PEG Coated Fe3O4/RGO Nano-Cube-Like Structures for Cancer Therapy via Magnetic Hyperthermia
Abstract
:1. Introduction
2. Experimental Methods
2.1. Fe3O4 Nanoparticles (SPIONs) Synthesis
2.2. Graphene Oxide (GO) Synthesis
2.3. Fe3O4/RGO Nanocomposites Synthesis
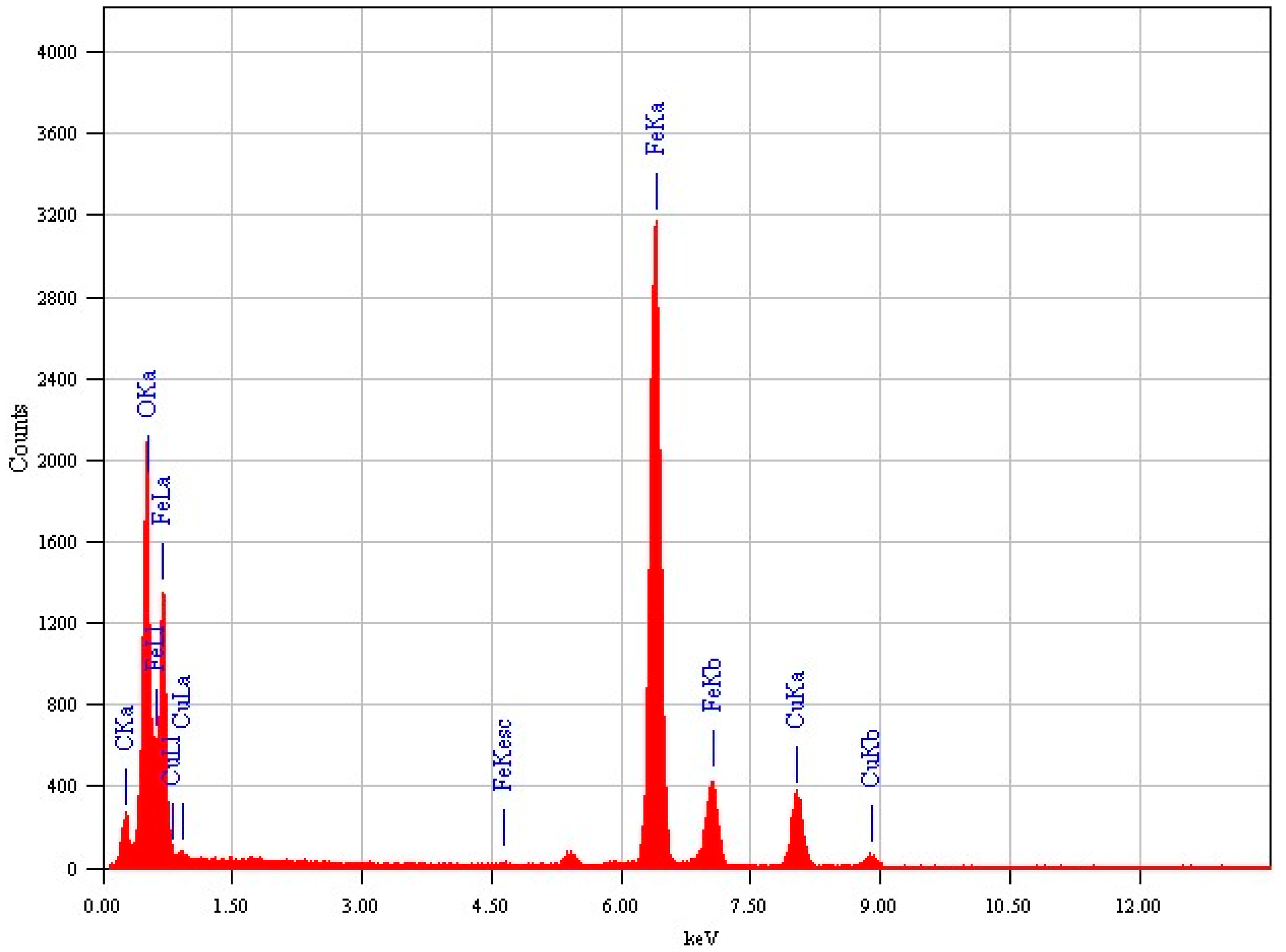
2.4. Characterization
2.5. Procedures for Application Testing
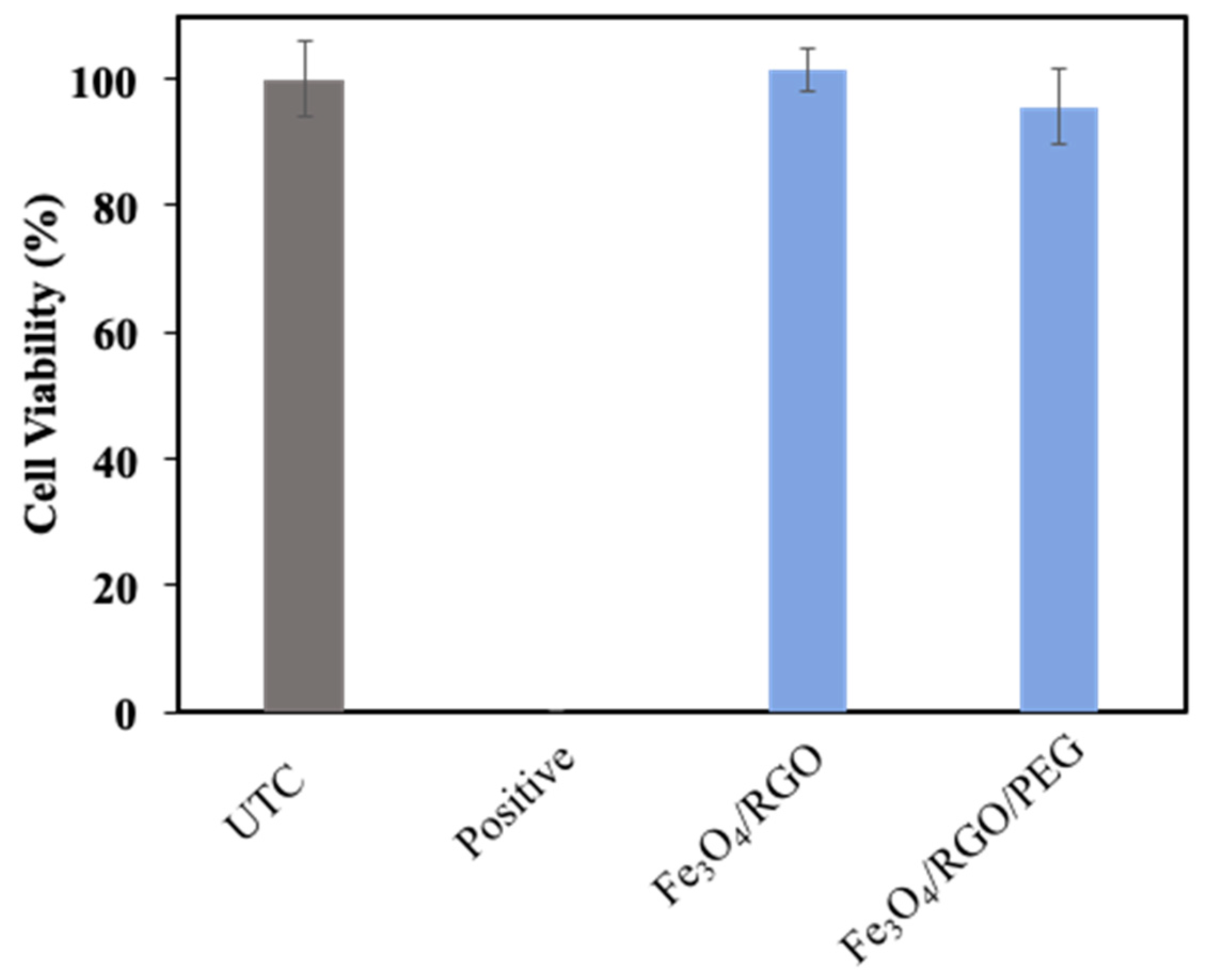
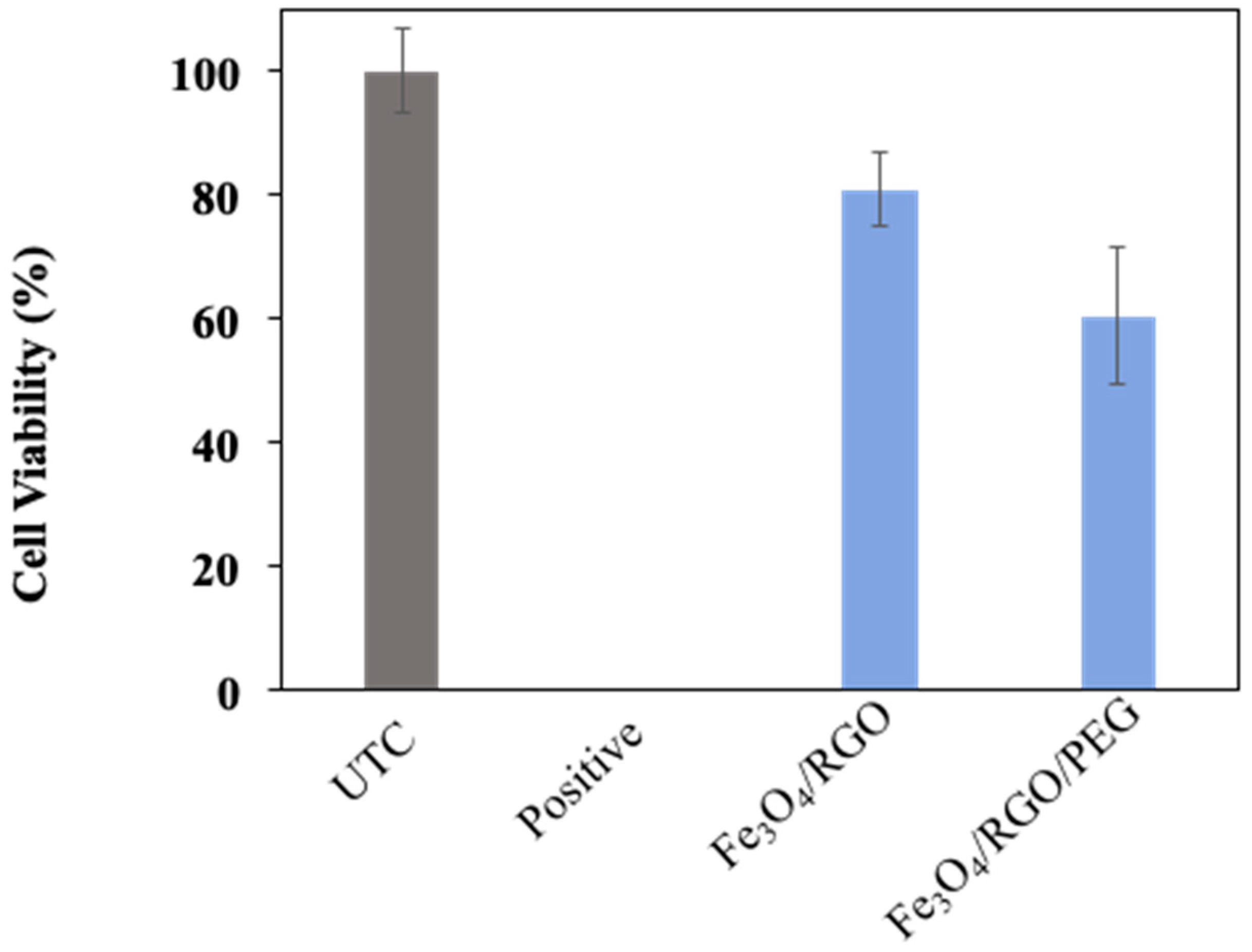
2.5.1. Cytotoxicity
2.5.2. Magnetic Hyperthermia
3. Results and Discussion
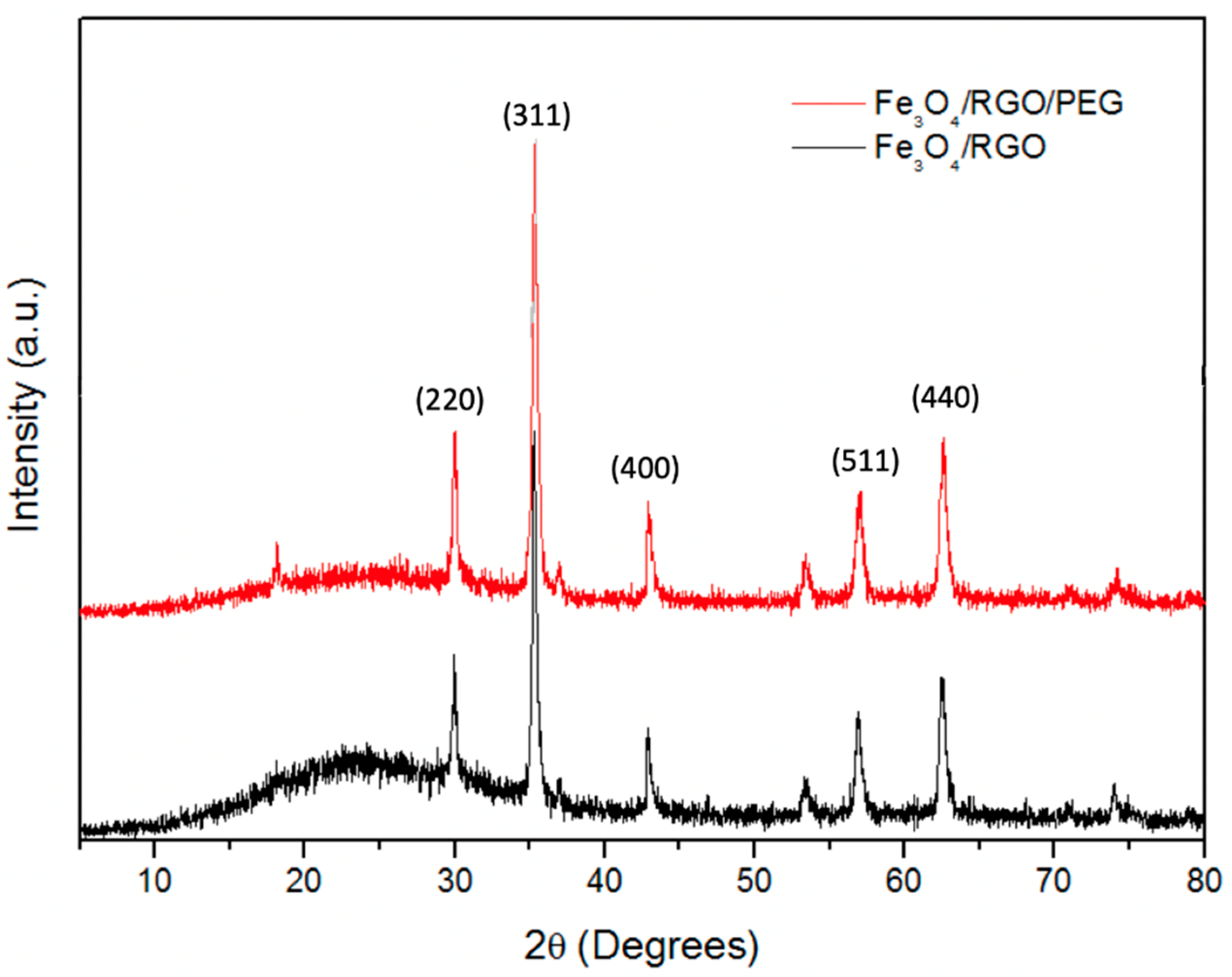
3.1. XRD
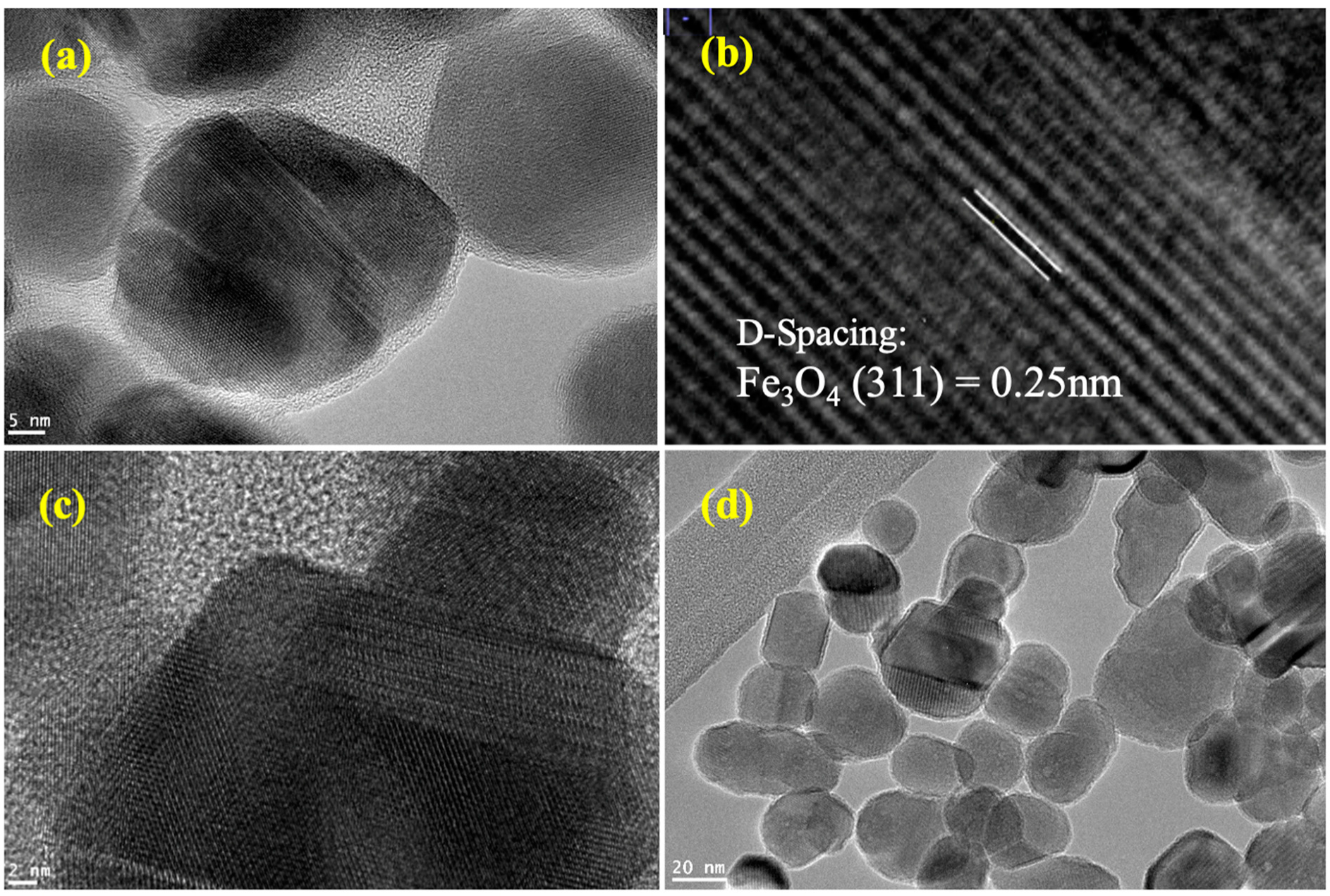
3.2. HR-TEM
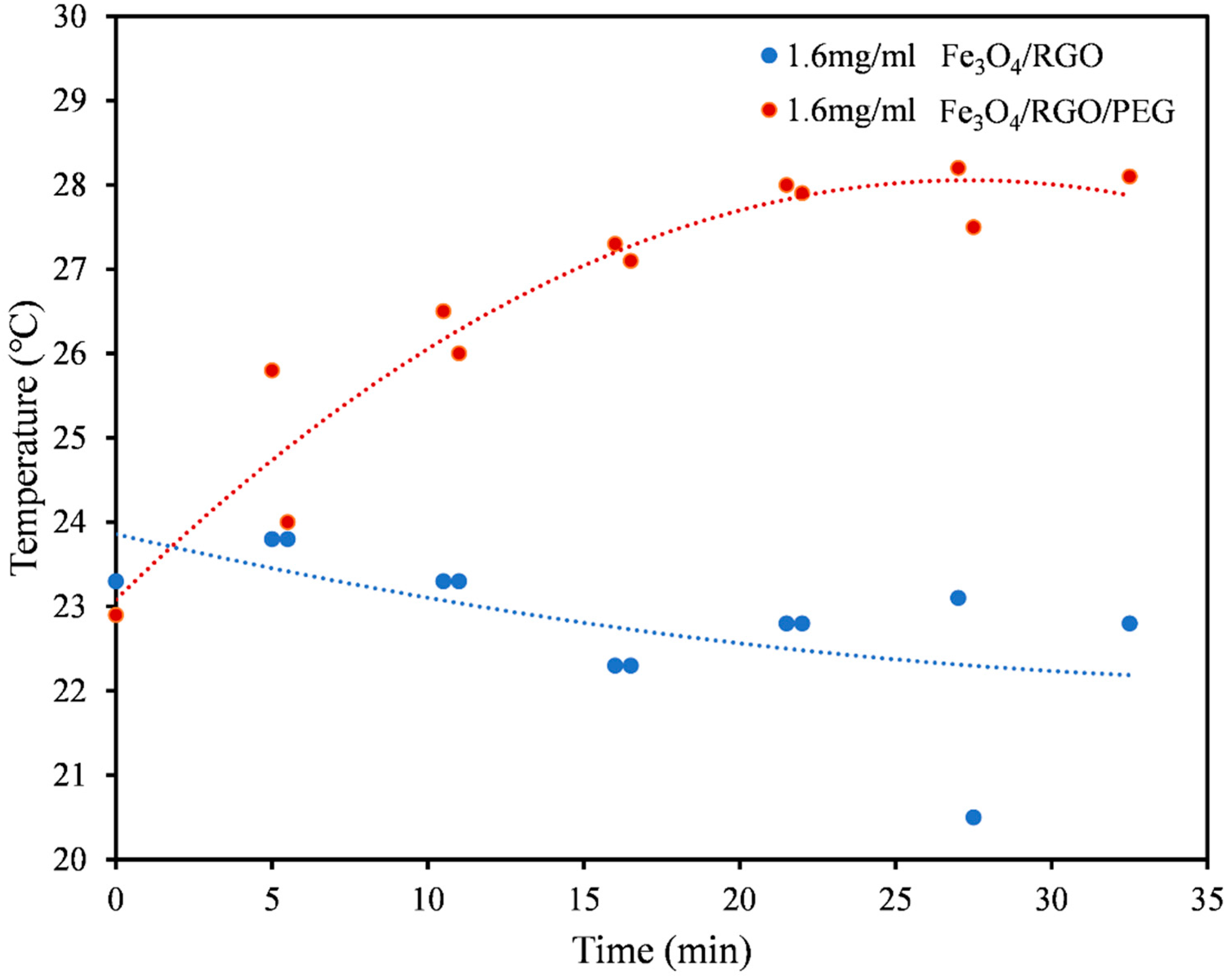
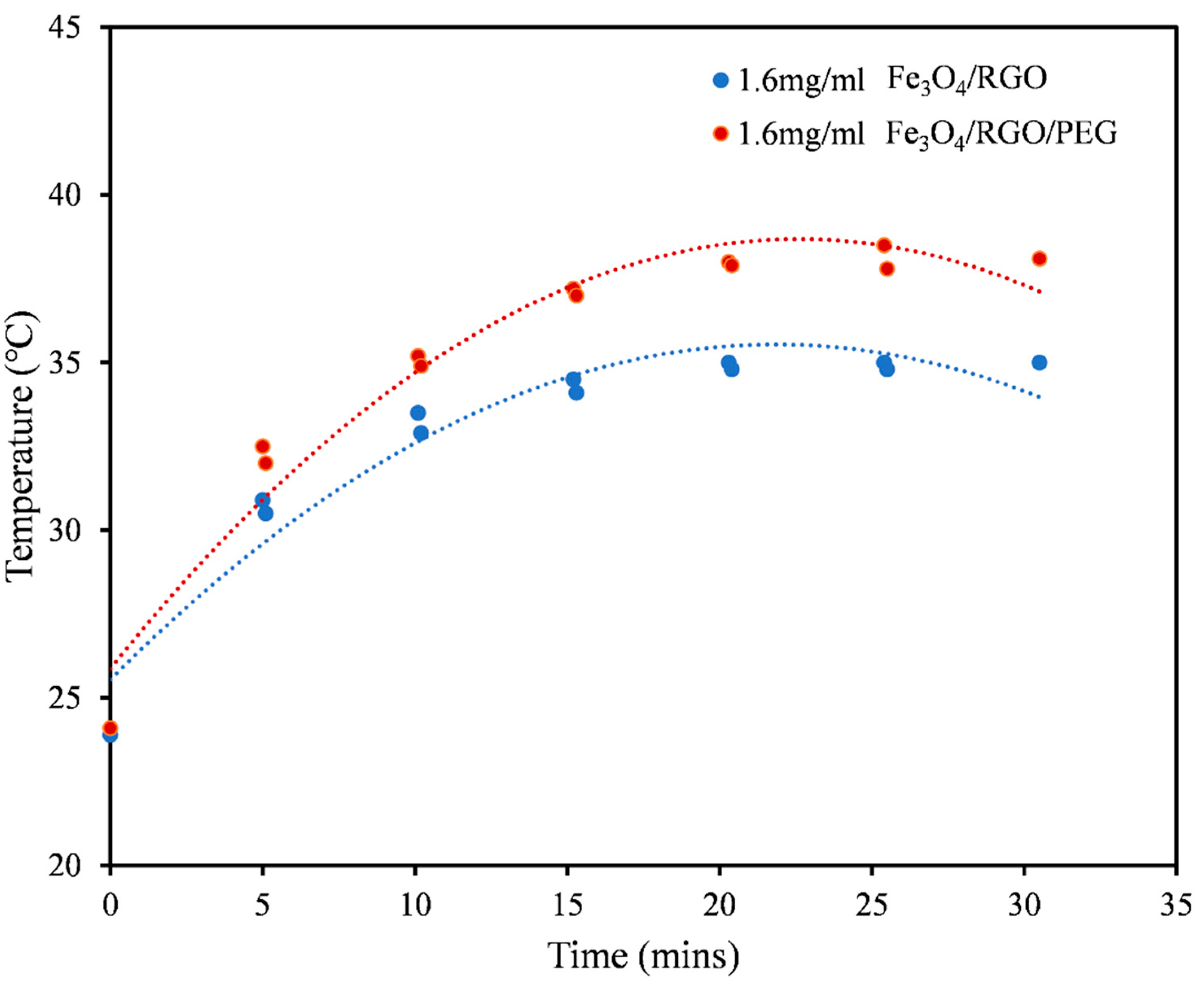
3.3. Magnetic Hyperthermia
3.4. Cytotoxicity
4. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nazir, S.; Hussain, T.; Ayub, A.; Rashid, U.; MacRobert, A. Nanomaterials in combating cancer: Therapeutic applications and developments. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Gao, J.; Ai, H.; Chen, X. Applications and Potential Toxicity of Magnetic Iron Oxide Nanoparticles. Small 2012, 9, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Shao, Y.; Peng, J.; Dai, X.; Li, H.; Wu, Q.; Shi, D. Near-infrared laser light mediated cancer therapy by photothermal effect of Fe3O4 magnetic nanoparticles. Biomaterials 2013, 34, 4078–4088. [Google Scholar] [CrossRef]
- Rauch, J.; Kolch, W.; Mahmoudi, M. Cell Type-Specific Activation of AKT and ERK Signaling Pathways by Small Negatively-Charged Magnetic Nanoparticles. Sci. Rep. 2012, 2, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beik, J.; Abed, Z.; Ghoreishi, F.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Nejadnik, H.; Daldrup-Link, H. Next-generation superparamagnetic iron oxide nanoparticles for cancer theranostics. Drug Discov. Today 2017, 22, 1421–1429. [Google Scholar] [CrossRef]
- Ohtake, M.; Umemura, M.; Sato, I.; Akimoto, T.; Oda, K.; Nagasako, A.; Kim, J.; Fujita, T.; Yokoyama, U.; Nakayama, T.; et al. Hyperthermia and chemotherapy using Fe(Salen) nanoparticles might impact glioblastoma treatment. Sci. Rep. 2017, 7, 42783. [Google Scholar] [CrossRef] [PubMed]
- Torres, T.E.; Lima, E.; Calatayud, M.P.; Sanz, B.; Ibarra, A.; Fernández-Pacheco, R.; Mayoral, A.; Marquina, C.; Ibarra, M.R.; Goya, G.F. The relevance of Brownian relaxation as power absorption mechanism in Magnetic Hyperthermia. Sci. Rep. 2019, 9, 3992. [Google Scholar] [CrossRef] [Green Version]
- Wahajuddin, S.A. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef] [Green Version]
- Kumar, C.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Kartikowati, C.; Horie, S.; Ogi, T.; Iwaki, T.; Okuyama, K. Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles. Sci. Rep. 2017, 7, 9894. [Google Scholar] [CrossRef]
- Abenojar, E.; Wickramasinghe, S.; Bas-Concepcion, J.; Samia, A. Structural effects on the magnetic hyperthermia properties of iron oxide nanoparticles. Prog. Nat. Sci. Mater. Int. 2016, 26, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Reddy, L.; Arias, J.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Yang, J.; Ramaraj, B.; Yoon, K. Preparation and characterization of superparamagnetic graphene oxide nanohybrids anchored with Fe3O4 nanoparticles. J. Alloys Compd. 2014, 583, 128–133. [Google Scholar] [CrossRef]
- Ramezani Farani, M.; Khadiv-Parsi, P.; Riazi, G.; Shafiee Ardestani, M.; Saligheh Rad, H. PEGylation of graphene/iron oxide nanocomposite: Assessment of release of doxorubicin, magnetically targeted drug delivery and photothermal therapy. Appl. Nanosci. 2020, 10, 1205–1217. [Google Scholar] [CrossRef]
- Ojha, K.; Anjaneyulu, O.; Ganguli, A.K. Graphene-based hybrid materials: Synthetic approaches and properties. Curr. Sci. 2014, 107, 397–418. [Google Scholar]
- Gupta, J.; Prakash, A.; Jaiswal, M.; Agarrwal, A.; Bahadur, D. Superparamagnetic iron oxide-reduced graphene oxide nanohybrid-a vehicle for targeted drug delivery and hyperthermia treatment of cancer. J. Magn. Magn. Mater. 2018, 448, 332–338. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, F.; Wu, L.; Jiang, X. Understanding the Synergic Mechanism of Weak Interactions between Graphene Oxide and Lipid Membrane Leading to the Extraction of Lipids. Langmuir 2019, 35, 14098–14107. [Google Scholar] [CrossRef] [PubMed]
- Singh, Z. Applications and toxicity of graphene family nanomaterials and their composites. Nanotechnol. Sci. Appl. 2016, 9, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Boubeta, C.; Simeonidis, K.; Makridis, A.; Angelakeris, M.; Iglesias, O.; Guardia, P.; Cabot, A.; Yedra, L.; Estradé, S.; Peiró, F.; et al. Learning from Nature to Improve the Heat Generation of Iron-Oxide Nanoparticles for Magnetic Hyperthermia Applications. Sci. Rep. 2013, 3, 1652. [Google Scholar] [CrossRef] [Green Version]
- Fultz, B.; Howe, J.M. Transmissiosn Electron Microscopy and Diffractometry of Materials, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, M.; Soler, E.; Torres, T.; Campos-Gonzalez, E.; Junquera, C.; Ibarra, M.; Goya, G. Cell damage produced by magnetic fluid hyperthermia on microglial BV2 cells. Sci. Rep. 2017, 7, 8627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Wang, L.; Cheng, R.; Mao, L.; Arnold, R.; Howerth, E.; Chen, Z.; Platt, S. Magnetic Nanoparticle-Based Hyperthermia for Head & Neck Cancer in Mouse Models. Theranostics 2012, 2, 113–121. [Google Scholar]
- Di Corato, R.; Espinosa, A.; Lartigue, L.; Tharaud, M.; Chat, S.; Pellegrino, T.; Ménager, C.; Gazeau, F.; Wilhelm, C. Magnetic hyperthermia efficiency in the cellular environment for different nanoparticle designs. Biomaterials 2014, 35, 6400–6411. [Google Scholar] [CrossRef]
- Chalopin, Y.; Bacri, J.; Gazeau, F.; Devaud, M. Nanoscale Brownian heating by interacting magnetic dipolar particles. Sci. Rep. 2017, 7, 1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotin, G.; Perton, F.; Blanco-Andujar, C.; Pichon, B.; Mertz, D.; Begin-Colin, S. Chapter 2 - Design of Anisotropic Iron-Oxide-Based Nanoparticles for Magnetic Hyperthermia. In Nanomaterials for Magnetic and Optical Hyperthermia Applications; Fratila, R.M., De La Fuente, J.M., Eds.; Micro and Nano Technologies; Elsevier Inc.: Strasbourg, France, 2019; pp. 41–60. [Google Scholar]
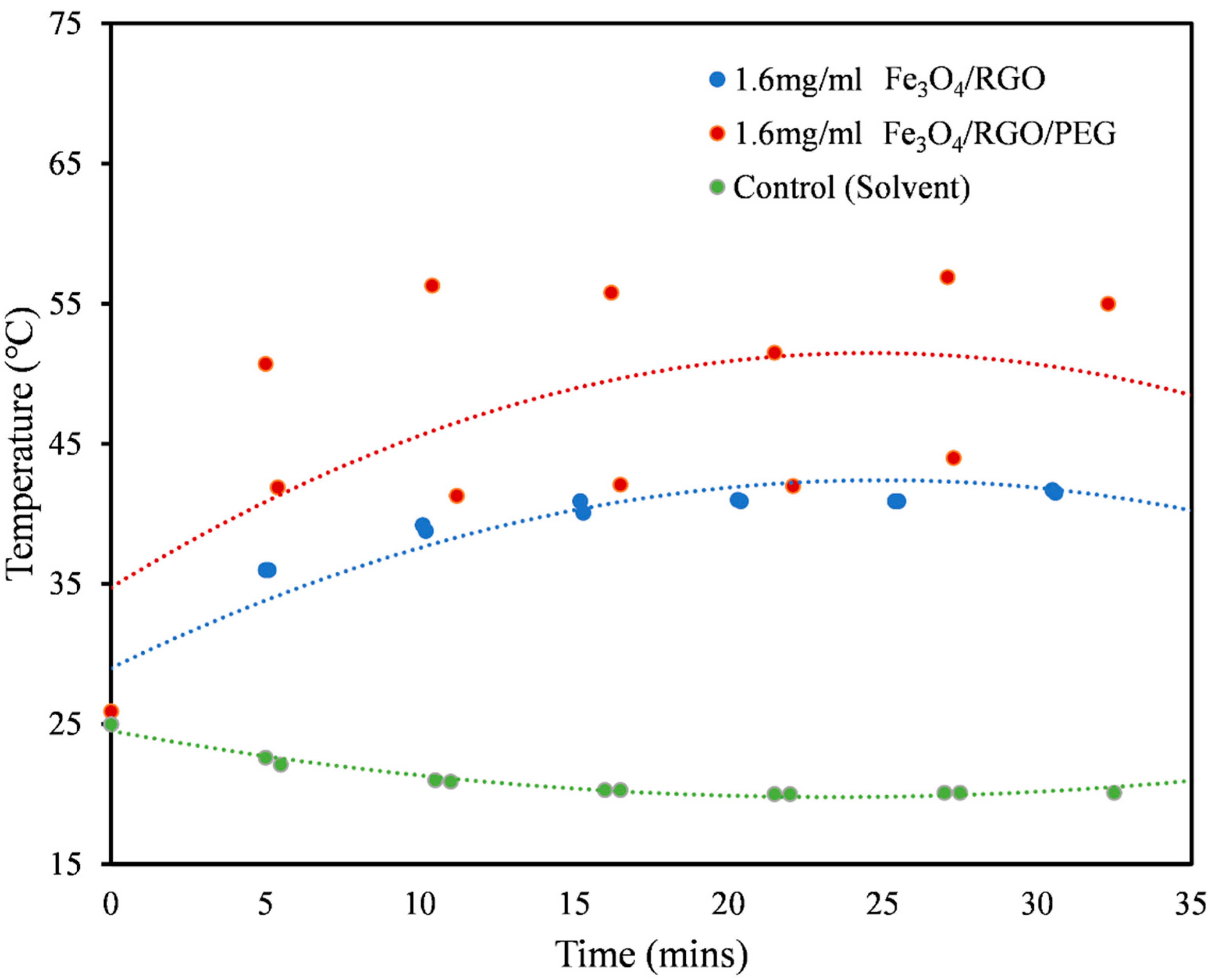

| Medium | Nanomaterial | SAR Values (W/g) |
|---|---|---|
| PBS (Figure 4) | Fe3O4/RGO | 6.54 |
| Fe3O4/RGO/PEG | 19.61 | |
| Aqueous DMSO (Figure 5) | Fe3O4/RGO | 60.70 |
| Fe3O4/RGO/PEG | 74.19 | |
| pH Buffer (Figure 6) | Fe3O4/RGO | 45.76 |
| Fe3O4/RGO/PEG | 58.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhayal, A.; Fathima, A.; Alhasan, A.H.; Alsharaeh, E.H. PEG Coated Fe3O4/RGO Nano-Cube-Like Structures for Cancer Therapy via Magnetic Hyperthermia. Nanomaterials 2021, 11, 2398. https://doi.org/10.3390/nano11092398
Alkhayal A, Fathima A, Alhasan AH, Alsharaeh EH. PEG Coated Fe3O4/RGO Nano-Cube-Like Structures for Cancer Therapy via Magnetic Hyperthermia. Nanomaterials. 2021; 11(9):2398. https://doi.org/10.3390/nano11092398
Chicago/Turabian StyleAlkhayal, Anoud, Arshia Fathima, Ali H. Alhasan, and Edreese H. Alsharaeh. 2021. "PEG Coated Fe3O4/RGO Nano-Cube-Like Structures for Cancer Therapy via Magnetic Hyperthermia" Nanomaterials 11, no. 9: 2398. https://doi.org/10.3390/nano11092398
APA StyleAlkhayal, A., Fathima, A., Alhasan, A. H., & Alsharaeh, E. H. (2021). PEG Coated Fe3O4/RGO Nano-Cube-Like Structures for Cancer Therapy via Magnetic Hyperthermia. Nanomaterials, 11(9), 2398. https://doi.org/10.3390/nano11092398






