Nanoplasmonic Strip Test for Salivary Glucose Monitoring
Abstract
:1. Introduction
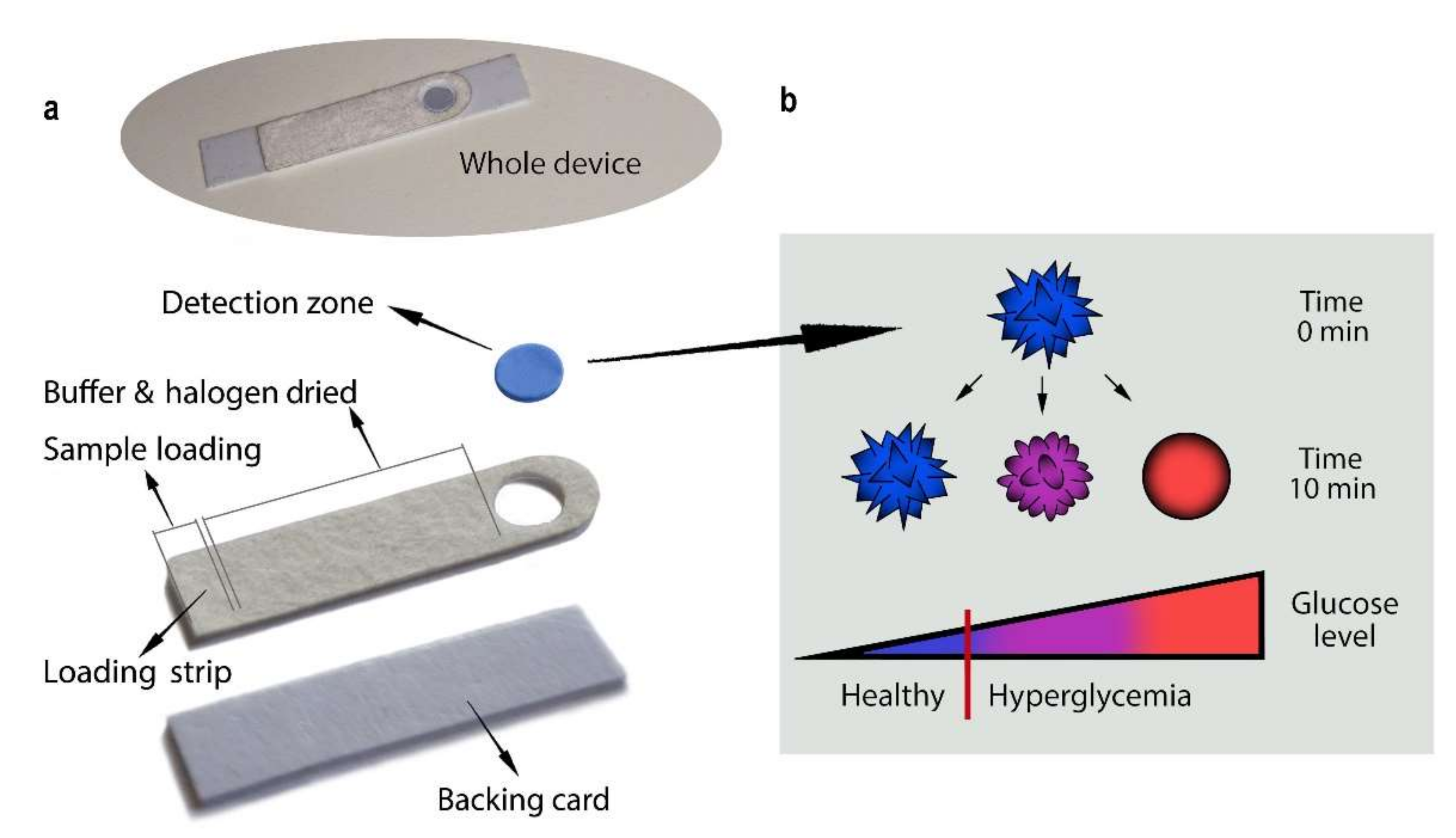
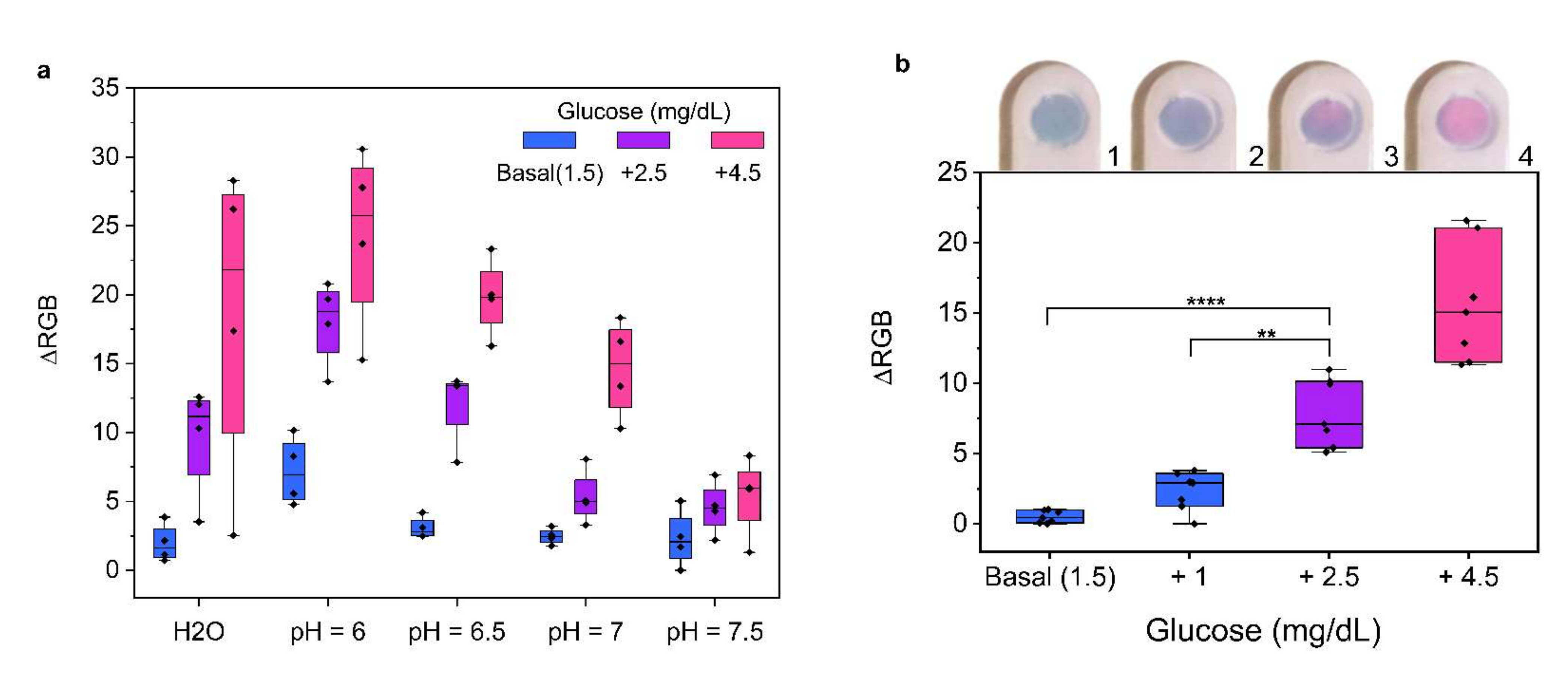
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 546–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, R.M.M.; Chua, Z.J.Y.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. From pre-diabetes to diabetes: Diagnosis, treatments and translational research. Medicina 2019, 55, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, F.; Pretty, C.G.; Signal, M.; Shaw, G.; Chase, J.G. Accuracy and performance of continuous glucose monitors in athletes. Biomed. Signal Process. Control 2017, 32, 124–129. [Google Scholar] [CrossRef]
- Flockhart, M.; Nilsson, L.C.; Tais, S.; Ekblom, B.; Apró, W.; Larsen, F.J. Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metab. 2021, 33, 957–970. [Google Scholar] [CrossRef]
- Todo, H. Continuous glucose monitoring can disclose glucose fluctuation in advanced Parkinsonian syndromes. Neurol. Int. 2018, 10, 107–110. [Google Scholar] [CrossRef] [Green Version]
- Land, K.J.; Boeras, D.I.; Chen, X.S.; Ramsay, A.R.; Peeling, R.W. REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat. Microbiol. 2019, 4, 46–54. [Google Scholar] [CrossRef]
- Gonzales, W.V.; Mobashsher, A.T.; Abbosh, A. The progress of glucose monitoring—A review of invasive to minimally and non-invasive techniques, devices and sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef] [Green Version]
- Zafra-Tanaka, J.H.; Beran, D.; Vetter, B.; Sampath, R.; Bernabe-Ortiz, A. Technologies for Diabetes Self-Monitoring: A Scoping Review and Assessment Using the REASSURED Criteria. J. Diabetes Sci. Technol. 2021, 4504–4511. [Google Scholar] [CrossRef]
- Avari, P.; Reddy, M.; Oliver, N. Is it possible to constantly and accurately monitor blood sugar levels, in people with Type 1 diabetes, with a discrete device (non-invasive or invasive)? Diabet. Med. 2020, 37, 532–544. [Google Scholar] [CrossRef]
- Bernabé-Ortiz, A.; Zafra-Tanaka, J.H.; Moscoso-Porras, M.; Sampath, R.; Vetter, B.; Miranda, J.J.; Beran, D. Diagnostics and monitoring tools for noncommunicable diseases: A missing component in the global response. Glob. Health 2021, 17, 1–5. [Google Scholar] [CrossRef]
- Aguirre-Romero, A.B.; Galeano-Valle, F.; Conde-Montero, E.; Velázquez-Tarjuelo, D.; de-la-Cueva-Dobao, P. Efficacy and safety of a rosehip seed oil extract in the prevention and treatment of skin lesions in the hands of patients with type 1 diabetes mellitus caused by finger prick blood glucose monitoring; a randomized, open-label, controlled clinical trial. Endocrinol. Diabetes Nutr. 2020, 67, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.K.; Thorsen, S.U.; Thyssen, J.P.; Zachariae, C.; Keiding, H.; Svensson, J. Cost of treating skin problems in patients with diabetes who use insulin pumps and/or glucose sensors. Diabetes Technol. Ther. 2020, 22, 658–665. [Google Scholar] [CrossRef]
- Deiss, D.; Irace, C.; Carlson, G.; Tweden, K.S.; Kaufman, F.R. Real-World Safety of an Implantable Continuous Glucose Sensor over Multiple Cycles of Use: A Post-Market Registry Study. Diabetes Technol. Ther. 2020, 22, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Seget, S.; Rusak, E.; Partyka, M.; Samulska, E.; Pyziak-Skupień, A.; Kamińska, H.; Skała-Zamorowska, E.; Jarosz-Chobot, P. Bacterial strains colonizing the sensor electrodes of a continuous glucose monitoring system in children with diabetes. Acta Diabetol. 2021, 58, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Hussain, J.N.; Mantri, N.; Cohen, M.M. Working up a good sweat—The challenges of standardising sweat collection for metabolomics analysis. Clin. Biochem. Rev. 2017, 38, 13–34. [Google Scholar]
- Pieczyński, J.; Szulc, U.; Harazna, J.; Szulc, A.; Kiewisz, J. Tear fluid collection methods: Review of current techniques. Eur. J. Ophthalmol. 2021, 31, 2245–2251. [Google Scholar] [CrossRef]
- Boselli, L.; Pomili, T.; Donati, P.; Pompa, P.P. Nanosensors for visual detection of glucose in biofluids: Are we ready for instrument-free home-testing? Materials 2021, 14, 1978. [Google Scholar] [CrossRef]
- Mosca, A.C.; Stieger, M.; Neyraud, E.; Brignot, H.; van de Wiel, A.; Chen, J. How are macronutrient intake, BMI, ethnicity, age, and gender related to the composition of unstimulated saliva? A case study. J. Texture Stud. 2019, 50, 53–61. [Google Scholar] [CrossRef]
- Chen, H.; Shi, Q.; Deng, G.; Chen, X.; Yang, Y.; Lan, W.; Hu, Y.; Zhang, L.; Xu, L.; Li, C.; et al. Rapid and highly sensitive colorimetric biosensor for the detection of glucose and hydrogen peroxide based on nanoporphyrin combined with bromine as a peroxidase-like catalyst. Sens. Actuators B Chem. 2021, 343, 130104. [Google Scholar] [CrossRef]
- Jung, D.G.; Jung, D.; Kong, S.H. A lab-on-a-chip-based non-invasive optical sensor for measuring glucose in saliva. Sensors 2017, 17, 2607. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.; Vázquez-Villegas, P.; Martínez-Chapa, S.O. Paper and fiber-based bio-diagnostic platforms: Current challenges and future needs. Appl. Sci. 2017, 7, 863. [Google Scholar] [CrossRef] [Green Version]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Donati, P.; Pomili, T.; Boselli, L.; Pompa, P.P. Colorimetric nanoplasmonics to spot hyperglycemia from saliva. Front. Bioeng. Biotechnol. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Parolo, C.; Sena-Torralba, A.; Bergua, J.F.; Calucho, E.; Fuentes-Chust, C.; Hu, L.; Rivas, L.; Álvarez-Diduk, R.; Nguyen, E.P.; Cinti, S.; et al. Tutorial: Design and fabrication of nanoparticle-based lateral-flow immunoassays. Nat. Protoc. 2020, 15, 3788–3816. [Google Scholar] [CrossRef]
- Maiorano, G.; Rizzello, L.; Malvindi, M.A.; Shankar, S.S.; Martiradonna, L.; Falqui, A.; Cingolani, R.; Pompa, P.P. Monodispersed and size-controlled multibranched gold nanoparticles with nanoscale tuning of surface morphology. Nanoscale 2011, 3, 2227–2232. [Google Scholar] [CrossRef]
- Pomili, T.; Donati, P.; Pompa, P.P. Paper-Based Multiplexed Colorimetric Device for the Simultaneous Detection of Salivary Biomarkers. Biosensors 2021, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sucre-Rosales, E.; Byram, A.; Hernandez, F.E.; Chen, G. Ultrasensitive Visual Detection of Glucose in Urine Based on the Iodide-Promoted Etching of Gold Bipyramids. ACS Appl. Mater. Interfaces 2020, 12, 49502–49509. [Google Scholar] [CrossRef]
- Yuan, H.; Janssen, K.P.F.; Franklin, T.; Lu, G.; Su, L.; Gu, X.; Uji-I, H.; Roeffaers, M.B.J.; Hofkens, J. Reshaping anisotropic gold nanoparticles through oxidative etching: The role of the surfactant and nanoparticle surface curvature. RSC Adv. 2015, 5, 49502–49509. [Google Scholar] [CrossRef]
- Kubala, E.; Strzelecka, P.; Grzegocka, M.; Lietz-Kijak, D.; Gronwald, H.; Skomro, P.; Kijak, E. A review of selected studies that determine the physical and chemical properties of saliva in the field of dental treatment. Biomed. Res. Int. 2018, 2018, 6572381. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torné-Morató, H.; Donati, P.; Pompa, P.P. Nanoplasmonic Strip Test for Salivary Glucose Monitoring. Nanomaterials 2022, 12, 105. https://doi.org/10.3390/nano12010105
Torné-Morató H, Donati P, Pompa PP. Nanoplasmonic Strip Test for Salivary Glucose Monitoring. Nanomaterials. 2022; 12(1):105. https://doi.org/10.3390/nano12010105
Chicago/Turabian StyleTorné-Morató, Helena, Paolo Donati, and Pier Paolo Pompa. 2022. "Nanoplasmonic Strip Test for Salivary Glucose Monitoring" Nanomaterials 12, no. 1: 105. https://doi.org/10.3390/nano12010105
APA StyleTorné-Morató, H., Donati, P., & Pompa, P. P. (2022). Nanoplasmonic Strip Test for Salivary Glucose Monitoring. Nanomaterials, 12(1), 105. https://doi.org/10.3390/nano12010105






