Construction of Electrostatic Self-Assembled 2D/2D CdIn2S4/g-C3N4 Heterojunctions for Efficient Visible-Light-Responsive Molecular Oxygen Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of the Photocatalysts
2.2.1. Synthesis of g-C3N4 Nanosheets and Protonated g-C3N4 Nanosheets
2.2.2. Preparation of CdIn2S4 Nanosheets
2.2.3. Synthesis of 2D/2D CdIn2S4/g-C3N4 Heterojunctions
2.3. Characterization
2.4. Electrochemical Analysis
2.5. Catalytic Experiments
2.6. Quantitative Analysis of •O2−
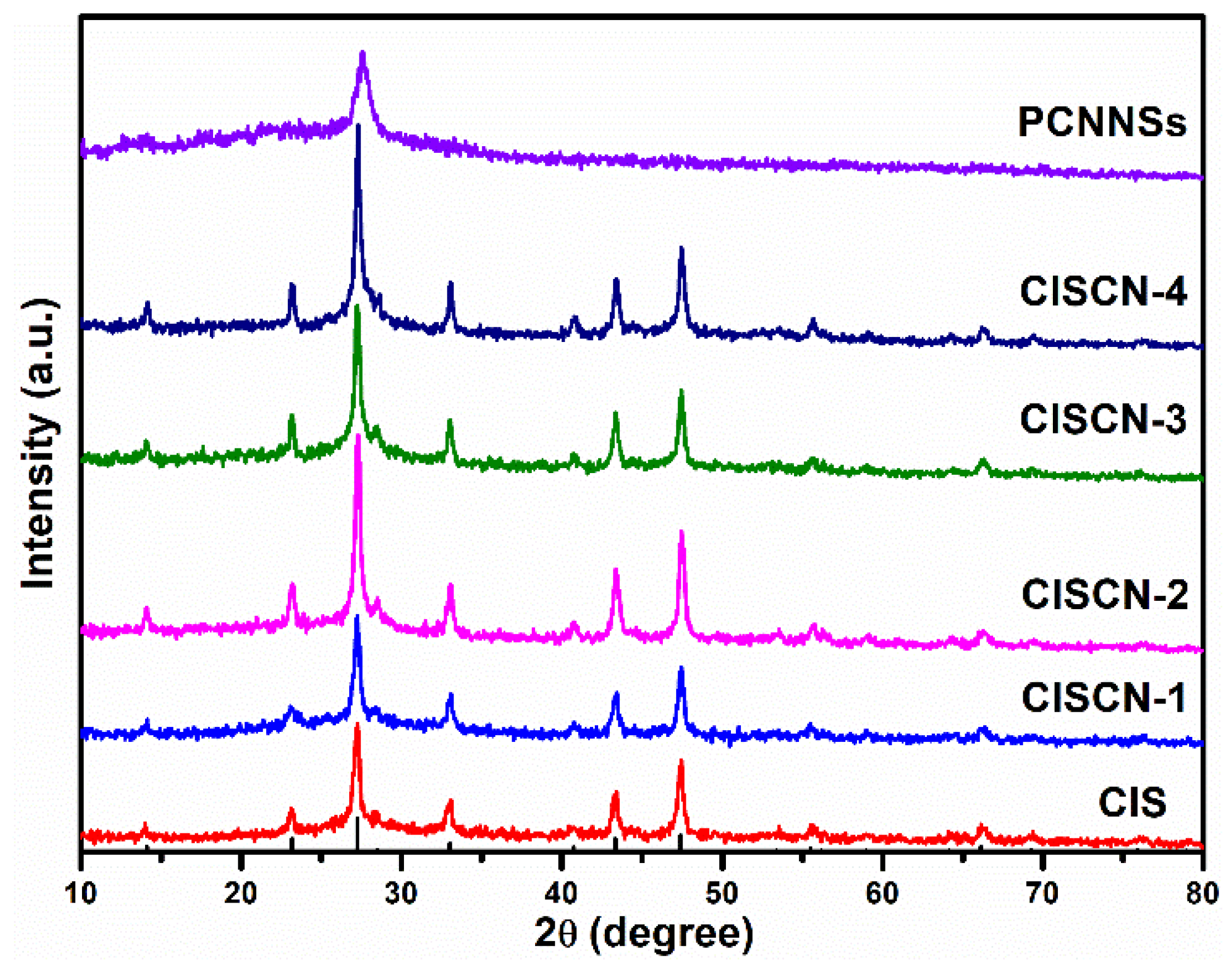
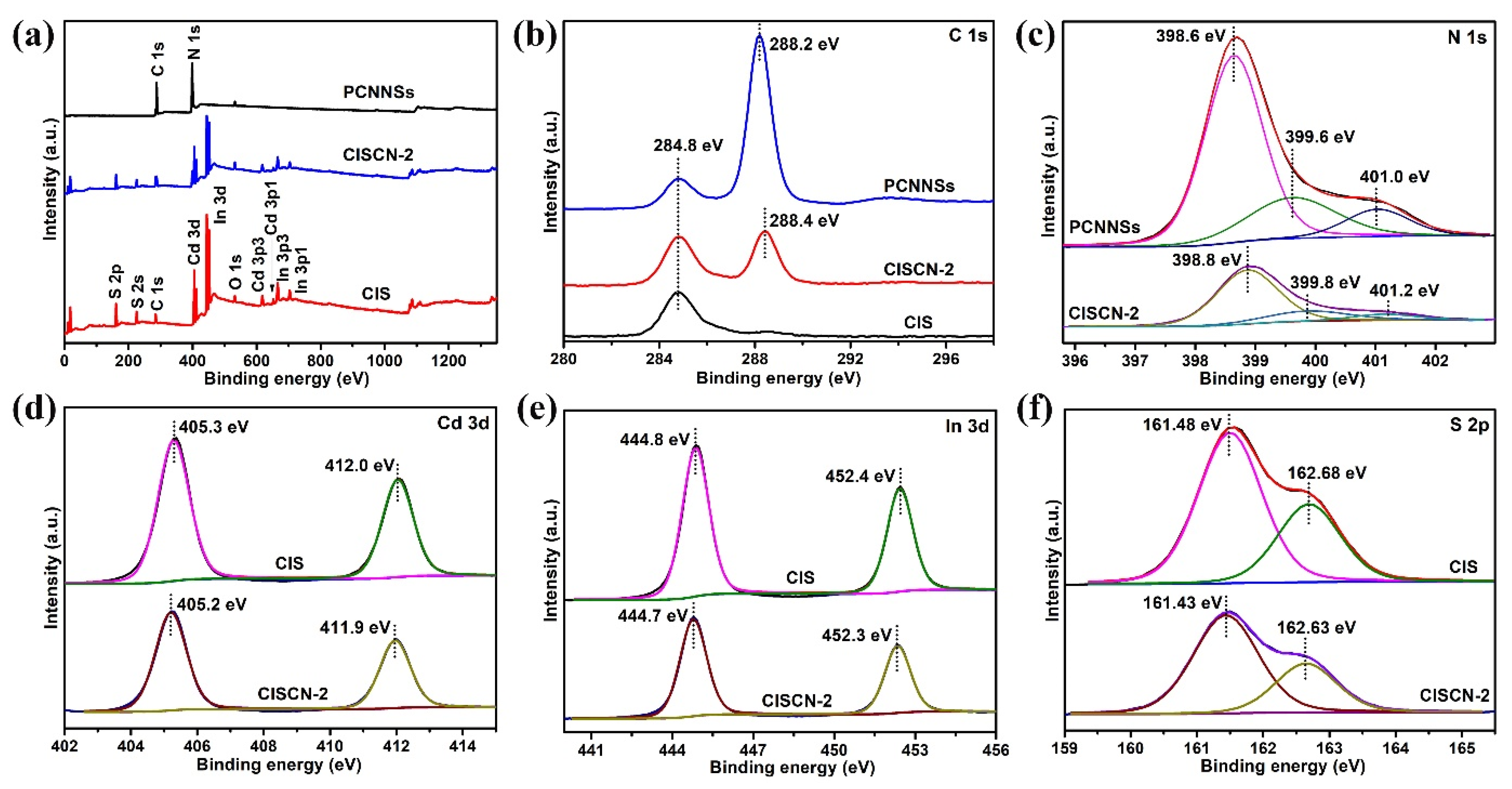
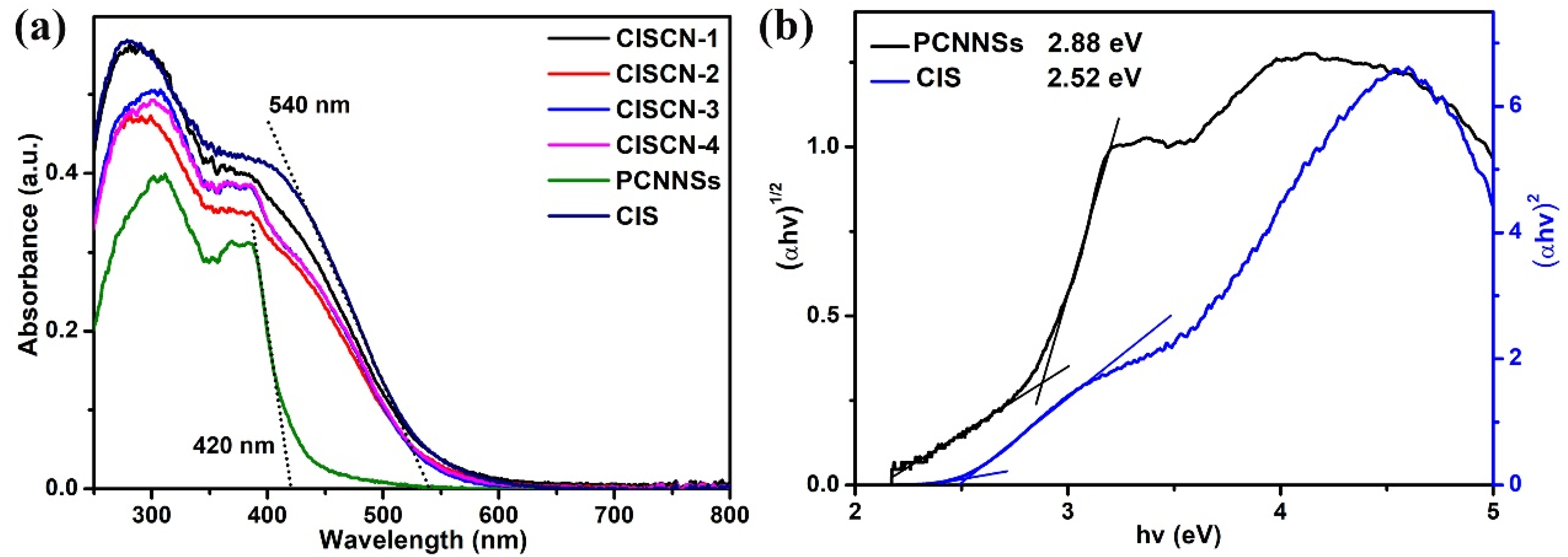
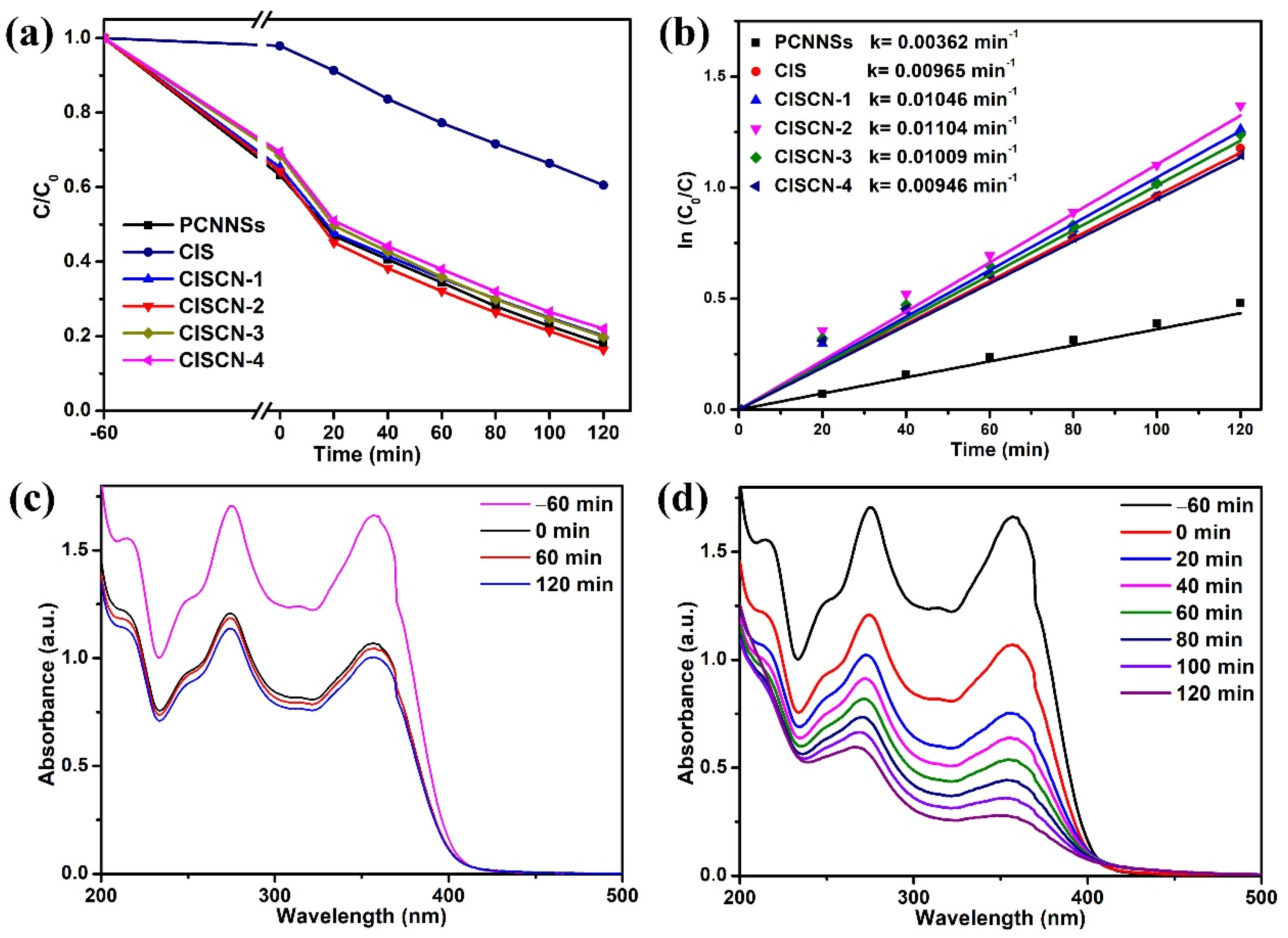
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Kadam, A.; Kim, H.; Lee, S. Low-temperature in situ fabrication of porous S-doped g-C3N4 nanosheets using gaseous-bubble template for enhanced visible-light photocatalysis. Ceram. Int. 2020, 46, 28481–28489. [Google Scholar] [CrossRef]
- Kadam, A.; Moniruzzaman, M.; Lee, S. Dual functional S-doped g-C3N4 pinhole porous nanosheets for selective fluorescence sensing of Ag+ and visible-light photocatalysis of dyes. Molecules 2019, 24, 450. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Huang, G.; Song, H.; Zhang, J. Efficient and stable charge transfer channels for photocatalytic water splitting activity of CdS without sacrificial agents. J. Mater. Chem. A 2020, 8, 20963–20969. [Google Scholar] [CrossRef]
- Dong, H.; Zuo, Y.; Song, N.; Hong, S.; Xiao, M.; Zhu, D.; Sun, J.; Chen, G.; Li, C. Bimetallic synergetic regulating effect on electronic structure in cobalt/vanadium co-doped carbon nitride for boosting photocatalytic performance. Appl. Catal. B Environ. 2021, 287, 119954. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, X.; Li, J.; Zhou, P.; Yu, S.; Song, N.; Liu, C.; Che, G.; Li, C. Construction of morphology-controlled nonmetal 2D/3D homojunction towards enhancing photocatalytic activity and mechanism insight. Appl. Catal. B Environ. 2020, 263, 118270. [Google Scholar] [CrossRef]
- Fang, H.; Guo, H.; Niu, C.; Liang, C.; Huang, D.; Tang, N.; Liu, H.; Yang, Y.; Li, L. Hollow tubular graphitic carbon nitride catalyst with adjustable nitrogen vacancy: Enhanced optical absorption and carrier separation for improving photocatalytic activity. Chem. Eng. J. 2020, 402, 126185. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Huang, D.; Zeng, G.; Huang, J.; Lai, C.; Zhou, C.; Wang, W.; Guo, H.; Xue, W.; et al. Boron nitride quantum dots decorated ultrathin porous g-C3N4: Intensified exciton dissociation and charge transfer for promoting visible-light-driven molecular oxygen activation. Appl. Catal. B Environ. 2019, 245, 87–99. [Google Scholar] [CrossRef]
- Kale, B.; Baeg, J.; Lee, S.; Chang, H.; Moon, S.; Lee, C. CdIn2S4 nanotubes and marigold nanostructures: A visible-light photocatalyst. Adv. Funct. Mater. 2006, 16, 1349–1354. [Google Scholar] [CrossRef]
- Li, L.; Peng, S.; Wang, N.; Srinivasan, M.; Mhaisalkar, S.G.; Yu, Q.; Ramakrishna, S. A general strategy toward carbon cloth-based hierarchical films constructed by porous nanosheets for superior photocatalytic activity. Small 2015, 11, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; An, H.; Yan, X.; Li, J.; Yang, B.; Wei, J.; Yang, G. Spatial charge separation and transfer in ultrathin CdIn2S4/rGO nanosheet arrays decorated by ZnS quantum dots for efficient visible-light-driven hydrogen evolution. Nano Energy 2017, 39, 513–523. [Google Scholar] [CrossRef]
- Mahadadalkar, M.; Gosavi, S.; Kale, B. Interstitial charge transfer pathways in a TiO2/CdIn2S4 heterojunction photocatalyst for direct conversion of sunlight into fuel. J. Mater. Chem. A 2018, 6, 16064–16073. [Google Scholar] [CrossRef]
- Chen, W.; Huang, T.; Hua, Y.; Liu, T.; Liu, X.; Chen, S. Hierarchical CdIn2S4 microspheres wrapped by mesoporous g-C3N4 ultrathin nanosheets with enhanced visible light driven photocatalytic reduction activity. J. Hazard. Mater. 2016, 320, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, J.; Ye, X.; Hui, Z.; Ye, L.; Wang, X.; Chen, S. Self-assembly of CdS/CdIn2S4 heterostructure with enhanced photocascade synthesis of schiff base compounds in an aromatic alcohols and nitrobenzene system with visible light. ACS Appl. Mater. Interfaces 2019, 11, 46735–46745. [Google Scholar] [CrossRef]
- Peng, Z.; Jiang, Y.; Wang, X.; Zhang, R.; Xu, H.; Xiao, Y.; Jing, X.; Zhang, J.; Liu, Y.; Ni, L. Novel CdIn2S4 nano-octahedra/TiO2 hollow hybrid heterostructure: In-situ synthesis, synergistic effect and enhanced dual-functional photocatalytic activities. Ceram. Int. 2019, 45, 15942–15953. [Google Scholar] [CrossRef]
- Xiao, Y.; Peng, Z.; Zhang, S.; Jiang, Y.; Jing, X.; Yang, X.; Zhang, J.; Ni, L. Z-scheme CdIn2S4/BiOCl nanosheet face-to-face heterostructure: In-situ synthesis and enhanced interfacial charge transfer for high-efficient photocatalytic performance. J. Mater. Sci. 2019, 54, 9573–9590. [Google Scholar] [CrossRef]
- Wang, J.; Yang, T.; He, R.; Xue, K.; Sun, R.; Wang, W.; Wang, J.; Yang, T.; Wang, Y. Silver-loaded In2S3-CdIn2S4@X(X=Ag, Ag3PO4, AgI) ternary heterostructure nanotubes treated by electron beam irradiation with enhanced photocatalytic activity. Sci. Total Environ. 2019, 695, 133884. [Google Scholar] [CrossRef]
- Jiang, W.; Yin, X.; Xin, F.; Bi, Y.; Liu, Y.; Li, X. Preparation of CdIn2S4 microspheres and application for photocatalytic reduction of carbon dioxide. Appl. Surf. Sci. 2014, 288, 138–142. [Google Scholar] [CrossRef]
- Wang, S.; Guan, B.; Lu, Y.; Lou, X. Formation of hierarchical In2S3-CdIn2S4 heterostructured nanotubes for efficient and stable visible light CO2 reduction. J. Am. Chem. Soc. 2017, 139, 17305–17308. [Google Scholar] [CrossRef]
- Bao, N.; Shen, L.; Takata, T.; Domen, K. Self-templated synthesis of nanoporous CdS nanostructures for highly efficient photocatalytic hydrogen production under visible light. Chem. Mater. 2008, 20, 110–117. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, Z.; Xu, J.; He, Y.; Xiao, G. Hydrothermal synthesis of Cu(X)-CdIn2S4 microspheres and photocatalytic reduction of aqueous Cr (VI) under visible light irradiation. Mater. Lett. 2014, 117, 17–20. [Google Scholar] [CrossRef]
- Peng, Z.; Jiang, Y.; Xiao, Y.; Xu, H.; Zhang, W.; Ni, L. CdIn2S4 surface-decorated Ta3N5 core-shell heterostructure for improved spatial charge transfer: In-situ growth, synergistic effect and efficient dual-functional photocatalytic performance. Appl. Surf. Sci. 2019, 487, 1084–1095. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Li, J.; Zhou, Y.; Sun, L.; Wang, H.; Huo, P.; Ma, C.; Yan, Y. Fabricated 2D/2D CdIn2S4/N-rGO muti-heterostructure photocatalyst for enhanced photocatalytic activity. Carbon 2019, 152, 565–574. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Li, J.; Sun, L.; Zhou, Y.; Guan, J.; Wang, H.; Huo, P.; Ma, C.; Yan, Y. Carbon dots modifying sphere-flower CdIn2S4 on N-rGO sheet muti-dimensional photocatalyst for efficient visible degradation of 2,4-dichlorophenol. J Taiwan Inst. Chem. E 2019, 99, 142–153. [Google Scholar] [CrossRef]
- Li, D.; Shi, F.; Jiang, D.; Chen, M.; Shi, W. CdIn2S4/g-C3N4 heterojunction photocatalysts: Enhanced photocatalytic performance and charge transfer mechanism. RSC Adv. 2017, 7, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Babu, B.; Koutavarapu, R.; Shim, J.; Yoo, K. Enhanced visible-light-driven photoelectrochemical and photocatalytic performance of Au-SnO2 quantum dot-anchored g-C3N4 nanosheets. Sep. Purif. Technol. 2020, 240, 116652. [Google Scholar] [CrossRef]
- Chen, W.; Huang, J.; He, Z.; Ji, X.; Zhang, Y.; Sun, H.; Wang, K.; Su, Z. Accelerated photocatalytic degradation of tetracycline hydrochloride over CuAl2O4/g-C3N4 p-n heterojunctions under visible light irradiation. Sep. Purif. Technol. 2021, 277, 119461. [Google Scholar] [CrossRef]
- Xiao, T.; Tang, Z.; Yang, Y.; Tang, L.; Zhou, Y.; Zou, Z. In situ construction of hierarchical WO3/g-C3N4 composite hollow microspheres as a Z-scheme photocatalyst for the degradation of antibiotics. Appl. Catal. B Environ. 2018, 220, 417–428. [Google Scholar] [CrossRef]
- Chen, D.; Wu, S.; Fang, J.; Lu, S.; Zhou, G.; Feng, W.; Yang, F.; Chen, Y.; Fang, Z. A nanosheet-like α-Bi2O3/g-C3N4 heterostructure modified by plasmonic metallic Bi and oxygen vacancies with high photodegradation activity of organic pollutants. Sep. Purif. Technol. 2018, 193, 232–241. [Google Scholar] [CrossRef]
- Hong, Y.; Li, C.; Zhang, G.; Meng, Y.; Yin, B.; Zhao, Y.; Shi, W. Efficient and stable Nb2O5 modified g-C3N4 photocatalyst for removal of antibiotic pollutant. Chem. Eng. J. 2016, 299, 74–84. [Google Scholar] [CrossRef]
- Xia, P.; Zhu, B.; Yu, J.; Cao, S.; Jaroniec, M. Ultra-thin nanosheet assemblies of graphitic carbon nitride for enhanced photocatalytic CO2 reduction. J. Mater. Chem. A 2017, 5, 3230–3238. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.; Shi, R.; Zhu, Y. Chemical exfoliation of graphitic carbon nitride for efficient heterogeneous photocatalysis. J. Mater. Chem. A 2013, 1, 14766–14772. [Google Scholar] [CrossRef]
- Lin, Q.; Li, L.; Liang, S.; Liu, M.; Bi, J.; Wu, L. Efficient synthesis of monolayer carbon nitride 2D nanosheet with tunable concentration and enhanced visible-light photocatalytic activities. Appl. Catal. B Environ. 2015, 163, 135–142. [Google Scholar] [CrossRef]
- Yang, S.; Gong, Y.; Zhang, J.; Zhan, L.; Ma, L.; Fang, Z.; Vajtai, R.; Wang, X.; Ajayan, P. Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light. Adv. Mater. 2013, 25, 2452–2456. [Google Scholar] [CrossRef]
- Han, Q.; Wang, B.; Gao, J.; Cheng, Z.; Zhao, Y.; Zhang, Z.; Qu, L. Atomically thin mesoporous nanomesh of graphitic C3N4 for high-efficiency photocatalytic hydrogen evolution. ACS Nano 2016, 10, 2745–2751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lai, C.; Li, B.; Huang, D.; Zeng, G.; Xu, P.; Qin, L.; Liu, S.; Liu, X.; Yi, H.; et al. Rational design 2D/2D BiOBr/CDs/g-C3N4 Z-scheme heterojunction photocatalyst with carbon dots as solid-state electron mediators for enhanced visible and NIR photocatalytic activity: Kinetics, intermediates, and mechanism insight. J. Catal. 2019, 369, 469–481. [Google Scholar] [CrossRef]
- Su, T.; Shao, Q.; Qin, Z.; Guo, Z.; Wu, Z. Role of interfaces in two-dimensional photocatalyst for water splitting. ACS Catal. 2018, 8, 2253–2276. [Google Scholar] [CrossRef]
- Yang, H.; Cao, R.; Sun, P.; Yin, J.; Zhang, S.; Xu, X. Constructing electrostatic self-assembled 2D/2D ultra-thin ZnIn2S4/protonated g-C3N4 heterojunctions for excellent photocatalytic performance under visible light. Appl. Catal. B Environ. 2019, 256, 117862. [Google Scholar] [CrossRef]
- Xie, L.; Ni, J.; Tang, B.; He, G.; Chen, H. A self-assembled 2D/2D-type protonated carbon nitride-modified graphene oxide nanocomposite with improved photocatalytic activity. Appl. Surf. Sci. 2018, 434, 456–463. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Bhardwaj, M.; Yadav, P.; Ogale, S. g-C3N4/NiAl-LDH 2D/2D hybrid heterojunction for high-performance photocatalytic reduction of CO2 into renewable fuels. ACS Appl. Mater. Interface 2018, 10, 2667–2678. [Google Scholar] [CrossRef] [Green Version]
- Ao, Y.; Wang, K.; Wang, P.; Wang, C.; Hou, J. Synthesis of novel 2D-2D pn hetero-junction BiOBr/La2Ti2O7 composite photocatalyst with enhanced photocatalytic performance under both UV and visible light irradiation. Appl. Catal. B Environ. 2016, 194, 157–168. [Google Scholar] [CrossRef]
- Ong, W.; Putri, L.; Tan, Y.; Tan, L.; Li, N.; Ng, Y.; Wen, X.; Chai, S. Unravelling charge carrier dynamics in protonated g-C3N4 interfaced with carbon nanodots as co-catalysts toward enhanced photocatalytic CO2 reduction: A combined experimental and first-principles DFT study. Nano Res. 2017, 10, 1673–1696. [Google Scholar] [CrossRef]
- Ong, W.; Tan, L.; Chai, S.; Yong, S.; Mohamed, A. Surface charge modification via protonation of graphitic carbon nitride (g-C3N4) for electrostatic self-assembly construction of 2D/2D reduced graphene oxide (rGO)/g-C3N4 nanostructures toward enhanced photocatalytic reduction of carbon dioxide to methane. Nano Energy 2015, 13, 757–770. [Google Scholar] [CrossRef]
- Yang, M.; Xu, Y.; Lu, W.; Zeng, K.; Zhu, H.; Xu, Q.; Ho, G. Self-surface charge exfoliation and electrostatically coordinated 2D hetero-layered hybrids. Nat. Commun. 2017, 8, 14224. [Google Scholar] [CrossRef]
- Han, Q.; Wang, B.; Zhao, Y.; Hu, C.; Qu, L. A graphitic-C3N4 seaweed architecture for enhanced hydrogen evolution. Angew. Chem. Int. Ed. 2015, 54, 11433–11437. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, P.; Borkar, R.; Bansiwal, A.; Labhsetwar, N.; Jain, S.L. Metal-organic hybrid: Photoreduction of CO2 using graphitic carbon nitride supported heteroleptic iridium complex under visible light irradiation. Carbon 2017, 123, 371–379. [Google Scholar] [CrossRef]
- Yu, H.; Shi, R.; Zhao, Y.; Bian, T.; Zhao, Y.; Zhou, C.; Waterhouse, G.; Wu, L.; Tung, C.; Zhang, T. Alkali-assisted synthesis of nitrogen deficient graphitic carbon nitride with tunable band structures for efficient visible-light-driven hydrogen evolution. Adv. Mater. 2017, 29, 1605148. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Liu, P.; Wang, D.; Li, Y.; Zhao, H. Cross-linked g-C3N4/rGO nanocomposites with tunable band structure and enhanced visible light photocatalytic activity. Small 2013, 9, 3336–3344. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Guo, R. Fabrication of novel CdIn2S4 hollow spheres via a facile hydrothermal process. J. Phys. Chem. C 2008, 112, 10700–10706. [Google Scholar] [CrossRef]
- Xiao, P.; Jiang, D.; Ju, L.; Jing, J.; Chen, M. Construction of RGO/CdIn2S4/g-C3N4 ternary hybrid with enhanced photocatalytic activity for the degradation of tetracycline hydrochloride. Appl. Surf. Sci. 2018, 433, 388–397. [Google Scholar] [CrossRef]
- Di, J.; Xiong, J.; Li, H.; Liu, Z. Ultrathin 2D photocatalysts: Electronic-structure tailoring, hybridization, and applications. Adv. Mater. 2018, 30, 1704548. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Bai, X.; Man, Z.; He, H.; Li, L.; Hu, J.; Alsaedi, A.; Hayat, T.; Yu, Z.; Zhang, W.; et al. Convincing synthesis of atomically thin, single-crystalline InVO4 sheets toward promoting highly selective and efficient solar conversion of CO2 into CO. J. Am. Chem. Soc. 2019, 141, 4209–4213. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Fan, T.; Cao, Y.; Li, P.; Yao, X.; Liu, X. Construction of three-dimensional MgIn2S4 nanoflowers/ two-dimensional oxygen-doped g-C3N4 nanosheets direct Z-scheme heterojunctions for efficient Cr(VI) reduction: Insight into the role of superoxide radicals. J. Hazard. Mater. 2021, 420, 126567. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Ma, S.; Du, X.; Zhao, C.; Fu, H.; Wang, P.; Wang, C. The facile fabrication of 2D/3D Z-scheme g-C3N4/UiO-66 heterojunction with enhanced photocatalytic Cr (VI) reduction performance under white light. Chem. Eng. J. 2019, 375, 121944. [Google Scholar] [CrossRef]
- Yin, H.; Cao, Y.; Fan, T.; Qiu, B.; Zhang, M.; Yao, J.; Li, P.; Liu, X.; Chen, S. Construction of carbon bridged TiO2/CdS tandem Z-scheme heterojunctions toward efficient photocatalytic antibiotic degradation and Cr (VI) reduction. J. Alloys Compd. 2020, 824, 153915. [Google Scholar] [CrossRef]

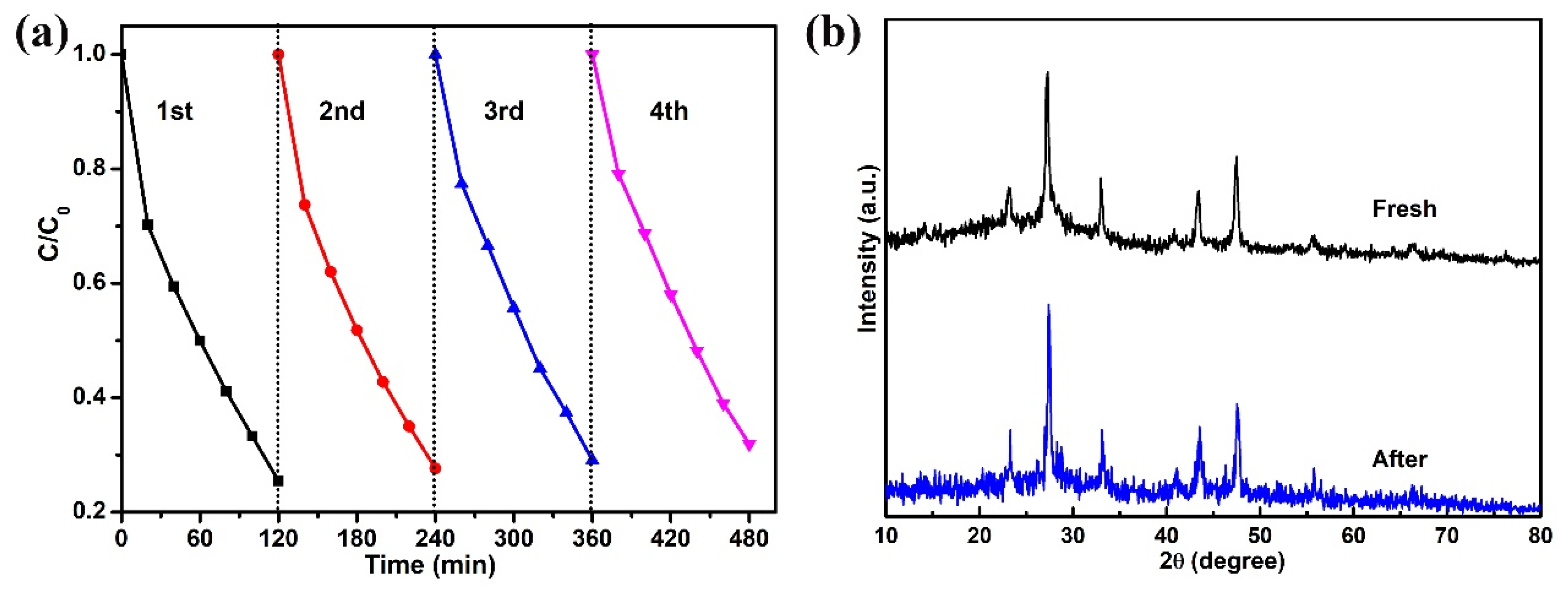
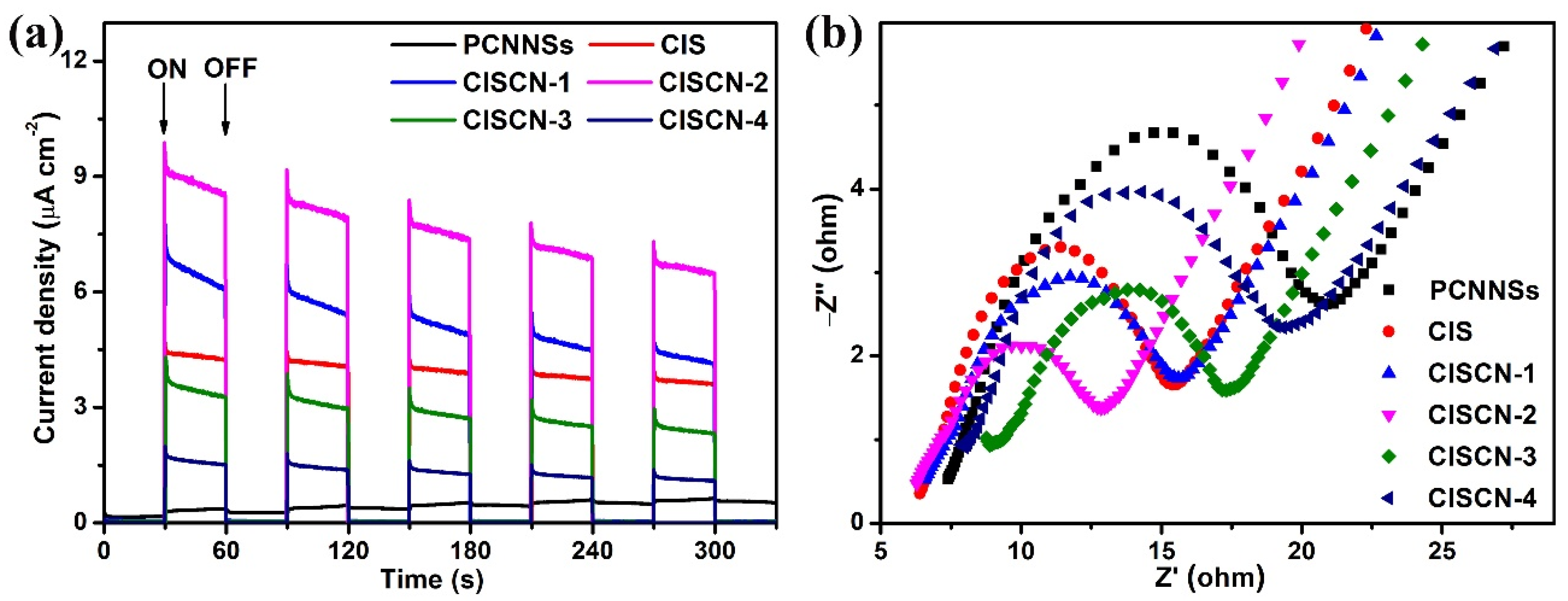
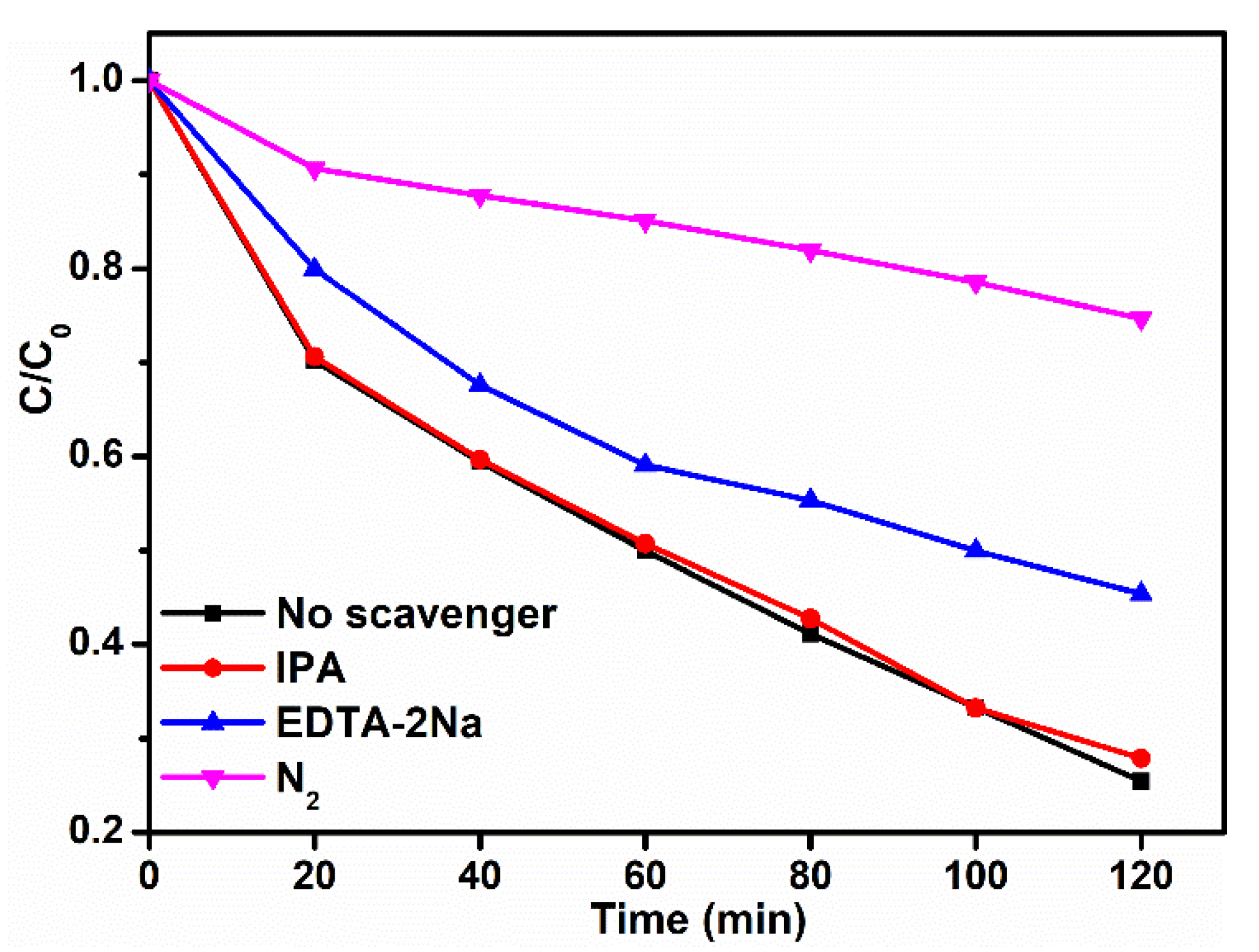
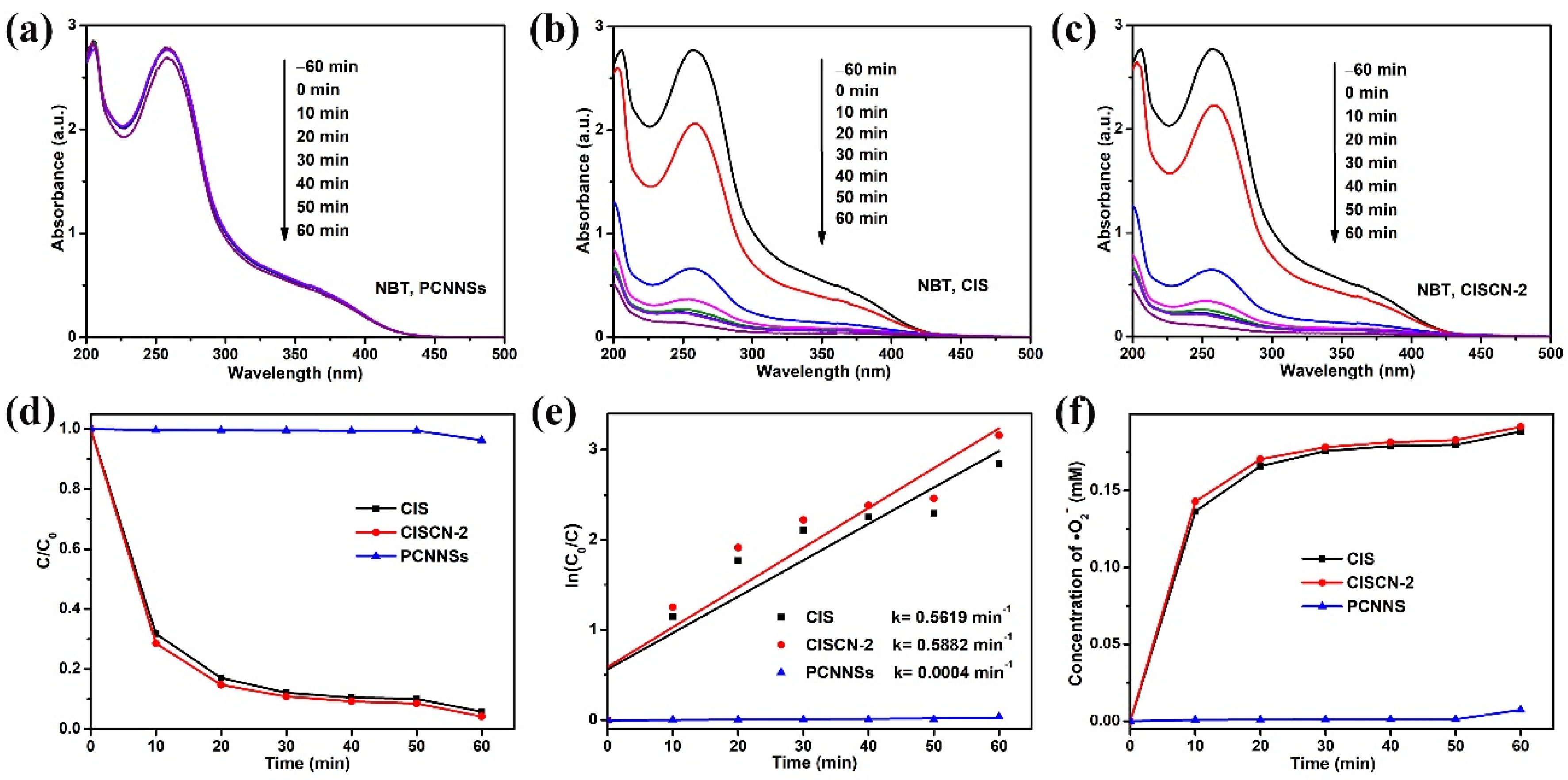

| Sample | TC (mg/L) | Dosage (g/L) | t (min) | Light Source | DE (%) | Refs. |
|---|---|---|---|---|---|---|
| Co/V-g-C3N4 | 10 | 0.5 | 120 | 250 W Xe lamp | 64.3 | [5] |
| 2D/3D g-C3N4 | 10 | 0.5 | 120 | 250 W Xe lamp | 69.6 | [6] |
| Nitrogen-deficient tubular g-C3N4 | 10 | 1.0 | 150 | 300 W Xe lamp | 84.3 | [7] |
| BN QDs/g-C3N4 | 10 | 1.0 | 60 | 300 W Xe lamp | 82 | [8] |
| WO3/g-C3N4 | 25 | 0.5 | 120 | 300 W Xe lamp | 70 | [28] |
| Bi/α-Bi2O3/g-C3N4 | 10 | 1.0 | 180 | 300 W Xe lamp | 91.2 | [29] |
| Nb2O5/g-C3N4 | 10 | 0.5 | 150 | 250 W Xe lamp | 76.2 | [30] |
| CdIn2S4/g-C3N4 | 50 | 0.6 | 120 | 300 W Xe lamp | 83.6 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, H.; Yuan, C.; Lv, H.; He, X.; Liao, C.; Liu, X.; Zhang, Y. Construction of Electrostatic Self-Assembled 2D/2D CdIn2S4/g-C3N4 Heterojunctions for Efficient Visible-Light-Responsive Molecular Oxygen Activation. Nanomaterials 2021, 11, 2342. https://doi.org/10.3390/nano11092342
Yin H, Yuan C, Lv H, He X, Liao C, Liu X, Zhang Y. Construction of Electrostatic Self-Assembled 2D/2D CdIn2S4/g-C3N4 Heterojunctions for Efficient Visible-Light-Responsive Molecular Oxygen Activation. Nanomaterials. 2021; 11(9):2342. https://doi.org/10.3390/nano11092342
Chicago/Turabian StyleYin, Hongfei, Chunyu Yuan, Huijun Lv, Xulin He, Cheng Liao, Xiaoheng Liu, and Yongzheng Zhang. 2021. "Construction of Electrostatic Self-Assembled 2D/2D CdIn2S4/g-C3N4 Heterojunctions for Efficient Visible-Light-Responsive Molecular Oxygen Activation" Nanomaterials 11, no. 9: 2342. https://doi.org/10.3390/nano11092342
APA StyleYin, H., Yuan, C., Lv, H., He, X., Liao, C., Liu, X., & Zhang, Y. (2021). Construction of Electrostatic Self-Assembled 2D/2D CdIn2S4/g-C3N4 Heterojunctions for Efficient Visible-Light-Responsive Molecular Oxygen Activation. Nanomaterials, 11(9), 2342. https://doi.org/10.3390/nano11092342






