g-C3N4: Properties, Pore Modifications, and Photocatalytic Applications
Abstract
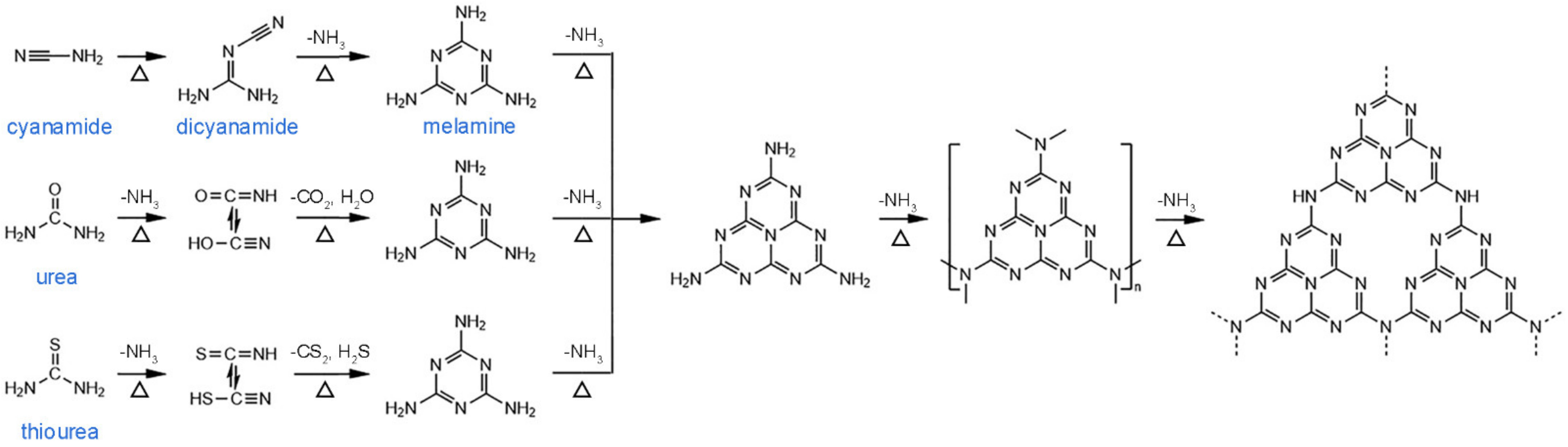
:1. Introduction
2. Pore Structure/Properties Relationship
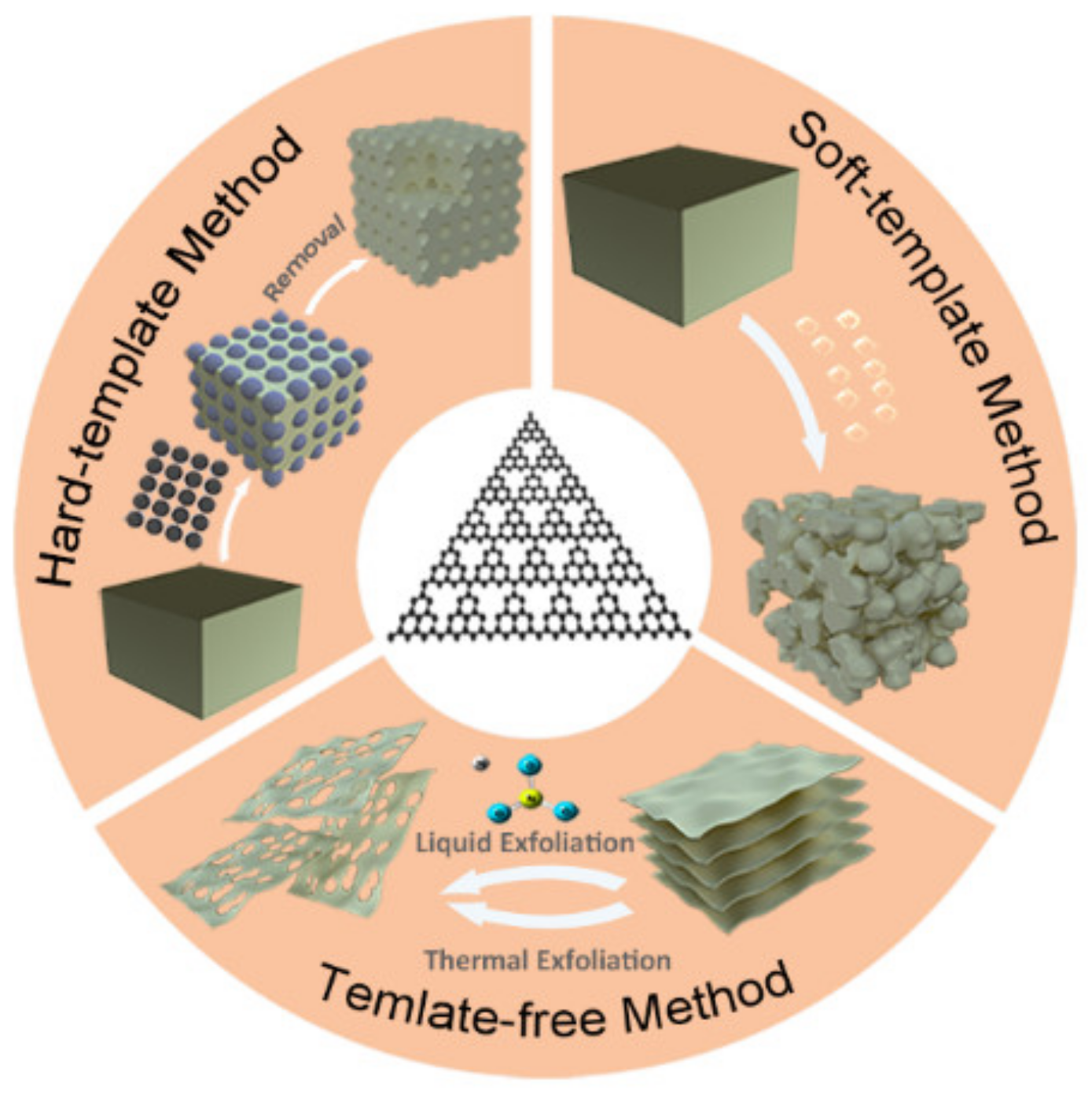
3. Pore Modifications over g-C3N4
3.1. Hard-Template Method
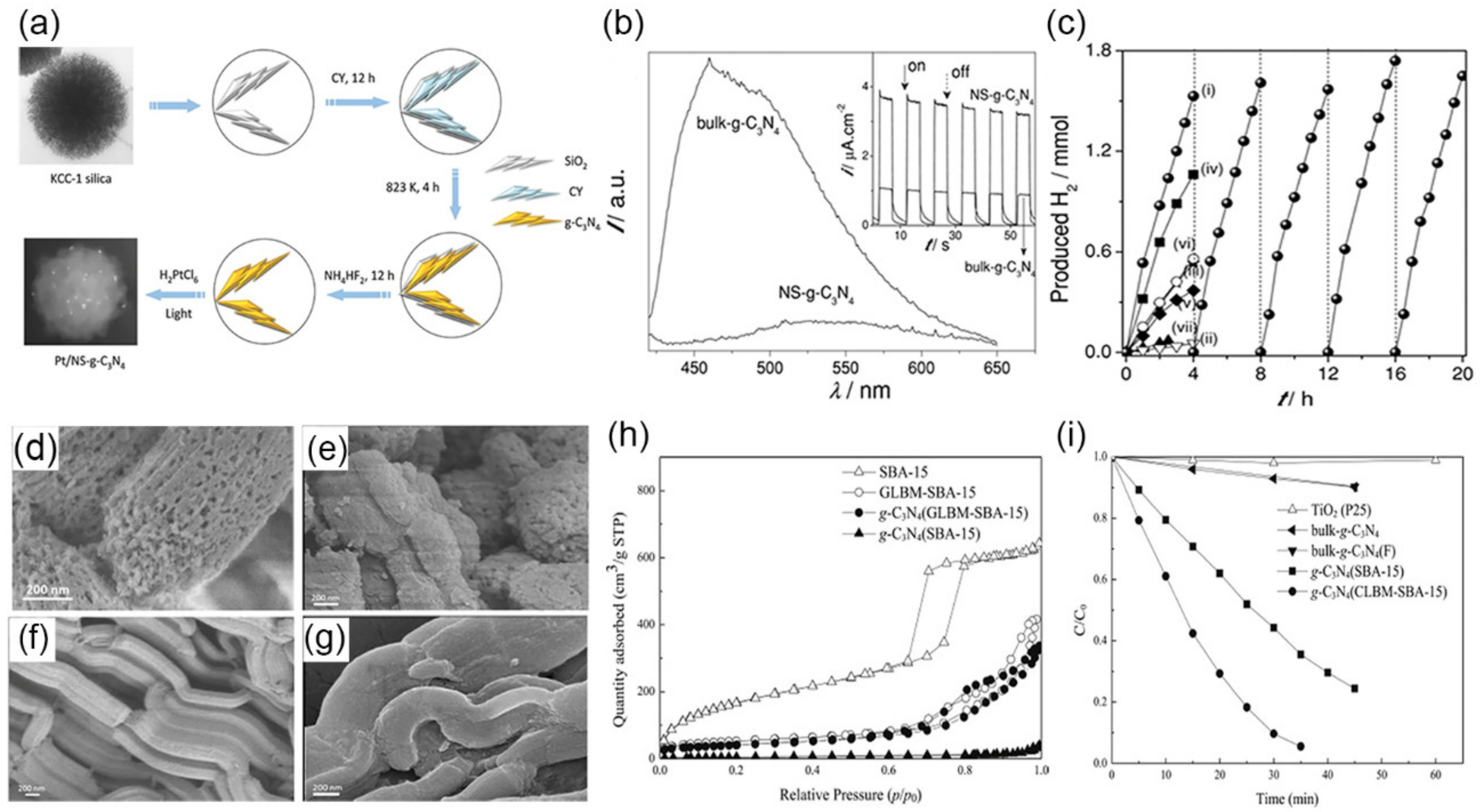
3.1.1. SiO2 as Template
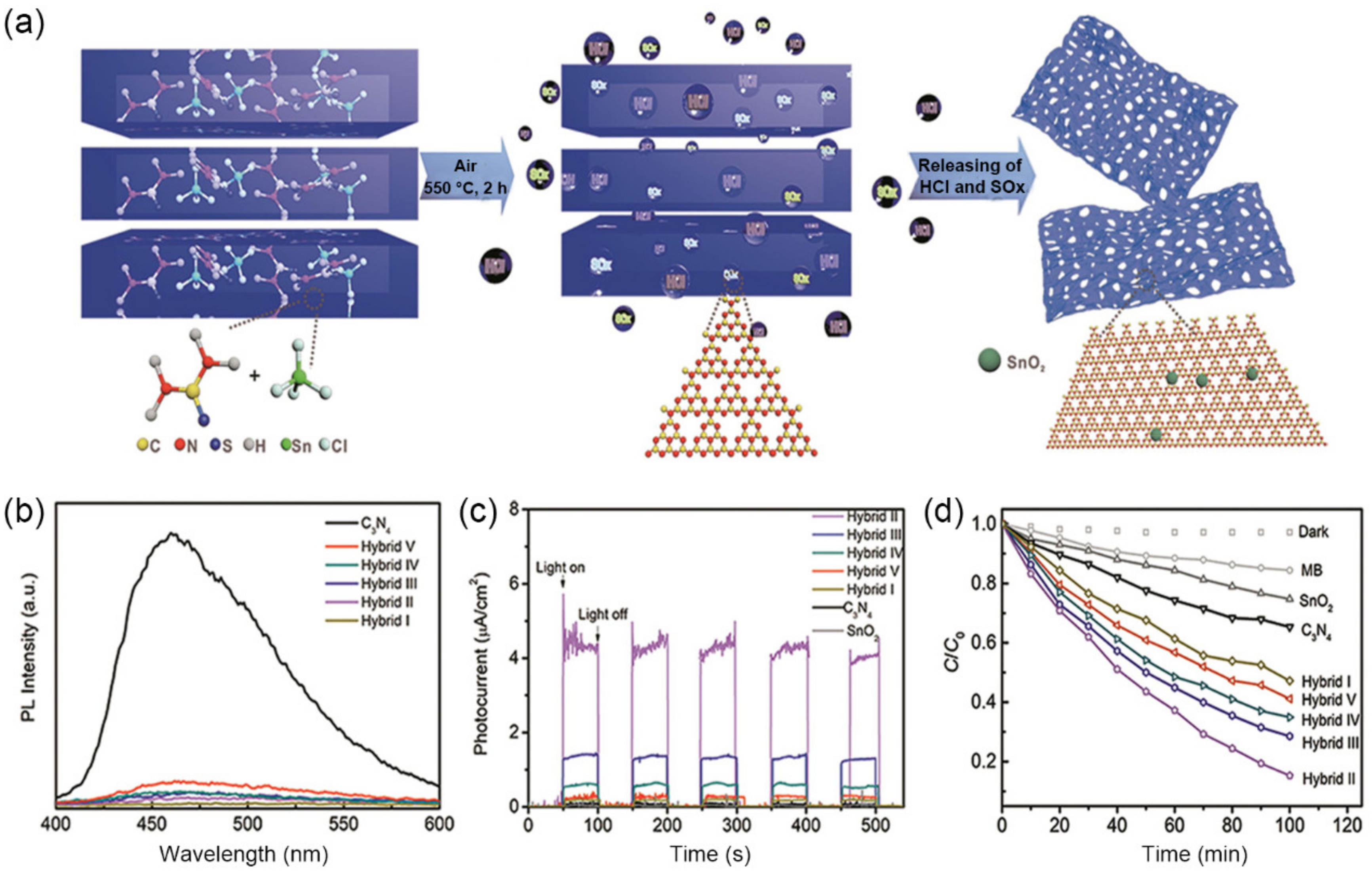
3.1.2. SiO2 Derivatives as Templates
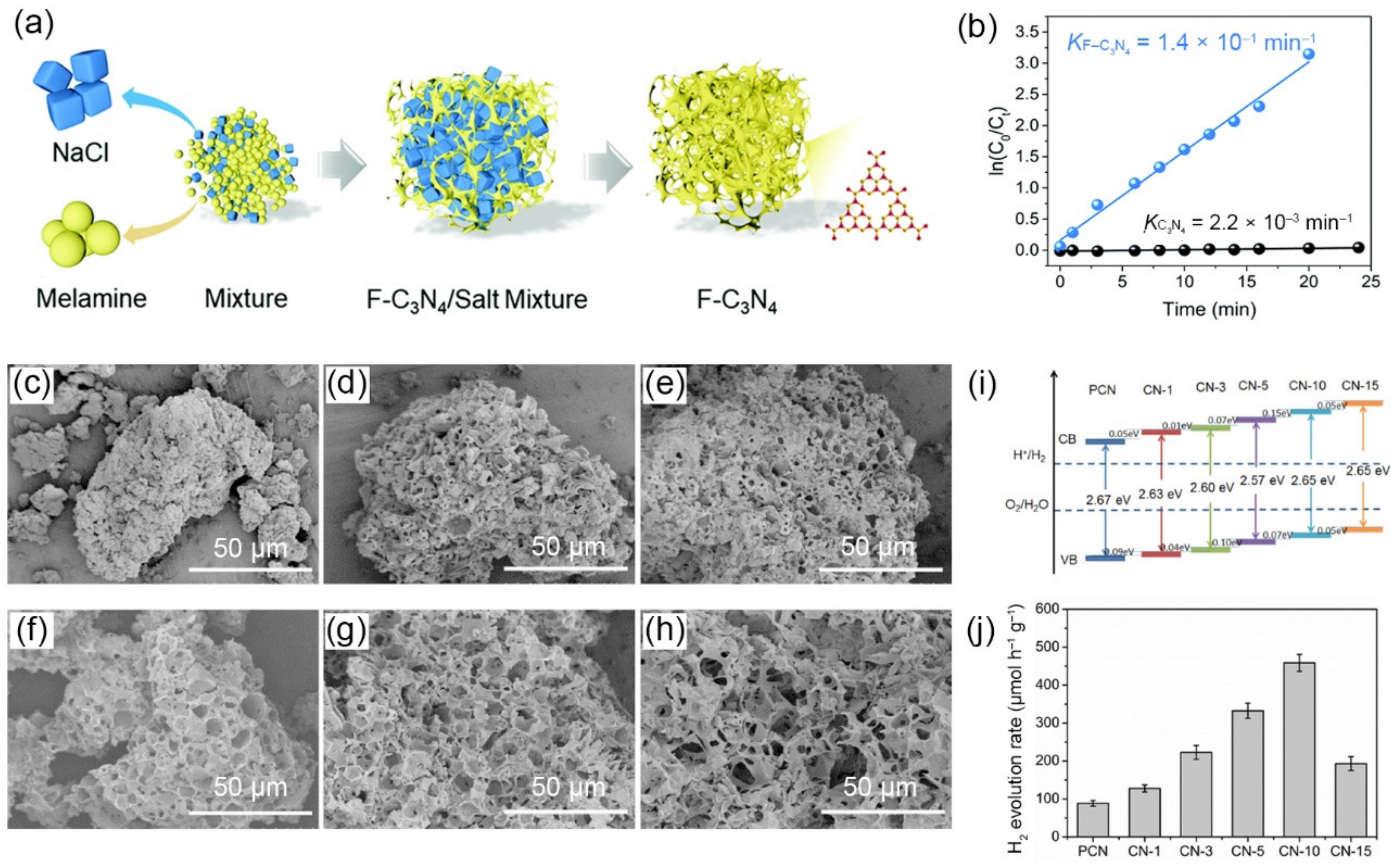
3.1.3. Salt as Templates
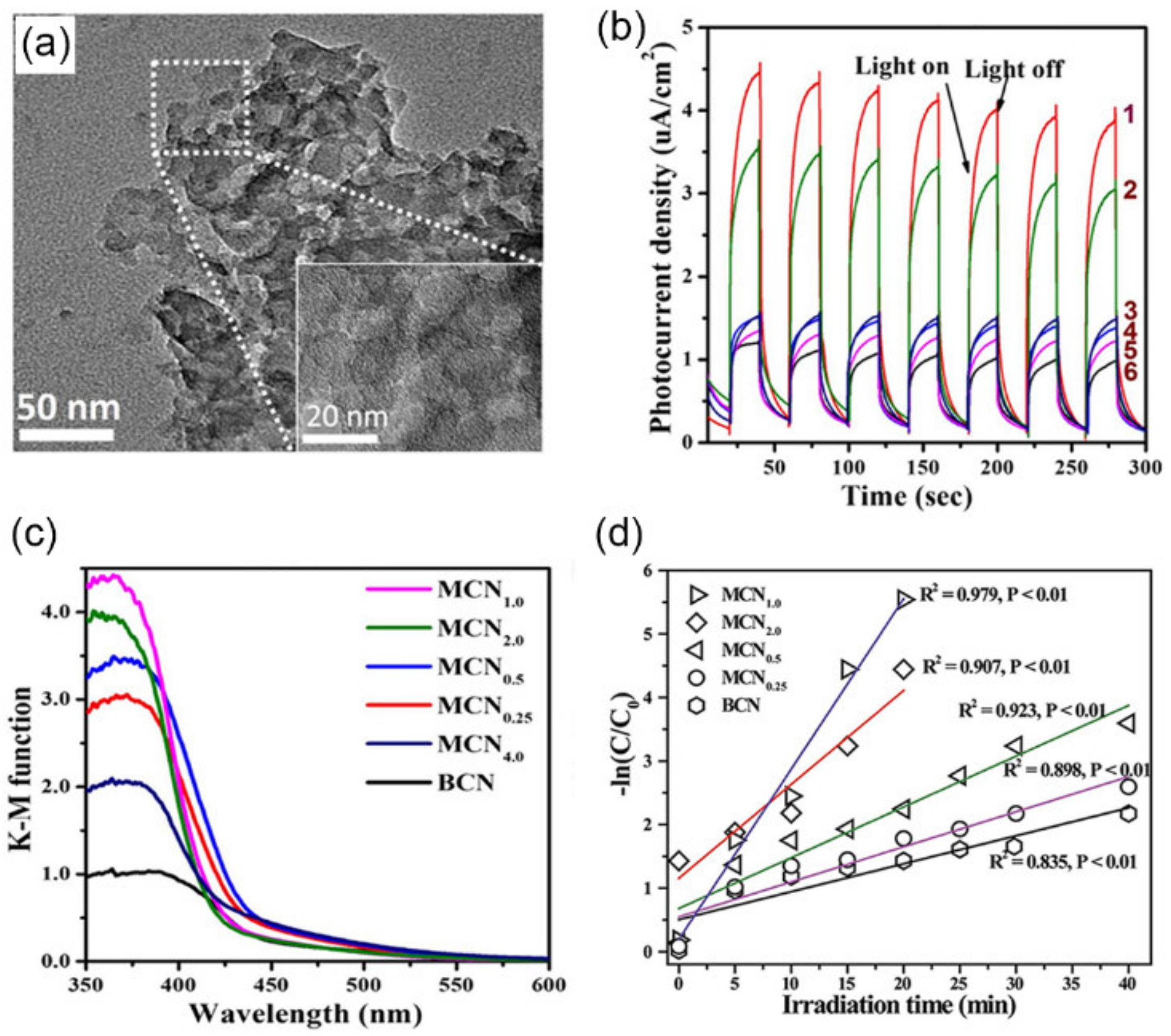
3.2. Soft-Template Method
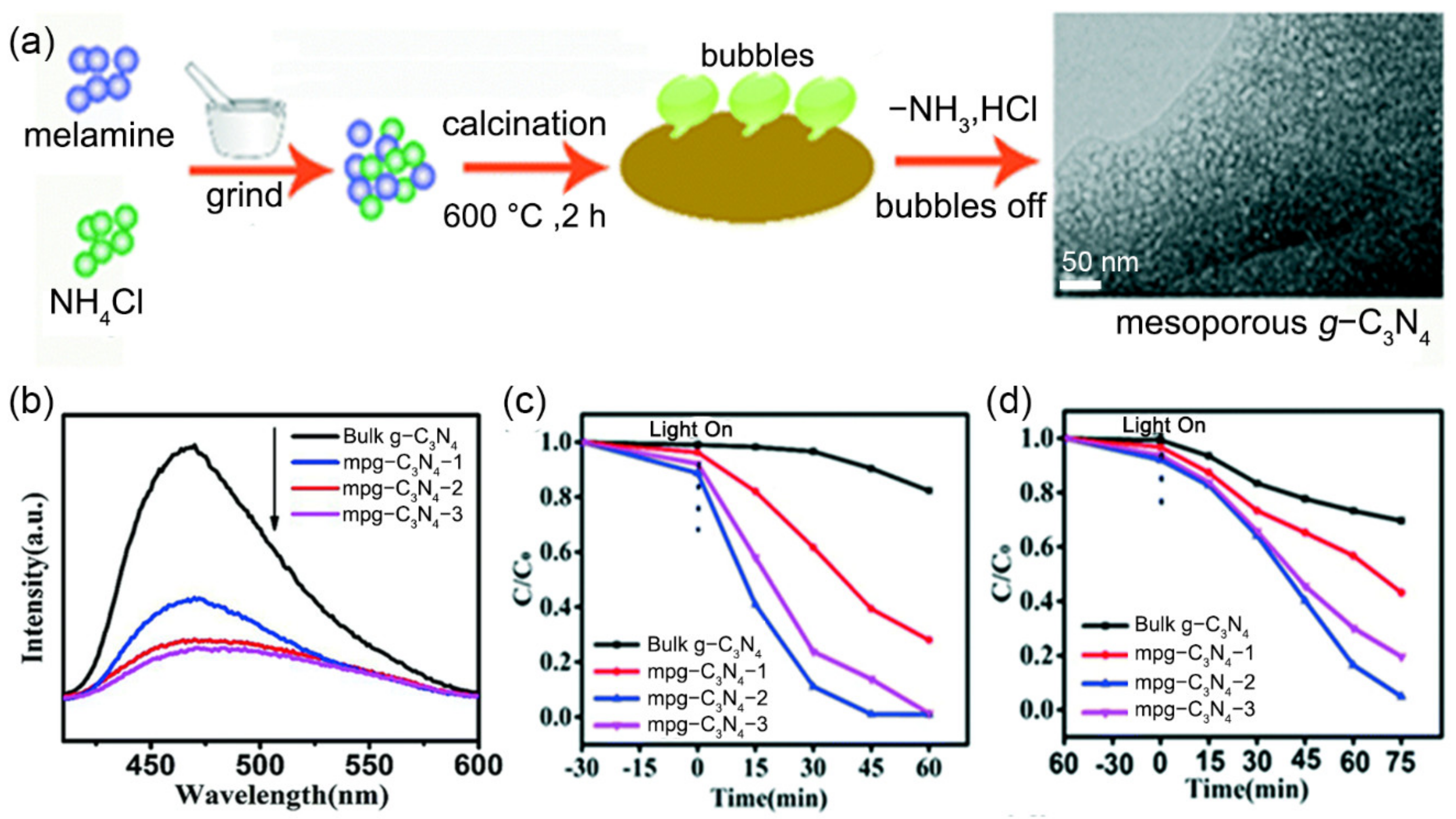
3.2.1. Surfactants as Templates
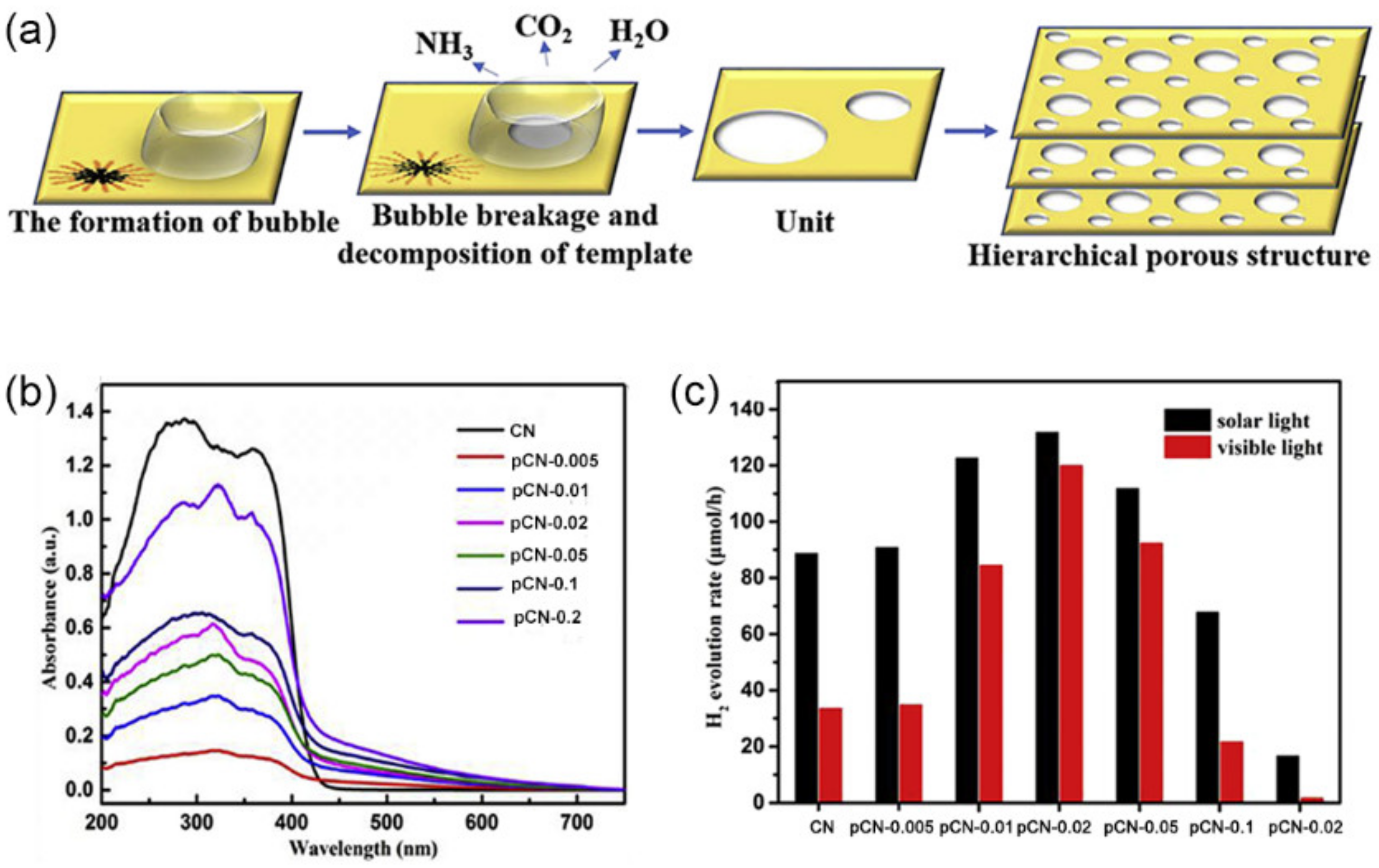
3.2.2. Bubbles as Templates
3.2.3. g-C3N4 Precursors as Templates
3.3. Template-Free Method
3.3.1. Pre-Etching of g-C3N4 Precursors
3.3.2. Supramolecular Precursors
3.3.3. Top-Down Approach
4. Application
4.1. Water Splitting
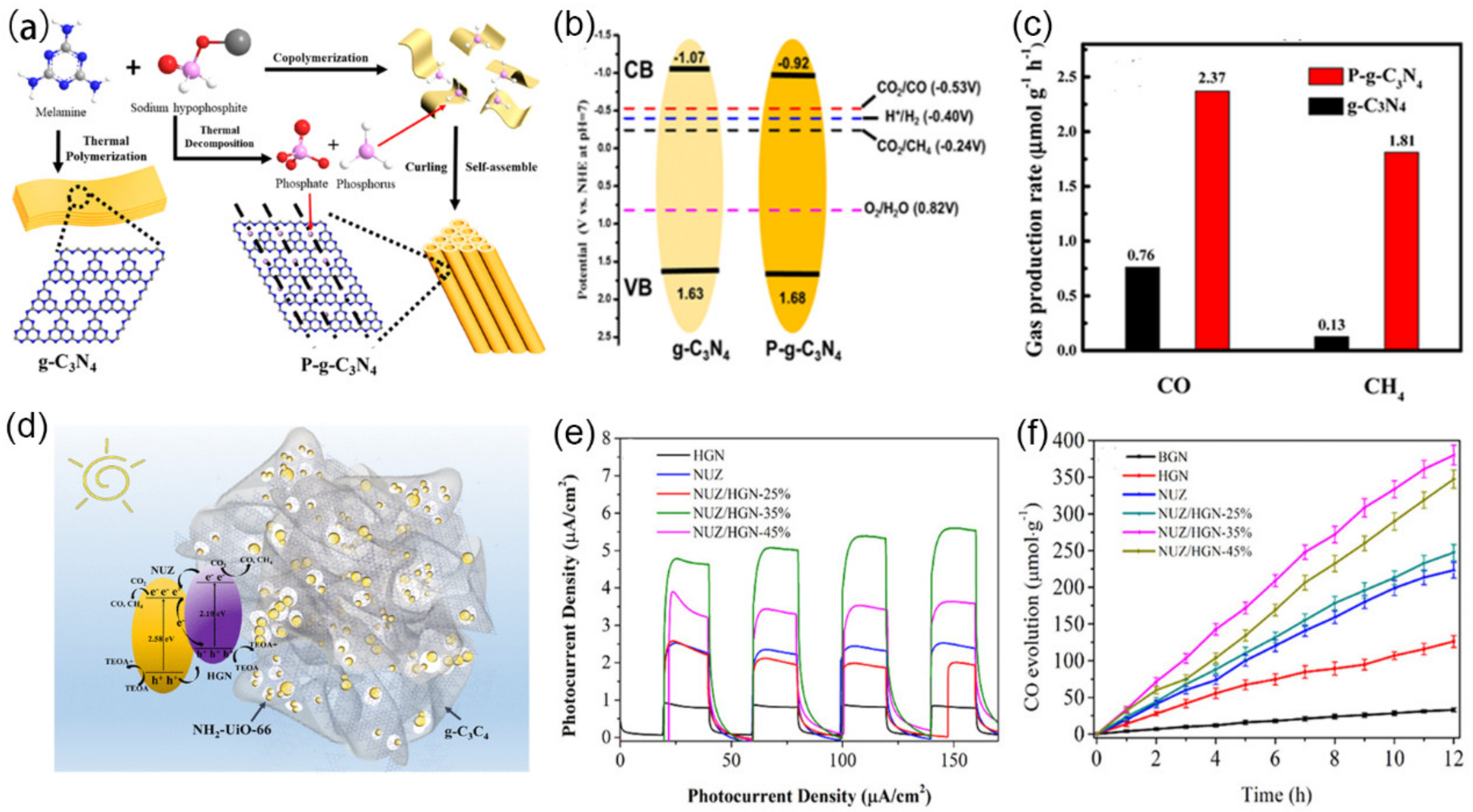
4.2. CO2 Reduction
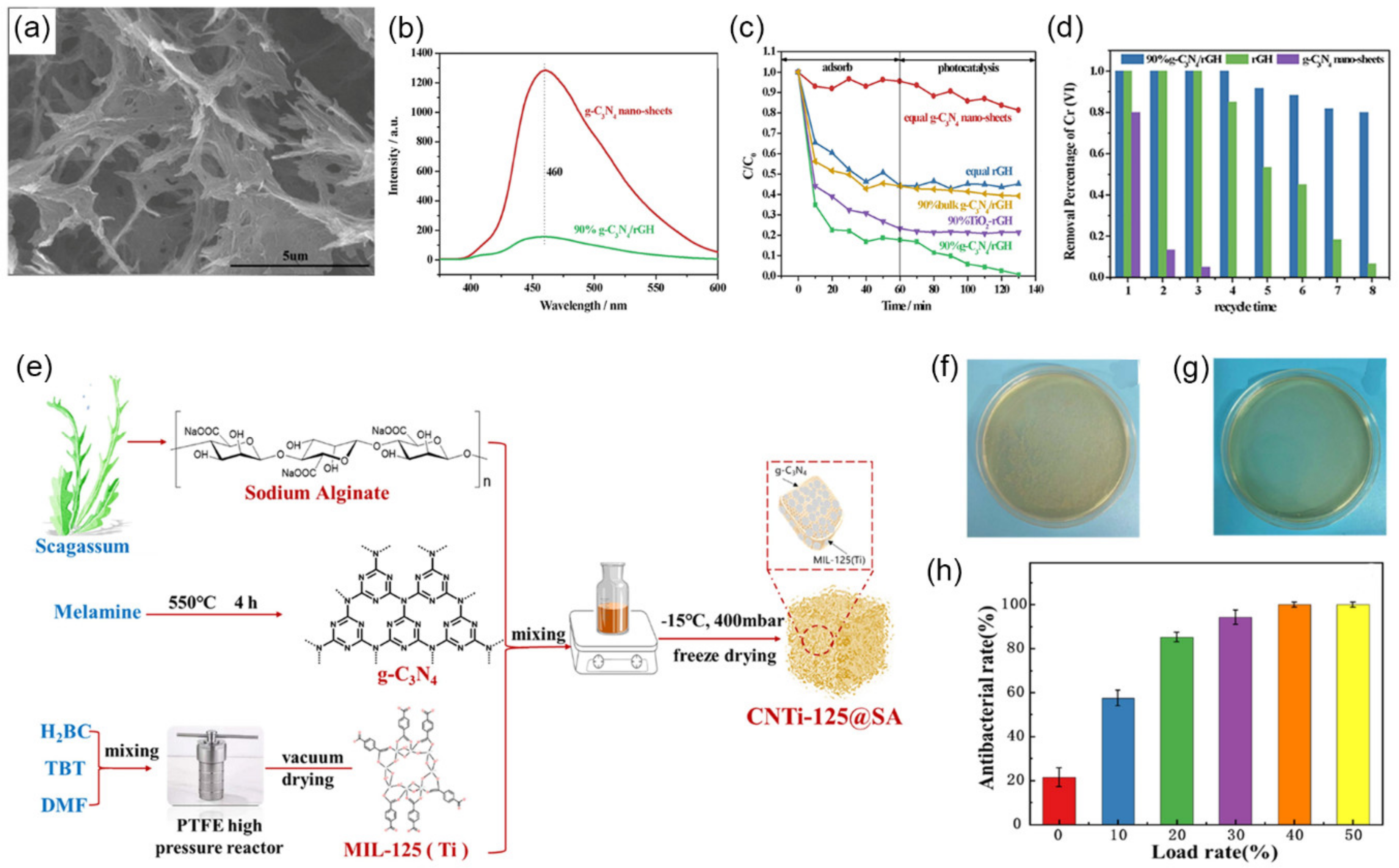
4.3. Wastewater Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Li, A.; Li, Q.; Hou, X.; Wang, L.-N.; Huang, Z.-H. Facile Fabrication of Three-Dimensional Interconnected Nanoporous N-TiO2 for Efficient Photoelectrochemical Water Splitting. J. Mater. Sci. Technol. 2018, 34, 955–960. [Google Scholar] [CrossRef]
- Osterloh, F.E. Inorganic Nanostructures for Photoelectrochemical and Photocatalytic Water Splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Z.; Li, W.-H.; Li, L.; Wang, L.-N. Progress in Organic Photocatalysts. Rare Met. 2018, 37, 1–12. [Google Scholar] [CrossRef]
- Chen, B.; Yu, J.; Wang, R.; Zhang, X.; He, B.; Jin, J.; Wang, H.; Gong, Y. Three-Dimensional Ordered Macroporous g-C3N4-Cu2O-TiO2 Heterojunction for Enhanced Hydrogen Production. Sci. China Mater. 2021, 6, 1–8. [Google Scholar] [CrossRef]
- Chen, M.; Bai, R.; Jin, P.; Li, J.; Yan, Y.; Peng, A.; He, J. A Facile Hydrothermal Synthesis of Few-Layer Oxygen-Doped g-C3N4 with Enhanced Visible Light-Responsive Photocatalytic Activity. J. Alloy. Compd. 2021, 869, 159292. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Liu, Y.; Alharbi, N.S.; Rabah, S.O.; Wang, S.; Wang, X. Synthesis and Fabrication of g-C3N4-Based Materials and Their Application in Elimination of Pollutants. Sci. Total Environ. 2020, 731, 139054. [Google Scholar] [CrossRef]
- Cheng, F.; Yin, H.; Xiang, Q. Low-Temperature Solid-State Preparation of Ternary CdS/g-C3N4/CuS Nanocomposites for Enhanced Visible-Light Photocatalytic H2 Production Activity. Appl. Surf. Sci. 2017, 391, 432–439. [Google Scholar] [CrossRef]
- Han, C.; Su, P.; Tan, B.; Ma, X.; Lv, H.; Huang, C.; Wang, P.; Tong, Z.; Li, G.; Huang, Y.; et al. Defective Ultra-Thin Two-Dimensional g-C3N4 Photocatalyst for Enhanced Photocatalytic H2 Evolution Activity. J. Colloid Interface Sci. 2021, 581, 159–166. [Google Scholar] [CrossRef]
- Das, K.K.; Patnaik, S.; Mansingh, S.; Behera, A.; Mohanty, A.; Acharya, C.; Parida, K.M. Enhanced Photocatalytic Activities of Polypyrrole Sensitized Zinc Ferrite/Graphitic Carbon Nitride n-n Heterojunction towards Ciprofloxacin Degradation, Hydrogen Evolution and Antibacterial Studies. J. Colloid Interface Sci. 2020, 561, 551–567. [Google Scholar] [CrossRef]
- Mohamed, F.; Abukhadra, M.R.; Shaban, M. Removal of Safranin Dye from Water Using Polypyrrole Nanofiber/Zn-Fe Layered Double Hydroxide Nanocomposite (Ppy NF/Zn-Fe LDH) of Enhanced Adsorption and Photocatalytic Properties. Sci. Total Environ. 2018, 640, 352–363. [Google Scholar] [CrossRef]
- Deng, X.; Chen, Y.; Wen, J.; Xu, Y.; Zhu, J.; Bian, Z. Polyaniline-TiO2 Composite Photocatalysts for Light-Driven Hexavalent Chromium Ions Reduction. Sci. Bull. 2020, 65, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Eskizeybek, V.; Sari, F.; Gulce, H.; Gulce, A.; Avci, A. Preparation of the New Polyaniline/ZnO Nanocomposite and Its Photocatalytic Activity for Degradation of Methylene Blue and Malachite Green Dyes under UV and Natural Sun Lights Irradiations. Appl. Catal. B Environ. 2012, 119, 197–206. [Google Scholar] [CrossRef]
- Zhang, K.; Li, H.; Shi, H.; Hong, W. Polyimide with Enhanced π Stacking for Efficient Visible-Light-Driven Photocatalysis. Catal. Sci. Technol. 2021, 11, 4889–4897. [Google Scholar] [CrossRef]
- Bao, H.; Chen, X.; Yuan, R.; Zhang, C.; Xu, S. A Dual Polymer Composite of Poly (3-Hexylthiophene) and Poly (3,4-Ethylenedioxythiophene) Hybrid Surface Heterojunction with g-C3N4 for Enhanced Photocatalytic Hydrogen Evolution. RSC Adv. 2021, 11, 32671–32679. [Google Scholar] [CrossRef]
- Tremblay, P.-L.; Xu, M.; Chen, Y.; Zhang, T. Nonmetallic Abiotic-Biological Hybrid Photocatalyst for Visible Water Splitting and Carbon Dioxide Reduction. iScience 2020, 23, 100784. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Tremblay, P.-L.; Jiang, L.; Zhang, T. Stimulating Bioplastic Production with Light Energy by Coupling Ralstonia Eutropha with the Photocatalyst Graphitic Carbon Nitride. Green Chem. 2019, 21, 2392–2400. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, B.; Li, X.; Fu, M.; Xie, S.; Deng, C.; Hu, Y. Progress in the Preparation of Nanomaterials Employing Template Method. Chem. Ind. Eng. Prog. 2010, 29, 94–99. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Shen, Y.; Liu, S.; Zhang, Y. Molecular engineering of polymeric carbon nitride: Advancing applications from photocatalysis to biosensing and more. Chem. Soc. Rev. 2018, 47, 2298–2321. [Google Scholar] [CrossRef]
- Wu, C.; Xue, S.; Qin, Z.; Nazari, M.; Yang, G.; Yue, S.; Tong, T.; Ghasemi, H.; Hernandez, F.C.R.; Xue, S.; et al. Making g-C3N4 ultra-thin nanosheets active for photocatalytic overall water splitting. Appl. Catal. B Environ. 2021, 282, 119557. [Google Scholar] [CrossRef]
- Tian, B.; Wu, Y.; Lu, G. Metal-Free Plasmonic Boron Phosphide/Graphitic Carbon Nitride with Core-Shell Structure Photocatalysts for Overall Water Splitting. Appl. Catal. B Environ. 2021, 280, 119410. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, Z.; Xiao, K.; Sun, H.; Chan, H.S.; Tsang, T.H.; Yang, S.; Wong, P.K. Photo-Assisted Separation of Noble-Metal-Free Oxidation and Reduction Cocatalysts for Graphitic Carbon Nitride Nanosheets with Efficient Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2021, 280, 119456. [Google Scholar] [CrossRef]
- Gu, J.-W.; Guo, R.-T.; Miao, Y.-F.; Liu, Y.-Z.; Wu, G.-L.; Duan, C.-P.; Pan, W.-G. Construction of Full Spectrum-Driven CsxWO3/g-C3N4 Heterojunction Catalyst for Efficient Photocatalytic CO2 Reduction. Appl. Surf. Sci. 2021, 540, 148316. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z.; Ding, C.; Xu, J. Boosting Charge Separation and Photocatalytic CO2 Reduction of CsPbBr3 Perovskite Quantum Dots by Hybridizing with P3HT. Chem. Eng. J. 2021, 419, 129543. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Gan, L.; Ji, X.; Chen, F.; Peng, X.; Zhang, R. 3D Macropore Carbon-Vacancy g-C3N4 Constructed Using Polymethylmethacrylate Spheres for Enhanced Photocatalytic H2 Evolution and CO2 Reduction. J. Energy Chem. 2021, 53, 139–146. [Google Scholar] [CrossRef]
- Liu, R.; Bie, Y.; Qiao, Y.; Liu, T.; Song, Y. Design of g-C3N4/TiO2 Nanotubes Heterojunction for Enhanced Organic Pollutants Degradation in Waste Water. Mater. Lett. 2019, 251, 126–130. [Google Scholar] [CrossRef]
- Chen, W.; Liu, M.; Wei, S.; Li, X.; Mao, L.; Shangguan, W. Solid-State Synthesis of Ultrathin MoS2 as a Cocatalyst on Mesoporous g-C3N4 for Excellent Enhancement of Visible Light Photoactivity. J. Alloy. Compd. 2020, 836, 155401. [Google Scholar] [CrossRef]
- Wu, M.; Yan, J.-M.; Zhang, X.-W.; Zhao, M. Synthesis of g-C3N4 with Heating Acetic Acid Treated Melamine and Its Photocatalytic Activity for Hydrogen Evolution. Appl. Surf. Sci. 2015, 354, 196–200. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Y.; Zhou, Y.; Wang, Y.; Qiu, K.; Zhang, C.; Fang, J.; Sheng, X. Facile One-Step Synthesis of Hollow Mesoporous g-C3N4 Spheres with Ultrathin Nanosheets for Photoredox Water Splitting. Carbon 2018, 126, 247–256. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Wang, Y.; Zhao, N.-D.; Zheng, M.; Guo, Y.-R.; Pan, Q.-J. Van Der Waals Enhanced Interfacial Interaction in Cellulose/Zinc Oxide Nanocomposite Coupled by Graphitic Carbon Nitride. Carbohydr. Polym. 2021, 268, 118235. [Google Scholar] [CrossRef]
- Liang, Q.; Li, Z.; Yu, X.; Huang, Z.-H.; Kang, F.; Yang, Q.-H. Macroscopic 3D Porous Graphitic Carbon Nitride Monolith for Enhanced Photocatalytic Hydrogen Evolution. Adv. Mater. 2015, 27, 4634–4639. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Li, Z.; Wang, Q. Renewable Biomass-Derived Carbon-Supported g-C3N4 Doped with Ag for Enhanced Photocatalytic Reduction of CO2. J. Colloid Interface Sci. 2022, 606, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kashyap, S.; Sharma, M.; Krishnan, V. Tuning the Surface and Optical Properties of Graphitic Carbon Nitride by Incorporation of Alkali Metals (Na, K, Cs and Rb): Effect on Photocatalytic Removal of Organic Pollutants. Chemosphere 2022, 287, 131988. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Chen, F.; Cao, F.; Zhao, X.; Meng, S.; Cui, Y. Facile Synthesis of Oxygen Doped Carbon Nitride Hollow Microsphere for Photocatalysis. Appl. Catal. B Environ. 2017, 206, 417–425. [Google Scholar] [CrossRef]
- Guo, S.; Tang, Y.; Xie, Y.; Tian, C.; Feng, Q.; Zhou, W.; Jiang, B. P-doped Tubular g-C3N4 with Surface Carbon Defects: Universal Synthesis and Enhanced Visible-Light Photocatalytic Hydrogen Production. Appl. Catal. B Environ. 2017, 218, 664–671. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Huang, D.; Zeng, G.; Huang, J.; Lai, C.; Zhou, C.; Wang, W.; Guo, H.; Xue, W.; et al. Boron Nitride Quantum Dots Decorated Ultrathin Porous g-C3N4: Intensified Exciton Dissociation and Charge Transfer for Promoting Visible-Light-Driven Molecular Oxygen Activation. Appl. Catal. B Environ. 2019, 245, 87–99. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Yang, Z.; Shen, R.; Li, H.; Luo, X.; Chen, X.; Li, X. Fabricating the Robust g-C3N4 Nanosheets/Carbons/NiS Multiple Heterojunctions for Enhanced Photocatalytic H2 Generation: An Insight into the Trifunctional Roles of Nanocarbons. ACS Sustain. Chem. Eng. 2017, 5, 2224–2236. [Google Scholar] [CrossRef]
- Arunraj, B.; Kaushik, U.S.G.; Rajesh, V.; Rajesh, N. Combinative Influence of Graphitic Carbon Nitride and Halomonas BVR1Bacteria Augment the Adsorptive Recovery of Precious ‘Euro’pium. Chem. Eng. J. 2021, 404, 126466. [Google Scholar] [CrossRef]
- Wei, F.; Kuang, S.; Rees, T.W.; Liao, X.; Liu, J.; Luo, D.; Wang, J.; Zhang, X.; Ji, L.; Chao, H. Ruthenium(II) Complexes Coordinated to Graphitic Carbon Nitride: Oxygen Self-Sufficient Photosensitizers Which Produce Multiple ROS for Photodynamic Therapy in Hypoxia. Biomaterials 2021, 276, 121064. [Google Scholar] [CrossRef]
- Sun, X.; An, X.; Zhang, S.; Li, Z.; Zhang, J.; Wu, W.; Wu, M. Physical Vapor Deposition (PVD): A Method to Fabricate Modified g-C3N4 Sheets. New J. Chem. 2019, 43, 6683–6687. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Zuo, Y.; Zhang, X.; Cui, L.-F. Simple Synthesis of Ordered Cubic Mesoporous Graphitic Carbon Nitride by Chemical Vapor Deposition Method Using Melamine. Mater. Lett. 2014, 136, 271–273. [Google Scholar] [CrossRef]
- Cao, M.; Wang, K.; Tudela, I.; Fan, X. Improve Photocatalytic Performance of g-C3N4 through Balancing the Interstitial and Substitutional Chlorine Doping. Appl. Surf. Sci. 2021, 536, 147784. [Google Scholar] [CrossRef]
- Zhou, J.; Ding, J.; Wan, H.; Guan, G. Boosting Photocatalytic Degradation of Antibiotic Wastewater by Synergy Effect of Heterojunction and Phosphorus Soping. J. Colloid Interface Sci. 2021, 582, 961–968. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, H.; Yan, Y.; Liu, C.; Hu, X.; Liu, E.; Fan, J. A Novel S-Scheme 1D/2D Bi2S3/g-C3N4 Heterojunctions with Enhanced H2 Evolution Activity. Colloid Surf. A Physicochem. Eng. Asp. 2021, 608, 125598. [Google Scholar] [CrossRef]
- Barrio, J.; Grafmueller, A.; Tzadikov, J.; Shalom, M. Halogen-Hydrogen Bonds: A General Synthetic Approach for Highly Photoactive Carbon Nitride with Tunable Properties. Appl. Catal. B Environ. 2018, 237, 681–688. [Google Scholar] [CrossRef]
- Trangwachirachai, K.; Chen, C.-H.; Huang, A.-L.; Lee, J.-F.; Chen, C.-L.; Lin, Y.-C. Conversion of Methane to Acetonitrile over GaN Catalysts Derived from Gallium Nitrate Hydrate Co-Pyrolyzed with Melamine, Melem, or g-C3N4: The Influence of Nitrogen Precursors. Catal. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Antonietti, M.; Zhang, Y. Facile One-Pot Synthesis of Nanoporous Carbon Nitride Solids by Using Soft Templates. ChemSusChem 2010, 3, 435–439. [Google Scholar] [CrossRef]
- Ji, H.; Chang, F.; Hu, X.; Qin, W.; Shen, J. Photocatalytic degradation of 2,4,6-trichlorophenol over g-C3N4 under visible light irradiation. Chem. Eng. J. 2013, 218, 183–190. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation Performance of g-C3N4 Fabricated by Directly Heating Melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Wu, G.; Chen, W. Porous graphitic carbon nitride synthesized via direct polymerization of urea for efficient sunlight-driven photocatalytic hydrogen production. Nanoscale 2012, 4, 5300–5303. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Zhang, M.; Wang, X. Polycondensation of thiourea into carbon nitride semiconductors as visible light photocatalysts. J. Mater. Chem. 2012, 22, 8083–8091. [Google Scholar] [CrossRef]
- Cheng, M.; Lv, P.; Zhang, X.; Xiong, R.; Guo, Z.; Wang, Z.; Zhou, Z.; Zhang, M. A New Active Species of Pd-N-x Synthesized by Hard-Template Method for Efficiently Catalytic Hydrogenation of Nitroarenes. J. Catal. 2021, 399, 182–191. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, W.; Su, Y.; Liu, Z.; Du, C. Halloysite Derived 1D Mesoporous Tubular g-C3N4: Synergy of Template Effect and Associated Carbon for Boosting Photocatalytic Performance toward Tetracycline Removal. Appl. Clay Sci. 2021, 213, 106238. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Y.; Lai, Y.; Li, Y. Mesoporous Graphitic Carbon Nitride for Adsorptive Desulfurization in an Isooctane Solution. J. Sulfur Chem. 2021, 10, 1985120. [Google Scholar] [CrossRef]
- Jiang, T.; Han, H.; Dong, M.; Zhao, Q. In Situ Construction of Porous g-C3N4 Isotype Heterojunction/BiOBr Nanosheets Ternary Composite Catalyst for Highly Efficient Visible-Light Photocatalytic Activity. ChemistrySelect 2021, 6, 6212–6222. [Google Scholar] [CrossRef]
- Ai, L.; Fan, H. CTAB-Melamine Molecular Crystals as Precursor for Synthesis of Layered Carbon Nitride Porous Nanostructures with Enhanced Photocatalytic Activity for Hydrogen Production. Mater. Today Commun. 2021, 29, 102780. [Google Scholar] [CrossRef]
- Li, H.; Ning, F.; Chen, X.; Shi, A. Effect of Carbon and Nitrogen Double Vacancies on the Improved Photocatalytic Hydrogen Evolution over Porous Carbon Nitride Nanosheets. Catal. Sci. Technol. 2021, 11, 3270–3278. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, L.; Zhuang, X.; Tang, Z.; Li, H.; Kang, W. Polyhedral Oligomeric Silsesquioxane as a Recyclable Soft Template to Synthesize Mesoporous Polymeric Carbon Nitride with Enhanced Photocatalytic Hydrogen Evolution. Sustain. Energy Fuels 2021, 5, 112–116. [Google Scholar] [CrossRef]
- Deng, P.; Hong, W.; Cheng, Z.; Zhang, L.; Hou, Y. Facile Fabrication of Nickel/Porous g-C3N4 by Using Carbon Dot as Template for Enhanced Photocatalytic Hydrogen Production. Int. J. Hydrog. Energy 2020, 45, 33543–33551. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, W.; Fang, J.; Chen, D.; Pan, T.; Feng, W.; Liang, Y.; Fang, Z. Soft-Template Assisted Construction of Superstructure TiO2/SiO2/g-C3N4 Hybrid as Efficient Visible-Light Photocatalysts to Degrade Berberine in Seawater via an Adsorption-Photocatalysis Synergy and Mechanism Insight. Appl. Catal. B Environ. 2020, 268, 118751. [Google Scholar] [CrossRef]
- Sharma, P.; Sarngan, P.P.; Lakshmanan, A.; Sarkar, D. One-step Synthesis of Highly Reactive g-C3N4. J. Mater. Sci. Mater. Electron. 2021, 10, 1–10. [Google Scholar] [CrossRef]
- Yang, X.; Cao, G.; Liu, H.; Wang, C.; Li, X.; Song, Z. Construction of Nesoporous C3N4/rGO Composite with Enhanced Visible Light Photocatalytic Activity. Optik 2021, 241, 167159. [Google Scholar] [CrossRef]
- Phoon, B.L.; Lai, C.W.; Pan, G.-T.; Yang, T.C.K.; Juan, J.C. Highly Mesoporous g-C3N4 with Uniform Pore Size Distribution via the Template-Free Method to Enhanced Solar-Driven Tetracycline Degradation. Nanomaterials 2021, 11, 2041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, Y.; Chang, X.; Zhang, P. Template-Free Synthesis of One-Dimensional g-C3N4 Chain Nanostructures for Efficient Photocatalytic Hydrogen Evolution. Front. Chem. 2021, 9, 652762. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Ordered Porous Materials for Merging Applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef]
- Fang, Z.; Bueken, B.; De Vos, D.E.; Fischer, R.A. Defect-Engineered Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2015, 54, 7234–7254. [Google Scholar] [CrossRef] [Green Version]
- Razavi, M.; Bordonaro, G.G.; Ferro, P.; Torgersen, J.; Berto, F. Porosity Effect on Tensile Behavior of Ti-6Al-4V Specimens Produced by Laser Engineered Net Shaping Technology. Proc. Inst. Mech. Eng. C J. Mech. Eng. Sci. 2018, 235, 1989–1996. [Google Scholar] [CrossRef] [Green Version]
- Tatarchuk, T.; Myslin, M.; Mironyuk, I.; Bououdina, M.; Pędziwiatr, A.T.; Gargula, R.; Bogacz, B.F.; Kurzydło, P. Synthesis, Morphology, Crystallite Size and Adsorption Properties of Nanostructured Mg-Zn Ferrites with Enhanced Porous Structure. J. Alloy. Compd. 2020, 819, 152945. [Google Scholar] [CrossRef]
- Zou, K.; Cai, P.; Wang, B.; Liu, C.; Li, J.; Qiu, T.; Zou, G.; Hou, H.; Ji, X. Insights into Enhanced Capacitive Behavior of Carbon Cathode for Lithium Ion Capacitors: The Coupling of Pore Size and Graphitization Engineering. Nano-Micro Lett. 2020, 12, 121. [Google Scholar] [CrossRef]
- Garcia-Mulero, A.; Rendon-Patino, A.; Asiri, A.M.; Primo, A.; Garcia, H. Band Engineering of Semiconducting Microporous Graphitic Carbons by Phosphorous Doping: Enhancing of Photocatalytic Overall Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 48753–48763. [Google Scholar] [CrossRef]
- Wang, D.; Jia, F.; Wang, H.; Chen, F.; Fang, Y.; Dong, W.; Zeng, G.; Li, X.; Yang, Q.; Yuan, X. Simultaneously Efficient Adsorption and Photocatalytic Degradation of Tetracycline by Fe-Based MOFs. J. Colloid Interface Sci. 2018, 519, 273–284. [Google Scholar] [CrossRef]
- Tian, N.; Zhang, Y.; Li, X.; Xiao, K.; Du, X.; Dong, F.; Waterhouse, G.I.N.; Zhang, T.; Huang, H. Precursor-Reforming Protocol to 3D Mesoporous g-C3N4 Established by Ultrathin Self-Doped Nanosheets for Superior Hydrogen Evolution. Nano Energy 2017, 38, 72–81. [Google Scholar] [CrossRef]
- Xiong, C.; Li, B.; Lin, X.; Liu, H.; Xu, Y.; Mao, J.; Duan, C.; Li, T.; Ni, Y. The Recent Progress on Three-dimensional Porous Graphene-Based Hybrid Structure for Supercapacitor. Compos. Part B Eng. 2019, 165, 10–46. [Google Scholar] [CrossRef]
- Zhao, X.; Pachfule, P.; Li, S.; Langenhahn, T.; Ye, M.; Schlesiger, C.; Praetz, S.; Schmidt, J.; Thomas, A. Macro/Microporous Covalent Organic Frameworks for Efficient Electrocatalysis. J. Am. Chem. Soc. 2019, 141, 6623–6630. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, X.; Yin, C.; Zhang, B.; Zhang, Q. Facile Fabrication of Hierarchical Porous ZIF-8 for Enhanced Adsorption of Antibiotics. J. Hazard. Mater. 2019, 367, 194–204. [Google Scholar] [CrossRef]
- Li, Y.; Jin, R.; Xing, Y.; Li, J.; Song, S.; Liu, X.; Li, M.; Jin, R. Macroscopic Foam-Like Holey Ultrathin g-C3N4 Nanosheets for Drastic Improvement of Visible-Light Photocatalytic Activity. Adv. Energy Mater. 2016, 6, 1601273. [Google Scholar] [CrossRef]
- Fang, B.; Kim, J.H.; Kim, M.-S.; Yu, J.-S. Hierarchical Nanostructured Carbons with Meso–Macroporosity: Design, Characterization, and Applications. ACS Appl. Mater. Interfaces 2013, 46, 1397–1406. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Wang, Q.; Meng, C. In-Situ Hydrothermal Growth of Zn4Si2O7(OH)2·H2O Anchored on 3D N, S-Enriched Carbon Derived from Plant Biomass for Flexible Solid-state Asymmetrical Supercapacitors. Chem. Eng. J. 2018, 352, 519–529. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Chen, L.-H.; Li, Y.; Rooke, J.C.; Sanchez, C.; Su, B.-L. Hierarchically Porous Materials: Synthesis Strategies and Structure Design. Chem. Soc. Rev. 2017, 46, 481–558. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Xiang, S.; Cao, S.; Zhang, J.; Ouyang, G.; Chen, L.; Su, C.-Y. A Synthetic Route to Ultralight Hierarchically Micro/Mesoporous Al(III)-Carboxylate Metal-Organic Aerogels. Nat. Commun. 2013, 4, 1774–1783. [Google Scholar] [CrossRef] [Green Version]
- Hao, R.; Wang, G.; Tang, H.; Sun, L.; Xu, C.; Han, D. Template-Free Preparation of Macro/Mesoporous g-C3N4/TiO2 Teterojunction Photocatalysts with Enhanced Visible Light Photocatalytic Activity. Appl. Catal. B Environ. 2016, 187, 47–58. [Google Scholar] [CrossRef]
- Wei, J.; Chen, Y.; Zhang, H.; Zhuang, Z.; Yu, Y. Hierarchically Porous S-Scheme CdS/UiO-66 Photocatalyst for Efficient 4-Nitroaniline Reduction. Chin. J. Catal. 2021, 42, 78–86. [Google Scholar] [CrossRef]
- Dong, G.; Zhang, Y.; Pan, Q.; Qiu, J. A Fantastic Graphitic Carbon Nitride (g-C3N4) Material: Electronic Structure, Photocatalytic and Photoelectronic Properties. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Zhao, H.-M.; Di, C.-M.; Wang, L.; Chun, Y.; Xu, Q.-H. Synthesis of Mesoporous Graphitic C3N4 Using Cross-Linked Bimodal Mesoporous SBA-15 as a Hard Template. Microporous Mesoporous Mater. 2015, 208, 98–104. [Google Scholar] [CrossRef]
- Wang, W.; Fang, J.; Chen, H. Nano-Confined g-C3N4 in Mesoporous SiO2 with Improved Quantum Size Effect and Tunable Structure for Photocatalytic Tetracycline Antibiotic Degradation. J. Alloy. Compd. 2020, 819, 153064. [Google Scholar] [CrossRef]
- Li, Y.; Qu, W.; Huang, L.; Li, P.; Zhang, F.; Yuan, D.; Wang, Q.; Xu, H.; Li, H. Porous-C3N4 with High Ability for Selective Adsorption and Photodegradation of Dyes Under Visible-Light. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1674–1682. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, E.; Shi, J.; Lin, X.; Sheng, L.; Zhang, M.; Wang, L.; Chen, J. A Direct One-Step Synthesis of Ultrathin g-C3N4 Nanosheets from Thiourea for Boosting Solar Photocatalytic H2 Evolution. Int. J. Hydrog. Energy 2019, 44, 7194–7204. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Wang, W.; Ding, Z.; Geng, R.; Li, P.; Pan, D.; Liang, J.; Qin, H.; Fan, Q. Tunable Mesoporous g-C3N4 Nanosheets as a Metal-Free Catalyst for Enhanced Visible-Light-Driven Photocatalytic Reduction of U(VI). Chem. Eng. J. 2020, 383, 123193. [Google Scholar] [CrossRef]
- Jiang, T.S.; Du, Y.; Dong, M.F.; Zhao, Q. The facile synthesis and enhanced photocatalytic activity of a graphitic carbon nitride isotype heterojunction with ordered mesopores. New J. Chem. 2019, 43, 10915–10925. [Google Scholar] [CrossRef]
- Liu, H.J.; Wu, H.N.; Lv, J.; Xu, G.Q.; Chen, X.; Zhang, X.Y.; Wu, Y.C. SBA-15 Templated Mesoporous Graphitic C3N4 for Remarkably Enhanced Photocatalytic Degradation of Organic Pollutants under Visible Light. Nano 2019, 14, 19501364. [Google Scholar] [CrossRef] [Green Version]
- Obregon, S.; Vazquez, A.; Ruiz-Gomez, M.A.; Rodriguez-Gonzalez, V. SBA-15 assisted preparation of mesoporous g-C3N4 for photocatalytic H2 production and Au3+ fluorescence sensing. Appl. Surf. Sci. 2019, 488, 205–212. [Google Scholar] [CrossRef]
- Gao, X.C.; Jiao, X.J.; Zhang, L.C.; Zhu, W.C.; Xu, X.H.; Ma, H.Y.; Chen, T. Cosolvent-free nanocasting synthesis of ordered mesoporous g-C3N4 and its remarkable photocatalytic activity for methyl orange degradation. RSC Adv. 2015, 5, 76963–76972. [Google Scholar] [CrossRef]
- Li, X.B.; Masters, A.F.; Maschmeyer, T. Photocatalytic Hydrogen Evolution from Silica-Templated Polymeric Graphitic Carbon Nitride-Is the Surface Area Important? ChemCatChem 2015, 7, 121–126. [Google Scholar] [CrossRef]
- Liu, J.Y.; Yan, J.; Ji, H.Y.; Xu, Y.G.; Huang, L.Y.; Li, Y.P.; Song, Y.H.; Zhang, Q.; Xu, H.; Li, H.M. Controlled synthesis of ordered mesoporous g-C3N4 with a confined space effect on its photocatalytic activity. Mater. Sci. Semicond. Process 2016, 46, 59–68. [Google Scholar] [CrossRef]
- Ovcharov, M.; Shcherban, N.; Filonenko, S.; Mishura, A.; Skoryk, M.; Shvalagin, V.; Granchak, V. Hard template synthesis of porous carbon nitride materials with improved efficiency for photocatalytic CO2 utilization. Mater. Sci. Eng. B 2015, 202, 1–7. [Google Scholar] [CrossRef]
- Esmati, M.; Allahresani, A.; Naghizadeh, A. Synthesis and characterization of Graphitic Carbon Nitride/Mesoporous Nano-Silica (g-C3N4/KCC-1) nanocomposite as a novel highly efficient and recyclable photocatalyst for degradation of antibiotic in aqueous solution. Res. Chem. Intermed. 2021, 47, 1447–1469. [Google Scholar] [CrossRef]
- Kadi, M.W.; Mohamed, R.M.; Ismail, A.A.; Bahnemann, D.W. Soft and hard templates assisted synthesis mesoporous CuO/g-C3N4 heterostructures for highly enhanced and accelerated Hg(II) photoreduction under visible light. J. Colloid Interface Sci. 2020, 580, 223–233. [Google Scholar] [CrossRef]
- Li, Q.; Yang, J.; Feng, D.; Wu, Z.; Wu, Q.; Park, S.S.; Ha, C.-S.; Zhao, D. Facile synthesis of porous carbon nitride spheres with hierarchical three-dimensional mesostructures for CO2 capture. Nano Res. 2010, 3, 632–642. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, M.; Yang, C.; Wang, X. Nanospherical Carbon Nitride Frameworks with Sharp Edges Accelerating Charge Collection and Separation at a Soft Photocatalytic Interface. Adv. Mater. 2014, 26, 4121–4126. [Google Scholar] [CrossRef]
- Duan, Y.; Cao, Y.; Shang, X.; Jia, D.; Li, C. Synthesis of g-C3N4/W-SBA-15 Composites for Photocatalytic Degradation of Tetracycline Hydrochloride. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2140–2149. [Google Scholar] [CrossRef]
- Xu, J.; Chen, T.; Wang, X.; Xue, B.; Li, Y.X. Preparation of mesoporous graphitic carbon nitride using hexamethylenetetramine as a new precursor and catalytic application in the transesterification of beta-keto esters. Catal. Sci. Technol. 2014, 4, 2126–2133. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Jun, Y.-S.; Takanabe, K.; Maeda, K.; Domen, K.; Fu, X.; Antonietti, M.; Wang, X. Ordered Mesoporous SBA-15 Type Graphitic Carbon Nitride: A Semiconductor Host Structure for Photocatalytic Hydrogen Evolution with Visible Light. Chem. Mater. 2009, 21, 4093–4095. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Li, F.; Wang, Y.; Pan, W.; Leung, D.Y.C. Salt-air Template Synthesis of Na and O Doped Porous Graphitic Carbon Nitride Nanorods with Exceptional Photocatalytic H2 Evolution Activity. Carbon 2021, 179, 42–52. [Google Scholar] [CrossRef]
- Tang, Q.; Niu, R.; Gong, J. Salt-Templated Synthesis of 3D Porous Foam-like C3N4 Towards High-Performance Photodegradation of Tetracyclines. New J. Chem. 2020, 44, 17405–17412. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, S.; Wu, Q.; He, F.; Zhao, N.; He, C.; Shi, C. Salt-assisted Synthesis of 3D Open Porous g-C3N4 Decorated with Cyano Groups for Photocatalytic Hydrogen Evolution. Nanoscale 2018, 10, 3008–3013. [Google Scholar] [CrossRef]
- Jing, H.; You, M.; Yi, S.; Li, T.; Ji, H.; Wang, Y.; Zhang, Z.; Zhang, R.; Chen, D.; Yang, H. Precursor-Engineering Coupled Microwave Molten-Salt Strategy Enhances Photocatalytic Hydrogen Evolution Performance of g-C3N4 Nanostructures. ChemSusChem 2020, 13, 827–837. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, J.; Lin, H.; Yuan, J. “Cooking carbon in a solid salt”: Synthesis of porous heteroatom-doped carbon foams for enhanced organic pollutant degradation under visible light. Appl. Mater. Today 2018, 12, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.D.; Li, Y.Y.; Zhou, J.; Ma, C.H.; Wang, Y.; Zhu, J.H. Facile synthesis of high photocatalytic active porous g-C3N4 with ZnCl2 template. J. Colloid Interface Sci. 2015, 451, 108–116. [Google Scholar] [CrossRef]
- Yang, F.; Liu, D.; Li, Y.; Cheng, L.; Ye, J. Salt-Template-Assisted Construction of Honeycomb-Like Structured g-C3N4 with Tunable Band Structure for Enhanced Photocatalytic H2 Production. Appl. Catal. B Environ. 2019, 240, 64–71. [Google Scholar] [CrossRef]
- Fei, B.; Tang, Y.; Wang, X.; Dong, X.; Liang, J.; Fei, X.; Xu, L.; Song, Y.; Zhang, F. One-Pot Synthesis of Porous g-C3N4 Nanomaterials with Different Morphologies and Their Superior Photocatalytic Performance. Mater. Res. Bull. 2018, 102, 209–217. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Yang, Q.; Zhang, Z.; Fang, X. Three-Dimensional g-C3N4 Aggregates of Hollow Bubbles with High Photocatalytic Degradation of Tetracycline. Carbon 2018, 136, 103–112. [Google Scholar] [CrossRef]
- Shahzeydi, A.; Ghiaci, M.; Farrokhpour, H.; Shahvar, A.; Sun, M.; Saraji, M. Facile and Green Synthesis of Copper Nanoparticles Loaded on the Amorphous Carbon Nitride for the Oxidation of Cyclohexane. Chem. Eng. J. 2019, 370, 1310–1321. [Google Scholar] [CrossRef]
- Yang, H.; Lv, K.; Zhu, J.; Li, Q.; Tang, D.; Ho, W.; Li, M.; Carabineiro, S.A.C. Effect of Mesoporous g-C3N4 Substrate on Catalytic Oxidation of CO over Co3O4. Appl. Surf. Sci. 2017, 401, 333–340. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, D.; Qin, Y.; Xia, J.; Jiang, D.; Chen, H.-Y. C3N4 Nanosheet Modified Microwell Array with Enhanced Electrochemiluminescence for Total Analysis of Cholesterol at Single Cells. Anal. Chem. 2017, 89, 2216–2220. [Google Scholar] [CrossRef]
- Ashrafi, H.; Akhond, M.; Absalan, G. Adsorption and Photocatalytic Degradation of Aqueous Methylene Blue Using Nanoporous Carbon Nitride. J. Photochem. Photobiol. A Chem. 2020, 396, 112533. [Google Scholar] [CrossRef]
- Shawky, A.; Albukhari, S.M.; Amin, M.S.; Zaki, Z.I. Mesoporous V2O5/g-C3N4 Nanocomposites for Promoted Mercury (II) Ions Reduction Under Visible Light. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4209–4221. [Google Scholar] [CrossRef]
- Qi, K.; Cui, N.; Zhang, M.; Ma, Y.; Wang, G.; Zhao, Z.; Khataee, A. Ionic Liquid-Assisted Synthesis of Porous Boron-Doped Graphitic Carbon Nitride for Photocatalytic Hydrogen Production. Chemosphere 2021, 272, 129953. [Google Scholar] [CrossRef]
- Li, P.; Liu, L.; An, W.; Wang, H.; Cui, W. Efficient Photothermal Catalytic CO2 Reduction to CH3CH2OH over Cu2O/g-C3N4 Assisted by Ionic Liquids. Appl. Surf. Sci. 2021, 565, 150448. [Google Scholar] [CrossRef]
- Chen, F.; Huang, S.; Xu, Y.; Huang, L.; Wei, W.; Xu, H.; Li, H. Novel Ionic Liquid Modified Carbon Nitride Fabricated by in Situ Pyrolysis of 1-Butyl-3-Methylimidazolium Cyanamide to Improve Electronic Structure for Efficiently Degradation of Bisphenol A. Colloid Surf. A Physicochem. Eng. Asp. 2021, 610, 125648. [Google Scholar] [CrossRef]
- Peer, M.; Lusardi, M.; Jensen, K.F. Facile Soft-Templated Synthesis of High-Surface Area and Highly Porous Carbon Nitrides. Chem. Mater. 2017, 29, 1496–1506. [Google Scholar] [CrossRef]
- Gao, F.; Hu, J.; Peng, C.; Liu, H.; Hu, Y. Synergic Effects of Imidazolium Ionic Liquids on P123 Mixed Micelles for Inducing Micro/Mesoporous Materials. Langmuir 2012, 28, 2950–2959. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Y.; Wang, Y.; Zhou, Y.; Qiu, K.; Zhang, C.; Fang, J.; Sheng, X. Ionic Liquid-Assisted Synthesis of Br-Modified g-C3N4 Semiconductors with High Surface Area and Highly Porous Structure for Photoredox Water Splitting. J. Power Sources 2017, 370, 106–113. [Google Scholar] [CrossRef]
- Yan, H. Soft-Templating Synthesis of Mesoporous Graphitic Carbon Nitride with Enhanced Photocatalytic H2 Evolution under Visible Light. Chem. Commun. 2012, 48, 3430–3432. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Chen, G.; Zhou, Y.; Yu, Y.; Zheng, Y.; Hao, S. The Facile Synthesis of Mesoporous g-C3N4 with Highly Enhanced Photocatalytic H2 Evolution Performance. Chem. Commun. 2015, 51, 16244–16246. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wang, N.; Tian, Y.; Ai, S.; Zhan, J. Acetic Acid Induced Synthesis of Laminated Activated Carbon Nitride Nanostructures. Carbon 2016, 107, 747–753. [Google Scholar] [CrossRef]
- Iqbal, W.; Dong, C.; Xing, M.; Tan, X.; Zhang, J. Eco-Friendly One-Pot Synthesis of Well-Adorned Mesoporous g-C3N4 with Efficiently Enhanced Visible Light Photocatalytic Activity. Catal. Sci. Technol. 2017, 7, 1726–1734. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Jiang, D.; Men, K.; Li, Z.; Li, L.; Sun, S.; Li, J.; Huang, Z.-H.; Wang, L.-N. Facile Synthesis of Bimodal Macroporous g-C3N4/SnO2 Nanohybrids with Enhanced Photocatalytic Activity. Sci. Bull. 2019, 64, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Shi, J.; Zhang, G.; Fang, Y.; Anpo, M.; Wang, X. The Facile Synthesis of Graphitic Carbon Nitride from Amino Acid and Urea for Photocatalytic H2 Production. Res. Chem. Intermed. 2017, 43, 5137–5152. [Google Scholar] [CrossRef]
- Ismael, M.; Wu, Y.; Taffa, D.H.; Bottke, P.; Wark, M. Graphitic Carbon Nitride Synthesized by Simple Pyrolysis: Role of Precursor in Photocatalytic Hydrogen Production. New J. Chem. 2019, 43, 6909–6920. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Takanabe, K.; Maeda, K.; Domen, K.; Epping, J.D.; Fu, X.; Antonietti, M.; Wang, X. Synthesis of a Carbon Nitride Structure for Visible-Light Catalysis by Copolymerization. Angew. Chem. Int. Ed. 2010, 49, 441–444. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, G.; Chen, X.; Lin, S.; Moehlmann, L.; Dolega, G.; Lipner, G.; Antonietti, M.; Blechert, S.; Wang, X. Co-Monomer Control of Carbon Nitride Semiconductors to Optimize Hydrogen Evolution with Visible Light. Angew. Chem. Int. Ed. 2012, 51, 3183–3187. [Google Scholar] [CrossRef]
- Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Peng, S.; Lu, G.; Li, S. Eosin Y-Sensitized Graphitic Carbon Nitride Fabricated by Heating Urea for Visible Light Photocatalytic Hydrogen Evolution: The Effect of the Pyrolysis Temperature of Urea. Phys. Chem. Chem. Phys. 2013, 15, 7657–7665. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, J.; Zong, R.; Zhu, Y. Enhancement of Visible Light Photocatalytic Activities via Porous Structure of g-C3N4. Appl. Catal. B Environ. 2014, 147, 229–235. [Google Scholar] [CrossRef]
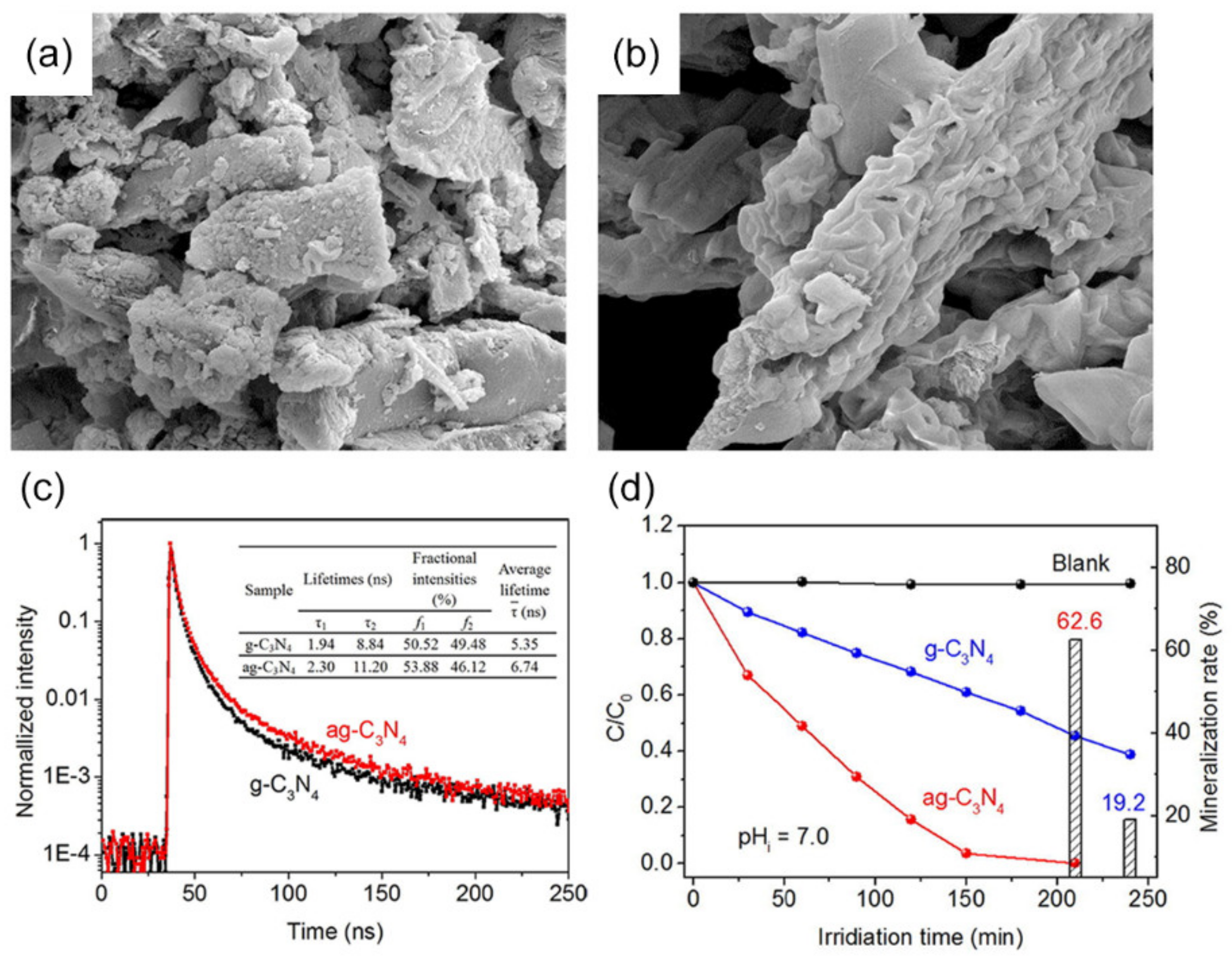
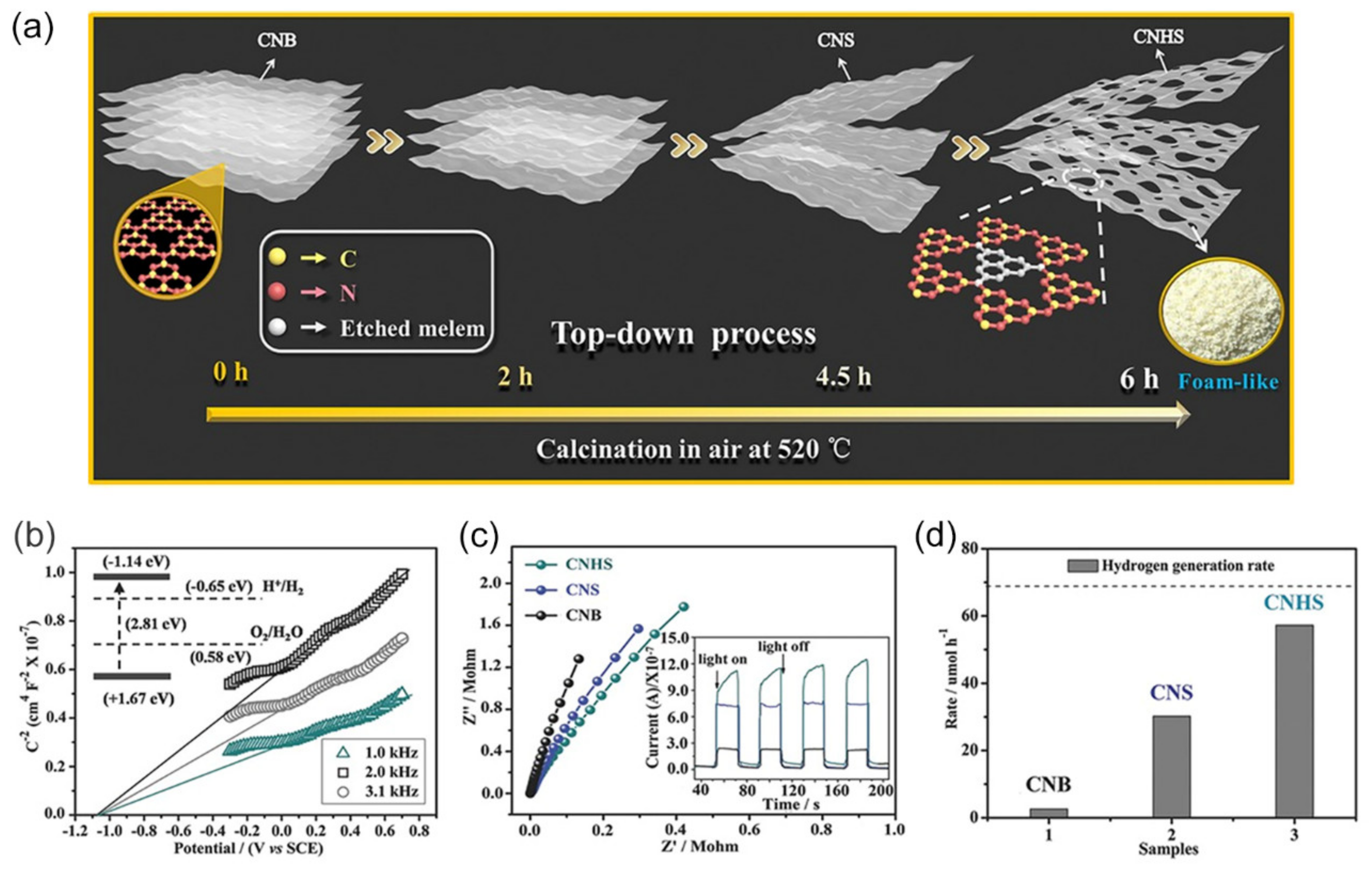
- Huang, H.; Xiao, K.; Tian, N.; Dong, F.; Zhang, T.; Du, X.; Zhang, Y. Template-Free Precursor-Surface-Etching Route to Porous, Thin g-C3N4 Nanosheets for Enhancing Photocatalytic Reduction and Oxidation Activity. J. Mater. Chem. A 2017, 5, 17452–17463. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Zhu, Y. Nanoporous Graphitic Carbon Nitride with Enhanced Photocatalytic Performance. Langmuir 2013, 29, 10566–10572. [Google Scholar] [CrossRef]
- Bai, J.; Yin, C.; Xu, H.; Chen, G.; Ni, Z.; Wang, Z.; Li, Y.; Kang, S.; Zheng, Z.; Li, X. Facile Urea-assisted Precursor Pre-treatment to Fabricate Porous g-C3N4 Nanosheets for Remarkably Enhanced Visible-light-driven Hydrogen Evolution. J. Colloid Interface Sci. 2018, 532, 280–286. [Google Scholar] [CrossRef]
- Liang, L.; Cong, Y.; Wang, F.; Yao, L.; Shi, L. Hydrothermal Pre-Treatment induced Cyanamide to Prepare Porous g-C3N4 with Boosted Photocatalytic Performance. Diam. Relat. Mater. 2019, 98, 107499. [Google Scholar] [CrossRef]
- Butt, F.K.; Hauenstein, P.; Kosiahn, M.; Garlyyev, B.; Dao, M.; Lang, A.; Scieszka, D.; Liang, Y.; Kreuzpaintner, W. An Innovative Microwave-Assisted Method for the Synthesis of Mesoporous Two Dimensional g-C3N4: A Revisited Insight into a Potential Electrode Material for Supercapacitors. Microporous Mesoporous Mater. 2020, 294, 109853. [Google Scholar] [CrossRef]
- Wang, L.; Hong, Y.; Liu, E.; Duan, X.; Lin, X.; Shi, J. A bottom-up acidification strategy engineered ultrathin g-C3N4 nanosheets towards boosting photocatalytic hydrogen evolution. Carbon 2020, 163, 234–243. [Google Scholar] [CrossRef]
- Shen, J.; Yang, H.; Shen, Q.; Feng, Y.; Cai, Q.; Yang, H. Template-Free Synthesis of Three-Dimensional Nanoporous Bulk Graphitic Carbon Nitride with Remarkably Enhanced Photocatalytic Activity and Good Separation Properties. Eur. J. Inorg. Chem. 2015, 2015, 2611–2618. [Google Scholar] [CrossRef]
- Li, B.; Si, Y.; Fang, Q.; Shi, Y.; Huang, W.-Q.; Hu, W.; Pan, A.; Fan, X.; Huang, G.-F. Hierarchical Self-assembly of Well-Defined Louver-Like P-Doped Carbon Nitride Nanowire Arrays with Highly Efficient Hydrogen Evolution. Nano-Micro Lett. 2020, 12, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, G.; Zhang, L. Porous Structure Dependent Photoreactivity of Graphitic Carbon Nitride under Visible Light. J. Mater. Chem. 2012, 22, 1160–1166. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Hu, J.-Y.; Jiang, H. Facile Modification of a Graphitic Carbon Nitride Catalyst to Improve Its Photoreactivity under Visible Light Irradiation. Chem. Eng. J. 2014, 256, 230–237. [Google Scholar] [CrossRef]
- Xie, L.; Ding, Y.; Wang, X.; Xu, W. Chlorine-Assisted Fabrication of Hybrid Supramolecular Structures via Electrostatic Interactions. Phys. Chem. Chem. Phys. 2019, 21, 9357–9361. [Google Scholar] [CrossRef]
- Shen, B.; Hong, Z.; Chen, Y.; Lin, B.; Gao, B. Template-Free Synthesis of a Novel Porous g-C3N4 with 3D Hierarchical Structure for Enhanced Photocatalytic H2 Evolution. Mater. Lett. 2014, 118, 208–211. [Google Scholar] [CrossRef]
- Liao, Y.; Zhu, S.; Ma, J.; Sun, Z.; Yin, C.; Zhu, C.; Lou, X.; Zhang, D. Tailoring the Morphology of g-C3N4 by Self-Assembly towards High Photocatalytic Performance. ChemCatChem 2014, 6, 3419–3425. [Google Scholar] [CrossRef]
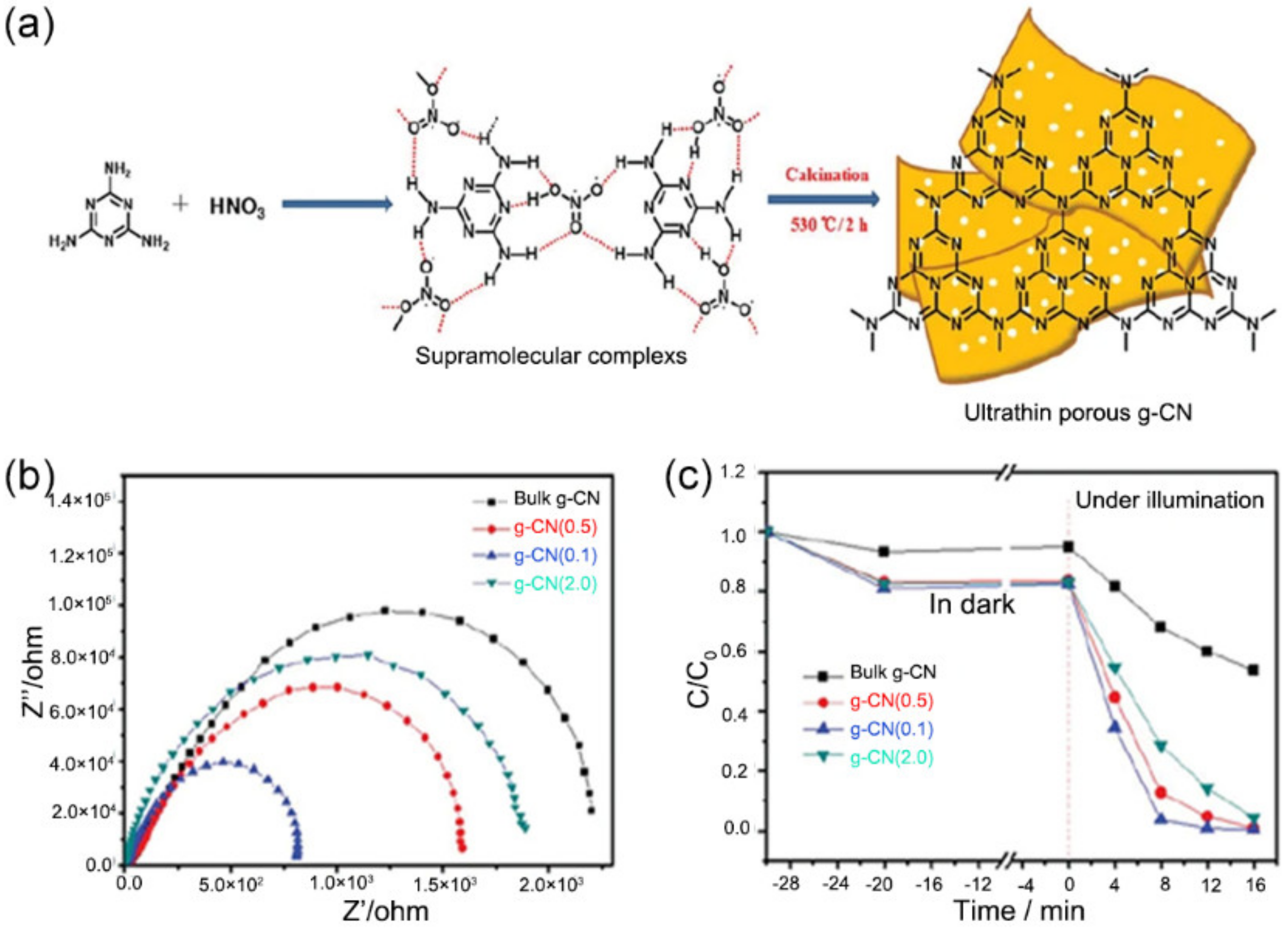
- Wang, X.; Zhou, C.; Shi, R.; Liu, Q.; Waterhouse, G.I.N.; Wu, L.; Tung, C.-H.; Zhang, T. Supramolecular Precursor Strategy for the Synthesis of Holey Graphitic Carbon Nitride Nanotubes with Enhanced Photocatalytic Hydrogen Evolution Performance. Nano Res. 2019, 12, 2385–2389. [Google Scholar] [CrossRef]
- Wu, M.; Gong, Y.; Nie, T.; Zhang, J.; Wang, R.; Wang, H.; He, B. Template-free synthesis of nanocage-like g-C3N4 with high surface area and nitrogen defects for enhanced photocatalytic H2 activity. J. Mater. Chem. A 2019, 7, 5324–5332. [Google Scholar] [CrossRef]
- Zhu, B.; Xu, G.; Li, X.; Wang, Z.; Lv, J.; Shu, X.; Huang, J.; Zheng, Z.; Wu, Y. Ultrathin Porous g-CN Nanosheets Fabricated by Direct Calcination of Pre-Treated Melamine for Enhanced Photocatalytic Performance. J. Mater. Res. 2019, 34, 3462–3473. [Google Scholar] [CrossRef]
- Piradashvili, K.; Alexandrino, E.M.; Wurm, F.R.; Landfester, K. Reactions and Polymerizations at the Liquid–Liquid Interface. Chem. Rev. 2016, 116, 2141–2169. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, S.; Song, Q.; Huang, Z.; Xu, J.-F.; Zhang, X. Supramolecular Interfacial Polymerization: A Controllable Method of Fabricating Supramolecular Polymeric Materials. Angew. Chem. Int. Ed. 2017, 56, 7639–7643. [Google Scholar] [CrossRef]
- Dolai, S.; Karjule, N.; Azoulay, A.; Barrio, J. Monomer sequence design at two solvent interface enables the synthesis of highly photoactive carbon nitride. RSC Adv. 2019, 9, 26091–26096. [Google Scholar] [CrossRef] [Green Version]
- Dolai, S.; Barrio, J.; Peng, G.; Grafmüller, A.; Shalom, M. Tailoring carbon nitride properties and photoactivity by interfacial engineering of hydrogen-bonded frameworks. Nanoscale 2019, 11, 5564–5570. [Google Scholar] [CrossRef]
- Azoulay, A.; Barrio, J.; Shalom, M. Modifying Crystallinity, Morphology, and Photophysical Properties of Carbon Nitride by Using Crystals as Reactants. Isr. J. Chem. 2020, 60, 544–549. [Google Scholar] [CrossRef]
- Barrio, J.; Barzilai, S.; Karjule, N.; Amo-Ochoa, P.; Zamora, F.; Shalom, M. Synergistic Doping and Surface Decoration of Carbon Nitride Macrostructures by Single Crystal Design. ACS Appl. Energy Mater. 2021, 4, 1868–1875. [Google Scholar] [CrossRef]
- Barrio, J.; Lin, L.; Amo-Ochoa, P.; Tzadikov, J.; Peng, G.; Sun, J.; Zamora, F.; Wang, X.; Shalom, M. Unprecedented Centimeter-Long Carbon Nitride Needles: Synthesis, Characterization and Applications. Small 2018, 14, 1800633. [Google Scholar] [CrossRef]
- Barrio, J.; Barzilai, S.; Karjule, N.; Amo-Ochoa, P.; Zamora, F.; Shalom, M. Fluorescent Carbon Nitride Macrostructures Derived from Triazine-Based Cocrystals. Adv. Opt. Mater. 2021, 9, 2100683. [Google Scholar] [CrossRef]
- Fang, X.; Gao, R.; Yang, Y.; Yan, D. A Cocrystal Precursor Strategy for Carbon-Rich Graphitic Carbon Nitride toward High-Efficiency Photocatalytic Overall Water Splitting. iScience 2019, 16, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Wang, J.; Qian, X.; Dong, Y.; Xu, H.; Song, R.; Yan, C.; Zhu, H.; Zhong, Q.; Qian, G.; et al. One-pot synthesis of copper-doped graphitic carbon nitride nanosheet by heating Cu-melamine supramolecular network and its enhanced visible-light-driven photocatalysis. J. Solid State Chem. 2015, 228, 60–64. [Google Scholar] [CrossRef]
- Zhu, J.-N.; Zhu, X.-Q.; Cheng, F.-F.; Li, P.; Wang, F.; Xiao, Y.-W.; Xiong, W.-W. Preparing copper doped carbon nitride from melamine templated crystalline copper chloride for Fenton-like catalysis. Appl. Catal. B Environ. 2019, 256, 117830. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, X.; Chen, H.-S.; Hu, X.; Yang, P. Formation of g-C3N4 Nanotubes towards Superior Photocatalysis Performance. ChemCatChem 2019, 11, 4558–4567. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.; Qian, X.; Dong, Y.; Gao, J.; Qian, G.; Yao, J. Direct Synthesis of Porous Nanorod-Type Graphitic Carbon Nitride/CuO Composite from Cu-Melamine Supramolecular Framework towards Enhanced Photocatalytic Performance. Chem. Asian J. 2015, 10, 1276–1280. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Q.; Zhuang, J.; Guan, S.; Li, B. Molten Salt Assisted In-Situ Synthesis of TiO2/g-C3N4 Composites with Enhanced Visible-Light-Driven Photocatalytic Activity and Adsorption Ability. J. Photochem. Photobiol. A Chem. 2018, 362, 1–13. [Google Scholar] [CrossRef]
- Dong, F.; Li, Y.; Wang, Z.; Ho, W.-K. Enhanced Visible Light Photocatalytic Activity and Oxidation Ability of Porous Graphene-Like g-C3N4 Nanosheets via Thermal Exfoliation. Appl. Surf. Sci. 2015, 358, 393–403. [Google Scholar] [CrossRef]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid Exfoliation of Layered Materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Fan, H.; Wang, J.; Zhao, Y.; Tian, H.; Dong, G. Water-Assisted Ions In Situ Intercalation for Porous Polymeric Graphitic Carbon Nitride Nanosheets with Superior Photocatalytic Hydrogen Evolution Performance. Appl. Catal. B Environ. 2016, 190, 93–102. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, S.; Li, Z.; Ma, D.; Xu, G.; Fang, B. Mesoporous g-C3N4 Nanosheets with Improved Photocatalytic Performance for Hydrogen Evolution. Mater. Charact. 2021, 174, 111031. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Wang, H.; Zhang, Q.; Xie, J.; Tian, Y.; Wang, J.; Xie, Y. Single-Layered Graphitic-C3N4 Quantum Dots for Two-Photon Fluorescence Imaging of Cellular Nucleus. Adv. Mater. 2014, 26, 4438–4443. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, Y.; Yan, Y.; Min, Y.; Fan, J.; Xu, Q. Holey Structured Graphitic Carbon Nitride Thin Sheets with Edge Oxygen Doping via Photo-Fenton Reaction with Enhanced Photocatalytic Activity. Appl. Catal. B Environ. 2016, 185, 315–321. [Google Scholar] [CrossRef]
- Yang, Z.; Xing, Z.; Feng, Q.; Jiang, H.; Zhang, J.; Xiao, Y.; Li, Z.; Chen, P.; Zhou, W. Sandwich-Like Mesoporous Graphite-Like Carbon Nitride (Meso-g-C3N4)/WP/Meso-g-C3N4 Laminated Heterojunctions Solar-Driven Photocatalysts. J. Colloid Interface Sci. 2020, 568, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hu, C.; Ji, H.; Zhang, L.; Li, F. Facile Synthesis of Nitrogen-Deficient Mesoporous Graphitic Carbon Nitride for Highly Efficient Photocatalytic Performance. Appl. Surf. Sci. 2019, 478, 304–312. [Google Scholar] [CrossRef]
- Wu, W.; Li, X.; Ruan, Z.; Li, Y.; Xu, X.; Yuan, Y.; Lin, K. Fabrication of a TiO2 trapped meso/macroporous g-C3N4 heterojunction photocatalyst and understanding its enhanced photocatalytic activity based on optical simulation analysis. Inorg. Chem. Front. 2018, 5, 481–489. [Google Scholar] [CrossRef]
- Xu, J.; Shen, K.; Xue, B.; Li, Y.-X.; Cao, Y. Synthesis of Three-Dimensional Mesostructured Graphitic Carbon Nitride Materials and their Application as Heterogeneous Catalysts for Knoevenagel Condensation Reactions. Catal. Lett. 2013, 143, 600–609. [Google Scholar] [CrossRef]
- Wu, X.; Ma, H.; Zhong, W.; Fan, J.; Yu, H. Porous Crystalline g-C3N4: Bifunctional NaHCO3 Template-Mediated Synthesis and Improved Photocatalytic H2 Evolution Rate. Appl. Catal. B Environ. 2020, 271, 118899. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, Q.; Liu, Y.; Liu, Y.; Wang, K.; Xu, N.; Yu, L.; Tong, Q.; Fan, Y. The Photocatalytic Redox Properties of Polymeric Carbon Nitride Nanocages (PCNCs) with Mesoporous Hollow Spherical Structures Prepared by a ZnO-Template Method. Microporous Mesoporous Mater. 2020, 292, 109639. [Google Scholar] [CrossRef]
- Zhao, S.; Fang, J.; Wang, Y.; Zhang, Y.; Zhou, Y.; Zhuo, S. Construction of Three-Dimensional Mesoporous Carbon Nitride with High Surface area for Efficient Visible-Light-Driven Hydrogen Evolution. J. Colloid Interface Sci. 2020, 561, 601–608. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, G.; Zhang, H.; Li, Z.; Wen, Y. One-Pot Synthesis of Porous g-C3N4 Nanosheets with Enhanced Photocatalytic Activity under Visible Light. Diam. Relat. Mater. 2021, 116, 108416. [Google Scholar] [CrossRef]
- Fei, T.; Qin, C.; Zhang, Y.; Dong, G.; Wang, Y.; Zhou, Y.; Cui, M. A 3D Peony-Like Sulfur-Doped Carbon Nitride Synthesized by Self-Assembly for Efficient Photocatalytic Hydrogen Production. Int. J. Hydrog. Energy 2021, 46, 20481–20491. [Google Scholar] [CrossRef]
- She, X.; Liu, L.; Ji, H.; Mo, Z.; Li, Y.; Huang, L.; Du, D.; Xu, H.; Li, H. Template-Free Synthesis of 2D Porous Ultrathin Nonmetal-Doped g-C3N4 Nanosheets with Highly Efficient Photocatalytic H2 Evolution from Water Under Visible Light. Appl. Catal. B Environ. 2016, 187, 144–153. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Z.; Zhu, Y. Enhanced Visible-Light-Driven Photocatalytic Disinfection Performance and Organic Pollutant Degradation Activity of Porous g-C3N4 Nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 27727–27735. [Google Scholar] [CrossRef]
- Mei, Z.; Wang, G.; Yan, S.; Wang, J. Rapid Microwave-Assisted Synthesis of 2D/1D ZnIn2S4/TiO2 S-Scheme Heterojunction for Catalyzing Photocatalytic Hydrogen Evolution. Acta Phys. Chim. Sin. 2021, 37, 2009097. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, J.; He, B.-B.; Wang, H.-W.; Wang, R.; Gong, Y.-S. In-Situ Construction of Coral-Like Porous P-Doped g-C3N4 Tubes with Hybrid 1D/2D Architecture and High Efficient Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2019, 241, 159–166. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Li, J.; Lu, Z.; Wang, X. Self-Assembled Synthesis of Oxygen-Doped g-C3N4 Nanotubes in Enhancement of Visible-Light Photocatalytic Hydrogen. J. Energy Chem. 2021, 54, 36–44. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K. State-of-the-Art Review of Morphological Advancements in Graphitic Carbon Nitride (g-CN) for Sustainable Hydrogen Production. Renew. Sustain. Energy Rev. 2021, 135, 110235. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Wang, X.; Wu, Y.; Su, Y.; Tang, H. 3D/2D Direct Z-Scheme Heterojunctions of Hierarchical TiO2 Microflowers/g-C3N4 Nanosheets with Enhanced Charge Carrier Separation for Photocatalytic H2 Evolution. Carbon 2019, 149, 618–626. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, R.; Huang, J.; Jiang, Y.; Zhao, C.; Yang, X. In Situ Construction of Protonated g-C3N4/Ti3C2 MXene Schottky Heterojunctions for Efficient Photocatalytic Hydrogen Production. Chin. J. Catal. 2021, 42, 107–114. [Google Scholar] [CrossRef]
- Ye, R.; Fang, H.; Zheng, Y.-Z.; Li, N.; Wang, Y.; Tao, X. Fabrication of CoTiO3/g-C3N4 Hybrid Photocatalysts with Enhanced H2 Evolution: Z-Scheme Photocatalytic Mechanism Insight. ACS Appl. Mater. Interfaces 2016, 8, 13879–13889. [Google Scholar] [CrossRef]
- Chen, X.; Shi, R.; Chen, Q.; Zhang, Z.; Jiang, W.; Zhu, Y.; Zhang, T. Three-Dimensional Porous g-C3N4 for Highly Efficient Photocatalytic Overall Water Splitting. Nano Energy 2019, 59, 644–650. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, J.; Yang, X.; Zhang, T.; Tang, H. 3D Reduced Graphene Oxide Aerogel-Mediated Z-Scheme Photocatalytic System for Highly Efficient Solar-Driven Water Oxidation and Removal of Antibiotics. Appl. Catal. B Environ. 2018, 232, 562–573. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Jiang, J.; Wan, Z.; Su, Y.; Tang, H. Insight into Charge Carrier Separation and Solar-Light Utilization: rGO Decorated 3D ZnO Hollow Microspheres for Enhanced Photocatalytic Hydrogen Evolution. J. Colloid Interface Sci. 2020, 564, 322–332. [Google Scholar] [CrossRef]
- Wen, P.; Sun, Y.; Li, H.; Liang, Z.; Wu, H.; Zhang, J.; Zeng, H.; Geyer, S.M.; Jiang, L. A Highly Ective Three-Dimensional Z-Scheme ZnO/Au/g-C3N4 Photocathode for Efficient Photoelectrochemical Water Splitting. Appl. Catal. B Environ. 2020, 263, 118180. [Google Scholar] [CrossRef]
- Niu, H.; Zhao, W.; Lv, H.; Yang, Y.; Cai, Y. Accurate Design of Hollow/Tubular Porous g-C3N4 from Melamine-Cyanuric Acid Supramolecular Prepared with Mechanochemical Method. Chem. Eng. J. 2021, 411, 128400. [Google Scholar] [CrossRef]
- Wang, T.; Meng, X.; Li, P.; Ouyang, S.; Chang, K.; Liu, G.; Mei, Z.; Ye, J. Photoreduction of CO2 over the Well-Crystallized Ordered Mesoporous TiO2 with the Confined Space Effect. Nano Energy 2014, 9, 50–60. [Google Scholar] [CrossRef]
- Elbanna, O.; Fujitsuka, M.; Majima, T. g-C3N4/TiO2 Mesocrystals Composite for H2 Evolution under Visible-Light Irradiation and Its Charge Carrier Dynamics. ACS Appl. Mater. Interfaces 2017, 9, 34844–34854. [Google Scholar] [CrossRef]
- Niu, P.; Yin, L.-C.; Yang, Y.-Q.; Liu, G.; Cheng, H.-M. Increasing the Visible Light Absorption of Graphitic Carbon Nitride (Melon) Photocatalysts by Homogeneous Self-Modification with Nitrogen Vacancies. Adv. Mater. 2014, 26, 8046–8052. [Google Scholar] [CrossRef]
- Liang, Q.; Li, Z.; Huang, Z.-H.; Kang, F.; Yang, Q.-H. Holey Graphitic Carbon Nitride Nanosheets with Carbon Vacancies for Highly Improved Photocatalytic Hydrogen Production. Adv. Funct. Mater. 2015, 25, 6885–6892. [Google Scholar] [CrossRef]
- Tu, W.; Xu, Y.; Wang, J.; Zhang, B.; Zhou, T.; Yin, S.; Wu, S.; Li, C.; Huang, Y.; Zhou, Y.; et al. Investigating the Role of Tunable Nitrogen Vacancies in Graphitic Carbon Nitride Nanosheets for Efficient Visible-Light-Driven H2 Evolution and CO2 Reduction. ACS Sustain. Chem. Eng. 2017, 5, 7260–7268. [Google Scholar] [CrossRef] [Green Version]
- Sagara, N.; Kamimura, S.; Tsubota, T.; Ohno, T. Photoelectrochemical CO2 Reduction by a P-Type Boron-Doped g-C3N4 Electrode under Visible Light. Appl. Catal. B Environ. 2016, 192, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, M.; Tahir, M.; Praserthdam, P. Effect of Nonmetals (B, O, P, and S) Doped with Porous g-C3N4 for Improved Electron Transfer Towards Photocatalytic CO2 Reduction with Water into CH4. Chemosphere 2022, 286, 131765. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, Y.; Yan, L.; Su, Z. DFT Study on Sulfur-Doped g-C3N4 Nanosheets as a Photocatalyst for CO2 Reduction Reaction. J. Phys. Chem. C 2018, 122, 7712–7719. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Liu, B.; Cheng, B.; Ho, W.; Yu, J. Sulfur-Doped g-C3N4 with Enhanced Photocatalytic CO2-Reduction Performance. Appl. Catal. B Environ. 2015, 176, 44–52. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, B.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical Porous O-Doped g-C3N4 with Enhanced Photocatalytic CO2 Reduction Activity. Small 2017, 13, 1603938. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, C.; Li, H.H.; Zhao, Y.F.; Gong, Y.Y.; Niu, L.Y.; Liu, X.J.; Wang, T. The Effects of Nonmetal Dopants on the Electronic, Optical and Chemical Performances of Monolayer g-C3N4 by First-Principles Study. Appl. Surf. Sci. 2017, 392, 966–974. [Google Scholar] [CrossRef]
- Liao, G.; Gong, Y.; Zhang, L.; Gao, H.; Yang, G.-J.; Fang, B. Semiconductor Polymeric Graphitic Carbon Nitride Photocatalysts: The “Holy Grail” for Photocatalytic Hydrogen Evolution Reaction under Visible Light. Energy Environ. Sci. 2019, 12, 2080–2147. [Google Scholar] [CrossRef]
- Liu, B.; Ye, L.; Wang, R.; Yang, J.; Zhang, Y.; Guan, R.; Tian, L.; Chen, X. Phosphorus-Doped Graphitic Carbon Nitride Nanotubes with Amino-rich Surface for Efficient CO2 Capture, Enhanced Photocatalytic Activity, and Product Selectivity. ACS Appl. Mater. Interfaces 2018, 10, 4001–4009. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Zeng, Y.; Guo, H.; Wan, S.; Ou, M.; Zhang, S.; Zhong, Q. Amino-Assisted NH2-UiO-66 Anchored on Porous g-C3N4 for Enhanced Visible-Light-Driven CO2 Reduction. ACS Appl. Mater. Interfaces 2019, 11, 30673–30681. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.-X.; Tang, J. Visible-Light Driven Heterojunction Photocatalysts for Water Splitting—A Critical Review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Zhang, P.; Song, T.; Wang, T.; Zeng, H. Effectively Extending Visible Light Absorption with a Broad Spectrum Sensitizer for Improving the H2 Evolution of In-Situ Cu/g-C3N4 Nanocomponents. Int. J. Hydrog. Energy 2017, 42, 14511–14521. [Google Scholar] [CrossRef]
- Shi, L.; Wang, T.; Zhang, H.; Chang, K.; Ye, J. Electrostatic Self-Assembly of Nanosized Carbon Nitride Nanosheet onto a Zirconium Metal-Organic Framework for Enhanced Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2015, 25, 5360–5367. [Google Scholar] [CrossRef]
- Wang, S.; He, F.; Zhao, X.; Zhang, J.; Ao, Z.; Wu, H.; Yin, Y.; Shi, L.; Xu, X.; Zhao, C.; et al. Phosphorous Doped Carbon Nitride Nanobelts for Photodegradation of Emerging Contaminants and Hydrogen Evolution. Appl. Catal. B Environ. 2019, 257, 117931. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Xie, Y. Photoresponsive Polymeric Carbon Nitride-Based Materials: Design and Application. Mater. Today 2019, 23, 72–86. [Google Scholar] [CrossRef]
- Huang, J.; Ho, W.; Wang, X. Metal-Free Disinfection Effects Induced by Graphitic Carbon Nitride Polymers under Visible Light Illumination. Chem. Commun. 2014, 50, 4338–4340. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Wang, X.; Liang, Y.; An, W.; Hu, J.; Zhu, Y.; Cui, W. Removal of Chromium (VI) by a Self-Regenerating and Metal free g-C3N4/Fraphene Hydrogel System via the Synergy of Adsorption and Photo-catalysis under Visible Light. Appl. Catal. B Environ. 2017, 219, 53–62. [Google Scholar] [CrossRef]
- Zhao, J.; Li, B.; Liu, Z.; Dai, D.; Li, Y.; Shi, R.; Zhang, H. A Novel Solar-Triggered MIL-125(Ti)/g-C3N4/SA Composite Aerogel with High Catalytic Activity for Degradation of Organic Contaminants. Sep. Purif. Technol. 2021, 279, 119696. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, W.; Liu, D.; Wang, J.; Liu, Y.; Zhu, Y.; Zhu, Y. Photodegradation of Phenol via C3N4-Agar Hybrid Hydrogel 3D Photocatalysts with Free Separation. Appl. Catal. B Environ. 2016, 183, 263–268. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, C.; Hao, D.; Wei, W.; Wang, L.; Ni, B.-J. Ultralight Biodegradable 3D-g-C3N4 Aerogel for Advanced Oxidation Water Treatment Driven by Oxygen Delivery Channels and Triphase Interfaces. J. Clean. Prod. 2021, 288, 125091. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, H.; Jin, D.; Yang, H.; He, D.; Zhang, Z.; Zhang, Y.; Qu, J.; Zhang, Y.-N. Effective Degradation of Norfloxacin on Ag3PO4/CNTs Photoanode: Z-Scheme Mechanism, Reaction Pathway, and Toxicity Assessment. Chem. Eng. J. 2022, 429, 132092. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, X.; Waterhouse, G.I.N.; Zhang, Z.-M.; Yu, L.-M. Efficient Photoelectrocatalytic Degradation of Azo-Dyes over Polypyrrole/Titanium Oxide/Reduced Graphene Oxide Electrodes under Visible Light: Performance Evaluation and Mechanism Insights. Chemosphere 2022, 288, 132509. [Google Scholar] [CrossRef]
- Liang, F.; Zhu, Y. Enhancement of Mineralization Ability for Phenol via Synergetic Effect of Photoelectrocatalysis of g-C3N4 Film. Appl. Catal. B Environ. 2016, 180, 324–329. [Google Scholar] [CrossRef]
- Qi, F.; Yang, B.; Wang, Y.; Mao, R.; Zhao, X. H2O2 Assisted Photoelectrocatalytic Oxidation of Ag-Cyanide Complexes at Metal-free g-C3N4 Photoanode with Simultaneous Ag Recovery. ACS Sustain. Chem. Eng. 2017, 5, 5001–5007. [Google Scholar] [CrossRef]
- Qiao, M.; Wu, X.; Zhao, S.; Djellabi, R.; Zhao, X. Peroxymonosulfate Enhanced Photocatalytic Decomposition of Silver-Cyanide Complexes Using g-C3N4 Nanosheets with Simultaneous Recovery of Silver. Appl. Catal. B Environ. 2020, 265, 118587. [Google Scholar] [CrossRef]
- Zhao, X.; Pan, D.; Chen, X.; Li, R.; Jiang, T.; Wang, W.; Li, G.; Leung, D.Y.C. g-C3N4 Photoanode for Photoelectrocatalytic Synergistic Pollutant Degradation and Hydrogen Evolution. Appl. Surf. Sci. 2019, 467-468, 658–665. [Google Scholar] [CrossRef]
- Wu, L.; Li, F.; Xu, Y.; Zhang, J.W.; Zhang, D.; Li, G.; Li, H. Plasmon-Induced Photoelectrocatalytic Activity of Au Nanoparticles Enhanced TiO2 Nanotube Arrays Electrodes for Environmental Remediation. Appl. Catal. B Environ. 2015, 164, 217–224. [Google Scholar] [CrossRef]


| Sample | Template | Removal Reagents | Pore Size (nm) | Pore Volume (cm3·g−1) | SSA (m2·g−1) | Photocatalytic Application (Efficiency) | References |
|---|---|---|---|---|---|---|---|
| Hard-template method | |||||||
| Meso-g-C3N4/WP/Meso-g-C3N4 | SiO2 | HF | 12 | - | 82 | H2 evolution (198.1 μmol·h−1·g−1) | [171] |
| Porous-C3N4 | SiO2 | HF | 5–40 | - | 209 | MB degradation (85% in 180 min) | [85] |
| mpg-C3N4-δ | SiO2 | NH4HF2 | 12.54 | 0.69 | 218.15 | RhB degradation (30.2% in 30 min) | [172] |
| mesoporous g-C3N4 | SiO2 | NH4HF2 | 12 | 0.52 | 190.7 | U(VI) reduction (73% in 60 min) | [87] |
| TiO2 trapped g-C3N4 | PSB | NH4HF2 | 27 | 0.30 | 37 | RhB degradation (100% in 50 min) | [173] |
| CN-MCF-0.4 | MCF | NH4HF2 | 5.3 | 1.36 | 498 | Knoevenagel condensation (93.6% in 240 min) | [174] |
| C3N4-MCF | MCF | HF | 5/20 | 0.30 | 70 | CO2 reduction (8.0 μmol·g1) | [94] |
| g-C3N4(GLBM-SBA-15) | SBA-15 | NH4HF2 | 11/90 | 0.43 | 145 | MO degradation (90% in 30 min) | [83] |
| macrostructure g-C3N4 | NaCl | H2O | - | - | 16.71 | H2 evolution (459 μmol·h1·g−1) | [109] |
| Na+ functionalized porous g-C3N4 nanorods | Na2S2O3 | H2O | 4.25 | - | 29.72 | H2 evolution (9796 μmol·h1·g−1) | [103] |
| Na2CO3-embedded porous crystalline g-C3N4 | NaHCO3 | H2O | 3/30 | 0.09 | 15.34 | H2 evolution (1010 μmol·h1·g1) | [175] |
| polymeric CN nanocages | ZnO | HCl and NH3·H2O | 16.8 | 0.103 | 32 | H2 evolution (227.23 μmol·h1·g−1) | [176] |
| Soft-template method | |||||||
| g-C3N4 | P123 | - | 20 | - | 90 | H2 evolution (60.6 μmol·h1) | [123] |
| 3D mesoporous CN | ionic liquid | - | 15 | 0.85 | 381 | H2 evolution (129.5 μmol·h−1) | [177] |
| hollow mesoporous g-C3N4 sphere | ionic liquid | - | 9.7 | - | 84 | H2 evolution (157 μmol·h−1) | [28] |
| g-C3N4/SnO2 | HCl, H2O | - | 100/430 | 2.638 | 44.3 | MB degradation (72% in 100 min) | [127] |
| porous g-C3N4 nanosheets | NH3, CO2 | - | 5/70 | - | 92.75 | H2 evolution (1437 μmol·h1·g−1) | [178] |
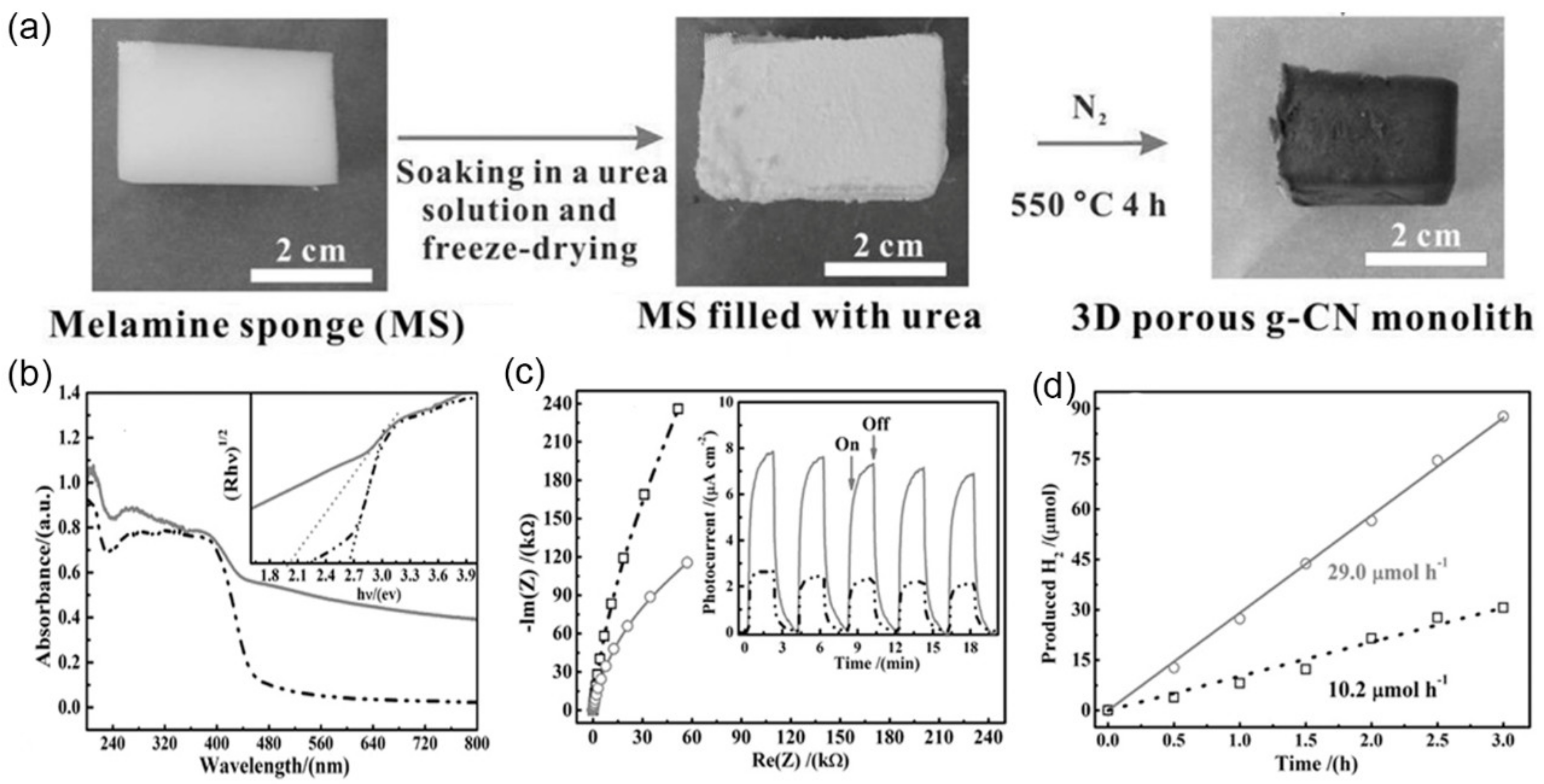
| Macroscopic 3D porous g-C3N4 monolith | melamine | - | 4/30–170 | 0.76 | 78 | H2 evolution (29 μmol·h−1) | [30] |
| Template-free method | |||||||
| Thin-layered g-C3N4 nanosheets | - | - | 4/20–100 | 0.752 | 92.8 | H2 evolution (1391 mmol·h1·g−1) | [86] |
| g-C3N4/TiO2 | - | - | 11 | 0.193 | 70.2 | RhB degradation (0.0478 min−1) | [80] |
| S-doped CN | - | - | 17.04 | 0.464 | 108.9 | H2 evolution (567.7 μmol·h1·g−1) | [179] |
| oxygen-doped g-C3N4 nanosheets | - | - | 5–100 | - | - | H2 evolution (189.3 μmol·h−1) | [180] |
| Porous g-C3N4 nanosheets | - | - | 5–25 | 0.61 | 190.1 | MB degradation (44.7% in 30 min) | [181] |
| mpg-C3N4/rGO | - | - | 10–80 | 0.419 | 85 | RhB degradation (99.7% in 40 min) | [61] |
| porous g-C3N4 | - | - | 4.5/40.5 | 0.211 | 44.2 | H2 evolution (99.1 μmol·h−1) | [135] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, J.; Zhang, Y.; Hussain, M.I.; Zhou, W.; Chen, Y.; Wang, L.-N. g-C3N4: Properties, Pore Modifications, and Photocatalytic Applications. Nanomaterials 2022, 12, 121. https://doi.org/10.3390/nano12010121
Dong J, Zhang Y, Hussain MI, Zhou W, Chen Y, Wang L-N. g-C3N4: Properties, Pore Modifications, and Photocatalytic Applications. Nanomaterials. 2022; 12(1):121. https://doi.org/10.3390/nano12010121
Chicago/Turabian StyleDong, Jiaqi, Yue Zhang, Muhammad Irfan Hussain, Wenjie Zhou, Yingzhi Chen, and Lu-Ning Wang. 2022. "g-C3N4: Properties, Pore Modifications, and Photocatalytic Applications" Nanomaterials 12, no. 1: 121. https://doi.org/10.3390/nano12010121
APA StyleDong, J., Zhang, Y., Hussain, M. I., Zhou, W., Chen, Y., & Wang, L.-N. (2022). g-C3N4: Properties, Pore Modifications, and Photocatalytic Applications. Nanomaterials, 12(1), 121. https://doi.org/10.3390/nano12010121





