Wettability Changes Due to Nanomaterials and Alkali—A Proposed Formulation for EOR
Abstract
1. Introduction
2. General Paper Approach
- Fluid/rock selection and characterization: As a continuation of the work previously performed by some of the authors [9,10,38], we have selected two surface-modified silica nanomaterials (characterized by means of ζ potential) and Na2CO3 alkali, two brines (differing in ion content), and two oils (differing in TAN). Outcrop rocks (differing in clay content) were selected/characterized by routine core analysis (RCA);
- Interfacial tension (IFT) measurements: IFT was measured as a function of time at 60 °C using a spinning drop tensiometer to define fluid–fluid interactions. Multiple concentrations and combinations were tested;
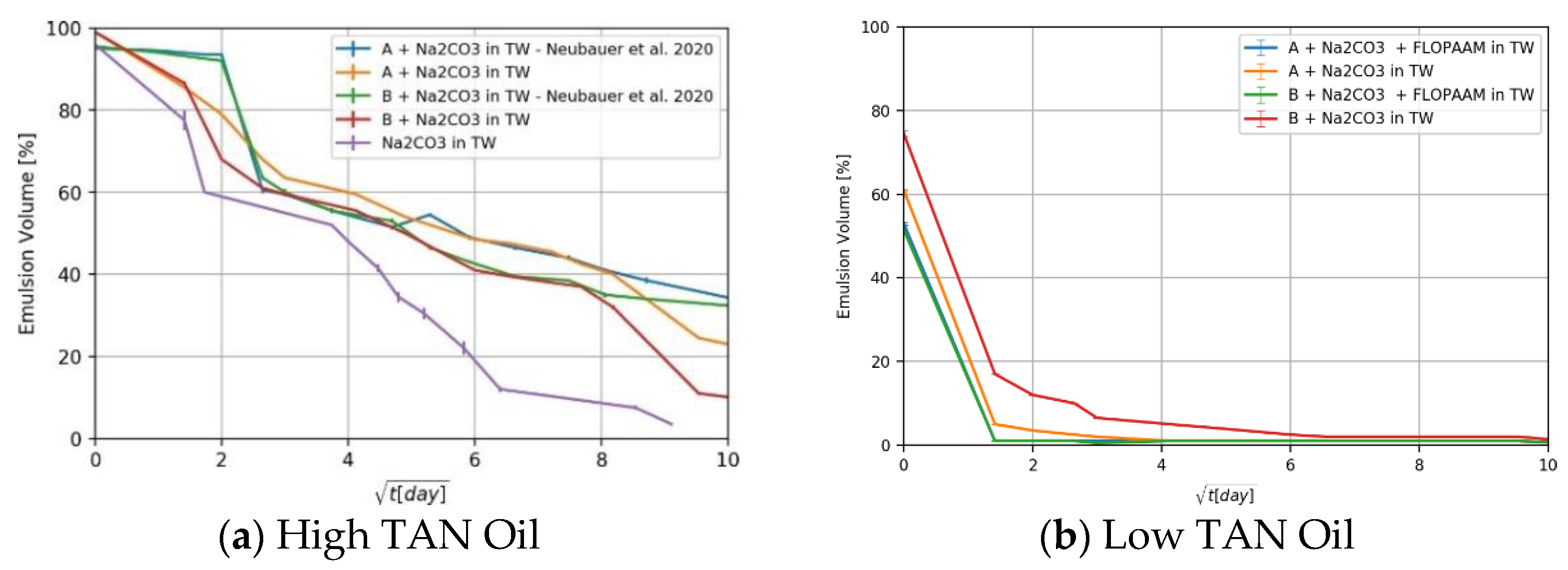
- Phase behavior experiments: These supported an understanding of the emulsion volumes generated by the fluid–fluid interactions. This work is complemented by the data presented by Neubauer et al. [9];
- Amott spontaneous imbibition experiments: Performed to evaluate wettability alteration and oil recovered over time by the imbibed fluid (nanoparticles/alkali). Data was assessed through detailed tracking of produced oil with time and final oil recovery;
- Modeling of selected data: Data was modelled by means of the capillary diffusion coefficient and the inverse Bond number to link the findings to literature data.
3. Materials and Methods
3.1. Fluids and Rock Material
3.1.1. Synthetic Brines
3.1.2. Crude Oil
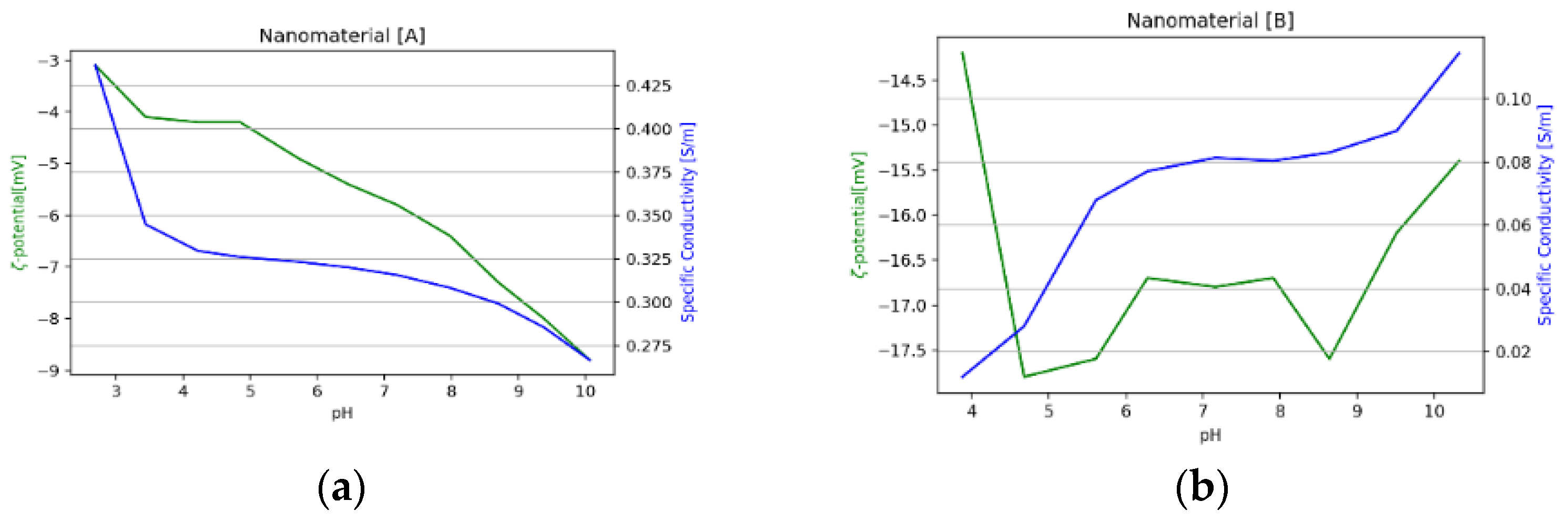
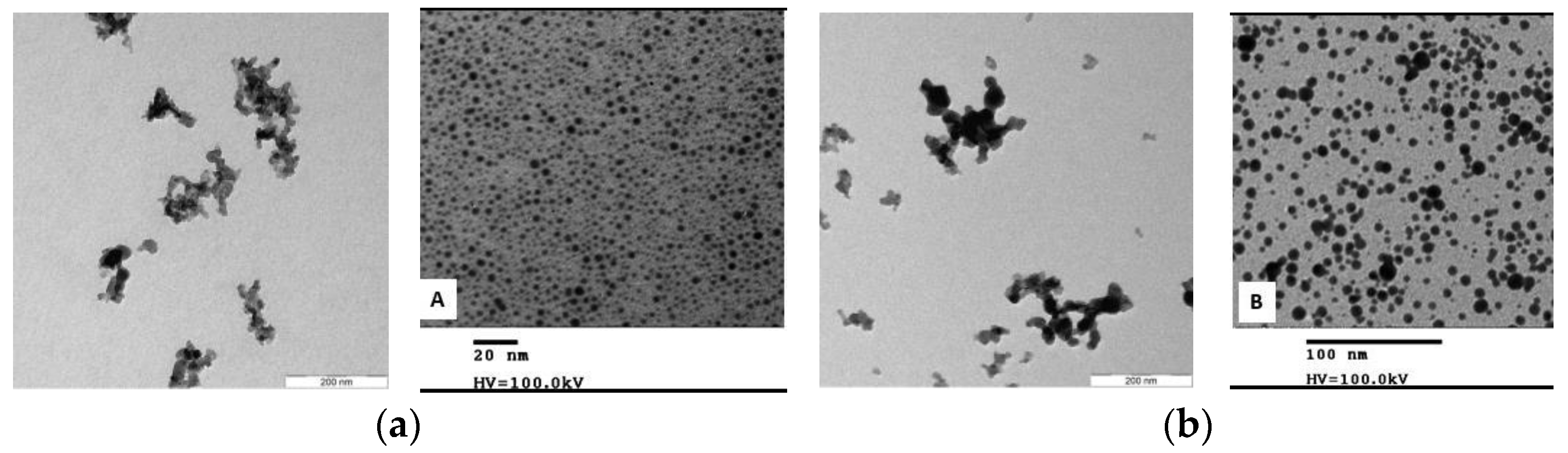
3.1.3. Nanomaterials
3.1.4. Nanomaterials Combined with Alkali—Combined Solutions
3.1.5. Core Plugs
3.2. Experimental Evaluations
3.2.1. Interfacial Tension (IFT) Measurements
3.2.2. Phase Behavior Tests
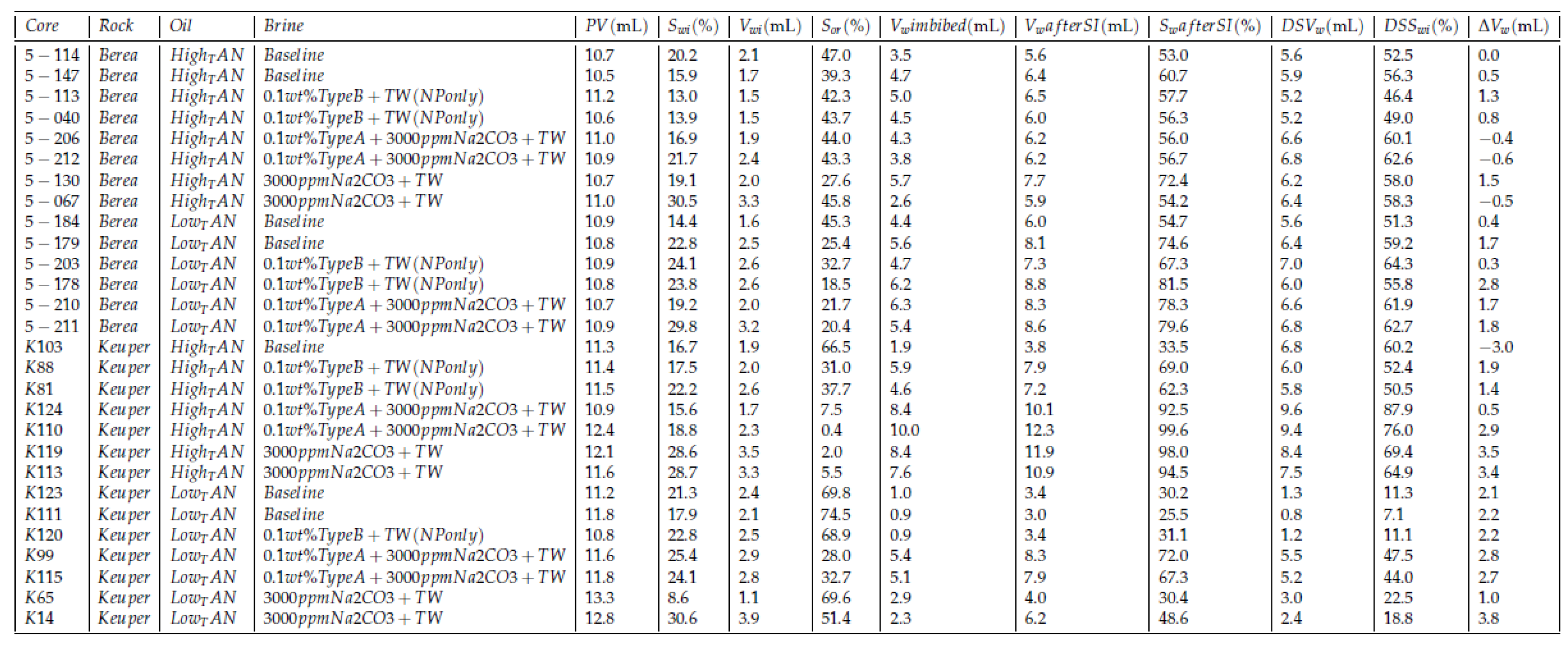
3.2.3. Amott Spontaneous Imbibition
3.3. Modeling Fundamentals and Approach
3.3.1. Capillary Diffusion Coefficient
3.3.2. Inverse Bond Number
4. Results and Discussion
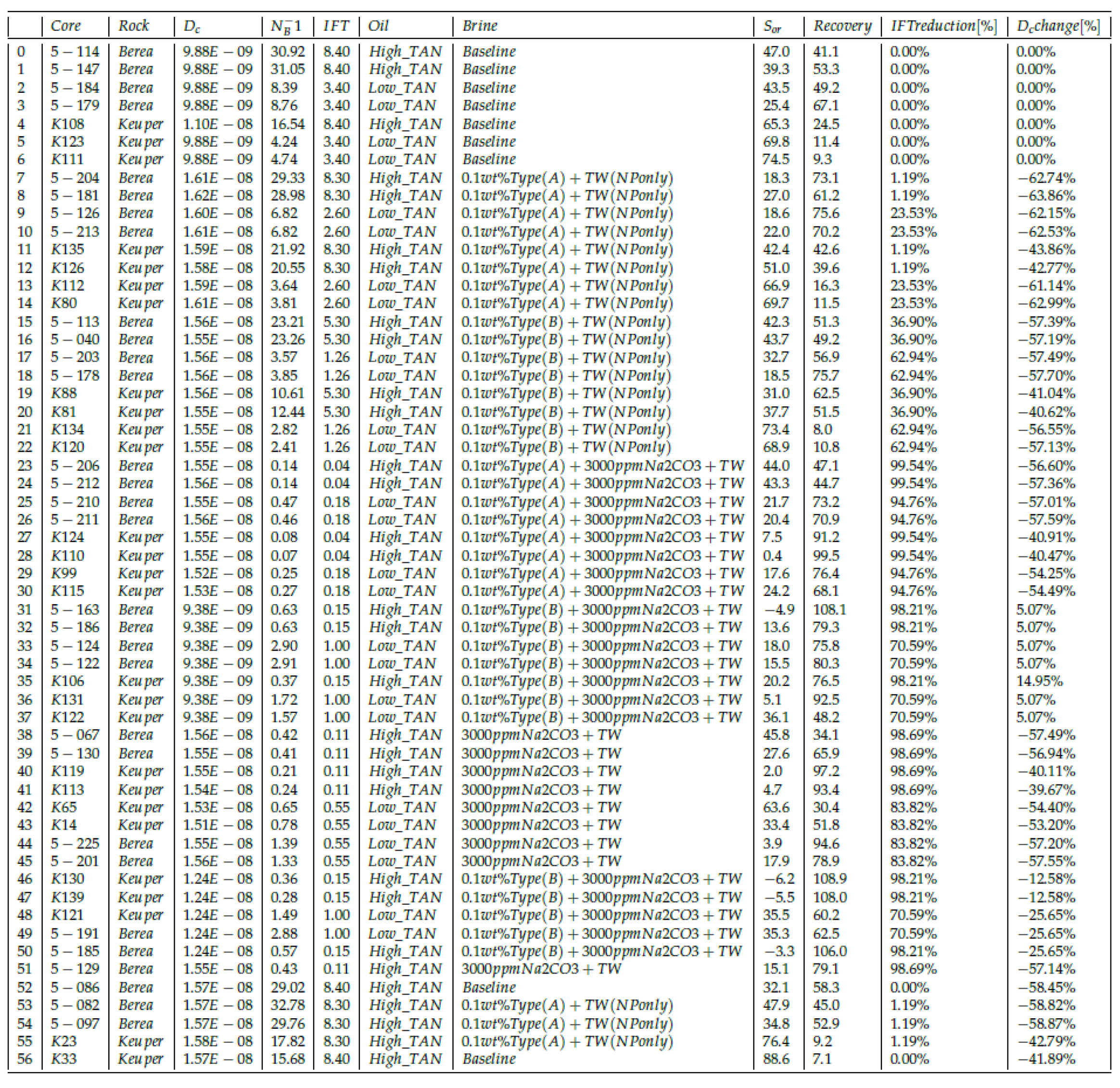
4.1. Interfacial Tension (IFT)
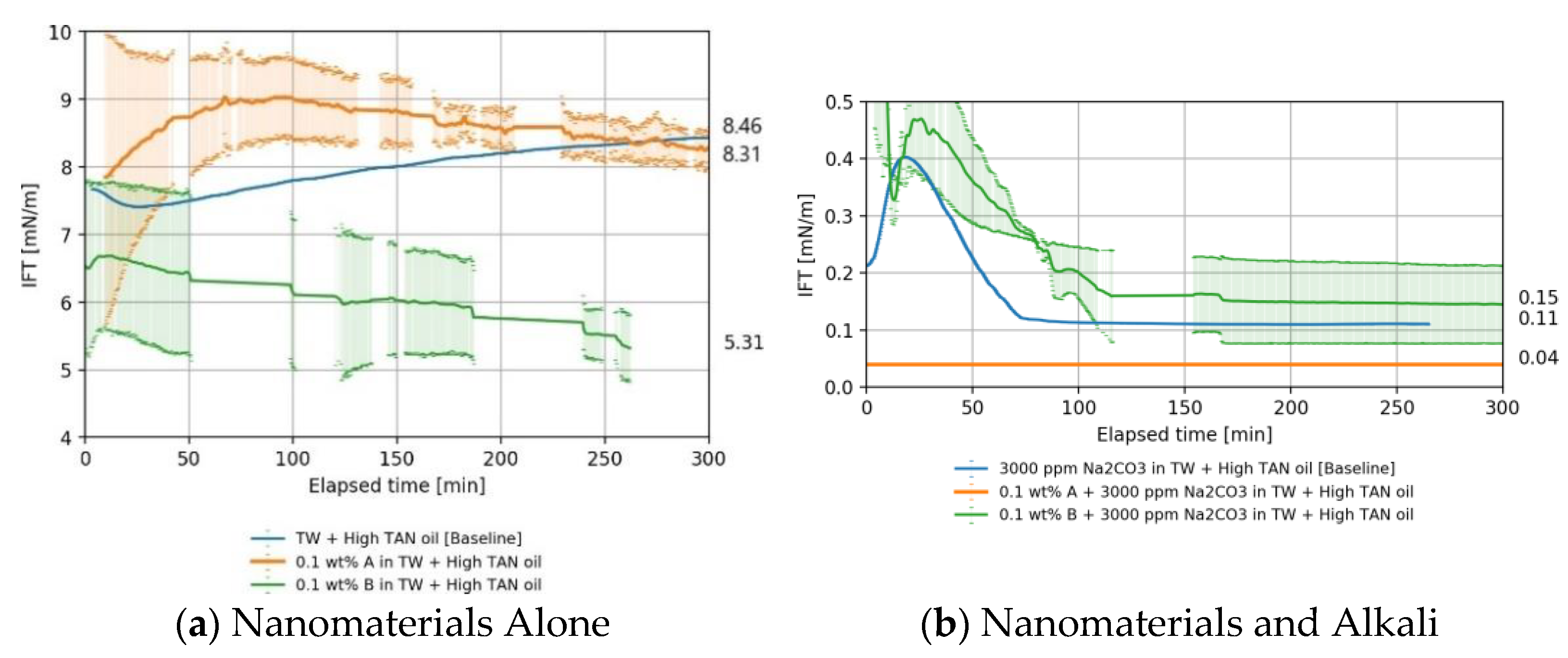
4.1.1. High TAN Oil
Nanomaterials as a Standalone EOR Agent in High TAN Oil
Nanomaterials and Alkali in High TAN Oil
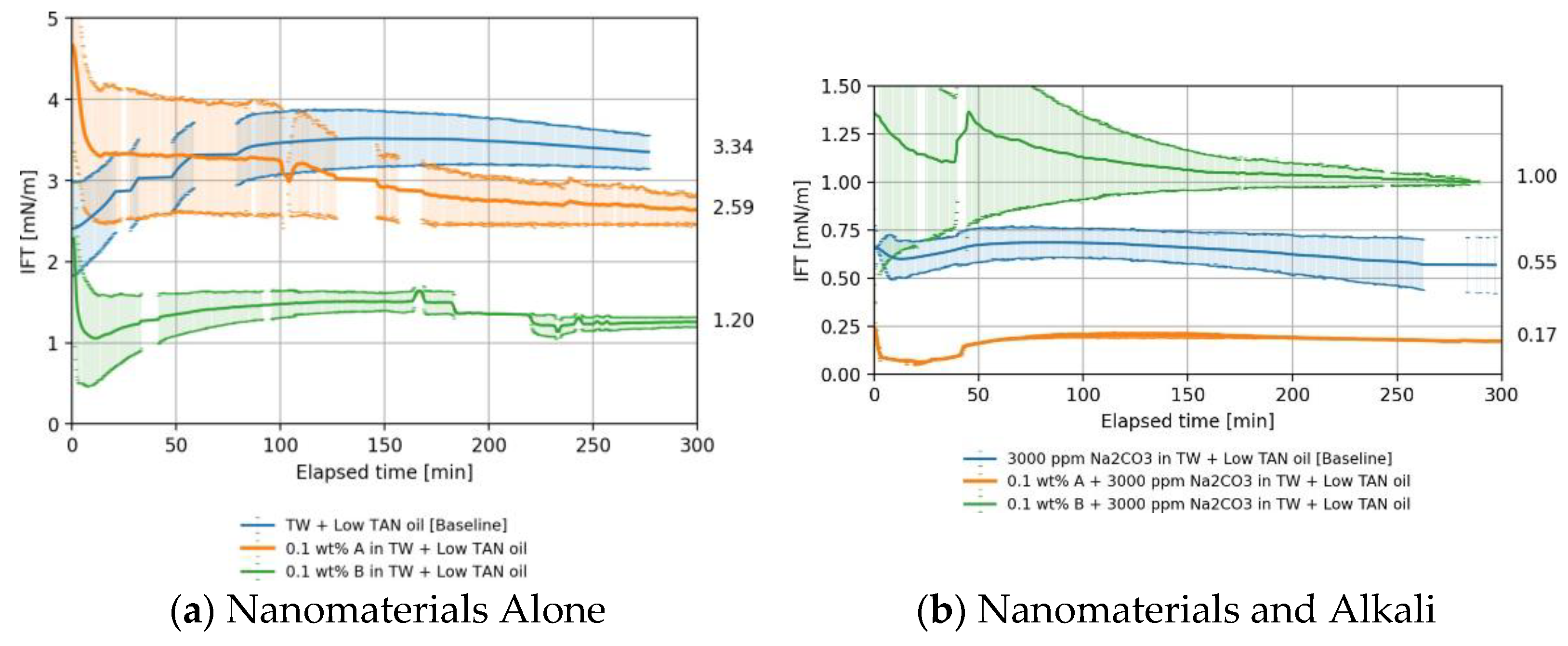
4.1.2. Low TAN Oil
Nanomaterials as a Standalone EOR Agent in Low TAN Oil
Nanomaterials and Alkali in Low TAN oil
4.1.3. Overall IFT Findings
4.2. Phase Behavior Observations
4.3. Amott Spontaneous Imbibition Evaluations
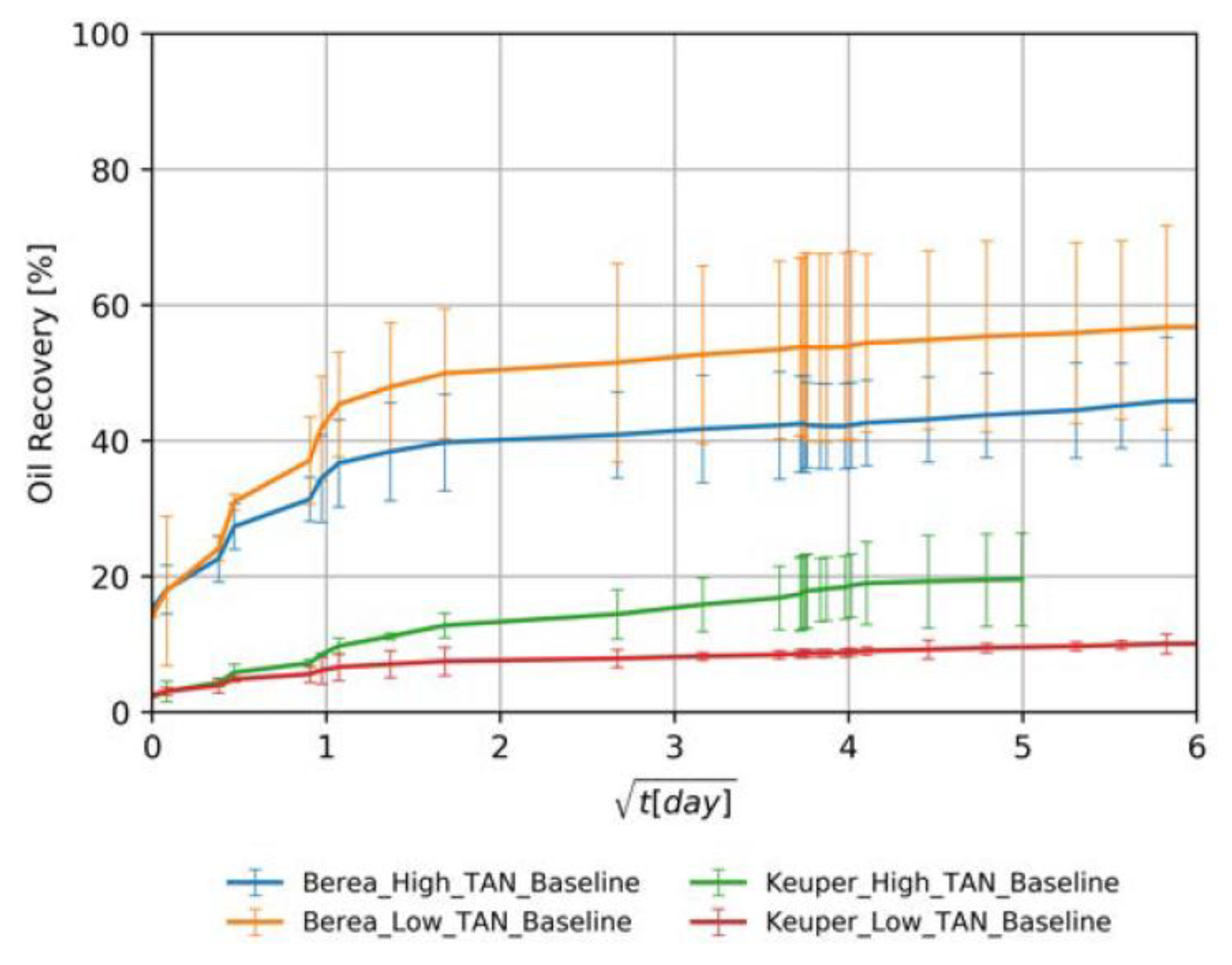
4.3.1. Baseline Experiments—Softened Brine
- Initial wettability state: The high recoveries from Berea samples for both oil types (orange and blue lines in Figure 7) can be attributed to a mixed-wet state. The recovered percentages for Berea and Keuper are consistent with the values reported by Neubauer et al. [10]. However, values obtained from the Keuper samples differ from Arekhov et al. [38]; we attribute it to a possible lack of consistency on core the saturation process.
- Core plugs permeability: Since the spontaneous imbibition is a capillary-driven mechanism, the recovery was higher/faster in cores with lower permeability (Berea, orange and blue lines in Figure 7) where the capillary forces tend to be more significant. This is in line with the behavior proposed by Morrow et al. [62].
- Visual observation of oil droplets: We observed that oil droplets were relatively large in volume (big oil droplets), which indicates a high IFT regime between the oil and brine. This observation was in accordance with the IFT data presented in Section 4.1.
- Effects of oil viscosity: There was a clear contrast of oil viscosities, with low TAN oil having significantly lower viscosity than high TAN oil (6 mPa∙s and 11.90 mPa∙s, respectively, refer to Table 3). In core plugs with low permeability, recovery values were higher for the low TAN oil (Berea, orange, and blue lines in Figure 7). Similar observations were reported by Schechter et al. [54], Zhou et al. [63] and Meng et al. [64], where high recovered oil was observed in low-permeability dolomite. The authors attributed that to the proportionality between ultimate oil recovery to the square root of dimensionless time, where wettability is highly dependent on IFT and the mobility ratio.
- Clay content in Keuper samples: Core plugs with large mineralogical heterogeneity like the Keuper cores containing goethite are believed to be more oil wet [65,66]. Sayyouh et al. [65] suggested that under low to moderate salinity the tendency towards oil-wet increases with increasing clay content. More oil wetness makes the water less favorable to imbibe the core, therefore recovery under spontaneous imbibition would be lower.
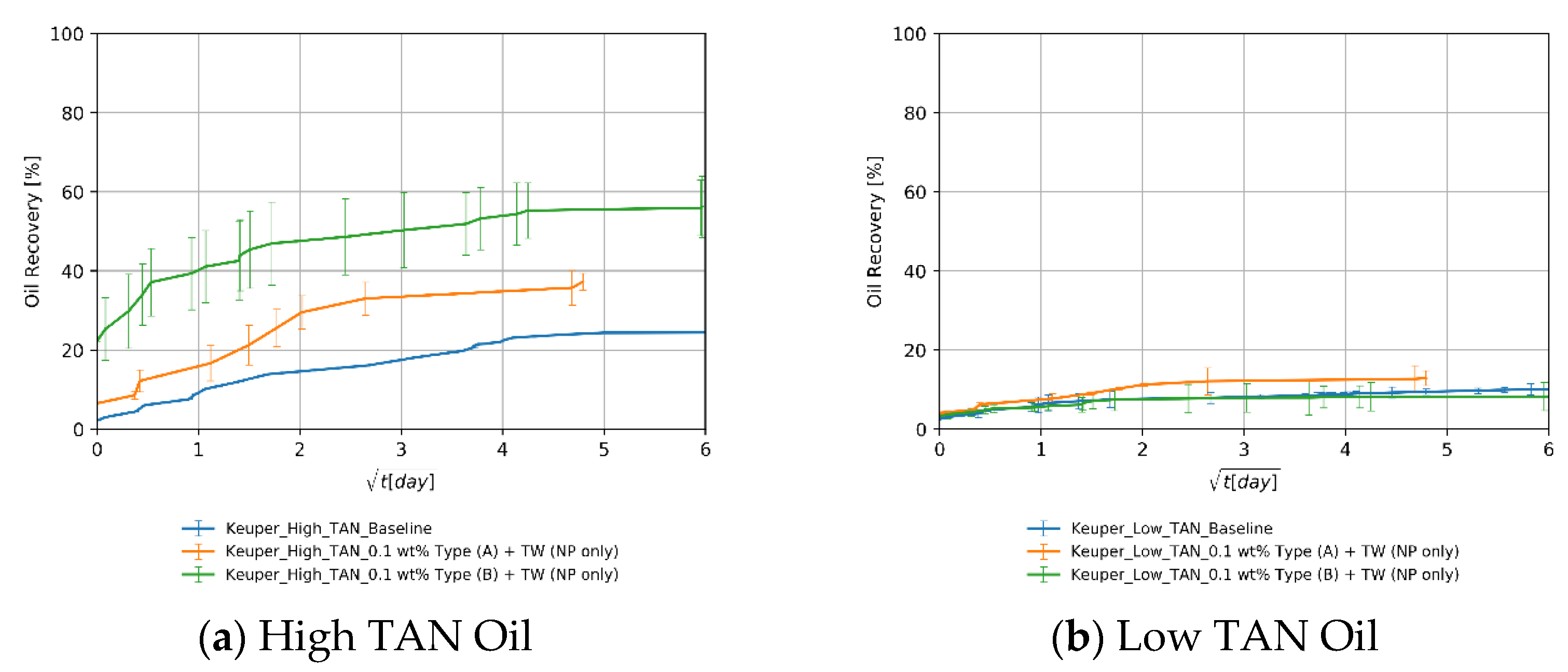
4.3.2. Influence of the Nanomaterials as a Standalone EOR Agent
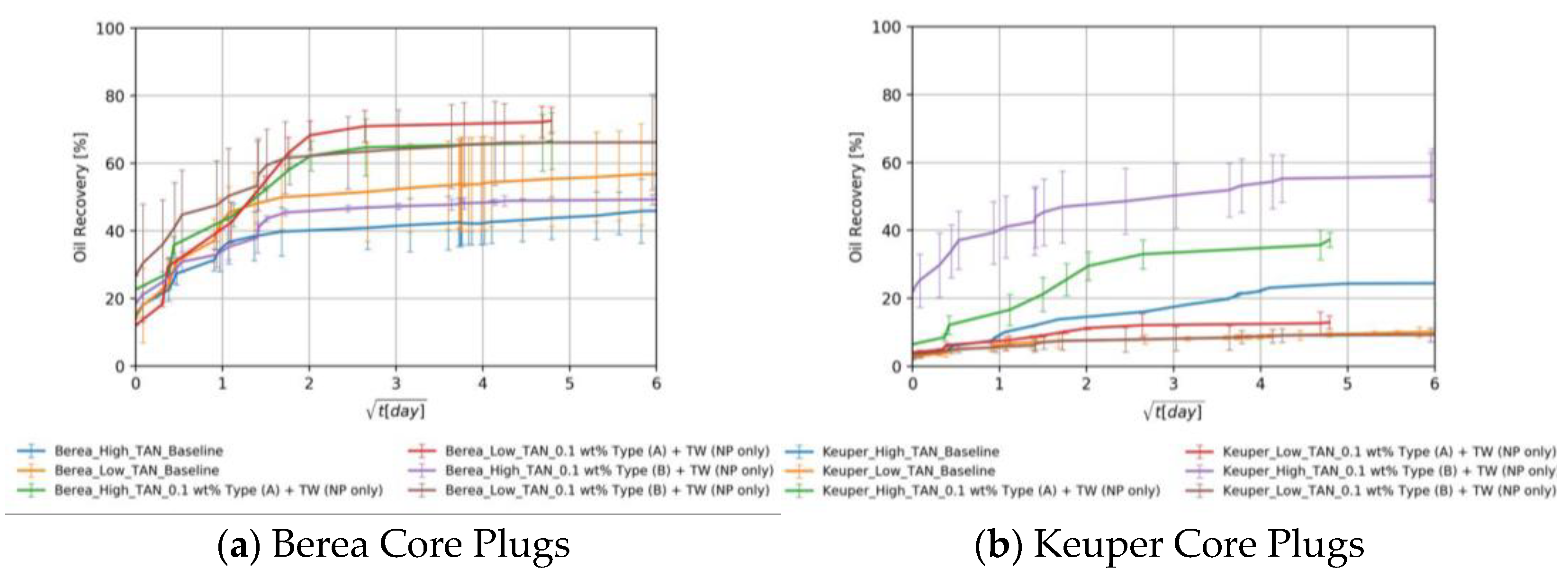
Berea Core Plugs
Keuper Core Plugs
4.3.3. Influence of the Oil—TAN
4.3.4. The Effect of Divalent Cations
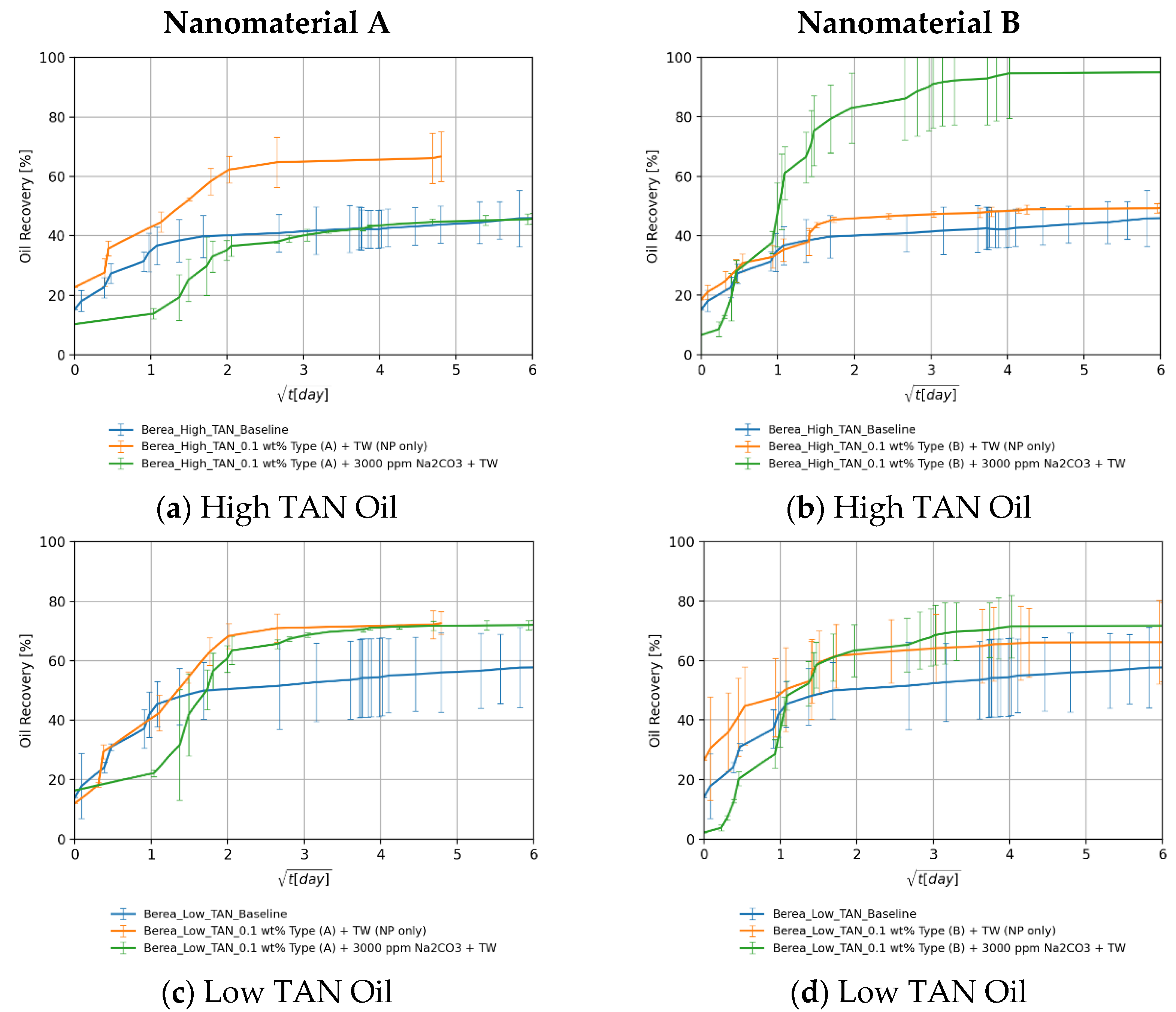
4.3.5. The Effect: Nanomaterials and Alkali
Berea Core Plugs
Keuper Core Plugs
4.4. Modeling Results
4.4.1. Shape of the Recovery Curve
4.4.2. Recovery vs. Inverse Bond Number (NB−1)
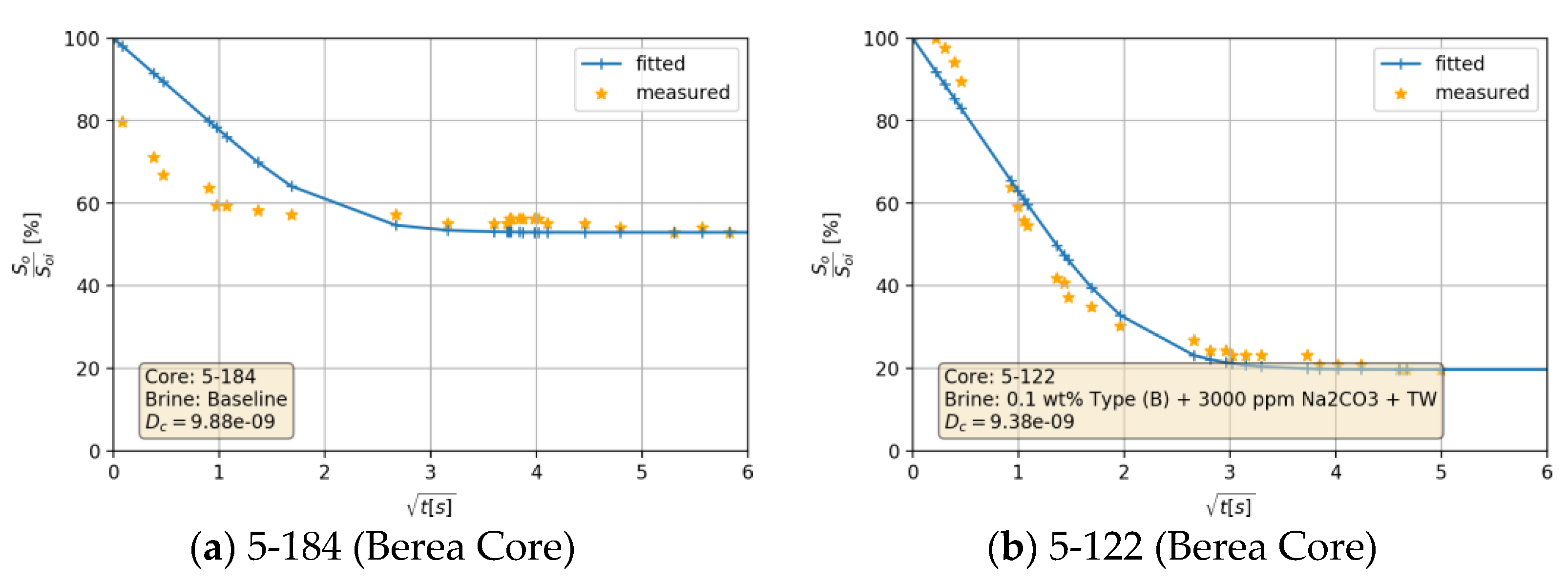
4.4.3. Capillary Diffusion Coefficient—An Example
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
Appendix C

References
- Jiang, R.; Li, K.; Horne, R. A Mechanism Study of Wettability and Interfacial Tension for EOR Using Silica Nanoparticles. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 9–11 October 2017. [Google Scholar] [CrossRef]
- Joonaki, E.; Ghanaatian, S. The Application of Nanofluids for Enhanced Oil Recovery: Effects on Interfacial Tension and Coreflooding Process. Pet. Sci. Technol. 2014, 32, 2599–2607. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Z.; Li, S.; Jiang, L.; Wang, J.; Wang, P. Utilization of Surfactant-Stabilized Foam for Enhanced Oil Recovery by Adding Nanoparticles. Energy Fuels 2014, 28, 2384–2394. [Google Scholar] [CrossRef]
- Roustaei, A.; Saffarzadeh, S.; Mohammadi, M. An evaluation of modified silica nanoparticles’ efficiency in enhancing oil recovery of light and intermediate oil reservoirs. Egypt. J. Pet. 2013, 22, 427–433. [Google Scholar] [CrossRef]
- Kamal, M.S.; Adewunmi, A.A.; Sultan, A.S.; Al-Hamad, M.F.; Mehmood, U. Recent Advances in Nanoparticles Enhanced Oil Recovery: Rheology, Interfacial Tension, Oil Recovery, and Wettability Alteration. J. Nanomater. 2017, 2017, 2473175. [Google Scholar] [CrossRef]
- Cheraghian, G.; Rostami, S.; Afrand, M. Nanotechnology in Enhanced Oil Recovery. Processes 2020, 8, 1073. [Google Scholar] [CrossRef]
- Rezk, M.Y.; Allam, N.K. Impact of Nanotechnology on Enhanced Oil Recovery: A Mini-Review. Ind. Eng. Chem. Res. 2019, 58, 16287–16295. [Google Scholar] [CrossRef]
- Kazemzadeh, Y.; Shojaei, S.; Riazi, M.; Sharifi, M. Review on application of nanoparticles for EOR purposes: A critical review of the opportunities and challenges. Chin. J. Chem. Eng. 2019, 27, 237–246. [Google Scholar] [CrossRef]
- Neubauer, E.; Hincapie, R.E.; Clemens, T.; Maximilian, C. Selection of Nanomaterials as Emulsion Stabilizers in Alkali-Polymer EOR of High-TAN Number Oil. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 31 August–4 September 2020. [Google Scholar] [CrossRef]
- Neubauer, E.; Hincapie, R.E.; Borovina, A.; Biernat, M.; Clemens, T.; Ahmad, Y.K. Influence of Nanofluids on Wettability Changes and Interfacial Tension Reduction. In Proceedings of the SPE Europec, Virtual, 1–3 December 2020. [Google Scholar] [CrossRef]
- Raffa, P.; Druetta, P. Chemical Enhanced Oil Recovery—Advances in Polymer Flooding and Nanotechnology; De Gruyter: Berlin, Germany; Boston, MA, USA, 2019. [Google Scholar] [CrossRef]
- ShamsiJazeyi, H.; Miller, C.A.; Wong, M.S.; Tour, J.M.; Verduzco, R. Polymer-Coated Nanoparticles for Enhanced Oil Recovery. J. Appl. Polym. Sci. 2014, 131, 40576. [Google Scholar] [CrossRef]
- Ali, J.A.; Kolo, K.; Manshad, A.K.; Mohammadi, A.H. Recent advances in application of nanotechnology in chemical enhanced oil recovery: Effects of nanoparticles on wettability alteration, interfacial tension reduction, and flooding. Egypt. J. Pet. 2018, 27, 1371–1383. [Google Scholar] [CrossRef]
- Singh, R.; Mohanty, K.K. Foam flow in a layered, heterogeneous porous medium: A visualization study. Fuel 2017, 197, 58–69. [Google Scholar] [CrossRef]
- Corredor, L.M.; Aliabadian, E.; Husein, M.; Chen, Z.; Maini, B.; Sundararaj, U. Heavy oil recovery by surface modified silica nanoparticle/HPAM nanofluids. Fuel 2019, 252, 622–634. [Google Scholar] [CrossRef]
- Sharma, T.; Kumar, G.S.; Chon, B.H.; Sangwai, J.S. Thermal stability of oil-in-water Pickering emulsion in the presence of nanoparticle, surfactant, and polymer. J. Ind. Eng. Chem. 2015, 22, 324–334. [Google Scholar] [CrossRef]
- Ahmed, A.; Saaid, I.M.; Ahmed, A.A.; Pilus, R.M.; Baig, M.K. Evaluating the potential of surface-modified silica nanoparticles using internal olefin sulfonate for enhanced oil recovery. Pet. Sci. 2020, 17, 722–733. [Google Scholar] [CrossRef]
- Kim, I.; Worthen, A.; Lotfollahi, M.; Johnston, K.; DiCarlo, D.; Chun Huh, C. Nanoparticle-Stabilized Emulsions for Improved Mobility Control for Adverse-mobility Waterflooding. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar] [CrossRef]
- Arab, D.; Kantzas, A.; Bryant, S. Nanoparticle stabilized oil in water emulsions: A critical review. J. Pet. Sci. Eng. 2018, 163, 217–242. [Google Scholar] [CrossRef]
- Perazzo, A.; Tomaiuolo, G.; Preziosi, V.; Guido, S. Emulsions in porous media: From single droplet behavior to applications for oil recovery. Adv. Colloid Interface Sci. 2018, 256, 305–325. [Google Scholar] [CrossRef]
- Binks, B.; Rodrigues, J.A. Enhanced Stabilization of Emulsions Due to Surfactant-Induced Nanoparticle Flocculation. Langmuir 2007, 23, 7436–7439. [Google Scholar] [CrossRef]
- Milad Kamkar, M.; Bazazi, P.; Kannan, A.; Chandran, V.S.; Hejazi, S.H.; Fuller, G.G.; Sundararaj, U. Polymeric-nanofluids stabilized emulsions: Interfacial versus bulk rheology. J. Colloid Interface Sci. 2020, 576, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Morejon, J.L.; Bertin, H.; Omari, A.; Hamon, G.; Cottin, C.; Bourdarot, G.; Morel, D. Spontaneous Imbibition as Indicator of Wettability Change During Polymer Flooding. In Proceedings of the IOR 2017—19th European Symposium on Improved Oil Recovery, Stavanger, Norway, 24–27 April 2017; EAGE, Ed.; EAGE Publications BV: Houten, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Chengara, A.; Nikolov, A.D.; Wasan, D.T.; Trokhymchuk, A.; Henderson, D. Spreading of nanofluids driven by the structural disjoining pressure gradient. J. Colloid Interface Sci. 2004, 280, 192–201. [Google Scholar] [CrossRef]
- Wasan, D.; Nikolov, A. Spreading of nanofluids on solids. Nature 2003, 423, 156–159. [Google Scholar] [CrossRef]
- Kuang, W.; Saraji, S.; Piri, M. A systematic experimental investigation on the synergistic effects of aqueous nanofluids on interfacial properties and their implications for enhanced oil recovery. Fuel 2018, 220, 849–870. [Google Scholar] [CrossRef]
- Sofla, S.J.D.; James, L.A.; Zhang, Y. Toward a mechanistic understanding of wettability alteration in reservoir rocks using silica nanoparticles. E3S Web Conf. 2019, 89, 03004. [Google Scholar] [CrossRef]
- Huh, C.; Daigle, H.; Prigiobbe, V.; Prodanović, M. Practical Nanotechnology for Petroleum Engineers, 1st ed.; Enhanced Oil Recovery: Wettability Alteration and Other Topics; CRC Press: Boca Raton, FL, USA, 2019; pp. 281–301. [Google Scholar] [CrossRef]
- Li, S.; Torsæter, O.; Lau, H.C.; Hadia, N.J.; Stubbs, L.P. The Impact of Nanoparticle Adsorption on Transport and Wettability Alteration in Water-Wet Berea Sandstone: An Experimental Study. Front. Phys. 2019, 7, 74. [Google Scholar] [CrossRef]
- Alvarez-Berrios, M.P.; Aponte-Reyes, L.M.; Aponte-Cruz, L.M.; Loman-Cortes, P.; Vivero-Escoto, J.L. Effect of the surface charge of silica nanoparticles on oil recovery: Wettability alteration of sandstone cores and imbibition experiments. Int. Nano Lett. 2018, 8, 181–188. [Google Scholar] [CrossRef]
- Bila, A.; Stensen, J.Å.; Torsæter, O. Experimental Investigation of Polymer-Coated Silica Nanoparticles for Enhanced Oil Recovery. Nanomaterials 2019, 9, 822. [Google Scholar] [CrossRef]
- Li, S.; Dan, D.; Lau, H.C.; Hadia, N.J.; Torsæter, O.; Stubbs, L.P. Investigation of Wettability Alteration by Silica Nanoparticles Through Advanced Surface-Wetting Visualization Techniques. In Proceedings of the SPE Annual Technical Conference and Exhibition, Calgary, AB, Canada, 30 September–2 October 2019. [Google Scholar] [CrossRef]
- Xu, D.; Bai, B.; Wu, H.; Hou, J.; Meng, Z.; Sun, R.; Li, Z.; Lu, Y.; Kang, W. Mechanisms of imbibition enhanced oil recovery in low permeability reservoirs: Effect of IFT reduction and wettability alteration. Fuel 2019, 244, 110–119. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, S.; Zhang, Z.; He, J. Effect of Nanoparticles on Spontaneous Imbibition of Water into Ultraconfined Reservoir Capillary by Molecular Dynamics Simulation. Energies 2017, 10, 506. [Google Scholar] [CrossRef]
- Sheng, J. Status of Alkaline Flooding Technology. J. Pet. Eng. Technol. 2015, 5, 44–50. [Google Scholar] [CrossRef]
- Sheng, J.J. Critical review of alkaline-polymer flooding. J. Pet. Explor. Prod. Technol. 2016, 7, 147–153. [Google Scholar] [CrossRef]
- Sheng, J.J. Modern Chemical Enhanced Oil Recovery; Gulf Professional Publishing: Houston, TX, USA, 2011; pp. 501–567. ISBN 9781856177450. [Google Scholar] [CrossRef]
- Arekhov, V.; Hincapie, R.E.; Clemens, T.; Tahir, M. Variations in Wettability and Interfacial Tension during Alkali–Polymer Application for High and Low TAN Oils. Polymers 2020, 12, 2241. [Google Scholar] [CrossRef]
- Schumi, B.; Clemens, T.; Wegner, J.; Ganzer, L.; Kaiser, A.; Leitenmüller, L.; Hincapie, R. Alkali-Co-Solvent-Polymer Flooding of High TAN Number Oil: Using Phase Experiments, Micro-Models and Corefloods for Injection Agent Selection. SPE Res. Eval. Eng. 2020, 23, 463–478. [Google Scholar] [CrossRef]
- Xu, X.; Saeedi, A. Evaluation and Optimization Study on a Hybrid EOR Technique Named as Chemical-Alternating-Foam Floods. Oil Gas Sci. Technol.—Rev. IFP 2017, 72, 1. [Google Scholar] [CrossRef][Green Version]
- Hu, X.; Li, M.; Peng, C.; Davarpanah, A. Hybrid Thermal-Chemical Enhanced Oil Recovery Methods; An Experimental Study for Tight Reservoirs. Symmetry 2020, 12, 947. [Google Scholar] [CrossRef]
- Hamza, M.F.; Sinnathambi, C.M.; Merican, Z.M. Recent advancement of hybrid materials used in chemical enhanced oil recovery (CEOR): A review. IOP Conf. Ser.: Mater. Sci. Eng. 2017, 206, 012007. Available online: https://iopscience.iop.org/article/10.1088/1757-899X/206/1/012007 (accessed on 5 November 2020). [CrossRef]
- Druetta, P.; Picchioni, F. Surfactant-Polymer Interactions in a Combined Enhanced Oil Recovery Flooding. Energies 2020, 13, 6520. [Google Scholar] [CrossRef]
- Druetta, P.; Picchioni, F. Polymer and nanoparticles flooding as a new method for Enhanced Oil Recovery. J. Pet. Sci. Eng. 2019, 177, 479–495. [Google Scholar] [CrossRef]
- Rock, A.; Hincapie, R.E.; Hoffmann, E.; Ganzer, L. Tertiary Low Salinity Waterflooding LSWF in Sandstone Reservoirs: Mechanisms, Synergies and Potentials in EOR Applications. In Proceedings of the SPE Europec featured at 80th EAGE Conference and Exhibition, Copenhagen, Denmark, 11–14 June 2018. [Google Scholar] [CrossRef]
- Maneeintr, K.; Meekoch, T.; Jongkittinarukorn, K.; Boonpramote, T. Interfacial Tension Measurement for Alkaline-Polymer Flooding Application for Oil from Fang Oilfield, Thailand. Chem. Eng. Trans. 2020, 78, 487–492. [Google Scholar] [CrossRef]
- Chevalier, T.; Labaume, J.; Delbos, A.; Clemens, T.; Waeger, V.M.; Bourbiaux, B.; Fleury, M. A Practical Methodology to Screen Oil Recovery Processes Involving Spontaneous Imbibition. Transp. Porous Media 2019, 127, 729–743. [Google Scholar] [CrossRef]
- Mortazavi, E.; Masihi, M.; Ghazanfari, M. An Influence of Polymer-Alkaline and Nanoparticles as Chemical Additives on the Immiscible Displacement and Phase Relative Permeability. Iran. J. Oil Gas Sci. Technol. 2016, 5, 14–31. Available online: http://ijogst.put.ac.ir/article_38523.html (accessed on 5 November 2020).
- French, T.; Burchfield, T. Design and Optimization of Alkaline Flooding Formulations. In Proceedings of the SPE/DOE Enhanced Oil Recovery Symposium, Tulsa, OK, USA, 22–25 April 1990. [Google Scholar] [CrossRef]
- Lüftenegger, M.; Clemens, T. Chromatography Effects in Alkali Surfactant Polymer Flooding. In Proceedings of the SPE Europec Featured at 79th EAGE Conference and Exhibition, Paris, France, 12–15 June 2017. [Google Scholar] [CrossRef]
- Bailey, H.R.; Gogarty, W.B. Diffusion Coefficients from Capillary Flow. SPE J. 1963, 3, 256–266. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford Science; Clarendon Press: Oxford, UK, 1979; ISBN 0198534116/9780198534112. [Google Scholar]
- Al-Quraishi, A.A. Oil Recovery by Dynamic Imbibition in Low Tension Aqueous Systems. Oil Gas Sci. Technol.—Rev. IFP 2004, 59, 267–273. [Google Scholar] [CrossRef]
- Schechter, D.; Zhou, D.; Orr, F.M. Low IFT drainage and imbibition. J. Pet. Sci. Eng. 1994, 11, 283–300. [Google Scholar] [CrossRef]
- Babadagli, T. Analysis of Oil Recovery by Spontaneous Imbibition of Surfactant Solution. Oil Gas Sci. Technol.—Rev. IFP 2005, 60, 697–710. [Google Scholar] [CrossRef]
- Chen, H.; Fan, H.; Zhang, Y.; Xu, X.; Liu, L.; Hou, Q. Investigations on the driving forces of the fluorocarbon surfactant-assisted spontaneous imbibition using thermogravimetric analysis (TGA). RSC Adv. 2018, 8, 38196–38203. [Google Scholar] [CrossRef]
- Vatanparast, H.; Shahabi, F.; Bahramian, A.; Javadi, A.; Miller, R. The Role of Electrostatic Repulsion on Increasing Surface Activity of Anionic Surfactants in the Presence of Hydrophilic Silica Nanoparticles. Sci. Rep. 2018, 8, 7251. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Jang, L.; Yen, T. Transient Interfacial Tension Behavior of Crude-Oil/Caustic Interfaces. SPE Reserv. Eng. 1989, 4, 228–236. [Google Scholar] [CrossRef]
- Spanos, N.; Koutsoukos, P.G. Kinetics of Precipitation of Calcium Carbonate in Alkaline pH at Constant Supersaturation. Spontaneous and Seeded Growth. J. Phys. Chem. B 1998, 102, 6679–6684. [Google Scholar] [CrossRef]
- Liesegang, M.; Milke, R.; Kranz, C.; Neusser, G. Silica nanoparticle aggregation in calcite replacement reactions. Sci. Rep. 2017, 7, 14550. [Google Scholar] [CrossRef]
- Magnabosco, G.; Polishchuk, I.; Palomba, F.; Rampazzo, E.; Prodi, L.; Aizenberg, J.; Pokroy, B.; Falini, G. Effect of Surface Chemistry on Incorporation of Nanoparticles within Calcite Single Crystals. Cryst. Growth Des. 2019, 19, 4429–4435. [Google Scholar] [CrossRef]
- Morrow, N.R.; Mason, G. Recovery of oil by spontaneous imbibition. Curr. Opin. Colloid Interface Sci. 2001, 6, 321–337. [Google Scholar] [CrossRef]
- Zhou, D.; Jia, L.; Kamath, J.; Kovscek, A.R. Scaling of counter-current imbibition processes in low-permeability porous media. J. Pet. Sci. Eng. 2002, 33, 61–74. [Google Scholar] [CrossRef]
- Meng, Q.; Liu, H.; Wang, J. A critical review on fundamental mechanisms of spontaneous imbibition and the impact of boundary condition, fluid viscosity and wettability. Adv. Geo-Energy Res. 2017, 1, 1–17. [Google Scholar] [CrossRef]
- Sayyouh, M.; Dahab, A.; Omar, A. Effect of clay content on wettability of sandstone reservoirs. J. Pet. Sci. Eng. 1990, 4, 119–125. [Google Scholar] [CrossRef]
- Mohammed, I.; Al Shehri, D.; Mahmoud, M.; Kamal, M.S.; Alade, O.S. Impact of Iron Minerals in Promoting Wettability Alterations in Reservoir Formations. ACS Omega 2021, 6, 4022–4033. [Google Scholar] [CrossRef] [PubMed]
- Omurlu, C.; Pham, H.; Nguyen, Q.P. Interaction of surface-modified silica nanoparticles with clay minerals. Appl. Nanosci. 2016, 6, 1167–1173. [Google Scholar] [CrossRef]
- Buckley, J.S. Asphaltene Precipitation and Crude Oil Wetting. SPE Adv. Technol. Ser. 1995, 3, 53–59. [Google Scholar] [CrossRef]
- Metin, C.O.; Lake, L.W.; Miranda, C.R.; Nguyen, Q.P. Stability of aqueous silica nanoparticle dispersions. J. Nanopart. Res. 2011, 13, 839–850. [Google Scholar] [CrossRef]
- Haagh, M.E.J.; Siretanu, I.; Duits, M.H.G.; Mugele, F. Salinity-Dependent Contact Angle Alteration in oil/Brine/Silicate Systems: The Critical Role of Divalent Cations. Langmuir 2017, 33, 3349–3357. Available online: https://pubs.acs.org/doi/abs/10.1021/acs.langmuir.6b04470 (accessed on 5 November 2020). [CrossRef] [PubMed]







| Formulation | Approach | Rock Type | Takeaways | Ref. |
|---|---|---|---|---|
| NPs/Surfact. | IFT, Contact Angle, Rheology, Core Floods | Sandstones/Carbonates | NPs decreased IFT, wettability changes. Effects of NPs in IFT and wettability. | [2,3,5] |
| NPs/Surfact/Polymers | IFT, Contact Angle and Core Floods | Sandstones | Strong IFT reduction in brine/oil system when using nanoparticles | [9,16,17,24,25,26,28,30] |
| Alkali/Alkali Polymer | IFT, Core Floods, Phase Behavior | Sandstones | Favorable findings using alkali combined with polymer/surfactant | [36,37] |
| Alkali Polymer | IFT, Phase Behavior, Amott Imbibition | Sandstones | Alkali of IFT reduction in high-TAN oils with clear wettability changes. | [38] |
| Alkali Polymer | IFT, pH, Rheology | -- | Alkali solution lowered IFT | [46] |
| Alkali Brine | IFT, NMR, Spontaneous Imbibition | Sandstones | Mixed impacts of alkali on the spontaneous imbibition kinetics | [47] |
| Alkali Polymer NPs | Unsteady State Displacement | Sandstones | Combination promoted favorable pressure gradient changes | [48] |
| Formulation | TW Softened Injection Brine | FW Synthetic Formation Brine |
|---|---|---|
| NaCl [g/L] | 18.96 | 19.75 |
| NaHCO3 [g/L] | 1.96 | - |
| CaCl2 · 2 H2O [g/L] | - | 0.40 |
| MgCl2 · 6 H2O [g/L] | - | 0.66 |
| NH4Cl [g/L] | - | 0.17 |
| SrCl2 · 6 H2O [g/L] | - | 0.06 |
| Property | High TAN | Low TAN |
|---|---|---|
| Reservoir/Well | 16 TH/Bockfliess 112 | St. Ulrich/St.U. 65 |
| TVD top [m] | 1622 | 1060 |
| TAN [mg KOH/g] | 1.61 | 0.39 |
| Saturates [%] | 39 | 55 |
| Aromatics [%] | 20 | 25.6 |
| Resins [%] | 39 | 18.6 |
| Asphaltene [%] | 2 | 0.8 |
| Saponifiable Acids [µmol/g] | 26 | n.m. |
| µ @ 60 °C [mPa∙s] | 11.9 | 6 |
| ρ @ 20°C/60 °C [g/cm3] | 0.917/0.884 | 0.866/0.842 |
| Property | Type A | Type B |
|---|---|---|
| Solid [wt. %] | 22.5 | 27.9 |
| m @ 10 1/s [mPa∙s] | 16 | 39 |
| Particle Size (d50) | 110 | 114 |
| pH | 9.5 | 3.2 |
| Fluid | Density 1 [g/cm3] | Ph 2 [-] | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| TW | 0.994 | 0.01 | 8.93 | 0.01 |
| FW | 0.995 | 0.01 | - | - |
| 0.1 wt.% Type (A) in TW | 0.997 | 0.01 | 8.56 | 0.02 |
| 0.1 wt.% Type (B) in TW | 0.984 | 0.01 | 8.76 | 0.02 |
| 0.1 wt.% Type (A) + 3000 ppm Na2CO3 in TW | 0.992 | 0.01 | 9.93 | 0.01 |
| 0.1 wt.% Type (B) + 3000 ppm Na2CO3 in TW | 1.001 | 0.01 | 9.92 | 0.01 |
| 3000 ppm Na2CO3 in TW | 0.998 | 0.01 | 9.98 | 0.01 |
| Parameter | Units | Berea 1 | Keuper 2 | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Length | cm | 6.97 | 0.02 | 8.12 | 0.09 |
| Diameter | 2.96 | 0.01 | 2.98 | 0.01 | |
| Bulk Volume | cm3 | 47.76 | 0.26 | 55.76 | 0.73 |
| Pore Volume | 10.77 | 0.19 | 12.75 | 0.22 | |
| Grain Volume | kg/cm3 | 37.00 | 0.31 | 42.98 | 0.67 |
| Porosity | % | 22.60 | 0.40 | 23.30 | 0.80 |
| N2 permeability (kg) | mD | 447.60 | 37.40 | 1425.20 | 349.60 |
| Water (Test Water) permeability (kw) | 223.90 | 17.90 | 890.00 | 193.90 | |
| Irreducible water saturation | % | 24.00 | 8.00 | 21.40 | 7.90 |
| Fluid | High TAN Oil [mN/m] | Low TAN Oil [mN/m] | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Baseline (TW) | 8.40 | - | 3.40 | 0.50 |
| 0.1 wt.% Nano A + TW | 8.30 | 0.15 | 2.60 | 0.13 |
| 0.1 wt.% Nano B + TW | 5.31 | 0.49 | 1.26 | 0.06 |
| 3000 ppm Na2CO3 + TW | 0.11 | 0.01 | 0.55 | 0.15 |
| 0.1 wt.% Nano A + 3000 ppm Na2CO3 + TW | 0.04 | 0.01 | 0.17 | 0.01 |
| 0.1 wt.% Nano B + 3000 ppm Na2CO3 + TW | 0.06 | 0.01 | 1.00 | 0.01 |
| Fluid | Berea Outcrop | Keuper Outcrop | ||||||
|---|---|---|---|---|---|---|---|---|
| High TAN Oil [%] | Low TAN Oil [%] | High TAN Oil [%] | Low TAN Oil [%] | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Baseline (TW) | 47.20 | 8.40 | 47.20 | 8.40 | 44.80 | - | 10.30 | 1.50 |
| 0.1 wt.% Nano A | 67.10 | 0.15 | 67.10 | 0.15 | 41.10 | 2.20 | 13.80 | 3.40 |
| 0.1 wt.% Nano B | 50.20 | 1.50 | 50.20 | 1.50 | 57.00 | 7.70 | 9.30 | 2.00 |
| 3000 ppm Na2CO3 | 59.70 | 23.10 | 59.70 | 23.10 | 95.30 | 2.60 | 41.00 | 15.10 |
| 0.1 wt.% Nano A + 3000 ppm Na2CO3 | 45.80 | 1.70 | 45.80 | 1.70 | 95.40 | 5.90 | 72.20 | 5.90 |
| 0.1 wt.% Nano B + 3000 ppm Na2CO3 | 97.70 | 16.01 | 97.70 | 16.01 | 97.50 | 19.01 | 66.80 | 22.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, S.; Neubauer, E.; Borovina, A.; Hincapie, R.E.; Clemens, T.; Ness, D. Wettability Changes Due to Nanomaterials and Alkali—A Proposed Formulation for EOR. Nanomaterials 2021, 11, 2351. https://doi.org/10.3390/nano11092351
Saleh S, Neubauer E, Borovina A, Hincapie RE, Clemens T, Ness D. Wettability Changes Due to Nanomaterials and Alkali—A Proposed Formulation for EOR. Nanomaterials. 2021; 11(9):2351. https://doi.org/10.3390/nano11092351
Chicago/Turabian StyleSaleh, Samhar, Elisabeth Neubauer, Ante Borovina, Rafael E. Hincapie, Torsten Clemens, and Daniel Ness. 2021. "Wettability Changes Due to Nanomaterials and Alkali—A Proposed Formulation for EOR" Nanomaterials 11, no. 9: 2351. https://doi.org/10.3390/nano11092351
APA StyleSaleh, S., Neubauer, E., Borovina, A., Hincapie, R. E., Clemens, T., & Ness, D. (2021). Wettability Changes Due to Nanomaterials and Alkali—A Proposed Formulation for EOR. Nanomaterials, 11(9), 2351. https://doi.org/10.3390/nano11092351








