Nitrogen-Containing Gas Sensing Properties of 2-D Ti2N and Its Derivative Nanosheets: Electronic Structures Insight
Abstract
:1. Introduction
2. Methods
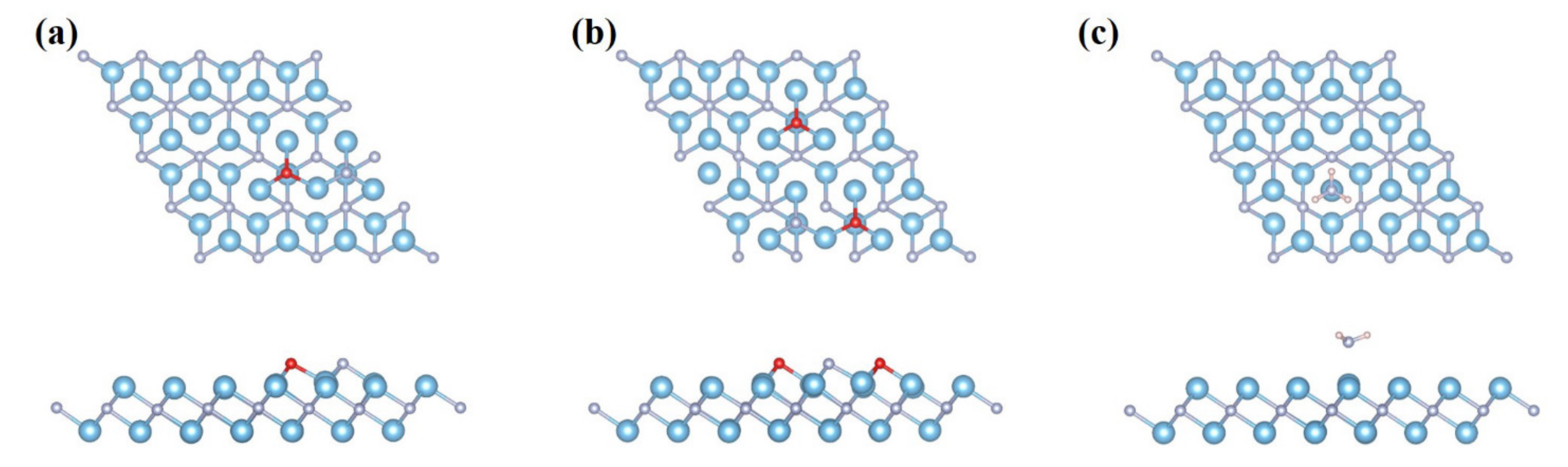
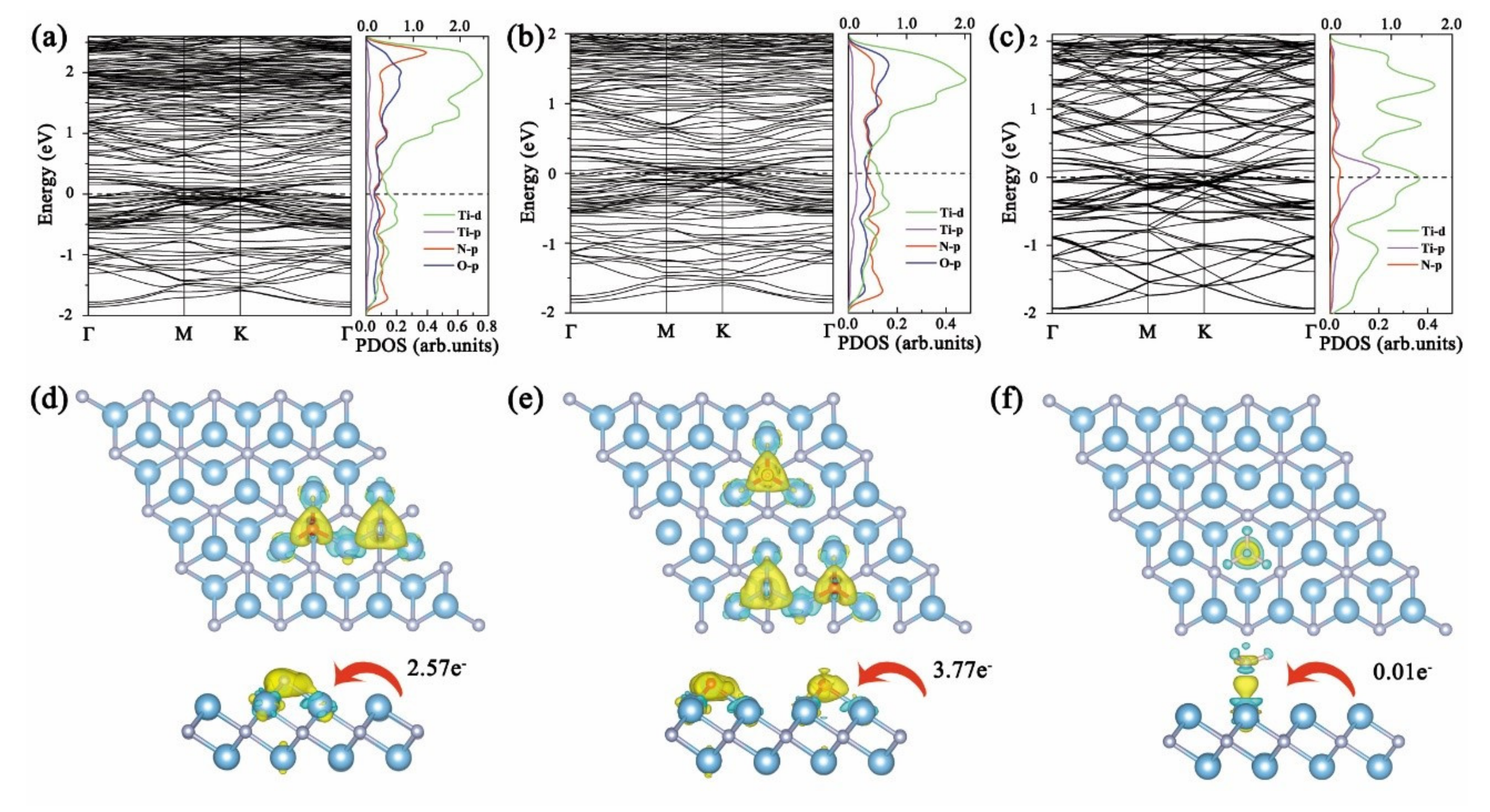
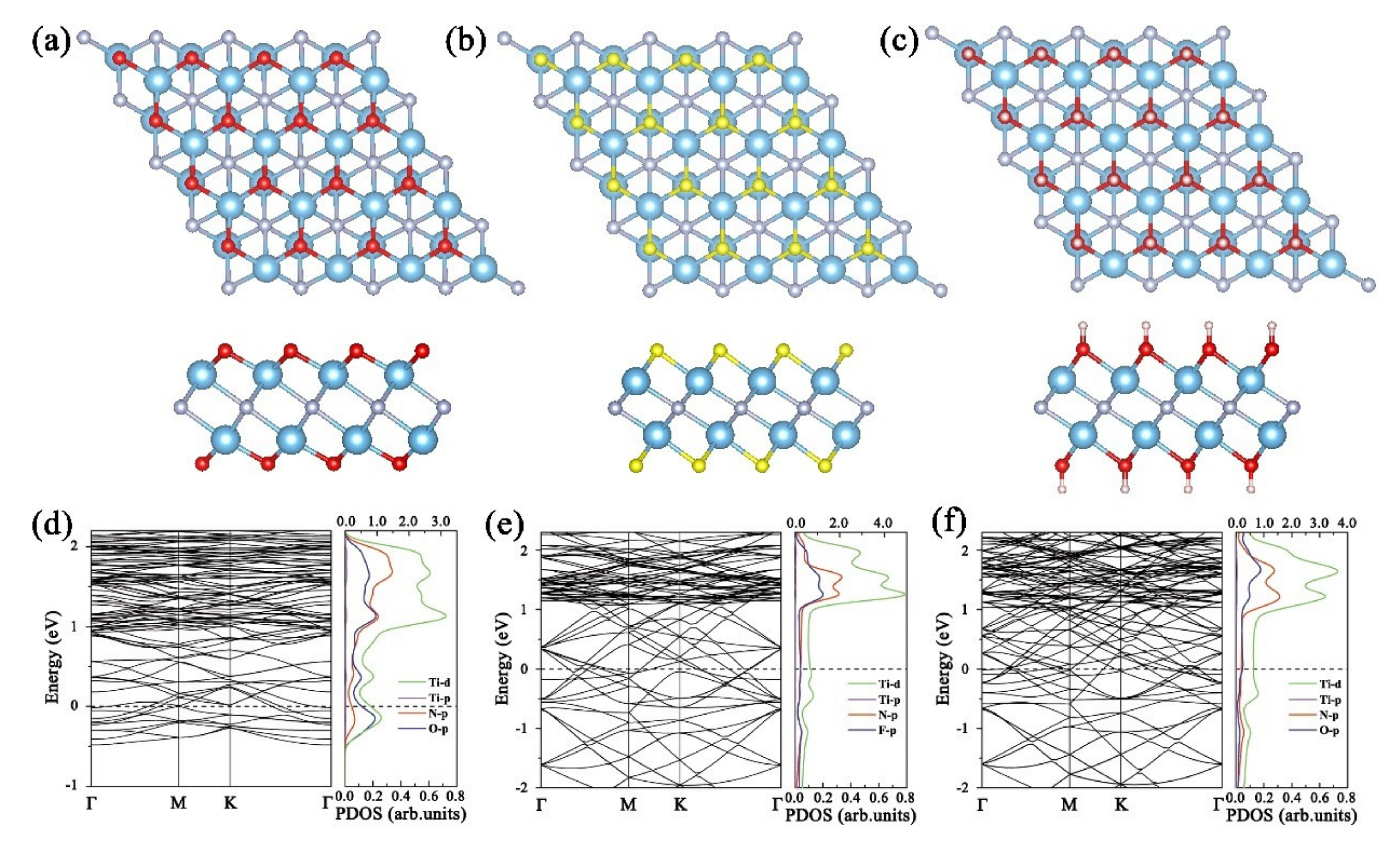
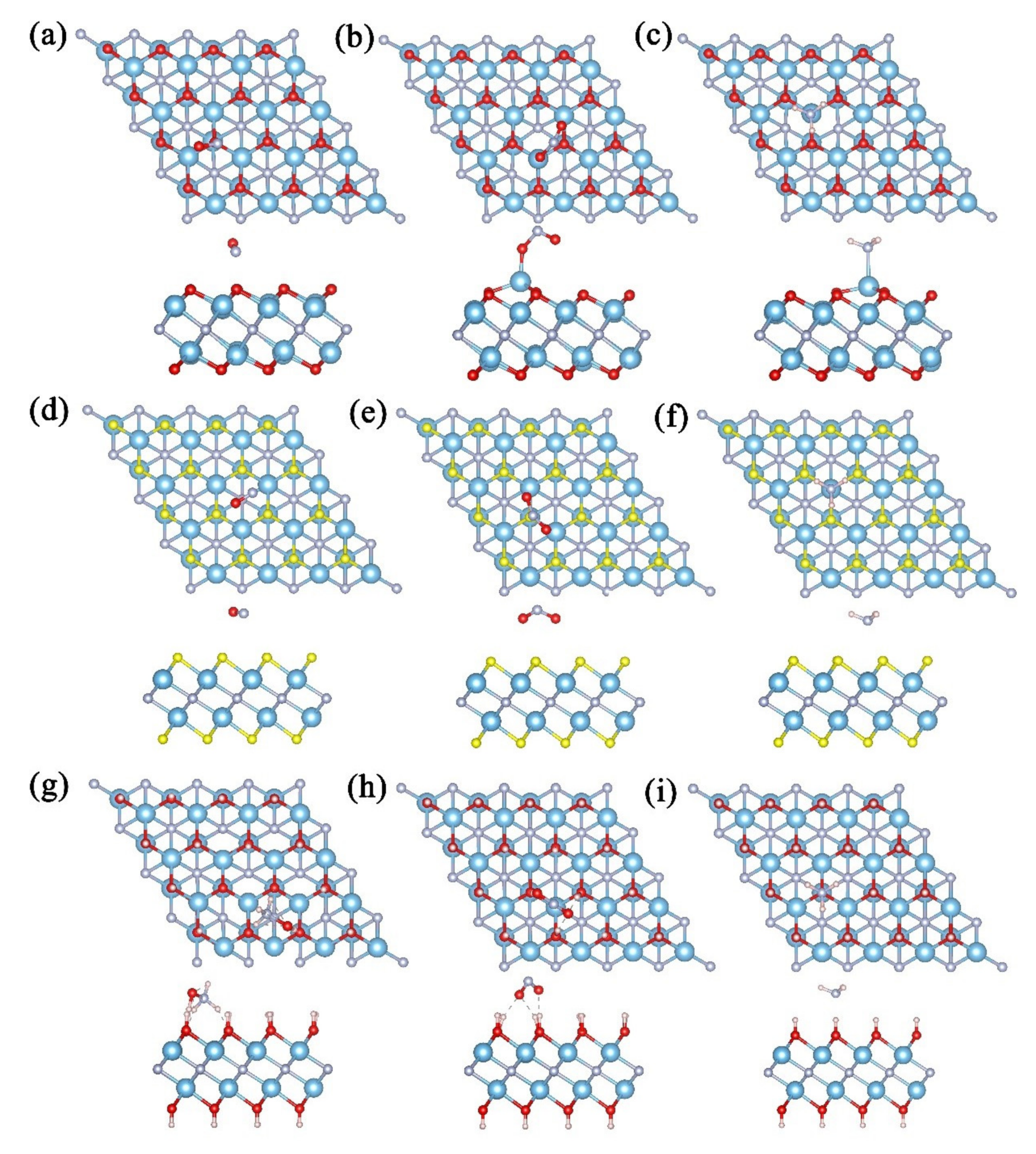
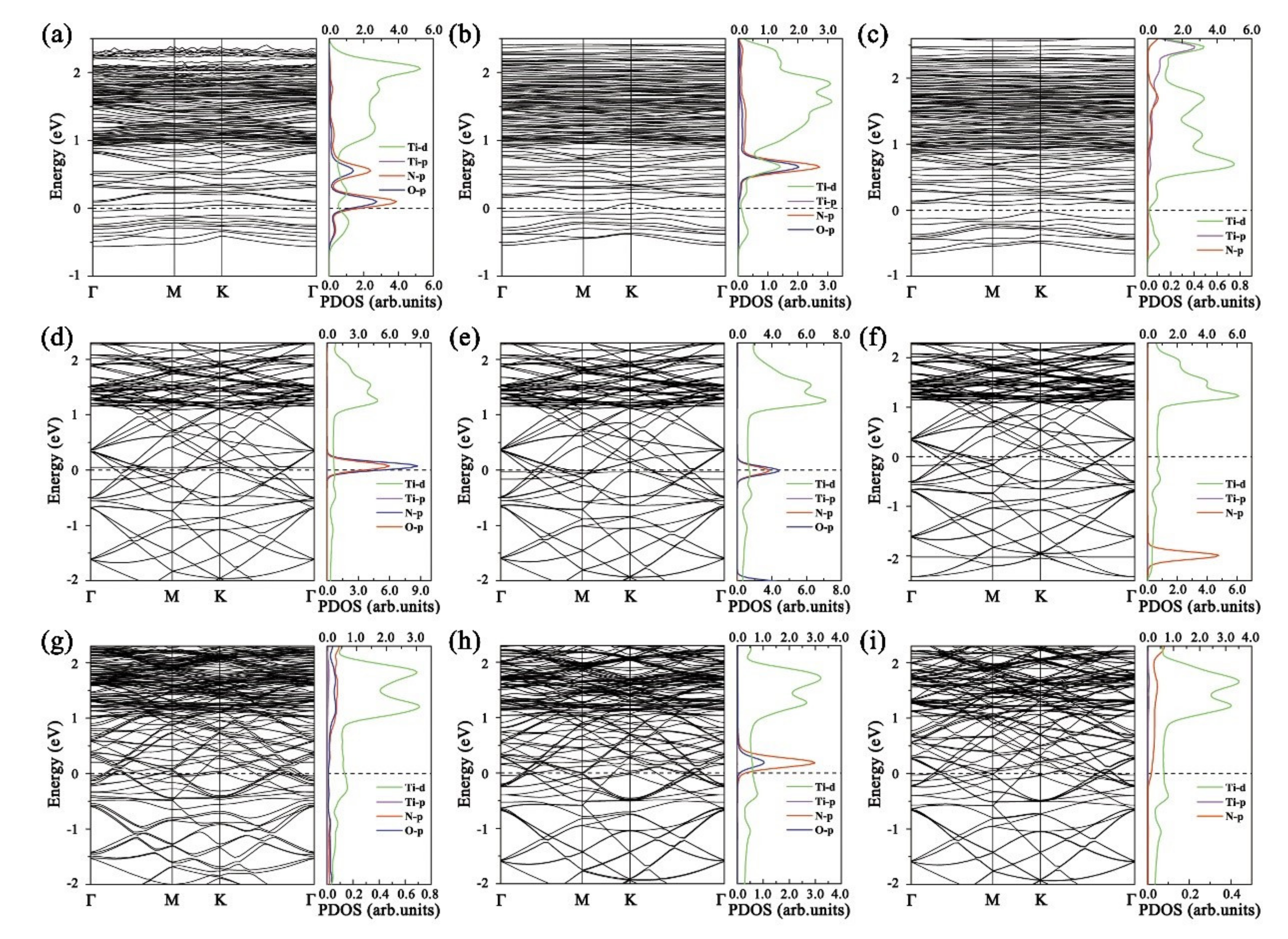
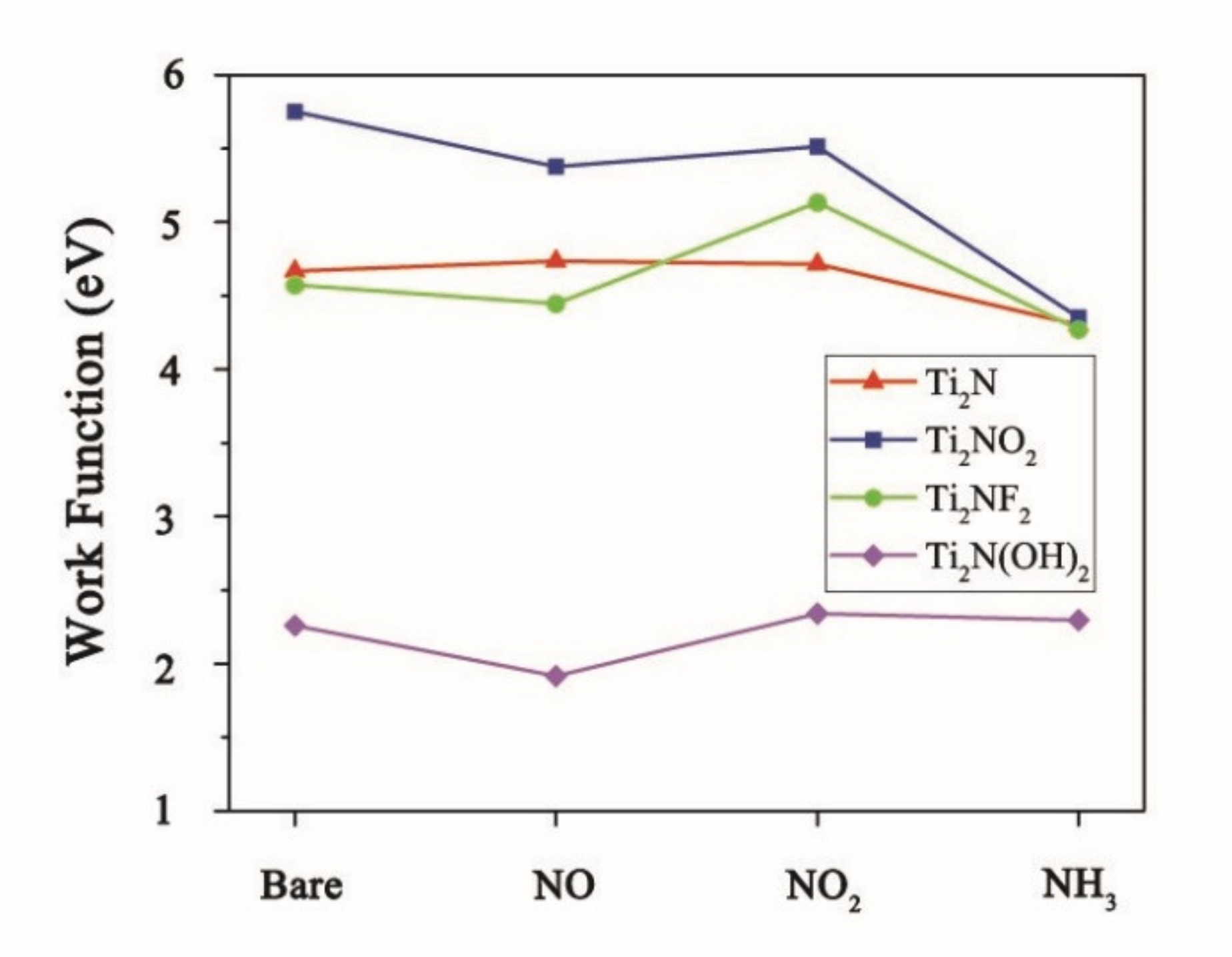
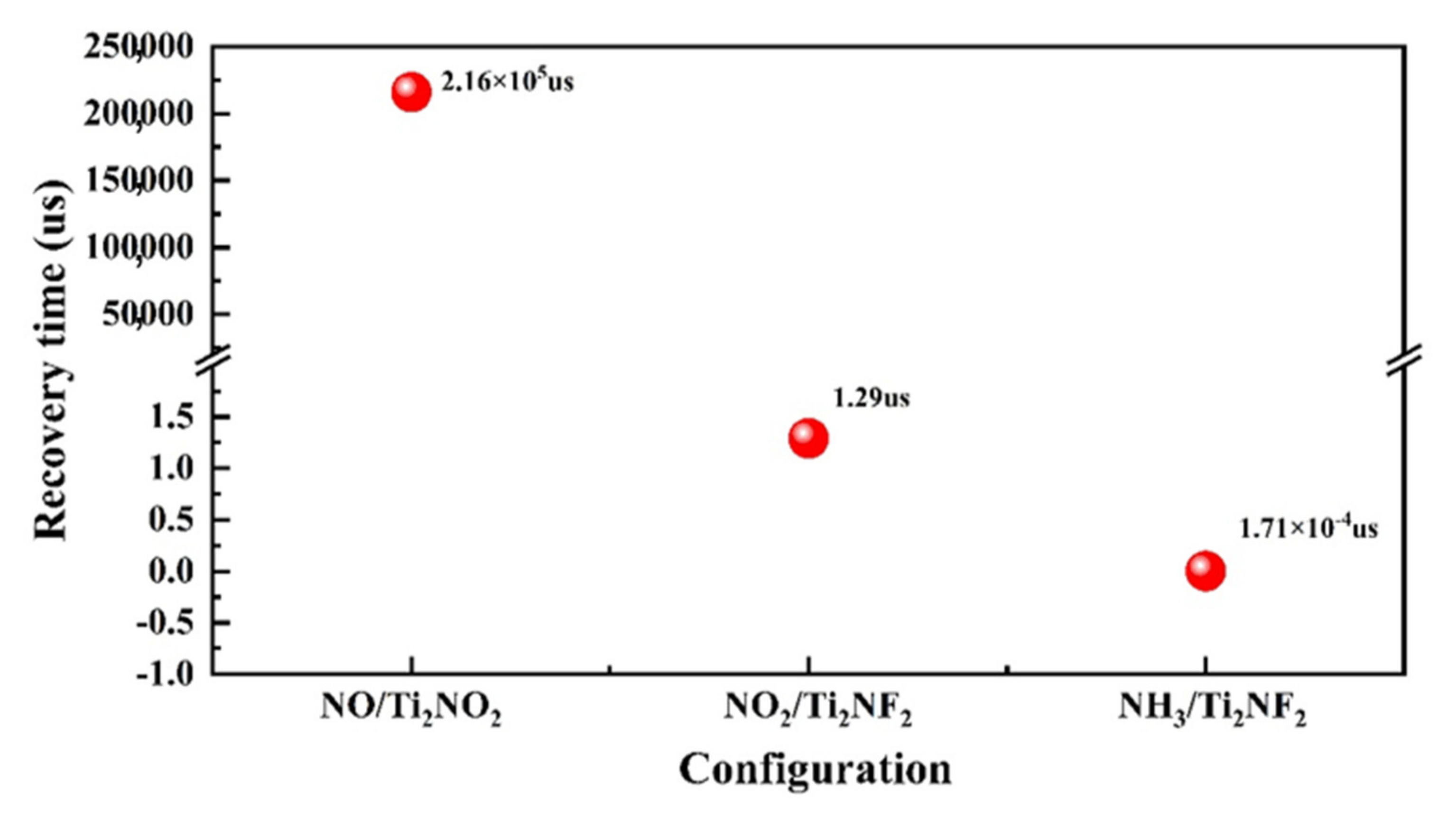
3. Results and Discussion
4. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramanathan, V.; Feng, Y. Air pollution, greenhouse gases and climate change: Global and regional perspectives. Atmos. Environ. 2009, 43, 37–50. [Google Scholar] [CrossRef]
- Koolen, C.D.; Rothenberg, G. Air pollution in Europe. ChemSusChem 2018, 12, 164–172. [Google Scholar] [CrossRef]
- Hasselblad, V.; Eddy, D.M.; Kotchmar, D.J. Synthesis of environmental evidence: Nitrogen dioxide epidemiology studies. J. Air Waste Manag. Assoc. 1992, 42, 662–671. [Google Scholar] [CrossRef]
- Garrett, M.H.; Hooper, M.A.; Hooper, B.M.; Abramson, M.J. Respiratory symptoms in children and indoor exposure to nitrogen dioxide and gas stoves. Am. J. Respir. Crit. Care Med. 1998, 158, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, T.; Qian, Z.; Ahuja, R.; Liu, X. First-principles investigation of CO adsorption on pristine, C-doped and N-vacancy defected hexagonal AlN nanosheets. Appl. Surf. Sci. 2018, 439, 196–201. [Google Scholar] [CrossRef]
- Liu, Y.; Parisi, J.; Sun, X.; Lei, Y. Solid-state gas sensors for high temperature applications—A review. J. Mater. Chem. A 2014, 2, 9919–9943. [Google Scholar] [CrossRef]
- Tang, X.; Du, A.; Kou, L. Gas sensing and capturing based on two-dimensional layered materials: Overview from theoretical perspective. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1361. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Chen, Y.-B.; Zhou, K.-G.; Liu, C.; Zeng, J.; Zhang, H.-L.; Peng, Y. Improving gas sensing properties of graphene by introducing dopants and defects: A first-principles study. Nanotechnology 2009, 20, 185504. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Yang, Q.; Wang, Z.; Ye, H.; Chen, X.; Fan, X.; Zhang, G. High Selective Gas Detection for small molecules based on Germanium selenide monolayer. Appl. Surf. Sci. 2018, 433, 575–581. [Google Scholar] [CrossRef]
- Li, F.; Shi, C. NO-sensing performance of vacancy defective monolayer MoS2 predicted by density function theory. Appl. Surf. Sci. 2018, 434, 294–306. [Google Scholar] [CrossRef]
- Yu, X.-F.; Li, Y.-C.; Cheng, J.-B.; Liu, Z.-B.; Li, Q.-Z.; Li, W.-Z.; Yang, X.; Xiao, B. Monolayer Ti2CO2: A Promising Candidate for NH3 Sensor or Capturer with High Sensitivity and Selectivity. ACS Appl. Mater. Interfaces 2015, 7, 13707–13713. [Google Scholar] [CrossRef]
- Xiao, B.; Li, Y.-C.; Yu, X.-F.; Cheng, J.-B. MXenes: Reusable materials for NH3 sensor or capturer by controlling the charge injection. Sens. Actuators B Chem. 2016, 235, 103–109. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Feng, A.; Yu, Y.; Wang, Y.; Jiang, F.; Yu, Y.; Mi, L.; Song, L. Two-dimensional MXene Ti3C2 produced by exfoliation of Ti3AlC2. Mater. Des. 2017, 114, 161–166. [Google Scholar] [CrossRef]
- Lashgari, H.; Abolhassani, M.; Boochani, A.; Elahi, S.; Khodadadi, J. Electronic and optical properties of 2D graphene-like compounds titanium carbides and nitrides: DFT calculations. Solid State Commun. 2014, 195, 61–69. [Google Scholar] [CrossRef]
- Weng, H.; Ranjbar, A.; Liang, Y.; Song, Z.; Khazaei, M.; Yunoki, S.; Arai, M.; Kawazoe, Y.; Fang, Z.; Dai, X. Large-gap two-dimensional topological insulator in oxygen functionalized MXene. Phys. Rev. B 2015, 92, 075436–0754367. [Google Scholar] [CrossRef] [Green Version]
- Pan, H. Electronic properties and lithium storage capacities of two-dimensional transition-metal nitride monolayers. J. Mater. Chem. A 2015, 3, 21486–21493. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Meng, X.; Wei, Y.; Zhao, Y.; Pang, Q.; Chen, G. Two-dimensional VS2 monolayers as potential anode materials for lithium-ion batteries and beyond: First-principles calculations. J. Mater. Chem. A 2017, 5, 21370–21377. [Google Scholar] [CrossRef]
- Kumar, H.; Frey, N.C.; Dong, L.; Anasori, B.; Gogotsi, Y.; Shenoy, V.B. Tunable magnetism and transport properties in nitride MXenes. ACS Nano 2017, 11, 7648–7655. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, Y.; Li, W.; Zhang, J. Investigation of two-dimensional hf-based MXenes as the anode materials for li/na-ion batteries: A DFT study. J. Comput. Chem. 2019, 40, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Ding, G.; Li, J.; Yao, K.; Wu, M.; Guangqian, D. Monolayer MXenes: Promising half-metals and spin gapless semiconductors. Nanoscale 2016, 8, 8986–8994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, J.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.; Rummeli, M.H. Applications of 2D MXenes in energy conversion and storage systems. Chem. Soc. Rev. 2018, 48, 72–133. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Jena, N.K.; Naqvi, S.R.; Luo, W.; Ahuja, R. Modelling high-performing batteries with Mxenes: The case of S-functionalized two-dimensional nitride Mxene electrode. Nano Energy 2019, 58, 877–885. [Google Scholar] [CrossRef]
- Gouveia, J.; Novell-Leruth, G.; Vines, F.; Illas, F.; Gomes, J. The Ti2CO2MXene as a nucleobase 2D sensor: A first-principles study. Appl. Surf. Sci. 2021, 544, 148946. [Google Scholar] [CrossRef]
- Gouveia, J.; Novell-Leruth, G.; Reis, P.M.L.S.; Viñes, F.; Illas, F.; Gomes, J. First-principles calculations on the adsorption behavior of amino acids on a titanium carbide MXene. ACS Appl. Biol. Mater. 2020, 3, 5913–5921. [Google Scholar] [CrossRef]
- Dolz, D.; Morales-García, A.; Viñes, F.; Illas, F. Exfoliation energy as a descriptor of MXenes synthesizability and surface chemical activity. Nanomaterials 2021, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Soundiraraju, B.; George, B.K. Two-dimensional Titanium Nitride (Ti2N) MXene: Synthesis, characterization, and potential application as surface-enhanced ramanscattering substrate. ACS Nano 2017, 11, 8892–8900. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Chen, W.; Jiao, Z.; Ma, S. Reversible hydrogen storage behaviors of Ti2NMXenes predicted by first-principles calculations. J. Mater. Sci. 2018, 54, 493–505. [Google Scholar] [CrossRef]
- Lin, H.; Yang, D.-D.; Lou, N.; Zhu, S.-G.; Li, H.-Z. Functionalized titanium nitride-based MXenes as promising host materials for lithium-sulfur batteries: A first principles study. Ceram. Int. 2018, 45, 1588–1594. [Google Scholar] [CrossRef]
- Urbankowski, P.; Anasori, B.; Makaryan, T.; Er, D.; Kota, S.; Walsh, P.L.; Zhao, M.; Shenoy, V.B.; Barsoum, M.W.; Gogotsi, Y. Synthesis of two-dimensional titanium nitride Ti4N3(MXene). Nanoscale 2016, 8, 11385–11391. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, H.; Jin, H.; Wu, M.; Fang, Y.; Sun, J.; Hu, Z.; Li, T.; Wu, J.; Huang, L.; et al. Salt-templated synthesis of 2D metallic MoN and other nitrides. ACS Nano 2017, 11, 2180–2186. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Y.; Liu, Y.; Jin, D.; Gogotsi, Y.; Meng, X.; Du, F.; Chen, G.; Wei, Y. First-principles calculations of Ti2N and Ti2NT2 (T=O, F, OH) monolayers as potential anode materials for lithium-ion batteries and beyond. J. Phys. Chem. C 2017, 121, 13025–13034. [Google Scholar] [CrossRef]
- Xie, Y.; Kent, P.R.C. Hybrid density functional study of structural and electronic properties of functionalized Tin+1Xn (X=C, N) monolayers. Phys. Rev. B 2013, 87, 235441. [Google Scholar] [CrossRef] [Green Version]
- Prasongkit, J.; Amorim, R.; Chakraborty, S.; Ahuja, R.; Scheicher, R.; Amornkitbamrung, V. Highly sensitive and selective gas detection based on silicene. J. Phys. Chem. C 2015, 119, 16934–16940. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B 2008, 77, 125416. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, J.D.; Viñes, F.; Illas, F.; Gomes, J.R.B. MXenes atomic layer stacking phase transitions and their chemical activity consequences. Phys. Rev. Mater. 2020, 4, 054003. [Google Scholar] [CrossRef]
- Ouyang, T.; Qian, Z.; Hao, X.; Ahuja, R.; Liu, X. Effect of defects on adsorption characteristics of AlN monolayer towards SO2 and NO2: Ab initio exposure. Appl. Surf. Sci. 2018, 462, 615–622. [Google Scholar] [CrossRef]
- Kou, L.; Frauenheim, T.; Chen, C. Phosphorene as a superior gas sensor: Selective adsorption and distinct I–V response. J. Phys. Chem. Lett. 2014, 5, 2675–2681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, Q.; Shao, Z.; Chang, S.; Li, J. Adsorption of gas molecules on monolayer MoS2 and effect of applied electric field. Nanoscale Res. Lett. 2013, 8, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, T.; Singh, D.; Gupta, S.K.; Karton, A.; Sonvane, Y.; Ahuja, R. Efficient and selective sensing of nitrogen-containing gases by Si2BN nanosheets under pristine and pre-oxidized conditions. Appl. Surf. Sci. 2018, 469, 775–780. [Google Scholar] [CrossRef]
- Gholizadeh, R.; Yu, Y.-X. Work functions of pristine and heteroatom-doped graphenes under different external electric fields: An ab initio DFT study. J. Phys. Chem. C 2014, 118, 28274–28282. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Nagarajan, V.; Chandiramouli, R. Investigation of NH3 adsorption behavior on graphdiyne nanosheet and nanotubes: A first-principles study. J. Mol. Liq. 2018, 249, 24–32. [Google Scholar] [CrossRef]
- Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 1935, 3, 107–115. [Google Scholar] [CrossRef]
- Hadipour, N.L.; Peyghan, A.A.; Soleymanabadi, H. Theoretical study on the Al-doped ZnO nanoclusters for CO chemical sensors. J. Phys. Chem. C 2015, 119, 6398–6404. [Google Scholar] [CrossRef]
- Ahmadi, A.; Hadipour, N.L.; Kamfiroozi, M.; Bagheri, Z. Theoretical study of aluminum nitride nanotubes for chemical sensing of formaldehyde. Sens. Actuators B Chem. 2012, 161, 1025–1029. [Google Scholar] [CrossRef]

| NCGs | Adsorption Energy (eV) | Charge Transfer (e−) | ||||||
|---|---|---|---|---|---|---|---|---|
| Ti2N | Ti2NO2 | Ti2NF2 | Ti2N(OH)2 | Ti2N | Ti2NO2 | Ti2NF2 | Ti2N(OH)2 | |
| NO | −9.81 | −0.68 | −0.15 | −5.55 | 2.57 | −0.24 | −0.07 | 0.50 |
| NO2 | −14.34 | −2.38 | −0.36 | −3.78 | 3.77 | 0.49 | 0.29 | 1.78 |
| NH3 | −1.29 | −1.16 | −0.13 | −0.57 | 0.01 | −0.18 | −0.03 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Du, W.; Zhang, J.; Ahuja, R.; Qian, Z. Nitrogen-Containing Gas Sensing Properties of 2-D Ti2N and Its Derivative Nanosheets: Electronic Structures Insight. Nanomaterials 2021, 11, 2459. https://doi.org/10.3390/nano11092459
Zhang H, Du W, Zhang J, Ahuja R, Qian Z. Nitrogen-Containing Gas Sensing Properties of 2-D Ti2N and Its Derivative Nanosheets: Electronic Structures Insight. Nanomaterials. 2021; 11(9):2459. https://doi.org/10.3390/nano11092459
Chicago/Turabian StyleZhang, Hongni, Wenzheng Du, Jianjun Zhang, Rajeev Ahuja, and Zhao Qian. 2021. "Nitrogen-Containing Gas Sensing Properties of 2-D Ti2N and Its Derivative Nanosheets: Electronic Structures Insight" Nanomaterials 11, no. 9: 2459. https://doi.org/10.3390/nano11092459
APA StyleZhang, H., Du, W., Zhang, J., Ahuja, R., & Qian, Z. (2021). Nitrogen-Containing Gas Sensing Properties of 2-D Ti2N and Its Derivative Nanosheets: Electronic Structures Insight. Nanomaterials, 11(9), 2459. https://doi.org/10.3390/nano11092459








