3. Results and Discussion
As shown in the schematic (
Figure 1a), the nanobelt K
2Ti
8O
17 is formed on the surface of the Ti foil via the facile hydrothermal reaction combined with a post-pyrolysis process.
Figure 1b displays the XRD patterns of the as-synthesized K
2Ti
8O
17 nanobelts. All diffraction peaks are indexed as a layered K
2Ti
8O
17 phase (JCPDF NO. 84-2057) with the space group of C2/m(12). The SEM image shows a network distribution of whiskers-like K
2Ti
8O
17 (
Figure 1c). The magnified image shows the nanoclusters aggregated with the intertwined nanobelts (
Figure 1d). The formed pore structure inside the nanoclusters facilitates the electrolyte to reach the crystal structure. The TEM image in
Figure 1e reveals an individual K
2Ti
8O
17 nanobelt with a length of several microns. The SAED pattern and HRTEM image (
Figure 1f,g) indicate a single crystal structure of the prepared K
2Ti
8O
17 nanobelt. The growth direction of the nanobelt is along the [010] direction. The lattice plane distances of 0.19 nm and 0.27 nm correspond to (020) and (
) lattice planes of K
2Ti
8O
17 (
Figure 1g), respectively. Even though a good contrast can be observed in the SAED pattern (
Figure 1f), the FFT (fast Fourier transform) pattern of the HRTEM image inserted in
Figure 1g reveals an elongation of the diffraction spot that may indicate the existence of defects in the single crystal.
The electrochemical properties of the as-prepared K
2Ti
8O
17 electrode were first investigated in 1 M of AlCl
3 aqueous electrolyte via cyclic voltammetry (CV) and galvanostatic charge–discharge (GCD) curves at room temperature (25 °C).
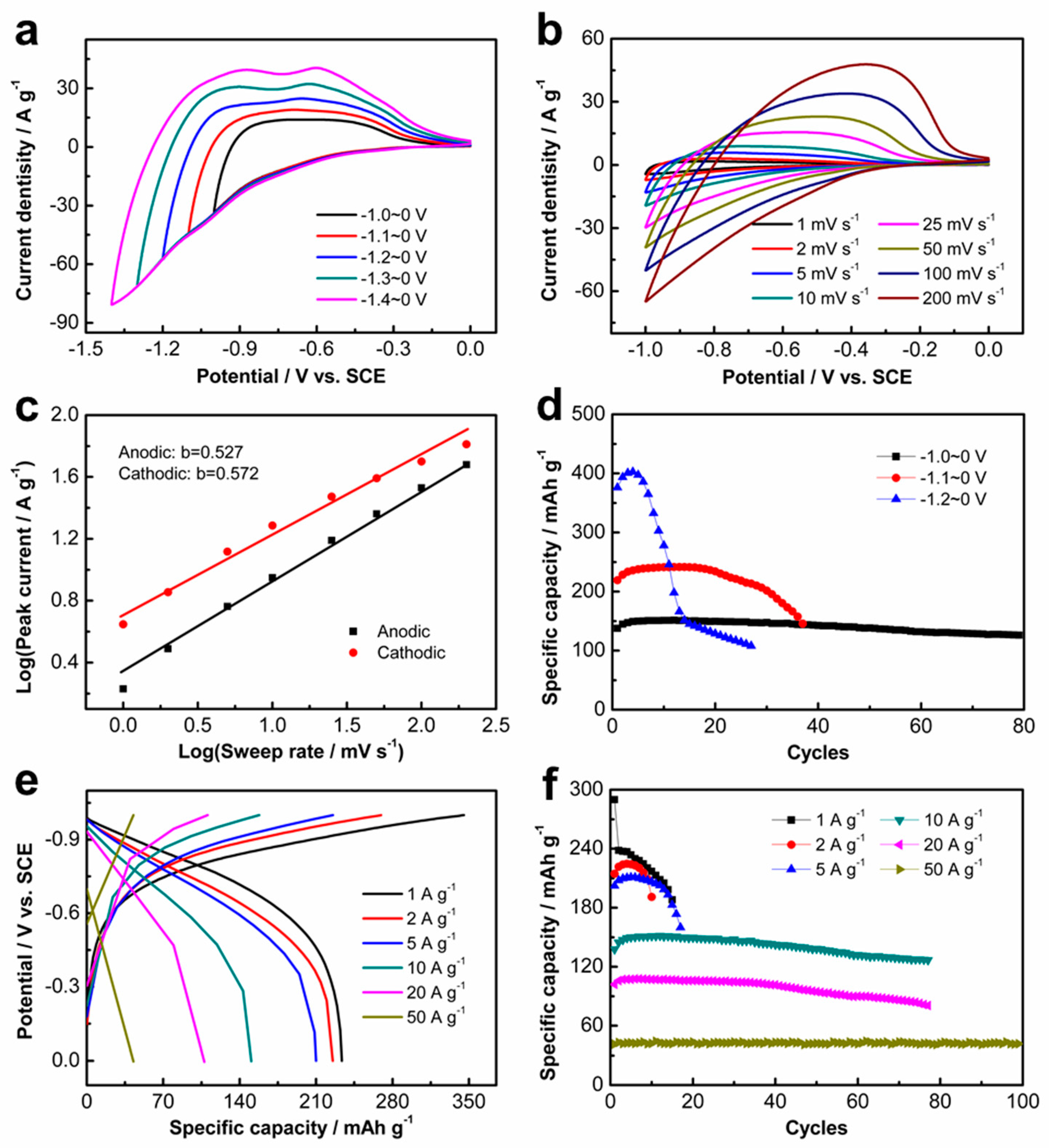
Figure 2a displays the CV curves at different potential ranges with a sweep rate of 25 mV s
−1, showing that the K
2Ti
8O
17 electrode can be operated at the negative potential range from 0.0 V to −1.4 V
VS. SCE. An ultra-high overpotential for the hydrogen evolution reaction (HER) is observed after the Al
3+ ions intercalation, indicating that the Al
3+ ions intercalation is prior to the HER process. The ultra-high hydrogen evolution overpotential is ascribed to the low catalytic activity of both K
2Ti
8O
17 nanobelts and Ti substrate for HER [
22]. Al
3+ ions with a smaller radius than that of Li
+ ions facilitate the intercalation/deintercalation reactions for the charge–discharge process, inducing the high reversibility of the insertion/extraction of Al
3+ ions into/from the anatase TiO
2 electrode [
15]. Similarly, it is possible that the Al
3+ ions could insert/extract into/from the K
2Ti
8O
17 electrode. Charge and discharge plateaus are observed in GCD curves at different potential ranges (
Figure S1a), in good agreement with the CV curves. CV measurements were performed at different scan rates, as shown in
Figure 2b. The peak currents of the K
2Ti
8O
17 electrode gradually increase with the scan rates. Under different sweep rates, the CV curves of the layered K
2Ti
8O
17 electrode exhibit similar quasi-symmetric shapes, illustrating the good reversibility of the Al
3+ ions insertion/extraction. The electrochemical behavior of Al
3+ ions in the K
2Ti
8O
17 electrode was evaluated by analyzing the collected CV data at various sweep rates according to the following equation:
where
i is the measured current and
v represents the sweep rate. Both
a and
b refer to the adjustable parameters. The
b-values are determined from the slope by plotting log
i vs. log
v. Well-defined
b values approaching 1 indicate capacitive characteristics, while
b values close to 0.5 represent diffusion-controlled characteristics of the electrode [
23]. Here, the
b values were determined as 0.527 and 0.572 from the redox peaks (
Figure 2c), indicating that the intercalation/deintercalation of Al
3+ ions in the K
2Ti
8O
17 electrode is a diffusion-controlled process.
Figure 2d presents the cycling performance of the K
2Ti
8O
17 electrode at different potential ranges with a current density of 10 A g
−1. As shown, the specific capacity increases with the potential ranges, while the stability decreases. The similar shape of the charge and discharge curves also reveals the good reversibility of the K
2Ti
8O
17 electrode (
Figure 2e). The coulombic efficiencies (CEs) of the electrode at the current density of 10 A g
−1 decrease with the cycling number (
Figure S1b), implying the degradation of the reversibility with the increase in cycles. The cycling stability under various current densities at −1.0~0 V
vs. SCE is shown in
Figure 2f. The K
2Ti
8O
17 electrode exhibits a specific capacity of 224 mAh g
−1 at the current density of 2 A g
−1 with the expense of cycling stability. The specific capacity of 107 mAh g
−1 is maintained at the current density of 20 A g
−1. It is surprising that the specific capacity is still 43 mAh g
−1 at the current density of 50 A g
−1 with a superior cycling performance. The CEs of the K
2Ti
8O
17 electrode reach up to approximately 100% at the high discharge current density of 50 A g
−1 (
Figure S1c). These results indicate the high-rate performance of the layered K
2Ti
8O
17 electrode, which should be ascribed to the layered structure for the fast diffusion of Al
3+ ions. Additionally, the morphology evolution process of the precursors of the K
2Ti
8O
17 electrode with the hydrothermal reaction time was investigated via changing the hydrothermal time (0 h, 4 h, 8 h, 12 h, and 24 h). The SEM and XRD results are presented in
Figure S2 in the supporting information. The compositional characterizations and electrochemical performance of the K
2Ti
8O
17 sample undergoing a 24 h hydrothermal process without the pyrolysis step are shown in
Figure S3 in the supporting information. Compared with the K
2Ti
8O
17 sample before and after undergoing the pyrolysis step, the obviously enhanced electrochemical performance is achieved for the one undergoing pyrolysis.
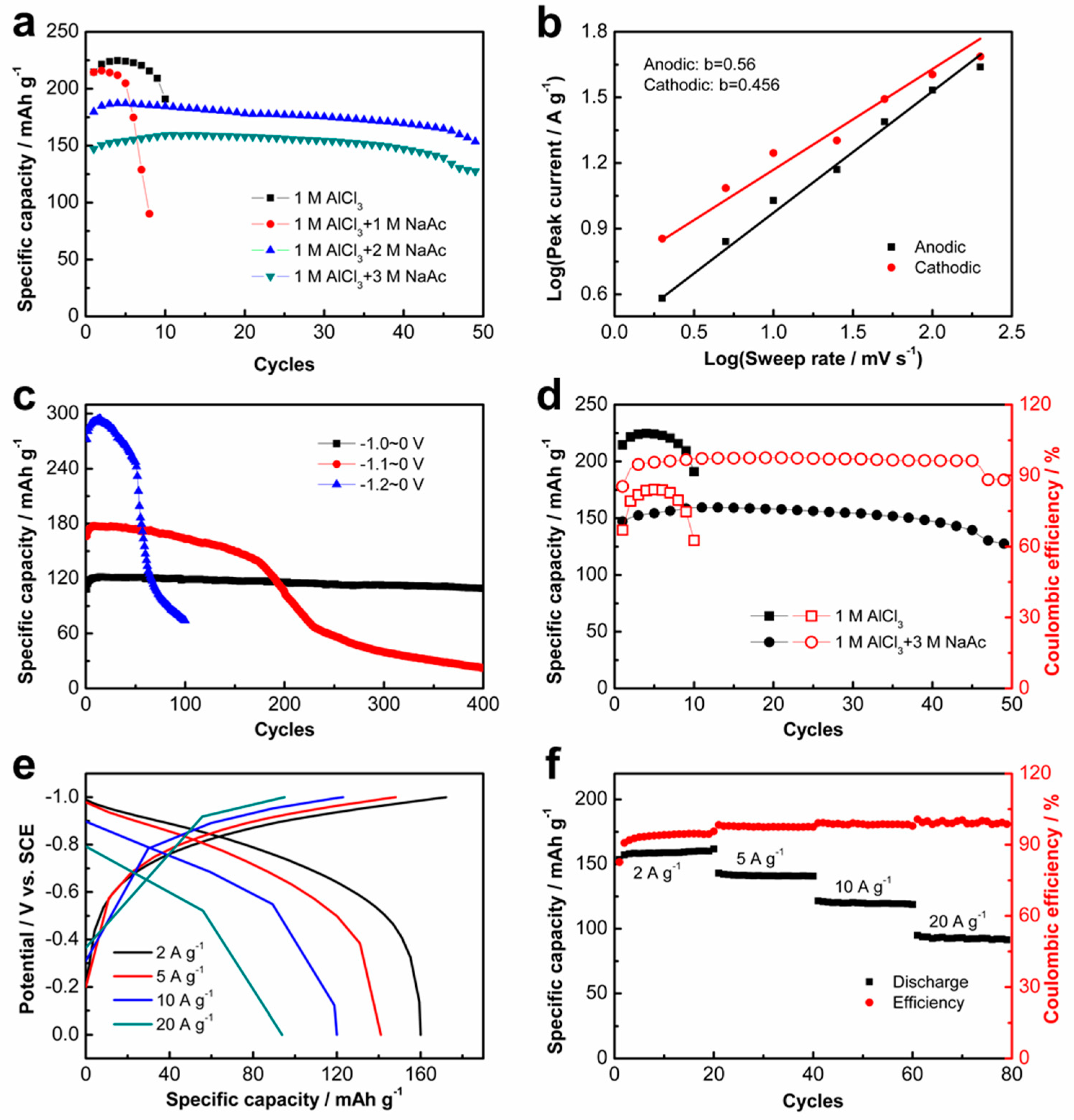
To alleviate the effect of the acidic AlCl
3 electrolyte on the electrode, various concentrations of the NaAc was added into the AlCl
3 electrolyte as a buffer agent. The electrochemical performance of the K
2Ti
8O
17 electrode in the hybrid AlCl
3/NaAc aqueous electrolyte was evaluated by CV and GCD measurements (
Figure 3 and
Figure S4). The CV curves at different potential ranges show the symmetric shapes (
Figure S4a), implying the good reversibility of the AIBs with the K
2Ti
8O
17 anode in the hybrid AlCl
3/NaAc aqueous electrolyte. Compared with the battery with pure AlCl
3 electrolyte, the cycling stability of the battery under a current density of 2 A g
−1 improves via the addition of NaAc with the concentrations of 2 M and 3 M (
Figure 3a). The corresponding CEs based on the GCD tests also improve with the addition of NaAc solution (
Figure S4b), although the discharge capacity of the battery slightly decreases (
Figure S4c). This demonstrates that the NaAc additive is a benefit for the rechargeable intercalation/deintercalation of Al
3+ ions. The b value plots for log i against log v of the K
2Ti
8O
17 anode were calculated on the basis of the CV curves at different scan rates (
Figure S4d) to obtain the b values via Equation 1. The calculated b values are 0.56 and 0.505 in the AlCl
3/NaAc hybrid electrolyte (
Figure 3b), suggesting a diffusion-controlled process in the K
2Ti
8O
17 electrode [
11,
24]. The cycling performance of the charge–discharge curves of the K
2Ti
8O
17 electrode in the aqueous AlCl
3/NaAc electrolyte shows a similar trend to that in the AlCl
3 electrolyte (
Figure 3c and
Figure S5a), exhibiting a decreasing trend with the potential ranges. In contrast, the cycling stability in the AlCl
3/NaAc hybrid electrolyte is improved compared with that in the single AlCl
3 electrolyte. To analyze the elemental composition of the cycled electrode, ICP-OES experiments for the cycled K
2Ti
8O
17 electrodes in both charge and discharge states, as well the tested electrolyte, were performed. The cycled K
2Ti
8O
17 electrode in the charge state was attained when it was charged to −1 V after charging/discharging for 10 cycles. The electrode in the discharge state was obtained when it was discharged to 0 V after charging/discharging for 10 cycles, and the tested electrolyte was collected simultaneously. All the samples were immersed into deionized water overnight and then repetitively washed before the ICP-OES test. From the experimental results, the molar ratio of Ti:K:Al:Na is 1:0.084:0.011:0.003 with the total cation charge of K, Al, and Na of around 0.96 in the charge state, while it is 1:0.087:0.016:0.002 with the total cation charge of K, Al, and Na of around 1.096 under the discharge state. The difference in K
+ ions under charge and discharge conditions indicates the contributions from K
+ insertion/deintercalation. Additionally, the difference of 0.136 in terms of the total cation charge of K, Al, and Na implies that the proton seems to also contribute to the charge/discharge process. Obviously, a decrease in the K
+ ion is detected in the cycled electrode, while the Al
3+ ion and a trace of the Na
+ ion are detected. Therefore, we can conclude that the ionic substitutions of K
+ ions by Al
3+/Na
+ ions occur in the K
2Ti
8O
17 electrode during the charge/discharge process. Simultaneously, the K
+ ions are detected in the cycled electrolyte, corresponding to the dissolution of K
+ from the electrode to electrolyte during the intercalation of Al
3+ and Na
+ ions. The ICP-OES measurement for the cycled K
2Ti
8O
17 electrode after washing shows the existence of Na
+ and Al
3+ ions. This indicates a possible mechanism of Na
+ and Al
3+ co-intercalation/deintercalation during the charge/discharge process. Although the specific capacitance of the K
2Ti
8O
17 electrode in the aqueous AlCl
3/NaAc electrolyte (159 mAh g
−1) is lower than that in the AlCl
3 electrolyte (234 mAh g
−1), the cycling stability and CEs during the cycling are significantly improved (
Figure 3d). Similar charge and discharge curve shapes reveal the excellent reversibility of the K
2Ti
8O
17 electrode in the AlCl
3/NaAc hybrid electrolyte (
Figure 3e). The K
2Ti
8O
17 electrode exhibits a superior rate capability and delivers good discharge capacities of 159, 141, 120, and 93 mAh g
−1 with increasing current densities of 2, 5, 10, and 20 A g
−1, respectively (
Figure 3f). The corresponding CEs at the current densities of 2, 5, 10, and 20 A g
−1 are approximately 90%, 93%, 94%, and 95%, respectively. These results demonstrate excellent rate-performance of the K
2Ti
8O
17 electrode in the AlCl
3/NaAc electrolyte. It is interesting that the cycling performance and CEs of the K
2Ti
8O
17 electrode are improved with the increase in the charge–discharge current density (
Figure S5b,c). The K
2Ti
8O
17 electrode exhibited good cycling stability when the electrode performed in the potential range of −1.0~0 V at a current density of 10 A g
−1 (
Figure S5d), implying the promising practical application of the electrode at high current densities. Definitely, the electrolyte also has a disparate influence on different anodes [
25,
26].
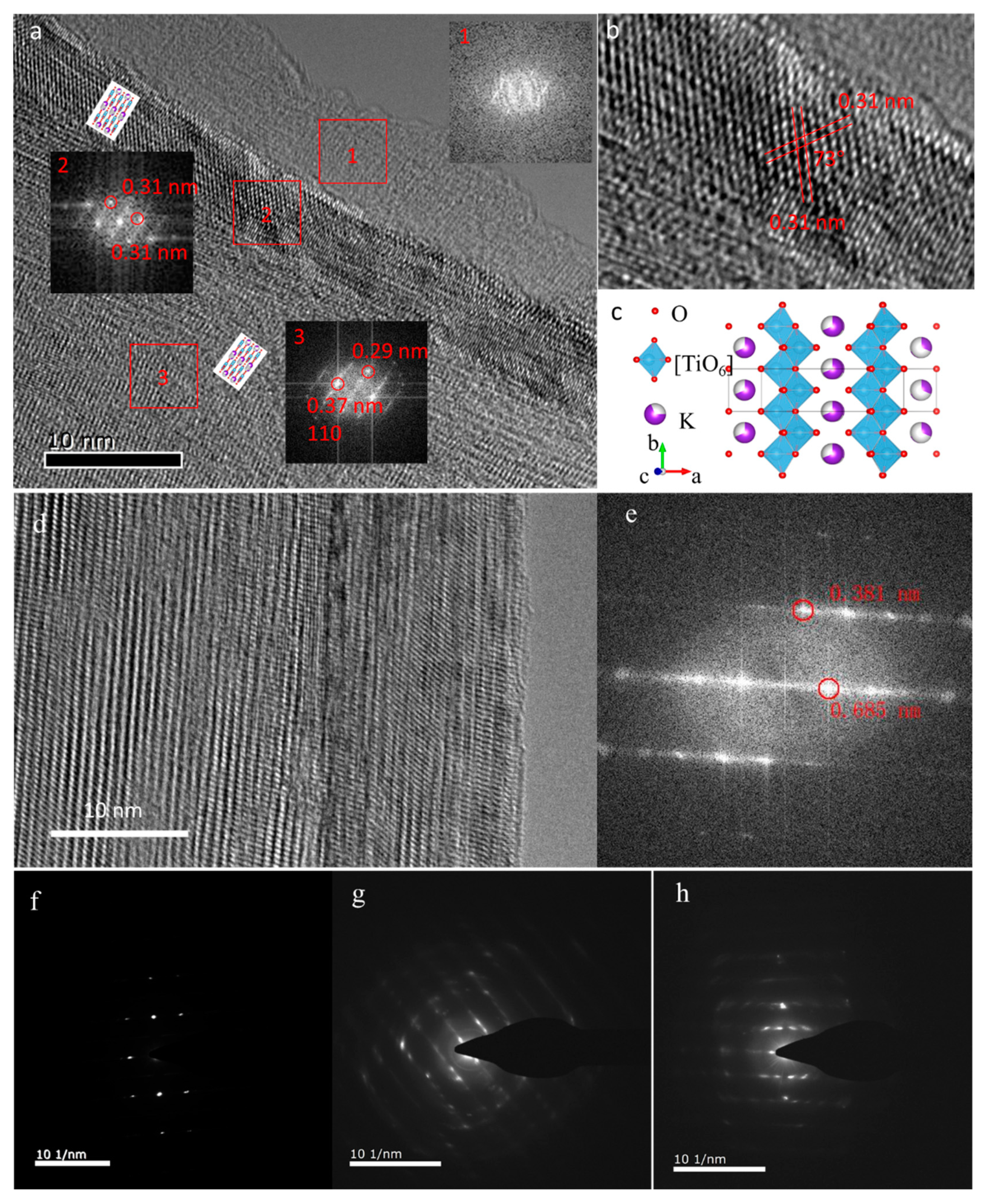
To further probe the microstructure evolution of the K
2Ti
8O
17 electrode during the charge–discharge cycling process in both the pure AlCl
3 and the AlCl
3/NaAc hybrid electrolytes, HRTEM measurements were performed and are shown in
Figure 4. Three well-defined regions are presented in the K
2Ti
8O
17 electrode after cycling in the aqueous AlCl
3 electrolyte (
Figure 4a). After electrochemical activation during the charge–discharge process, the phase transition occurs in the surface region due to the Na
+/Al
3+ intercalation/deintercalation in the K
2Ti
8O
17 lattice, forming an amorphous phase (region 1 in
Figure 4a). The corresponding fast Fourier transform (FFT) pattern confirms the amorphous structure (inset 1 of
Figure 4a). Meanwhile, the well-defined crystalline lattice is still observed in the bulk of the K
2Ti
8O
17 (region 3 in
Figure 4a). The calculated lattice fringe distance of 0.37 nm via FFT corresponds to the (110) plane of the layered K
2Ti
8O
17 phase. Between region 1 and 3, there is a crystallized layer that reveals a different FFT pattern from that of the bulk (region 2 in
Figure 4a). The difference could be the orientation change of the K
2Ti
8O
17 phase during the charge–discharge cycling process. The lattice plane distances of 0.31 nm, as well as the 73° cross angle of the lattice plane shown in
Figure 4b, indicate that the lattice planes in region 2 are (310) and (−310) planes of the K
2Ti
8O
17 phase. In the sample without the charge–discharge cycling process, no such layered structure is observed (see
Figure 1f). The amorphization in region 1 and the reorientation in region 2 are ascribed to the irreversible phase transition of K
2Ti
8O
17 during the charge–discharge process. The irreversible phase transition-induced amorphization of the surface layer of K
2Ti
8O
17 primarily causes the capacity to fade. Analogously, the Al
3+ ions intercalation and irreversible phase transition caused capacity degradation, which were also present in the transition-metal chalcogen for AIBs [
12,
27].
For comparison, the amorphous layer is not present in the K
2Ti
8O
17 electrode after cycling in the hybrid AlCl
3/NaAc electrolyte. Accordingly, the clear lattice fringe in the HRTEM image and the corresponding FFT plot (
Figure 4g) indicate the relative high crystallinity in the sample, referring to the layered K
2Ti
8O
17 phase. Selected-area electron diffraction (SAED) was employed to probe the phase structure of the K
2Ti
8O
17 nanobelt before and after cycling. The three SAED images in
Figure 4f–h show the pristine K
2Ti
8O
17, as well as the K
2Ti
8O
17 after cycling in the AlCl
3 aqueous electrolyte and in the AlCl
3/NaAc hybrid electrolyte, respectively. The pristine K
2Ti
8O
17 nanobelt is a single crystal with high crystallinity (
Figure 4f). After charge/discharge cycling, defects are introduced in the K
2Ti
8O
17, causing local structure distortions. As shown in
Figure 4g,h, the diffraction patterns become blurred after cycling in both AlCl
3 and AlCl
3/NaAc electrolytes, confirming the structure distortions. The distortion extent of the K
2Ti
8O
17 electrode in the AlCl
3/NaAc electrolyte is lower than that in the AlCl
3 electrolyte. The trend of polycrystallization is also confirmed by the appearance of a diffraction ring. This could be attributed to the Na
+/Al
3+ intercalation into the layered K
2Ti
8O
17 crystal structure during the charge–discharge process. In addition, the low-magnification SEM, energy-dispersive X-ray spectroscopy (EDS), and element mapping images of the pristine and reacted K
2Ti
8O
17 electrodes provide information on the homogenous distribution of Al into the K
2Ti
8O
17 phase (
Figure S6). These results suggest that the Al
3+ ions are successfully inserted into the layered K
2Ti
8O
17 structure after the charge/discharge behaviors. However, no amorphous layer is found in the reacted K
2Ti
8O
17 electrode in the hybrid AlCl
3/NaAc electrolyte, implying the contributions of the additive NaAc on maintaining the crystallinity of the K
2Ti
8O
17 electrode during the electrochemical process. Simultaneously, a significantly improved cycling performance of the K
2Ti
8O
17 electrode in the hybrid AlCl
3/NaAc electrolyte is obtained in comparison with that in the single AlCl
3 electrolyte (
Figure 3d). It should be ascribed to the ability of alkaline NaAc to change the AlCl
3 aqueous electrolyte from neutral or acid conditions into alkaline conditions. As is known, the hydroxyl has a capacity to eliminate the formation of a Al
2O
3 passivation layer under alkaline conditions, while the formed Al
2O
3 passivation layer in neutral solutions hinders the electrode reactions, and a severe HER and self-discharge possibly occur under acidic conditions [
12]. Therefore, we speculate that the NaAc additive eliminates the adverse effects of low pH values from the solo AlCl
3 aqueous electrolyte, thus improving the electrochemical characteristics of the K
2Ti
8O
17 electrode. Further evidence on the effect of the NaAc additive, however, is recommended in a study in the near future. As a comparison, the Na
2Ti
9O
19 sample was synthesized using the same experimental method with K
2Ti
8O
17. SEM, XRD, and electrochemical performance tests for the Na
2Ti
9O
19 sample as an electrode in the AlCl
3 electrolyte were conducted, as shown in
Figure S7 in the supporting information. Compared to the K
2Ti
8O
17 sample, the Na
2Ti
9O
19 sample exhibits worse electrochemical performance from the CV curves and rate-capacity curves.
To gain insight into the reversibility of the electrode, an ex situ X-ray absorption near-edge structure (XANES) technique was employed here to identify the structural evolution of the K
2Ti
8O
17 electrode materials during charge/discharge.
Figure 5a displays the Ti L-edge XANES features undergoing the background subtraction and normalization procedures, for the pristine and tested K
2Ti
8O
17 under various charge/discharge states. Four observed peaks located at 458.4 eV (a’), 460.7 eV (b’), 463.9 eV (c’), and 462.1 eV (d’) are ascribed to the excitation of Ti 2p
3/2 (peaks t
2g (L3) and e
g (L3)) and Ti 2p
1/2 (peaks t
2g (L2) and e
g (L2)) core levels into empty Ti 3d states, respectively [
28,
29,
30]. As reported, the peak t
2g (L3) is related to the lower oxidation state of the cation, which has been detected on the TiO
2 nanosheet with respect to the reduced Ti
4+ surface specie [
30]. The peak e
g (L3) is related to the higher oxidation state of the cation, corresponding to the Ti
4+ species herein. The decreased intensity ratio of e
g(L3)/t
2g(L3) peaks (
Table S1) after the discharge of the electrode indicate the partial reduction of Ti
4+ during the discharge process. Moreover, differences between the pristine and the charged/discharged K
2Ti
8O
17 electrodes are observed in the O K-edge XANES spectra (
Figure 5b), reflecting the effects of Na
+/Al
3+ intercalation/deintercalation in the layered K
2Ti
8O
17 electrode in the charge/discharge process. According to molecular orbital theory, the O K-edge features originate from a transition of the O 1s electron to the various partially occupied and unoccupied molecular orbitals of the oxides, considering the crystal-field splitting effects [
31]. As shown in the O K-edge XANES spectrum of the pristine K
2Ti
8O
17 sample, the major peaks located at about 532.6 eV and 534.1 eV are assigned to excitations varying from the O 1s core level to the Ti 3d-related conduction band, splitting into t
2g and e
g subbands [
32]. This result confirms the partial reduction of Ti
4+ during the discharge of the K
2Ti
8O
17 electrode.
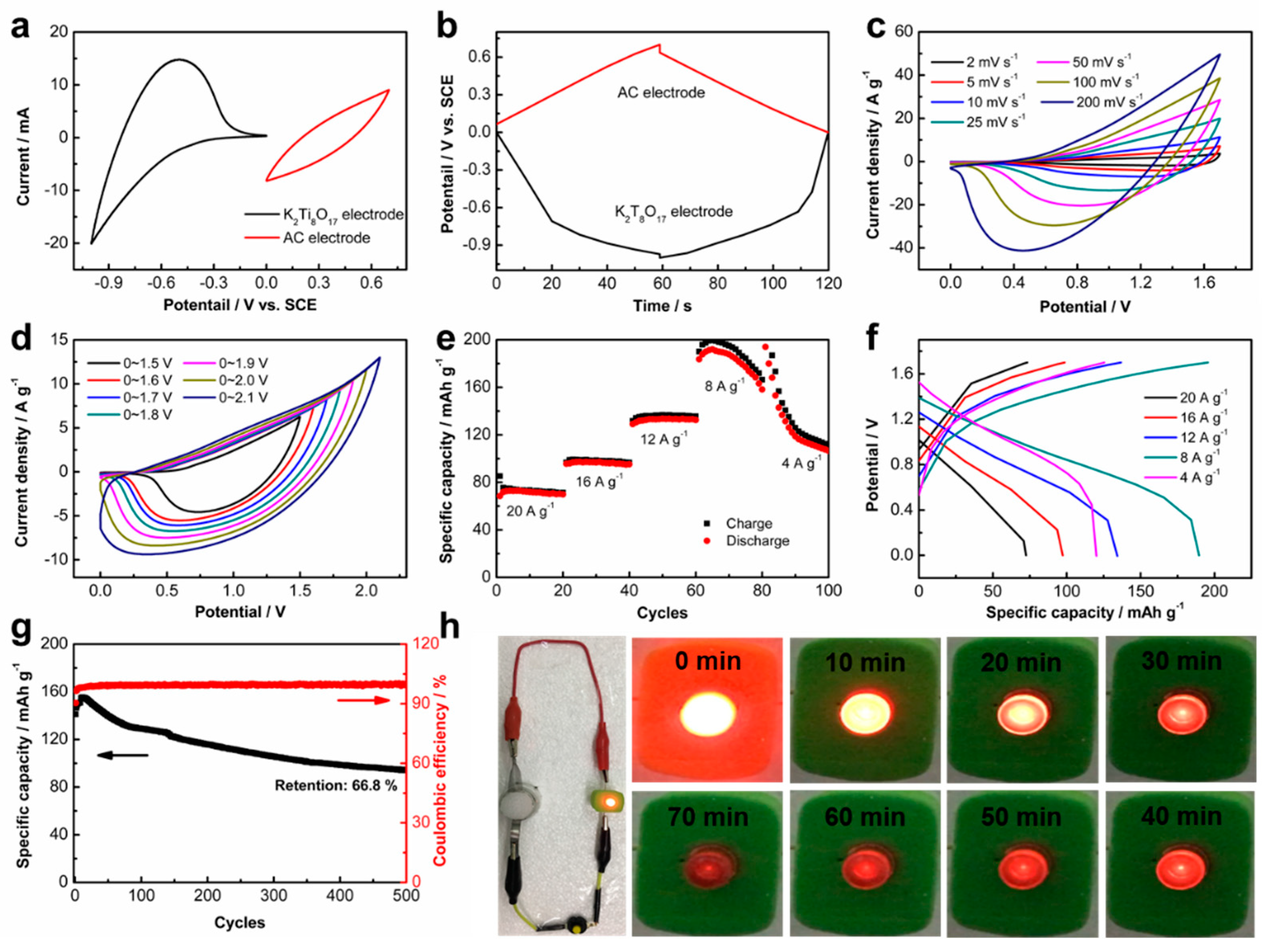
To further explore the potential practical application of the K
2Ti
8O
17 electrode, a full cell of AIB was assembled using the carbon coated on the Ti mesh (AC-Ti), K
2Ti
8O
17 electrode, and the aqueous AlCl
3/NaAc hybrid solution as the cathode, anode, and electrolyte, respectively. The working potential range of the AC-Ti and K
2Ti
8O
17 electrodes was measured by CV and GCD curves. The electrochemical properties of the AC-Ti electrode are given in
Figure S8, demonstrating that AC-Ti is a suitable cathode for the aqueous AIB.
Figure 6a presents the CV curves of both the cathode and anode. As shown, the AC-Ti electrode displays typical capacitance characteristics in the voltage range of 0~0.7 V
VS. SCE, while the K
2Ti
8O
17 electrode demonstrates the capacitance in the potential of −1~0 V
VS. SCE in the hybrid AlCl
3/NaAc electrolyte. The AC-Ti cathode and K
2Ti
8O
17 anode were also evaluated by GCD tests (
Figure 6b). The potential ranges for the K
2Ti
8O
17 and AC-Ti electrodes are consistent with the CV measurements. The maximal voltage of the as-assembled AIB device reaches up to 1.6 V, as shown in
Figure 6c. The CV curves without any obvious shape distortions at various sweeping rates, even at a high sweeping rate of 200 mV s
−1, indicate its superior rate capability. No gas evolution peaks are observed in the CV curves even at the potential range of 0~2.0 V
VS. SCE (
Figure 6d). The specific capacities of the K
2Ti
8O
17// AlCl
3/NaAc//AC-Ti battery are 189.6, 134.3, 97.3, and 72.4 mAh g
−1 at high scan rates of 8, 12, 16, and 20 A g
−1, respectively (
Figure 6d,f). Additionally, the AIB exhibits a capacity retention of approximately 66.8% after 500 cycles at the current density of 20 A g
−1 (
Figure 6g), demonstrating its superior cycling stability. Noticeably, a slightly larger discharge capacity is observed in the first 10 cycles and then decreases. Irreversible changes likely occurred in the electrode at the early stage [
33,
34]. Furthermore, the as-assembled AIB has the power to light-up a commercial light-emitting diode (LED) lasting more than one hour (
Figure 6h), confirming its promising practical applications for energy storage.