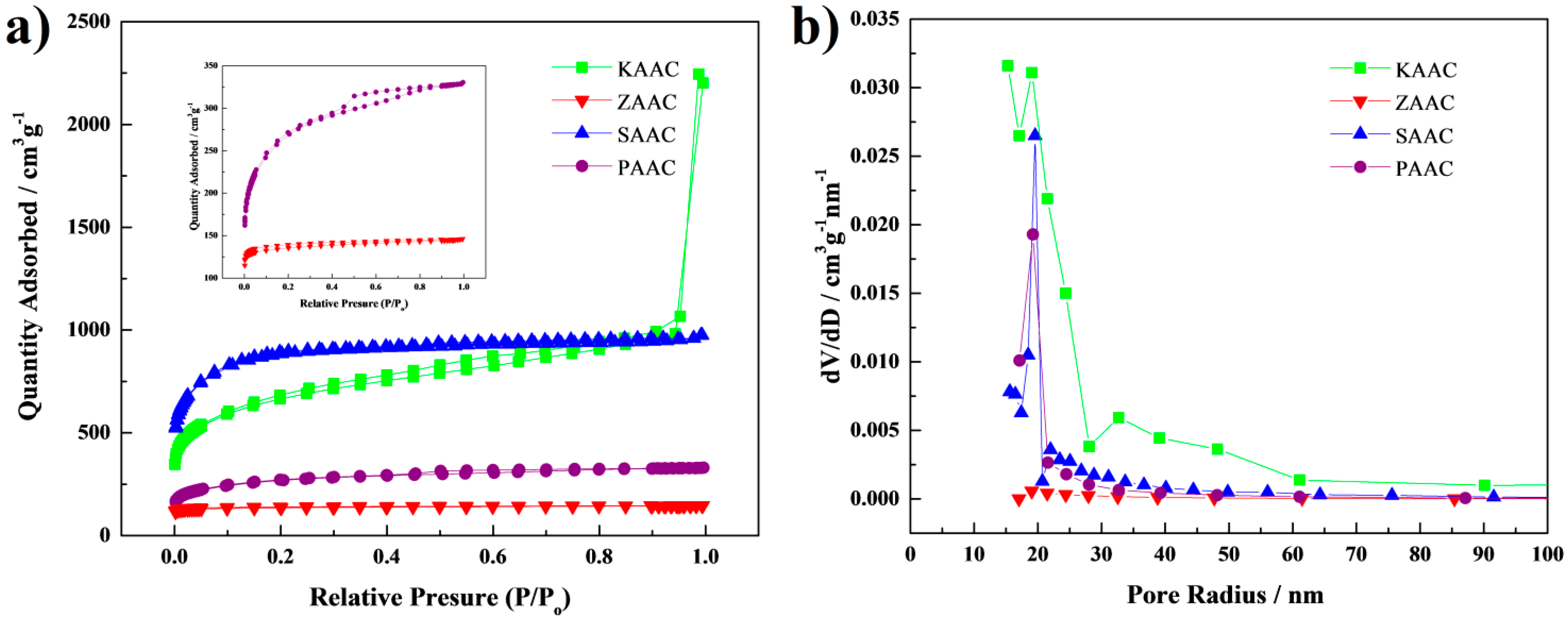
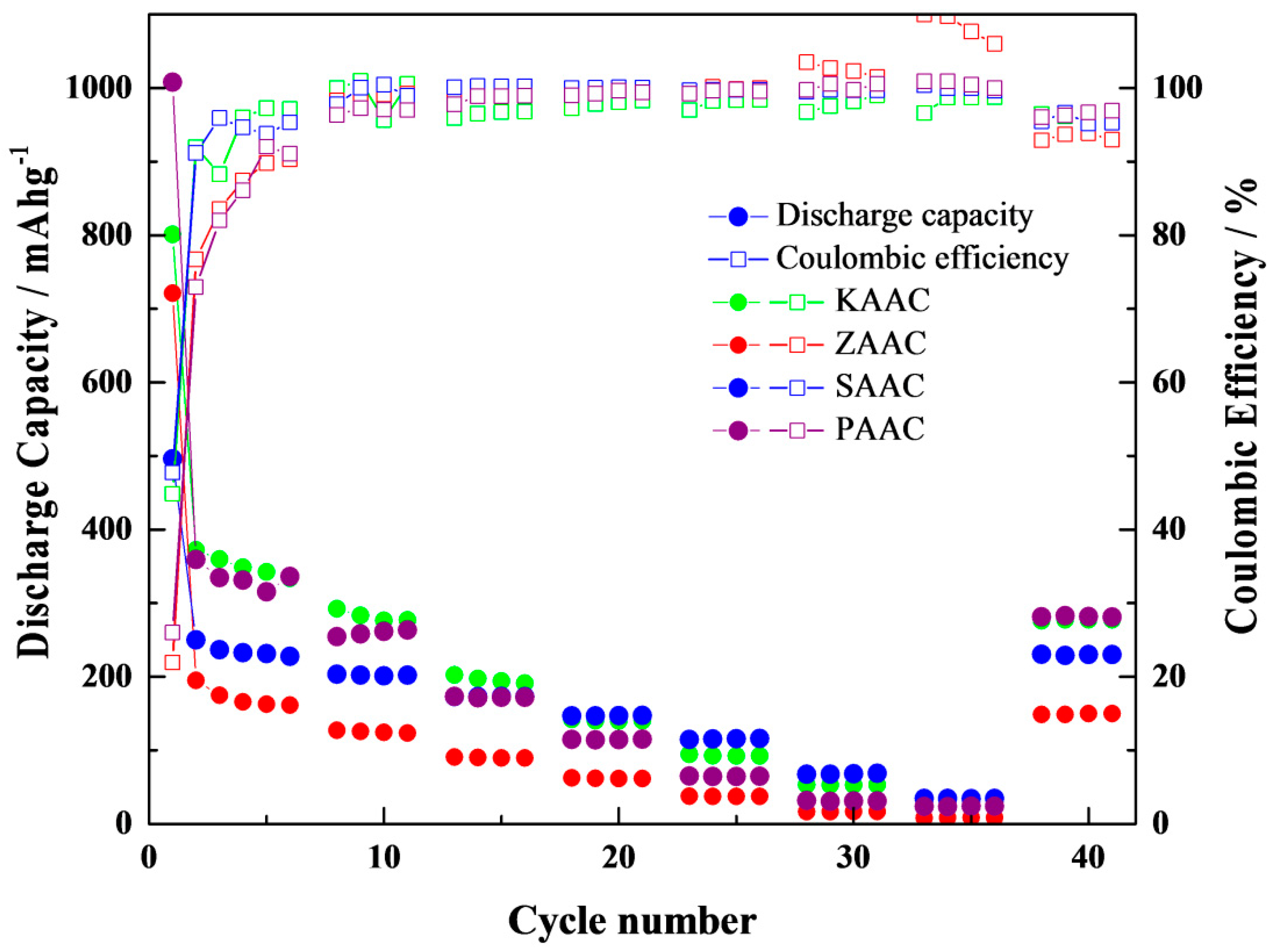
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kang, K.; Meng, Y.S.; Breger, J.; Grey, C.P.; Ceder, G. Electrodes with High Power and High Capacity for Rechargeable Lithium Batteries. Science 2006, 311, 977–980. [Google Scholar] [CrossRef]
- Lecce, D.D.; Verrelli, R.; Hassoun, J. Lithium-ion batteries for sustainable energy storage: Recent advances towards new cell configurations. Green Chem. 2017, 19, 3442–3467. [Google Scholar] [CrossRef]
- Hu, M.; Pang, X.; Zhou, Z. Recent progress in high-voltage lithium ion batteries. J. Power Sources 2013, 237, 229–242. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Z.; Wang, R.; Wu, Z.; Liang, H.; Shao, L.; Shu, J.; Wang, Z. High performance Na-doped lithium zinc titanate as anode material for Li-ion batteries. RSC Adv. 2015, 5, 49890–49898. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, K.; Tian, N.; Qin, A.; Liao, L.; Du, R.; Wei, C. Biomass carbon derived from sisal fiber as anode material for lithium-ion batteries. Mater. Lett. 2015, 142, 193–196. [Google Scholar] [CrossRef]
- Scrosati, B.; Hassoun, J.; Sun, Y.K. Lithium-ion batteries. A look into the future. Energy Environ. Sci. 2011, 4, 3287–3295. [Google Scholar] [CrossRef]
- Yu, K.; Wang, J.; Wang, X.; Liang, J.; Liang, C. Sustainable application of biomass by-products: Corn straw-derived porous carbon nanospheres using as anode materials for lithium ion batteries. Mater. Chem. Phys. 2020, 243, 122644. [Google Scholar] [CrossRef]
- Kamali, A.R.; Fray, D.J. Tin-based materials as advanced anode materials for lithium ion batteries: A review. Rev. Adv. Mater. Sci. 2011, 27, 14–24. [Google Scholar]
- Nurpeissova, A.; Adi, A.; Aishova, A.; Mukanova, A.; Kim, S.S.; Bakenov, Z. Synergistic effect of 3D current collector structure and Ni inactive matrix on the electrochemical performances of Sn-based anodes for lithium-ion batteries. Mater. Today Energy 2020, 16, 100397. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, H.; Zhang, H.; Yang, J.; Hartono, S.B.; Qian, K.; Zou, J.; Yu, C. Cheap and scalable synthesis of α-Fe2O3 multi-shelled hollow spheres as high-performance anode materials for lithium ion batteries. Chem. Commun. 2013, 49, 8695–8697. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Li, X.; Yan, B.; Xiong, D.; Li, D.; Lawes, S.; Sun, X.L. Recent Developments and Understanding of Novel Mixed Transition-Metal Oxides as Anodes in Lithium Ion Batteries. Adv. Energy Mater. 2016, 6, 1502175. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, S.; Lu, Y. 3D Porous Cu Current Collector/Li-Metal Composite Anode for Stable Lithium-Metal Batteries. Adv. Funct. Mater. 2017, 27, 1606422. [Google Scholar] [CrossRef]
- Liu, S.; Hou, H.; Liu, X.; Hu, W.; Yan, C.; Duan, J.; Meng, R. High-performance hierarchical homologous scale-like CuCl/Cu foam anode for lithium ion battery. Ceram. Int. 2016, 42, 8310–8315. [Google Scholar] [CrossRef]
- Nurpeissova, A.; Mukanova, A.; Kalimuldina, G.; Umirov, N. Onion-Structured Si Anode Constructed with Coating by Li4Ti5O12 and Cyclized-Polyacrylonitrile for Lithium-Ion Batteries. Nanomaterials 2020, 10, 1995. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Lee, H.R.; Lee, Y.K.; Lee, G.B.; Lee, S.; Kim, H.J.; Joh, H.I. Stable fast-charging electrodes derived from hierarchical porous carbon for lithium-ion batteries. Int. J. Energy Res. 2021, 45, 4718–4726. [Google Scholar] [CrossRef]
- Gao, F.; Geng, C.; Xiao, N.; Qu, J.; Qiu, J. Hierarchical porous carbon sheets derived from biomass containing an activation agent and in-built template for lithium ion batteries. Carbon 2018, 139, 1085–1092. [Google Scholar] [CrossRef]
- Titirici, M.M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; Monte, F.D.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Huang, J.; Li, J.; Cao, L.; Zhong, X.; Yu, A.; Lu, G. Nitrogen-doped porous hard carbons derived from shaddock peel for high-capacity lithium-ion battery anodes. J. Electroanal. Chem. 2020, 862, 114044. [Google Scholar] [CrossRef]
- Alam, M.M.; Hossain, M.A.; Hossain, M.D.; Johir, M.A.H.; Hossen, J.; Rahman, M.S.; Zhou, J.L.; Hasan, A.T.M.; Karmakar, A.K.; Ahmed, M.B. The potentiality of rice husk-derived activated carbon: From synthesis to application. Processes 2020, 8, 203. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Chen, F.; Bai, T.; Long, B.; Liao, Q.; Ren, Y.; Yang, J. Interconnected highly graphitic carbon nanosheets derived from wheat stalk as high performance anode materials for lithium ion batteries. Green Chem. 2016, 18, 2078–2088. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Graphitic carbon nanostructures from cellulose. Chem. Phys. Lett. 2010, 490, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Sekar, S.; Lee, Y.; Kim, D.Y.; Lee, S. Substantial LIB anode performance of graphitic carbon nanoflakes derived from biomass green-tea waste. Nanomaterials 2019, 9, 871. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Yi, H.; Zhu, C.; Wang, X.; Fan, H.J. Functionalized highly porous graphitic carbon fibers for high-rate supercapacitive electrodes. Nano Energy 2015, 13, 658–669. [Google Scholar] [CrossRef]
- Zhu, L.; Jiang, H.; Yang, Q.; Yao, S.; Shen, X.; Tu, F. An effective porous activated carbon derived from puffed corn employed as the separator coating in a lithium–sulfur battery. Energy Technol. 2019, 7, 1900752. [Google Scholar] [CrossRef]
- Yang, J.; Zuo, S. Facile synthesis of graphitic mesoporous carbon materials from sucrose. Diam. Relat. Mater. 2019, 95, 1–4. [Google Scholar] [CrossRef]
- Zaini, M.A.A.; Zhi, L.L.; Hui, T.S.; Amano, Y.; Machida, M. Effects of physical activation on pore textures and heavy metals removal of fiber-based activated carbons. Mater. Today Proc. 2020, 39, 917–921. [Google Scholar] [CrossRef]
- Nahil, M.A.; Williams, P.T. Pore characteristics of activated carbons from the phosphoric acid chemical activation of cotton stalks. Biomass Bioenergy 2012, 37, 142–149. [Google Scholar] [CrossRef]
- Wang, A.; Sun, K.; Xu, R.; Sun, Y.; Jiang, J. Cleanly synthesizing rotten potato-based activated carbon for supercapacitor by self-catalytic activation. J. Clean. Prod. 2021, 283, 125385. [Google Scholar] [CrossRef]
- Ao, W.; Fu, J.; Mao, X.; Kang, Q.; Ran, C.; Liu, Y.; Zhang, H.; Gao, Z.; Li, J.; Liu, G.; et al. Microwave assisted preparation of activated carbon from biomass: A review. Renew. Sustain. Energy Rev. 2018, 92, 958–979. [Google Scholar] [CrossRef]
- Kong, L.; Peng, H.J.; Huang, J.Q.; Zhang, Q. Review of nanostructured current collectors in lithium–sulfur batteries. Nano Res. 2017, 10, 4027–4054. [Google Scholar] [CrossRef]
- Dou, Y.; Liu, X.; Wang, X.; Yu, K.; Liang, C. Jute fiber based micro-mesoporous carbon: A biomass derived anode material with high-performance for lithium-ion batteries. Mater. Sci. Eng. B 2021, 265, 115015. [Google Scholar] [CrossRef]
- Sankar, S.; Saravanan, S.; Ahmed, A.T.A.; Inamdar, A.I.; Im, H.; Lee, S.; Kim, D.Y. Spherical activated-carbon nanoparticles derived from biomass green tea wastes for anode material of lithium-ion battery. Mater. Lett. 2019, 240, 189–192. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Dai, Y.Q.; Li, G.C.; Li, X.H.; Guo, H.J.; Wang, Z.X.; Yan, G.C.; Wang, J.X. Ultrathin porous graphitic carbon nanosheets activated by alkali metal salts for high power density lithium-ion capacitors. Rare Met. 2020, 39, 1364–1373. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, H.; Sun, H.; Cao, F.; Chen, Y.; Chen, G.Z. Molecular level one-step activation of agar to activated carbon for high performance supercapacitors. Carbon 2018, 132, 573–579. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, J.; Zhang, L.; Li, Y.; Chen, M.; Chen, Y.; Shen, Z. Activated carbon by one-step calcination of deoxygenated agar for high voltage lithium ion supercapacitor. ACS Sustain. Chem. Eng. 2020, 8, 3637–3643. [Google Scholar] [CrossRef]
- Han, S.W.; Jung, D.W.; Jeong, J.H.; Oh, E.S. Effect of pyrolysis temperature on carbon obtained from green tea biomass for superior lithium ion battery anodes. Chem. Eng. J. 2014, 254, 597–604. [Google Scholar] [CrossRef]
- Yang, Z.; Gleisner, R.; Mann, D.H.; Xu, J.; Jiang, J.; Zhu, J.Y. Lignin Based Activated Carbon Using H3PO4 Activation. Polymers 2020, 12, 2829. [Google Scholar] [CrossRef]
- Laksaci, H.; Khelifi, A.; Trari, M.; Addoun, A. Synthesis and characterization of microporous activated carbon from coffee grounds using potassium hydroxides. J. Clean. Prod. 2017, 147, 254–262. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Liu, S. Hydrothermal synthesis, characterization, and KOH activation of carbon spheres from glucose. Carbohydr. Res. 2011, 346, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.L.; Seo, J.S.; Lee, H.Y.; Lee, J.W. Activated carbon with hierarchical micro–mesoporous structure obtained from rice husk and its application for lithium–sulfur batteries. RSC Adv. 2017, 7, 4144–4151. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.W.L.; Yilmaz, G.; Ong, W.L.; Ho, G.W. One-step activation towards spontaneous etching of hollow and hierarchical porous carbon nanospheres for enhanced pollutant adsorption and energy storage. Appl. Catal. B Environ. 2018, 220, 533–541. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Feng, N.; Qiao, L.; Li, X.; He, D. A new carbonaceous material derived from biomass source peels as an improved anode for lithium ion batteries. J. Anal. Appl. Pyrolysis 2013, 100, 181–185. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, J.; Ai, W.; Fan, Z.; Shen, X.; Zou, C.; Liu, J.; Zhang, H.; Yu, T. Evolution of disposable bamboo chopsticks into uniform carbon fibers: A smart strategy to fabricate sustainable anodes for Li-ion batteries. Energy Environ. Sci. 2014, 7, 2670–2679. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Wang, G.; Xu, L.; Lian, J.; Bao, J.; Zhao, Y.; Qiu, J.; Li, H. Defect-rich N-doped porous carbon derived from soybean for high rate lithium-ion batteries. Appl. Surf. Sci. 2018, 451, 298–305. [Google Scholar] [CrossRef]
- Ou, J.; Yang, L.; Zhang, Z.; Xi, X. Nitrogen-doped porous carbon derived from horn as an advanced anode material for sodium ion batteries. Microporous Mesoporous Mater. 2017, 237, 23–30. [Google Scholar] [CrossRef]
- Hernández-Rentero, C.; Marangon, V.; Olivares-Marín, M.; Gómez-Serrano, V.; Caballero, Á.; Morales, J.; Hassoun, J. Alternative lithium-ion battery using biomass-derived carbons as environmentally sustainable anode. J. Colloid Interface Sci. 2020, 573, 396–408. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Meng, Y.; Xie, J.; Guo, Y.; Xiao, D. Lithium and sodium storage in highly ordered mesoporous nitrogen-doped carbons derived from honey. J. Power Sources 2016, 335, 20–30. [Google Scholar] [CrossRef]
- Hong, K.L.; Qie, L.; Zeng, R.; Yi, Z.Q.; Zhang, W.; Wang, D.; Yin, W.; Wu, C.; Fan, Q.J.; Zhang, W.X.; et al. Biomass derived hard carbon used as a high performance anode material for sodium ion batteries. J. Mater. Chem. A 2014, 2, 12733–12738. [Google Scholar] [CrossRef]
- Byon, H.R.; Gallant, B.M.; Lee, S.W.; Shao-Horn, Y. Role of oxygen functional groups in carbon nanotube/graphene freestanding electrodes for high performance lithium batteries. Adv. Funct. Mater. 2013, 23, 1037–1045. [Google Scholar] [CrossRef]

| AC Precursor | Activating Agent | Measurement Conditions | Initial Capacity (mAh g−1) | Reversible Capacity (mAh g−1) | References |
|---|---|---|---|---|---|
| Agar | KOH | 0.1 C | 931 | 320 at 0.1 C after 100 cycles | This work |
| Sisal fiber | KOH | 0.1 C | 646 | Capacity loss of 363 compared to initial capacity | [5] |
| Corn straw | CaCl2 | 0.2 C | 1534 | 546 at 0.2 after 100 cycles | [7] |
| Shaddock peel | KOH | 50 mAg−1 | 1284 | 673 at t 50 mAg−1 after 100 cycles | [19] |
| Wheat stalk | KOH | 0.1 C | 502 | 139.6 at 10 C after 3000 cycles | [21] |
| Green tea wastes | KOH | 0.1 A/g | 706 | 400 at 0.1 A/g after 100 cycles | [23] |
| Jute fiber | ZnCl2 | 0.2 C | 2117 | 742.7 at 0.2 C after 100 cycles | [32] |
| Pomelo peels | - | 90 mAg−1 | 756 | 452 at 90 mAg−1 after 200 cycles | [44] |
| Bamboo chopsticks | KOH | ∼0.37 C | ∼500 | ∼360 at ∼0.37 C after 100 cycles | [45] |
| Cherry pit | KOH | C/3 | - | 210 at C/3 after 200 cycles | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Issatayev, N.; Kalimuldina, G.; Nurpeissova, A.; Bakenov, Z. Biomass-Derived Porous Carbon from Agar as an Anode Material for Lithium-Ion Batteries. Nanomaterials 2022, 12, 22. https://doi.org/10.3390/nano12010022
Issatayev N, Kalimuldina G, Nurpeissova A, Bakenov Z. Biomass-Derived Porous Carbon from Agar as an Anode Material for Lithium-Ion Batteries. Nanomaterials. 2022; 12(1):22. https://doi.org/10.3390/nano12010022
Chicago/Turabian StyleIssatayev, Nurbolat, Gulnur Kalimuldina, Arailym Nurpeissova, and Zhumabay Bakenov. 2022. "Biomass-Derived Porous Carbon from Agar as an Anode Material for Lithium-Ion Batteries" Nanomaterials 12, no. 1: 22. https://doi.org/10.3390/nano12010022
APA StyleIssatayev, N., Kalimuldina, G., Nurpeissova, A., & Bakenov, Z. (2022). Biomass-Derived Porous Carbon from Agar as an Anode Material for Lithium-Ion Batteries. Nanomaterials, 12(1), 22. https://doi.org/10.3390/nano12010022







