Effect of Morphology and Plasmonic on Au/ZnO Films for Efficient Photoelectrochemical Water Splitting
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Fabrication of Pre-Layer ZnO
2.3. Synthesis of Hierarchical ZnO Thin Film
2.4. Decoration of ZnO Films with Au NPs
2.5. Characterization Techniques
2.6. Photoelectrochemical (PEC) Measurements
3. Results and Discussion
3.1. Characterization of the Fabricated ZnO Nanostructured Thin Film
3.1.1. Structural Properties
3.1.2. Chemical Composition
3.1.3. Morphological Analysis
3.1.4. Optical Properties
Effect of Time Deposition
Effect of Coating Au Thickness
3.2. Photo-Electrochemical (PEC) Studies
3.3. Mechanism of PEC
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bian, X.; Zhang, S.; Zhao, Y.; Shi, R.; Zhang, T. Layered Double Hydroxide-Based Photocatalytic Materials toward Renewable Solar Fuels Production. InfoMat 2021, 3, 719–738. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. Decarbonization of Fossil Fuels as a Strategy to Control Global Warming. Renew. Sustain. Energy Rev. 2011, 15, 1828–1834. [Google Scholar] [CrossRef]
- Omri, A.; Nguyen, D.K. On the Determinants of Renewable Energy Consumption: International Evidence. Energy 2014, 72, 554–560. [Google Scholar] [CrossRef]
- Shaban, M.; Rabia, M.; El-Sayed, A.M.A.; Ahmed, A.; Sayed, S. Photocatalytic Properties of PBS/Graphene Oxide/Polyaniline Electrode for Hydrogen Generation. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- She, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining Theory and Experiment in Electrocatalysis: Insights into Materials Design. Science 2017, 355. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Yang, N.; Huang, X.; Peng, L.; Tong, C.; Deng, M.; Tang, X.; Li, L.; Liao, Q.; Wei, Z. Synthesis of Ammonia: Via Electrochemical Nitrogen Reduction on High-Index Faceted Au Nanoparticles with a High Faradaic Efficiency. Chem. Commun. 2019, 55, 14482–14485. [Google Scholar] [CrossRef]
- Jamesh, M.I. Recent Progress on Earth Abundant Hydrogen Evolution Reaction and Oxygen Evolution Reaction Bifunctional Electrocatalyst for Overall Water Splitting in Alkaline Media. J. Power Sources 2016, 333, 213–236. [Google Scholar] [CrossRef]
- Wang, W.; Chen, M.; Huang, D.; Zeng, G.; Zhang, C.; Lai, C.; Zhou, C.; Yang, Y.; Cheng, M.; Hu, L.; et al. An Overview on Nitride and Nitrogen-Doped Photocatalysts for Energy and Environmental Applications. Compos. Part B Eng. 2019, 172, 704–723. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, S.; Sun, M.; Yang, L.; Luo, S.; Crittenden, J.C. A Critical Review on Energy Conversion and Environmental Remediation of Photocatalysts with Remodeling Crystal Lattice, Surface, and Interface. ACS Nano 2019, 13, 9811–9840. [Google Scholar] [CrossRef]
- Bonomo, M.; Naponiello, G.; Venditti, I.; Zardetto, V.; Di Carlo, A.; Dini, D. Electrochemical and Photoelectrochemical Properties of Screen-Printed Nickel Oxide Thin Films Obtained from Precursor Pastes with Different Compositions. J. Electrochem. Soc. 2017, 164, 137–147. [Google Scholar] [CrossRef]
- Ma, X.X.; Chen, L.; Zhang, Z.; Tang, J.L. Electrochemical Performance Evaluation of CuO@Cu2O Nanowires Array on Cu Foam as Bifunctional Electrocatalyst for Efficient Water Splitting. Chin. J. Anal. Chem. 2020, 48, e20001–e20012. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Erfan, N.A.; Mohammed, A.A.; Mohamed, S.E.I. Ag-Decorated TiO2 Nanofibers as Arrhenius Equation-Incompatible and Effective Photocatalyst for Water Splitting under Visible Light Irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125307. [Google Scholar] [CrossRef]
- Ali, H.; Brahimi, R.; Outemzabet, R.; Bellal, B.; Trari, M. Comparative Analysis of Structural and Photoelectrochemical Properties of Pure and Sb Doped SnO2 Functional Electrode. Thin Solid Film. 2018, 649, 225–231. [Google Scholar] [CrossRef]
- Vidyarthi, V.S.; Hofmann, M.; Savan, A.; Sliozberg, K.; König, D.; Beranek, R.; Schuhmann, W.; Ludwig, A. Enhanced Photoelectrochemical Properties of WO3 Thin Films Fabricated by Reactive Magnetron Sputtering. Int. J. Hydrogen Energy 2011, 36, 4724–4731. [Google Scholar] [CrossRef]
- Ng, J.; Xu, S.; Zhang, X.; Yang, H.Y.; Sun, D.D. Hybridized Nanowires and Cubes: A Novel Architecture of a Heterojunctioned TiO2/SrTiO3 Thin Film for Efficient Water Splitting. Adv. Funct. Mater. 2010, 20, 4287–4294. [Google Scholar] [CrossRef]
- Rahman, G.; Najaf, Z.; Shah, A.u.H.A.; Mian, S.A. Investigation of the Structural, Optical, and Photoelectrochemical Properties of α-Fe2O3 Nanorods Synthesized via a Facile Chemical Bath Deposition. Optik 2020, 200, 163454. [Google Scholar] [CrossRef]
- Luo, H.; Mueller, A.H.; McCleskey, T.M.; Burrell, A.K.; Bauer, E.; Jia, Q.X. Structural and Photoelectrochemical Properties of BiVO4 Thin Films. J. Phys. Chem. C 2008, 112, 6099–6102. [Google Scholar] [CrossRef]
- Takahara, Y.; Kondo, J.N.; Takata, T.; Lu, D.; Domen, K. Mesoporous Tantalum Oxide. 1. Characterization and Photocatalytic Activity for the Overall Water Decomposition. Chem. Mater. 2001, 13, 1194–1199. [Google Scholar] [CrossRef]
- Chen, Y.H.; Shen, Y.M.; Wang, S.C.; Huang, J.L. Fabrication of One-Dimensional ZnO Nanotube and Nanowire Arrays with an Anodic Alumina Oxide Template via Electrochemical Deposition. Thin Solid Films 2014, 570, 303–309. [Google Scholar] [CrossRef]
- Zaraska, L.; Mika, K.; Syrek, K.; Sulka, G.D. Formation of ZnO Nanowires During Anodic Oxidation of Zinc in Bicarbonate Electrolytes. J. Electroanal. Chem. 2017, 801, 511–520. [Google Scholar] [CrossRef]
- Zaraska, L.; Mika, K.; Hnida, K.E.; Gajewska, M.; Łojewski, T.; Jaskuła, M.; Sulka, G.D. High Aspect-Ratio Semiconducting ZnO Nanowires Formed by Anodic Oxidation of Zn Foil and Thermal Treatment. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2017, 226, 94–98. [Google Scholar] [CrossRef]
- Quang Dang, V.; Kim, D.I.; Thai Duy, L.; Kim, B.Y.; Hwang, B.U.; Jang, M.; Shin, K.S.; Kim, S.W.; Lee, N.E. Piezoelectric Coupling in a Field-Effect Transistor with a Nanohybrid Channel of ZnO Nanorods Grown Vertically on Graphene. Nanoscale 2014, 6, 15144–15150. [Google Scholar] [CrossRef]
- Dang, V.Q.; Trung, T.Q.; Duy, L.T.; Kim, B.Y.; Siddiqui, S.; Lee, W.; Lee, N.E. High-Performance Flexible Ultraviolet (UV) Phototransistor Using Hybrid Channel of Vertical ZnO Nanorods and Graphene. ACS Appl. Mater. Interfaces 2015, 7, 11032–11040. [Google Scholar] [CrossRef] [PubMed]
- Dang, V.Q.; Trung, T.Q.; Kim, D.I.; Duy, L.T.; Hwang, B.U.; Lee, D.W.; Kim, B.Y.; Toan, L.D.; Lee, N.E. Ultrahigh Responsivity in Graphene-ZnO Nanorod Hybrid UV Photodetector. Small 2015, 11, 3054–3065. [Google Scholar] [CrossRef] [PubMed]
- Dang, V.Q.; Byun, M.; Kang, J.; Kim, C.; Jung, P.H.; Kim, Y.D.; Lee, N.E.; Lee, H. Low Temperature Fabrication of Hybrid Solar Cells Using Co-Sensitizer of Perovskite and Lead Sulfide Nanoparticles. Org. Electron. 2017, 50, 247–254. [Google Scholar] [CrossRef]
- Bauer, C.; Boschloo, G.; Mukhtar, E.; Hagfeldt, A. Electron Injection and Recombination in Ru(dcbpy)2(NCS)2 Sensitized Nanostructured Zno. J. Phys. Chem. B 2001, 105, 5585–5588. [Google Scholar] [CrossRef]
- Wang, Z.L. Zinc Oxide Nanostructures: Growth, Properties and Applications. J. Phys. Condens. Matter 2004, 16, R829. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide-From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A Review on Green Synthesis of Zinc Oxide Nanoparticles-An Eco-Friendly Approach. Resour. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Lee, H.Y.; Cheng, C.Y.; Lee, C.T. Bottom Gate Thin-Film Transistors Using Parallelly Lateral ZnO Nanorods Grown by Hydrothermal Method. Mater. Sci. Semicond. Process. 2020, 119, 105223. [Google Scholar] [CrossRef]
- Thongsuksai, W.; Panomsuwan, G.; Rodchanarowan, A. Fast and Convenient Growth of Vertically Aligned ZnO Nanorods via Microwave Plasma-Assisted Thermal Evaporation. Mater. Lett. 2018, 224, 50–53. [Google Scholar] [CrossRef]
- Ding, J.; Chen, S.; Han, N.; Shi, Y.; Hu, P.; Li, H.; Wang, J. Aerosol Assisted Chemical Vapour Deposition of Nanostructured ZnO Thin Films for NO2 and Ethanol Monitoring. Ceram. Int. 2020, 46, 15152–15158. [Google Scholar] [CrossRef]
- Fang, L.; Li, H.; Ma, X.; Song, Q.; Chen, R. Optical Properties of Ultrathin ZnO Films Fabricated by Atomic Layer Deposition. Appl. Surf. Sci. 2020, 527, 146818. [Google Scholar] [CrossRef]
- Song, H.; Ma, Y.; Ko, D.; Jo, S.; Hyun, D.C.; Kim, C.S.; Oh, H.J.; Kim, J. Influence of Humidity for Preparing Sol-Gel ZnO Layer: Characterization and Optimization for Optoelectronic Device Applica-Tions. Appl. Surf. Sci. 2020, 512, 145660. [Google Scholar] [CrossRef]
- Shabani, E.; Abdekhodaie, M.J.; Mousavi, S.A.; Taghipour, F. ZnO Nanoparticle/Nanorod-Based Label-Free Electrochemical Immunoassay for Rapid Detection of MMP-9 Biomarker. Biochem. Eng. J. 2020, 164, 107772. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Mwafy, E.A. SYnthesis of ZnO and Au@ZnO Core/Shell Nano-Catalysts by Pulsed Laser Ablation in Different Liquid Media. J. Mater. Res. Technol. 2020, 9, 3241–3248. [Google Scholar] [CrossRef]
- Qiu, Y.; Yan, K.; Deng, H.; Yang, S. Secondary Branching and Nitrogen Doping of ZnO Nanotetrapods: Building a Highly Active Network for Photoelectrochemical Water Splitting. Nano Lett. 2012, 12, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Sangle, A.; Zhang, S.; Yuan, S.; Zhao, Y.; Shi, L.; Hoye, R.L.Z.; Cho, S.; Li, D.; Macmanus-Driscoll, J.L. Photoelectrochemical Water Splitting Strongly Enhanced in Fast-Grown ZnO Nanotree and Nanocluster Structures. J. Mater. Chem. A 2016, 4, 10203–10211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rokade, A.; Rondiya, S.; Sharma, V.; Prasad, M.; Pathan, H.; Jadkar, S. Electrochemical Synthesis of 1D ZnO Nanoarchitectures and Their Role in Efficient Photoelectrochemical Splitting of Water. J. Solid State Electrochem. 2017, 21, 2639–2648. [Google Scholar] [CrossRef]
- Sohila, S.; Rajendran, R.; Yaakob, Z.; Teridi, M.A.M.; Sopian, K. Photoelectrochemical Water Splitting Performance of Flower Like ZnO Nanostructures Synthesized by a Novel Chemical Method. J. Mater. Sci. Mater. Electron. 2016, 27, 2846–2851. [Google Scholar] [CrossRef]
- Wang, T.; Lv, R.; Zhang, P.; Li, C.; Gong, J. Au Nanoparticle Sensitized ZnO Nanopencil Arrays for Photoelectrochemical Water Splitting. Nanoscale 2015, 7, 77–81. [Google Scholar] [CrossRef]
- Wang, X.; Liow, C.; Bisht, A.; Liu, X.; Sum, T.C.; Chen, X.; Li, S. Engineering Interfacial Photo-Induced Charge Transfer Based on Nanobamboo Array Architecture for Efficient Solar-to-Chemical Energy Conversion. Adv. Mater. 2015, 27, 2207–2214. [Google Scholar] [CrossRef]
- Dom, R.; Govindarajan, S.; Joshi, S.V.; Borse, P.H. A Solar-Responsive Zinc Oxide Photoanode for Solar-Photon-Harvester Photoelectrochemical (PEC) Cells. Nanoscale Adv. 2020, 2, 3350–3357. [Google Scholar] [CrossRef]
- Bera, S.; Ghosh, S.; Basu, R.N. Fabrication of Bi2S3/ZnO Heterostructures: An Excellent Photocatalyst for Visible-Light-Driven Hydrogen Generation and Pho-Toelectrochemical Properties. New J. Chem. 2018, 42, 541–554. [Google Scholar] [CrossRef]
- Hsu, Y.K.; Chen, Y.C.; Lin, Y.G. Novel ZnO/Fe2O3 Core-Shell Nanowires for Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2015, 7, 14157–14162. [Google Scholar] [CrossRef]
- Kim, H.; Oh, M.H.; Yang, B.L. Photocorrosion of Polyaniline-ZnS–ZnO Photoelectrode for Water Splitting. Thin Solid Films 2020, 693, 137678. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Kang, Z. 3D Branched ZnO Nanowire Arrays Decorated with Plasmonic Au Nanoparticles for High-Performance Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2014, 6, 4480–4489. [Google Scholar] [CrossRef] [PubMed]
- Mageshwari, K.; Nataraj, D.; Pal, T.; Sathyamoorthy, R.; Park, J. Improved Photocatalytic Activity of ZnO Coupled CuO Nanocomposites Synthesized by Reflux Condensation Method. J. Alloys Compd. 2015, 625, 362–370. [Google Scholar] [CrossRef]
- Ping, T.; Mihua, S.; Chengwen, S.; Shuaihua, W.; Murong, C. Enhanced Photocatalytic Activity of Cu2O/Cu Heterogeneous Nanoparticles Synthesized in Aqueous Colloidal Solutions on Degradation of Methyl Orange. Rare Met. Mater. Eng. 2016, 45, 2214–2218. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Zhou, Y.; Wu, Z.; Zheng, G.; He, J.; Zhou, Y. Improved Photocatalytic Activity of Nanocrystalline ZnO by Coupling with CuO. J. Phys. Chem. Solids 2017, 106, 29–36. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, H.; Hu, S.; Li, J. Preparation and Enhanced Photoelectrochemical Performance of Coupled Bicomponent ZnO-TiO2 Nanocomposites. J. Phys. Chem. C 2008, 112, 117–122. [Google Scholar] [CrossRef]
- Sambandam, B.; Michael, R.J.V.; Manoharan, P.T. Oxygen Vacancies and Intense Luminescence in Manganese Loaded Zno Microflowers for Visible Light Water Splitting. Nanoscale 2015, 7, 13935–13942. [Google Scholar] [CrossRef] [PubMed]
- Martha, S.; Reddy, K.H.; Parida, K.M. Fabrication of In2O3 Modified Zno for Enhancing Stability, Optical Behaviour, Electronic Properties and Photocatalytic Activity for Hydrogen Production Under Visible Light. J. Mater. Chem. A 2014, 2, 3621–3631. [Google Scholar] [CrossRef]
- Shi, R.; Cao, Y.; Bao, Y.; Zhao, Y.; Waterhouse, G.I.N.; Fang, Z.; Wu, L.-Z.; Tung, C.-H.; Yin, Y.; Zhang, T. Self-Assembled Au/CdSe Nanocrystal Clusters for Plasmon-Mediated Photocatalytic Hydrogen Evolution. Adv. Mater. 2017, 29, 1700803. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Shang, L.; Yu, H.; Bian, T.; Wu, L.Z.; Tung, C.H.; Zhang, T. Mesoporous Plasmonic Au-Loaded Ta2O5 Nanocomposites for Efficient Visible Light Photocatalysis. Catal. Today 2014, 225, 158–163. [Google Scholar] [CrossRef]
- Ping, M.M.; Qiu, S.J.; Wei, G.J.; Liu, J.X.; Wang, Z.J.; Wang, S.T.; An, C.H. Monolith Free-Standing Plasmonic PAN/Ag/AgX (X = Br, I) Nanofiber Mat as Easily Recoverable Visible-Light-Driven Photo-Catalyst. Rare Met. 2019, 38, 361–368. [Google Scholar] [CrossRef]
- Machín, A.; Cotto, M.; Duconge, J.; Arango, J.C.; Morant, C.; Pinilla, S.; Soto-Vázquez, L.; Resto, E.; Márquez, F. Hydrogen Production via Water Splitting Using Different Au@ZnO Catalysts Under UV–Vis Irradiation. J. Photochem. Photobiol. A Chem. 2018, 353, 385–394. [Google Scholar] [CrossRef]
- Machín, A.; Arango, J.C.; Fontánez, K.; Cotto, M.; Duconge, J.; Soto-Vázquez, L.; Resto, E.; Ion, F.; Morant, C.; Márquez, F. Biomimetic Catalysts Based on Au@ZnO-Graphene Composites for the Generation of Hydrogen by Water Splitting. Biomimetics 2020, 5, 39. [Google Scholar] [CrossRef]
- Warren, S.C.; Thimsen, E. Plasmonic Solar Water Splitting. Energy Environ. Sci. 2012, 5, 5133–5146. [Google Scholar] [CrossRef]
- Bertoni, G.; Fabbri, F.; Villani, M.; Lazzarini, L.; Turner, S.; Van Tendeloo, G.; Calestani, D.; Gradečak, S.; Zappettini, A.; Salviati, G. Nanoscale Mapping of Plasmon and Exciton in ZnO Tetrapods Coupled with Au Nanoparticles. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Khan, W.; Ajmal, H.M.S.; Khan, F.; Ul Huda, N.; Kim, S.D. Induced Photonic Response of ZnO Nanorods Grown on Oxygen Plasma-Treated Seed Crystallites. Nanomaterials 2018, 8, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourshaban, E.; Abdizadeh, H.; Golobostanfard, M.R. ZnO Nanorods Array Synthesized by Chemical Bath Deposition: Effect of Seed Layer Sol Concentration. Procedia Mater. Sci. 2015, 11, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Mariappan, R.; Ponnuswamy, V.; Suresh, P.; Suresh, R.; Ragavendar, M. Nanostructured GdxZn1-xO Thin Films by Nebulizer Spray Pyrolysis Technique: Role of Doping Concentration on the Structural and Optical Properties. Superlattices Microstruct. 2013, 59, 47–59. [Google Scholar] [CrossRef]
- Soylu, M.; Savas, O. Electrical and Optical Properties of ZnO/Si Heterojunctions as a Function of the Mg Dopant Content. Mater. Sci. Semicond. Process. 2015, 29, 76–82. [Google Scholar] [CrossRef]
- Das, N.C.; Biswas, S.; Sokol, P.E. The Photovoltaic Performance of ZnO Nanorods in Bulk Heterojunction Solar Cells. J. Renew. Sustain. Energy 2011, 3, 033105. [Google Scholar] [CrossRef]
- Sreeja Sreedharan, R.; Vinodkumar, R.; Navas, I.; Prabhu, R.; Mahadevan Pillai, V.P. Influence of Pr Doping on the Structural, Morphological, Optical, Luminescent and Non-linear Optical Properties of RF-Sputtered ZnO Films. JOM 2016, 68, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Basyooni, M.A.; Shaban, M.; El Sayed, A.M. Enhanced Gas Sensing Properties of Spin-Coated Na-Doped ZnO Nanostructured Films. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Mary, J.A.; Vijaya, J.J.; Dai, J.H.; Bououdina, M.; John Kennedy, L.; Song, Y. Experimental and First-Principles DFT Studies of Electronic, Optical and Magnetic Properties of Cerium-Manganese Codoped Zinc Oxide Nanostructures. Mater. Sci. Semicond. Process. 2015, 34, 27–38. [Google Scholar] [CrossRef]
- Shaban, M.; El Sayed, A.M. Influences of Lead and Magnesium Co-Doping on the Nanostructural, Optical Properties and Wettability of Spin Coated Zinc Oxide Films. Mater. Sci. Semicond. Process. 2015, 39, 136–147. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Norton, M.G.; Suryanarayana, C.; Norton, M.G. X-rays and Diffraction. In X-Ray Diffraction; Springer: New York, NY, USA, 1998; pp. 3–19. [Google Scholar]
- Cao, B.; Cai, W. From ZnO Nanorods to Nanoplates: Chemical Bath Deposition Growth and Surface-Related Emissions. J. Phys. Chem. C 2008, 112, 680–685. [Google Scholar] [CrossRef]
- Shaban, M.; Mustafa, M.; El Sayed, A.M. Structural, Optical, and Photocatalytic Properties of the Spray Deposited Nanoporous CdS Thin Films; Influence of Copper Doping, Annealing, and Deposition Parameters. Mater. Sci. Semicond. Process. 2016, 56, 329–343. [Google Scholar] [CrossRef]
- Shah, A.; Ahmad, M.; Rahmanuddin; Khan, S.; Aziz, U.; Ali, Z.; Khan, A.; Mahmood, A. The Role of Al Doping on ZnO Nanowire Evolution and Optical Band Gap Tuning. Appl. Phys. A Mater. Sci. Process. 2019, 125, 713. [Google Scholar] [CrossRef]
- Davis, K.; Yarbrough, R.; Froeschle, M.; White, J.; Rathnayake, H. Band Gap Engineered Zinc Oxide Nanostructures: Via a Sol-Gel Synthesis of Solvent Driven Shape-Controlled Crystal Growth. RSC Adv. 2019, 9, 14638–14648. [Google Scholar] [CrossRef] [Green Version]
- Seok Jo, H.; Samuel, E.; Kwon, H.J.; Joshi, B.; Kim, M.W.; Kim, T.G.; Swihart, M.T.; Yoon, S.S. Highly Flexible Transparent Substrate-Free Photoanodes Using ZnO Nanowires on Nickel Microfibers. Chem. Eng. J. 2019, 363, 13–22. [Google Scholar] [CrossRef]
- Muskens, O.L.; Rivas, J.G.; Algra, R.E.; Bakkers, E.P.A.M.; Lagendijk, A. Design of Light Scattering in Nanowire Materials for Photovoltaic Applications. Nano Lett. 2008, 8, 2638–2642. [Google Scholar] [CrossRef]
- Tang, Y.B.; Chen, Z.H.; Song, H.S.; Lee, C.S.; Cong, H.T.; Cheng, H.M.; Zhang, W.J.; Bello, I.; Lee, S.T. Vertically Aligned P-Type Single-Crystalline GaN Nanorod Arrays on N-Type Si for Heterojunction Photovoltaic Cells. Nano Lett. 2008, 8, 4191–4195. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Yan, R.; Fardy, M. Semiconductor Nanowire: Whats Next? Nano Lett. 2010, 10, 1529–1536. [Google Scholar] [CrossRef]
- Vayssieres, L. (Ed.) On Solar Hydrogen & Nanotechnology; John Wiley & Sons, Ltd.: Chichester, UK, 2010; ISBN 9780470823996. [Google Scholar]
- Zhang, H.; Zhao, Y.; Geng, X.; Huang, Y.; Li, Y.; Liu, H.; Liu, Y.; Li, Y.; Wang, X.; Tian, H.; et al. Au Nanoparticles-Decorated Surface Plasmon Enhanced ZnO Nanorods Ultraviolet Photodetector on Flexible Transparent Mica Substrate. IEEE J. Electron Devices Soc. 2019, 7, 191–195. [Google Scholar] [CrossRef]
- Atwater, H.A.; Polman, A. Erratum: Plasmonics for Improved Photovoltaic Devices. Nat. Mater. 2010, 9, 865. [Google Scholar] [CrossRef] [Green Version]
- Meškinis, Š.; Peckus, D.; Vasiliauskas, A.; Čiegis, A.; Gudaitis, R.; Tamulevičius, T.; Yaremchuk, I.; Tamulevičius, S. Photovoltaic Properties and Ultrafast Plasmon Relaxation Dynamics of Diamond-Like Carbon Nanocomposite Films with Embedded Ag Nanoparticles. Nanoscale Res. Lett. 2017, 12, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousa, A.M.; Ismail, R.A.; Amin, M.H. Hybrid P-Au@PbI2/N-Si Heterojunction Photodetector Prepared by Pulsed Laser Ablation in Liquid. Optik 2019, 183, 933–941. [Google Scholar] [CrossRef]
- Wang, L.; Chen, R.; Ren, Z.F.; Ge, C.W.; Liu, Z.X.; He, S.J.; Yu, Y.Q.; Wu, C.Y.; Luo, L.B. Plasmonic Silver Nanosphere Enhanced ZnSe Nanoribbon/Si Heterojunction Optoelectronic Devices. Nanotechnology 2016, 27, 215202. [Google Scholar] [CrossRef] [PubMed]
- Bora, T.; Kyaw, H.H.; Sarkar, S.; Pal, S.K.; Dutta, J. Highly Efficient ZnO/Au Schottky Barrier Dye-Sensitized Solar Cells: Role of Gold Nanoparticles on the Charge-Transfer Process. Beilstein J. Nanotechnol. 2011, 2, 681–690. [Google Scholar] [CrossRef] [Green Version]
- Low, J.; Qiu, S.; Xu, D.; Jiang, C.; Cheng, B. Direct Evidence and Enhancement of Surface Plasmon Resonance Effect on Ag-Loaded TiO2 Nanotube Arrays for Photocatalytic CO2 Reduction. Appl. Surf. Sci. 2018, 434, 423–432. [Google Scholar] [CrossRef]
- Mahala, C.; Sharma, M.D.; Basu, M. Near-Field and Far-Field Plasmonic Effects of Gold Nanoparticles Decorated on ZnO Nanosheets for Enhanced Solar Water Splitting. ACS Appl. Nano Mater. 2020, 3, 1153–1165. [Google Scholar] [CrossRef]
- Basyooni, M.A.; Ahmed, A.M.; Shaban, M. Plasmonic Hybridization between Two Metallic Nanorods. Optik 2018, 172, 1069–1078. [Google Scholar] [CrossRef]
- Zhang, C.; Shao, M.; Ning, F.; Xu, S.; Li, Z.; Wei, M.; Evans, D.G.; Duan, X. Au Nanoparticles Sensitized ZnO Nanorod@nanoplatelet Core-Shell Arrays for Enhanced Photoelectrochemical Water Splitting. Nano Energy 2015, 12, 231–239. [Google Scholar] [CrossRef]
- Abdullayeva, N.; Altaf, C.T.; Mintas, M.; Ozer, A.; Sankir, M.; Kurt, H.; Sankir, N.D. Investigation of Strain Effects on Photoelectrochemical Performance of Flexible ZnO Electrodes. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.Q.; Hu, Y.; Sun, R.N.; Fu, H.; Peng, K.Q. Gold-Sensitized Silicon/ZnO Core/Shell Nanowire Array for Solar Water Splitting. Front. Chem. 2019, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, J.; Zhao, Y.; Zhai, J.; Jiang, L.; Song, Y.; Zhu, D. Photonic Crystal Concentrator for Efficient Output of Dye-Sensitized Solar Cells. J. Mater. Chem. 2008, 18, 2650–2652. [Google Scholar] [CrossRef]
- Kwon, J.; Cho, H.; Jung, J.; Lee, H.; Hong, S.; Yeo, J.; Han, S.; Ko, S.H. ZnO/CuO/M (M = Ag, Au) Hierarchical Nanostructure by Successive Photoreduction Process for Solar Hydrogen Generation. Nanomaterials 2018, 8, 323. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.P.; Hanumantha, P.J.; Velikokhatnyi, O.I.; Datta, M.K.; Hong, D.; Gattu, B.; Poston, J.A.; Manivannan, A.; Kumta, P.N. Nitrogen and Cobalt Co-Doped Zinc Oxide Nanowires-Viable Photoanodes for Hydrogen Generation via Photoelectrochemical Water Splitting. J. Power Sources 2015, 299, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.C.; Lin, Y.G.; Hsu, Y.K.; Chen, Y.C.; Chen, S.Y.; Chen, K.H. Visible-Light-Driven Photocatalytic Carbon-Doped Porous ZnO Nanoarchitectures for Solar Water-Splitting. Nanoscale 2012, 4, 6515–6519. [Google Scholar] [CrossRef]
- Wei, Y.; Ke, L.; Kong, J.; Liu, H.; Jiao, Z.; Lu, X.; Du, H.; Sun, X.W. Enhanced Photoelectrochemical Water-Splitting Effect with a Bent ZnO Nanorod Photoanode Decorated with Ag Nanoparticles. Nanotechnology 2012, 23, 235401. [Google Scholar] [CrossRef]
- Kushwaha, A.; Aslam, M. ZnS Shielded ZnO Nanowire Photoanodes for Efficient Water Splitting. Electrochim. Acta 2014, 130, 222–231. [Google Scholar] [CrossRef]
- Shi, M.; Pan, X.; Qiu, W.; Zheng, D.; Xu, M.; Chen, H. Si/ZnO Core-Shell Nanowire Arrays for Photoelectrochemical Water Splitting. Int. J. Hydrogen Energy 2011, 36, 15153–15159. [Google Scholar] [CrossRef]
- Sheikh, A.; Yengantiwar, A.; Deo, M.; Kelkar, S.; Ogale, S. Near-Field Plasmonic Functionalization of Light Harvesting Oxide-Oxide Heterojunctions for Efficient Solar Photoelectrochemical Water Splitting: The AuNP/ZnFe2O4/ZnO System. Small 2013, 9, 2091–2096. [Google Scholar] [CrossRef]
- Sreedhar, A.; Reddy, I.N.; Hoai Ta, Q.T.; Namgung, G.; Cho, E.; Noh, J.S. Facile Growth of Novel Morphology Correlated Ag/Co-Doped ZnO Nanowire/Flake-Like Composites for Superior Photoelectro-Chemical Water Splitting Activity. Ceram. Int. 2019, 45, 6985–6993. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Zeng, J.; Shi, Y.; Lin, S.; Huang, G.; Wang, H.; Kong, Z.; Xi, J.; Ji, Z. MnS Coupled With Ultrathin MoS2nanolayers As Heterojunction Photocatalyst for High Photocatalytic and Photoelectrochemical Activities. J. Alloys Compd. 2019, 771, 364–372. [Google Scholar] [CrossRef]
- Majumder, T.; Dhar, S.; Chakraborty, P.; Mondal, S.P. Growth of Carbon-Functionalized, Carbon-Doped ZnO/C Core-Shell Nanorods for Photoelectrochemical Solar Energy Conversion. ChemistrySelect 2018, 3, 4082–4094. [Google Scholar] [CrossRef]
- Dom, R.; Baby, L.R.; Kim, H.G.; Borse, P.H. Enhanced Solar Photoelectrochemical Conversion Efficiency of ZnO:Cu Electrodes for Water-Splitting Application. Int. J. Photoenergy 2013, 2013, 18–20. [Google Scholar] [CrossRef] [Green Version]
- Huynh, H.Q.; Pham, K.N.; Phan, B.T.; Tran, C.K.; Lee, H.; Dang, V.Q. Enhancing Visible-Light-Driven Water Splitting of ZnO Nanorods by Dual Synergistic Effects of Plasmonic Au Nanoparticles and Cu Dopants. J. Photochem. Photobiol. A Chem. 2020, 399, 112639. [Google Scholar] [CrossRef]
- Misra, M.; Kapur, P.; Nayak, M.K.; Singla, M. Synthesis and Visible Photocatalytic Activities of a Au@Ag@ZnO Triple Layer Core–Shell Nanostructure. New J. Chem. 2014, 38, 4197–4203. [Google Scholar] [CrossRef]
- Xia, J.; Diao, K.; Zheng, Z.; Cui, X. Porous Au/ZnO Nanoparticles Synthesised through a Metal Organic Framework (MOF) Route for Enhanced Acetone Gas-Sensing. RSC Adv. 2017, 7, 38444–38451. [Google Scholar] [CrossRef] [Green Version]
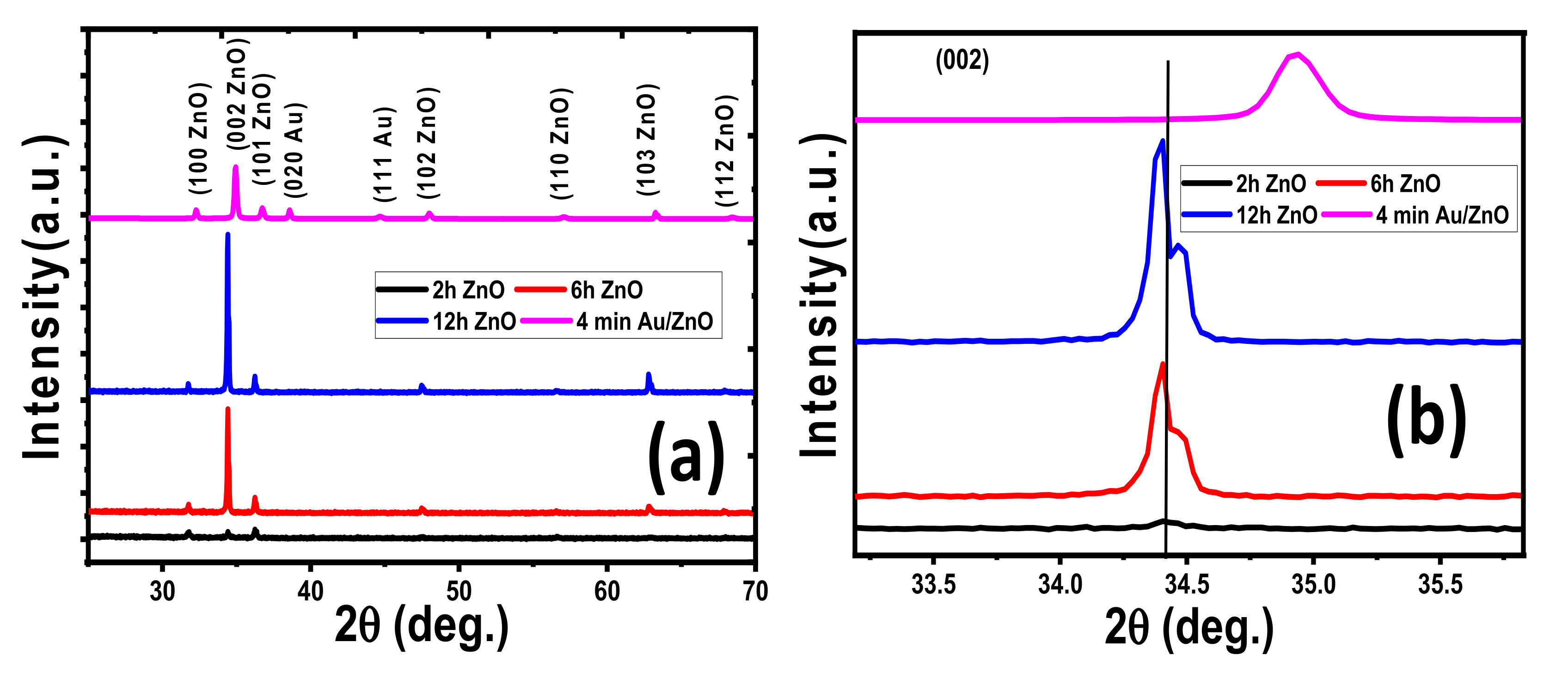
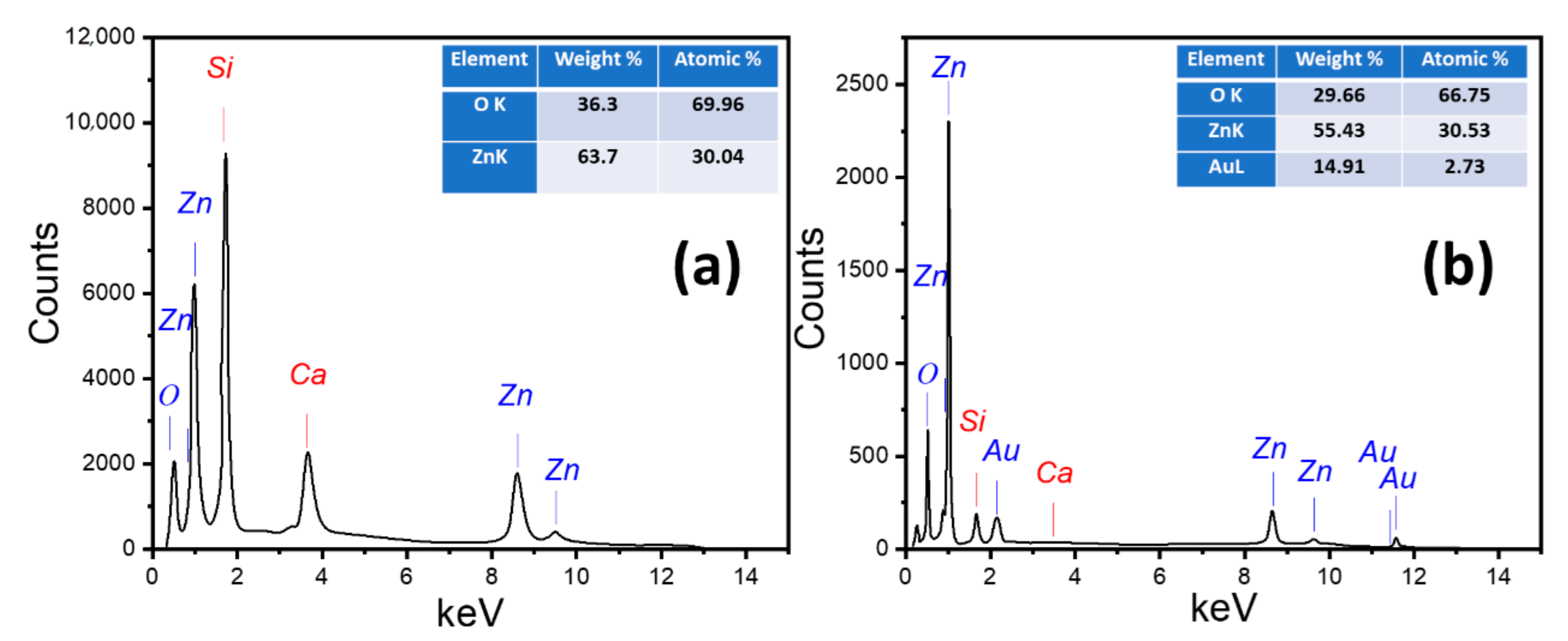

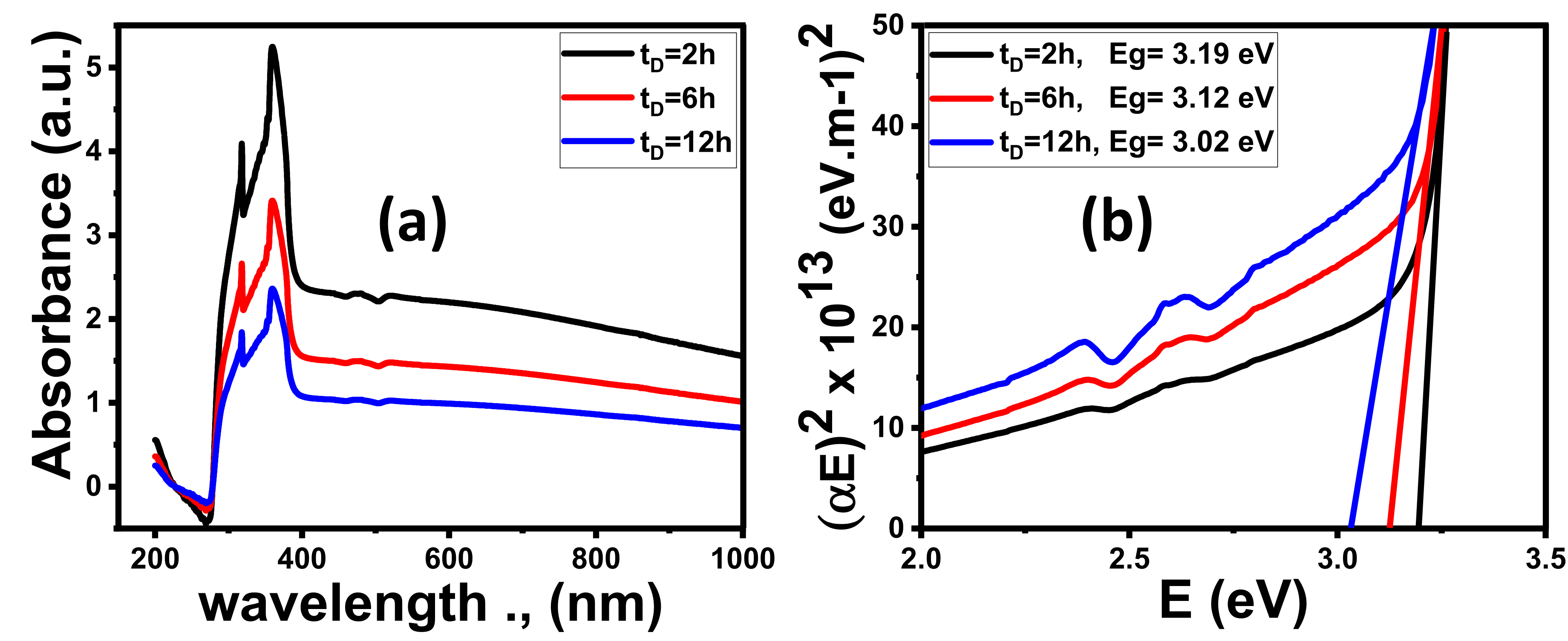
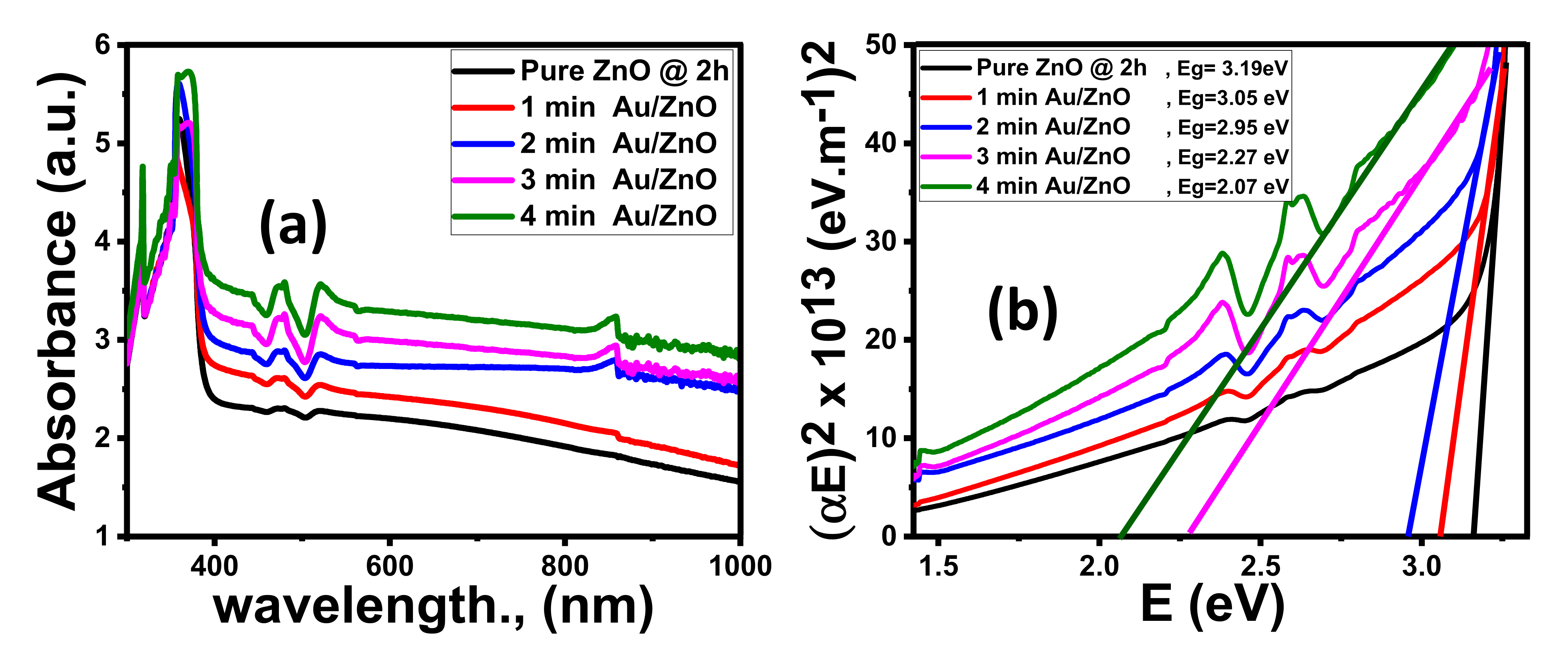
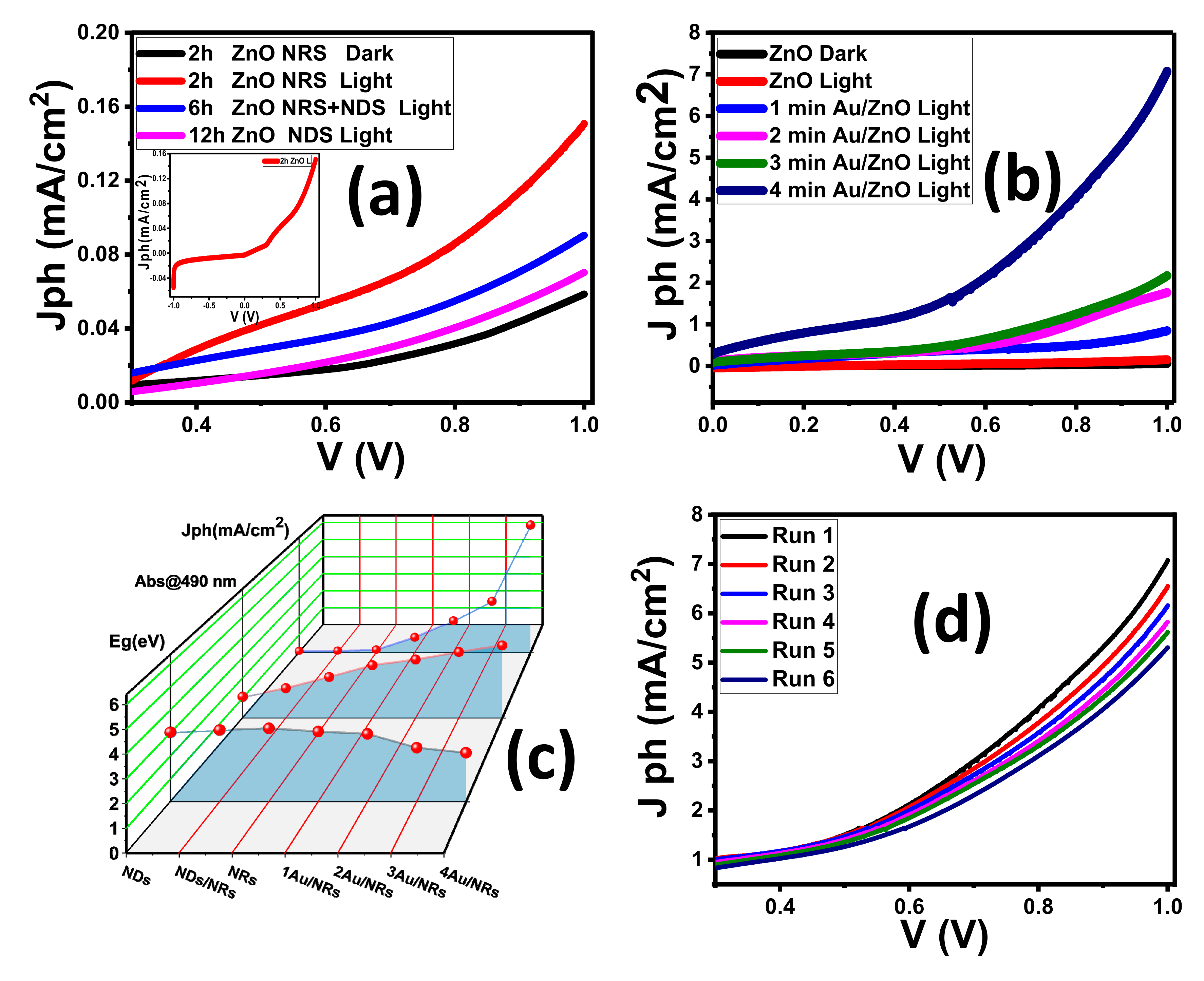

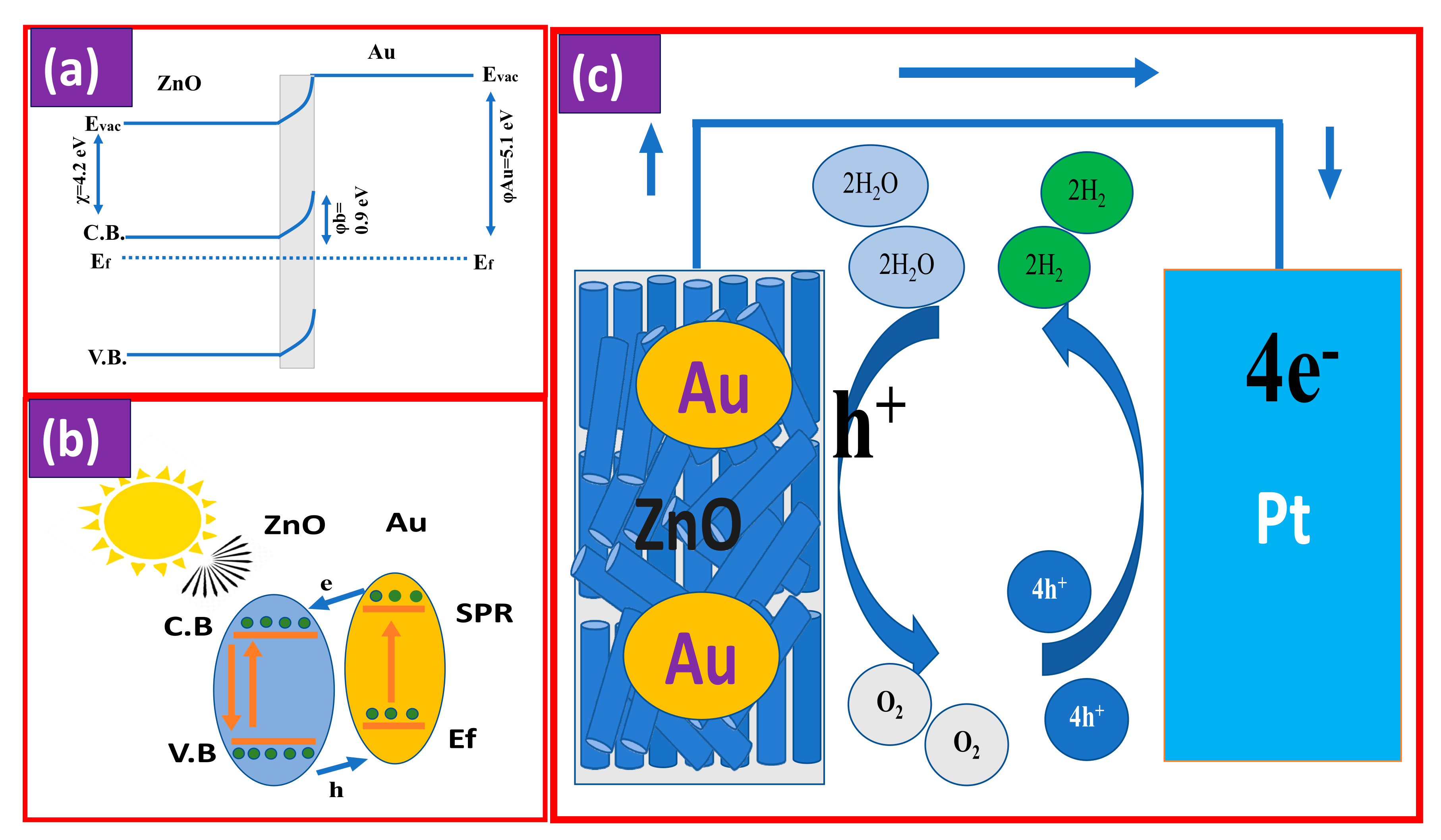
| Parameter | ZnO @ 2 h | ZnO @ 6 h | ZnO @ 12 h | 4 min Au/ZnO @ 2 h |
|---|---|---|---|---|
| Plane (hkl) | (002) | (002) | (002) | (002) |
| 2θ | 34.41 | 34.40 | 34.39 | 34.93 |
| d-spacing (Å) | 5.2976 | 5.2992 | 5.3010 | 2.57 |
| D (nm) | 79 | 79 | 77 | 65 |
| A (Å) | 3.2459 | 3.2357 | 3.2386 | 3.2046 |
| C (Å) | 5.2078 | 5.2653 | 5.2488 | 5.1405 |
| V (Å3) | 47.5176 | 47.7392 | 47.6748 | 45.7176 |
| L (Å) | 1.9757 | 1.9785 | 1.9776 | 2.2339 |
| U | 0.37936 | 0.37576 | 0.37678 | 0.3795 |
| σ∗109 (N/m2) | −0.2091 | −5.17566 | –3.74764 | 5.6131 |
| Catalyst | Jph (mA/cm2) | IPCE % | ABPE % | Ref. |
|---|---|---|---|---|
| ZnO/Ag | 0.616 | 0.5 @ 390 nm | 0.81 | [94] |
| (ZnO/Co)O:N nanowires | 8.78 at 0.78 VRHE | 15 @ 500 nm | 1.39 | [95] |
| C-doped ZnO porous nanoarchitectures | 1 at 1 VAg/AgCl | 0.75 | - | [96] |
| Au-coated 3D ZnO nanowires | 1.45 at 1 VRHE | 35 | 0.52 | [48] |
| ZnO nanorods | 0.19 at 1 VSCE | - | 0.084 | [97] |
| ZnO-ZnS core–shell | 0.534 at 1 VAg/AgCl | - | 0.38 | [98] |
| Si/ZnO core–shell | 4 at 1 VAg/AgCl | - | 0.47 | [99] |
| ZnFe2O4/ZnO system | 1.1 at 0.8 VAg/AgCl | 0.15@500 nm | 0.15 | [100] |
| Ag/Co-doped ZnO nanowire | 0.7 at 0.39 VAg/AgCl | 2.6 | 0.73 | [101] |
| MoS2-decorated ZnO nanowires | 0.22 at 1 VAg/AgCl | - | 3.18 | [102] |
| ZnO/C Core-Shell Nanorods | 0.82 at 1 VAg/AgCl | 3.5 at 500 nm | 0.79 | [103] |
| ZnO:Cu photoanode | 2.5 at 1 VSCE | 42 at 360 nm | 0.28 | [104] |
| Au decorated ZnO:Cu NRs | 0.9 at 1 VAg/AgCl | 40 | 0.59 | [105] |
| Au/ZnONRs | 7.7 | 14.2@490 nm | 2.05 | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zayed, M.; Nasser, N.; Shaban, M.; Alshaikh, H.; Hamdy, H.; Ahmed, A.M. Effect of Morphology and Plasmonic on Au/ZnO Films for Efficient Photoelectrochemical Water Splitting. Nanomaterials 2021, 11, 2338. https://doi.org/10.3390/nano11092338
Zayed M, Nasser N, Shaban M, Alshaikh H, Hamdy H, Ahmed AM. Effect of Morphology and Plasmonic on Au/ZnO Films for Efficient Photoelectrochemical Water Splitting. Nanomaterials. 2021; 11(9):2338. https://doi.org/10.3390/nano11092338
Chicago/Turabian StyleZayed, Mohamed, Nourhan Nasser, Mohamed Shaban, Hind Alshaikh, Hany Hamdy, and Ashour M. Ahmed. 2021. "Effect of Morphology and Plasmonic on Au/ZnO Films for Efficient Photoelectrochemical Water Splitting" Nanomaterials 11, no. 9: 2338. https://doi.org/10.3390/nano11092338
APA StyleZayed, M., Nasser, N., Shaban, M., Alshaikh, H., Hamdy, H., & Ahmed, A. M. (2021). Effect of Morphology and Plasmonic on Au/ZnO Films for Efficient Photoelectrochemical Water Splitting. Nanomaterials, 11(9), 2338. https://doi.org/10.3390/nano11092338








