Low Toxicological Impact of Commercial Pristine Multi-Walled Carbon Nanotubes on the Yeast Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Results and Discussion
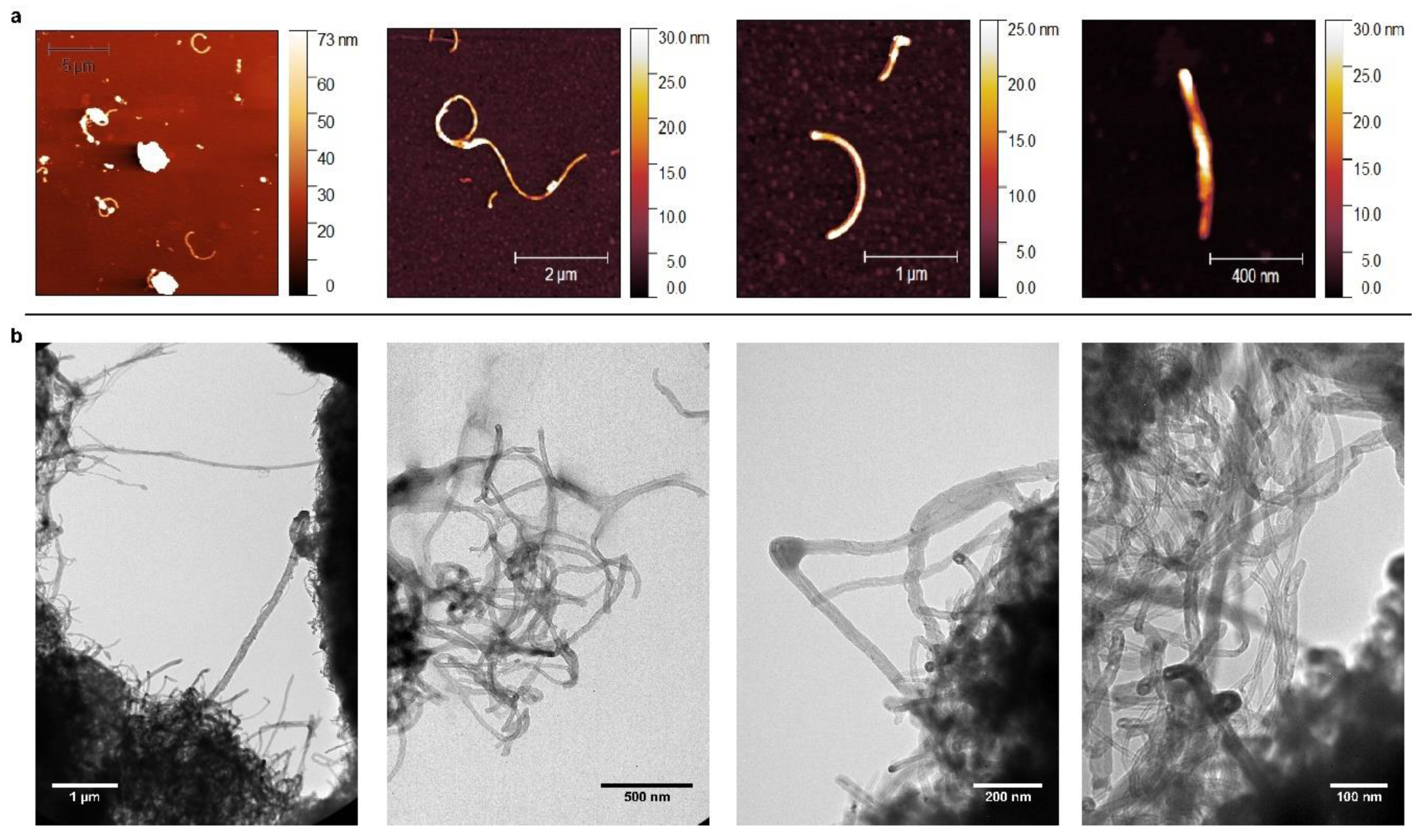
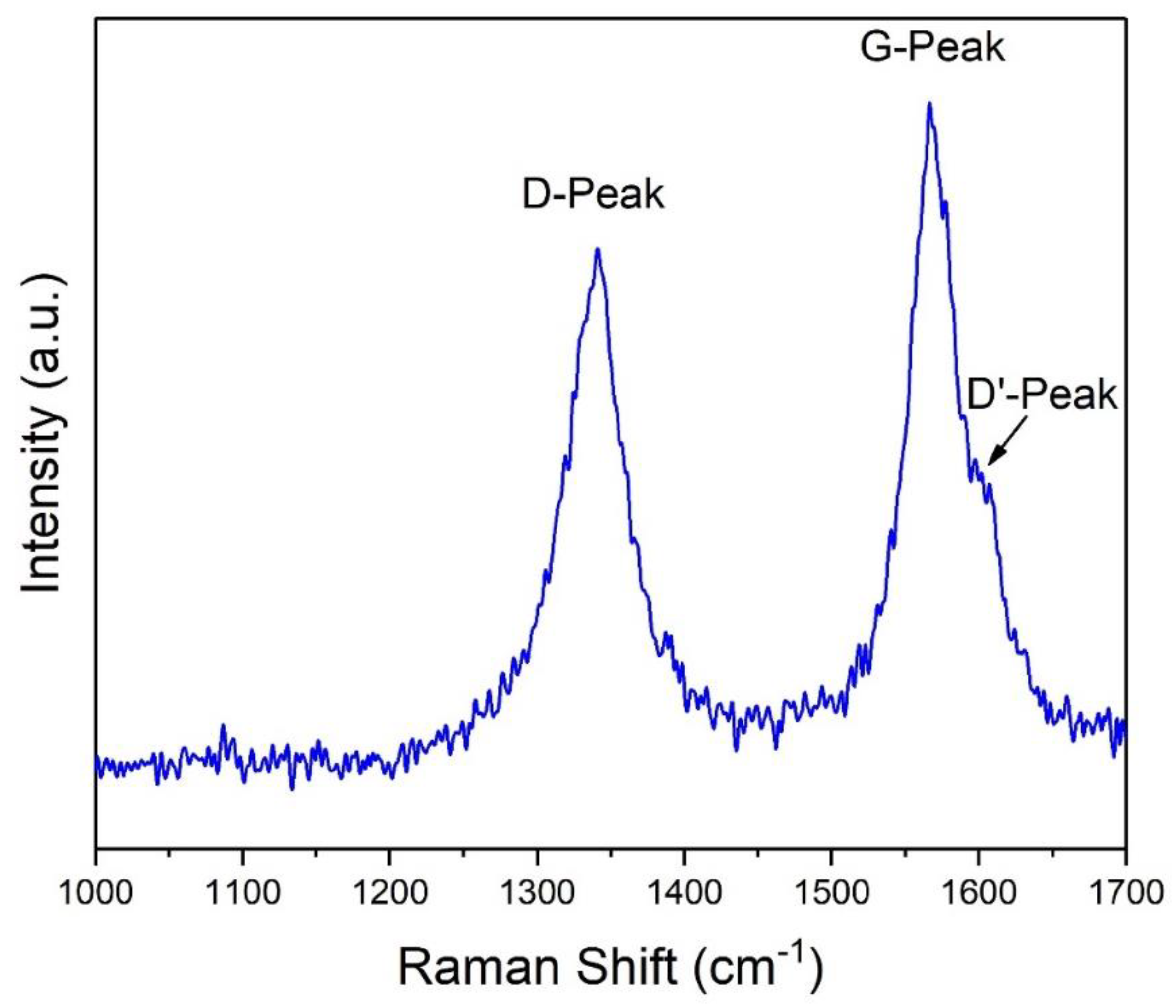
2.1. Carbon Nanotubes Selection and Characterization
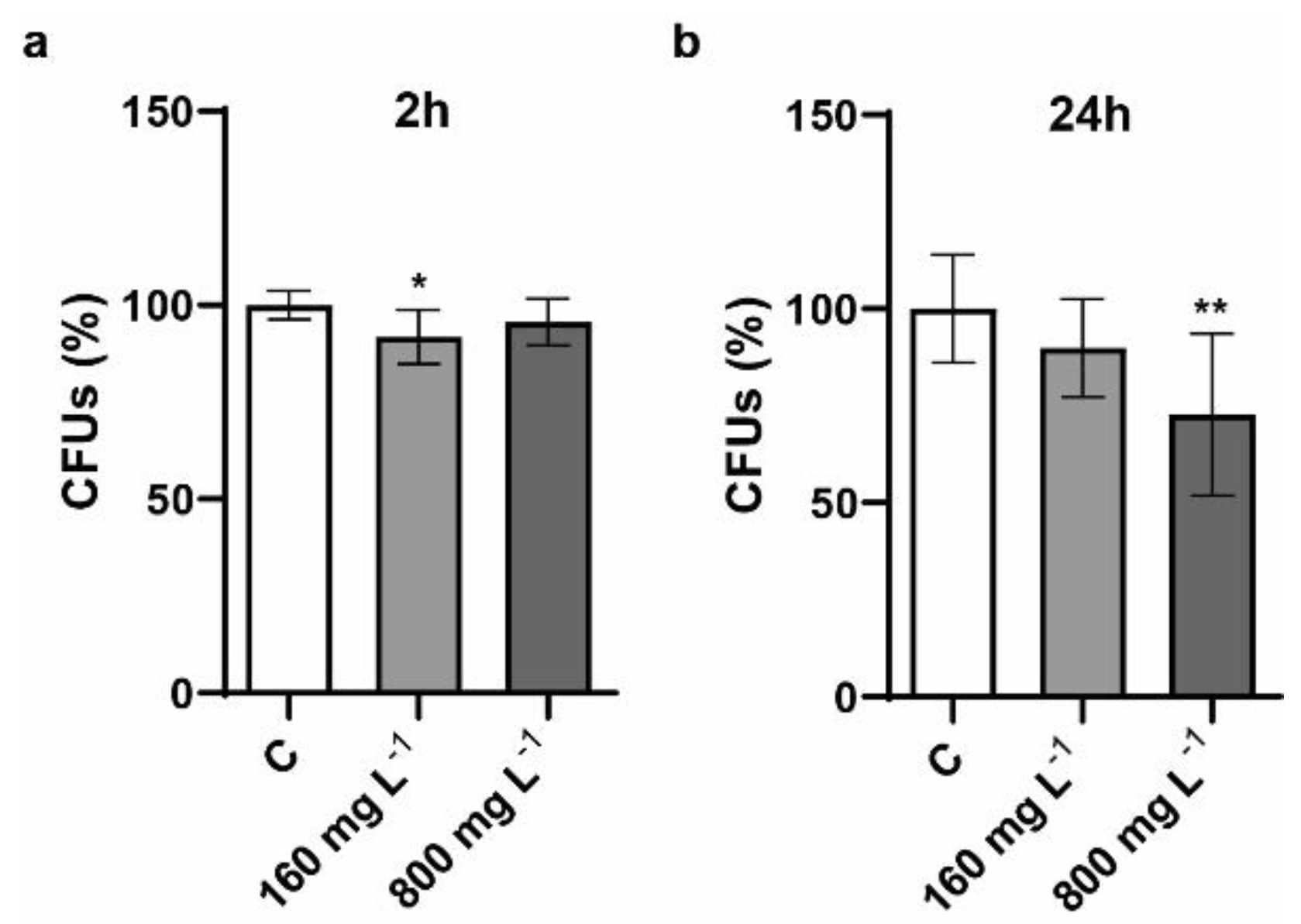
2.2. Determination of Viability of S. cerevisiae Cells Exposed to Different Concentration of CNTs
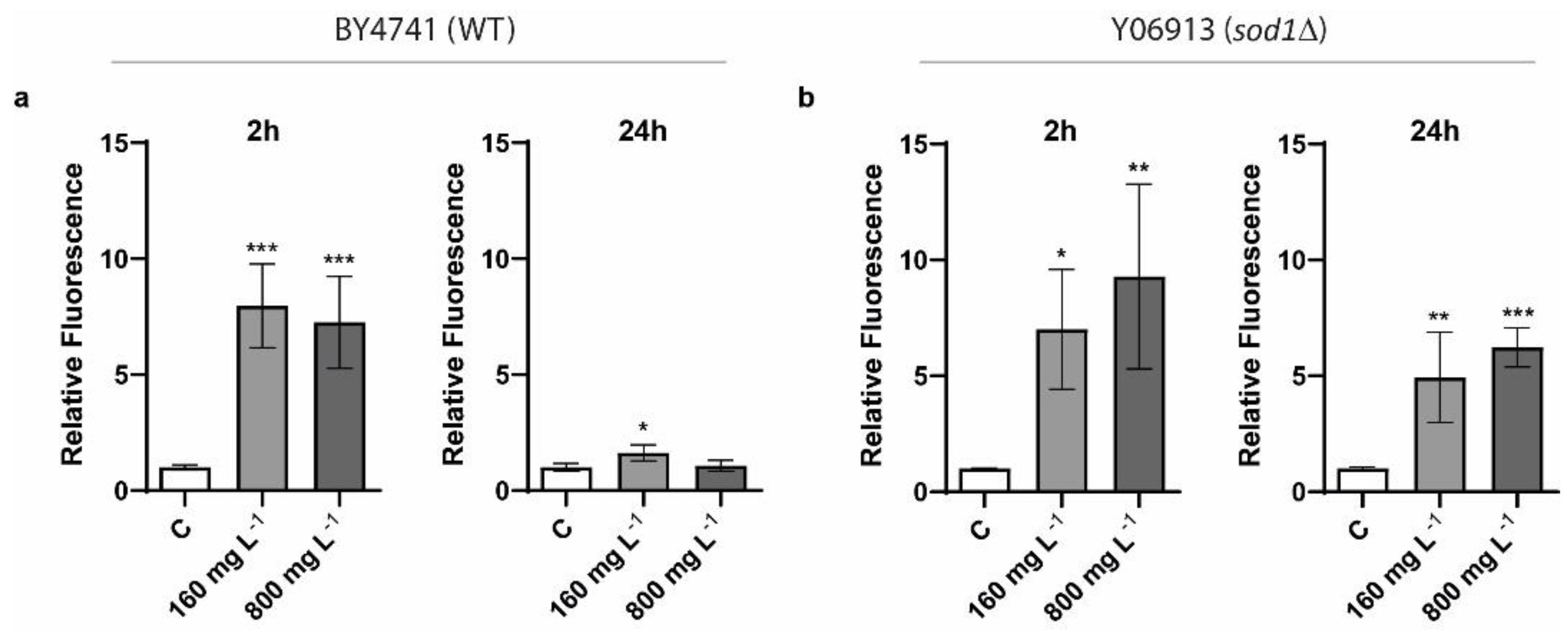
2.3. Determination of Oxidative Stress
2.4. Transcriptional Response of S. cerevisiae Cells to Different CN Concentrations
3. Materials and Methods
3.1. Materials, Reagents and Strains
3.2. ICP-MS Analysis
3.3. Raman Analysis
3.4. Yeast Viability Assays Determination
3.5. ROS Determination
3.6. RNA Isolation, Quality Control and Sequencing
3.7. RNA-Seq Data Processing and Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| TEM RNAseq CNTs MWCNTs DWCNTs | Transition electron microscopy Ribonucleic acid sequencing Carbon nanotubes Multi-walled carbon nanotubes Double-walled carbon nanotubes |
| SWCNTs | Single-walled carbon nanotubes |
| SD CM-H2DCFDA | Standard deviation Chloromethyl 2,7-dichlorofluorescin diacetate |
| ANOVA | One-way analysis of variance |
| RIN | RNA integrity number |
| DPBS | Dulbecco’s phosphate-buffered saline |
References
- Rahman, G.; Najaf, Z.; Mehmood, A.; Bilal, S.; Shah, A.U.H.A.; Mian, S.A.; Ali, G.; Ali, G. An Overview of the Recent Progress in the Synthesis and Applications of Carbon Nanotubes. C 2019, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon nanotube A review on Synthesis, Properties and plethora of applications in the field of biomedical science. Sens. Int. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Pirard, S.L.; Douven, S.; Pirard, J.-P. Large-scale industrial manufacturing of carbon nanotubes in a continuous inclined mobile-bed rotating reactor via the catalytic chemical vapor deposition process. Front. Chem. Sci. Eng. 2017, 11, 280–289. [Google Scholar] [CrossRef]
- Bjorkland, R.; Tobias, D.A.; Petersen, E.J. Increasing evidence indicates low bioaccumulation of carbon nanotubes. Environ. Sci. Nano 2017, 4, 747–766. [Google Scholar] [CrossRef] [Green Version]
- Laux, P.; Riebeling, C.; Booth, A.M.; Brain, J.D.; Brunner, J.; Cerrillo, C.; Creutzenberg, O.; Estrela-Lopis, I.; Gebel, T.; Johanson, G.; et al. Challenges in characterizing the environmental fate and effects of carbon nanotubes and inorganic nanomaterials in aquatic systems. Environ. Sci. Nano 2018, 5, 48–63. [Google Scholar] [CrossRef] [Green Version]
- Liné, C.; Larue, C.; Flahaut, E. Carbon nanotubes: Impacts and behaviour in the terrestrial ecosystem A review. Carbon 2017, 123, 767–785. [Google Scholar] [CrossRef] [Green Version]
- Francis, A.P.; Devasena, T. Toxicity of carbon nanotubes: A review. Toxicol. Ind. Health 2018, 34, 200–210. [Google Scholar] [CrossRef]
- Kobashi, K.; Ata, S.; Yamada, T.; Futaba, D.N.; Okazaki, T.; Hata, K. Classification of Commercialized Carbon Nanotubes into Three General Categories as a Guide for Applications. ACS Appl. Nano Mater. 2019, 2, 4043–4047. [Google Scholar] [CrossRef]
- Simon, J.; Flahaut, E.; Golzio, M. Overview of Carbon Nanotubes for Biomedical Applications. Materials 2019, 12, 624. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Jia, B.; Li, Y.; Ren, B.; Liang, H.; Yan, D.; Xie, H.; Zhang, X.; Liang, H. Effects of multi-walled carbon nanotubes on the enantioselective toxicity of the chiral insecticide indoxacarb toward zebrafish (Danio rerio). J. Hazard. Mater. 2020, 397, 122724. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, M.L.; Hills, C.R.; Miglionico, C.J. Growth of graphite filaments. Carbon 1971, 9, 633–635. [Google Scholar] [CrossRef]
- Patel, D.K.; Kim, H.-B.; Dutta, S.D.; Ganguly, K.; Lim, K.-T. Carbon Nanotubes-Based Nanomaterials and Their Agricultural and Biotechnological Applications. Materials 2020, 13, 1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 1–27. [Google Scholar] [CrossRef]
- Maksimova, Y.G. Microorganisms and Carbon Nanotubes: Interaction and Applications (Review). Appl. Biochem. Microbiol. 2019, 55, 1–12. [Google Scholar] [CrossRef]
- Mortimer, M.; Devarajan, N.; Li, D.; Holden, P.A. Multiwall Carbon Nanotubes Induce More Pronounced Transcriptomic Responses in Pseudomonas aeruginosa PG201 than Graphene, Exfoliated Boron Nitride, or Carbon Black. ACS Nano 2018, 12, 2728–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.; Herzberg, M.; Rodrigues, D.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter. Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef]
- Ming, Z.; Feng, S.; Yilihamu, A.; Yang, S.; Ma, Q.; Yang, H.; Bai, Y.; Yang, S.-T. Toxicity of carbon nanotubes to white rot fungus Phanerochaete chrysosporium. Ecotoxicol. Environ. Saf. 2018, 162, 225–234. [Google Scholar] [CrossRef]
- Gorczyca, A.; Kasprowicz, M.J.; Lemek, T. The physiological effects of multi-walled carbon nanotubes (MWCNTs) on conidia and the development of the entomopathogenic fungus, Metarhizium Anisopliae (Metsch.) Sorok. J. Environ. Sci. Health Part A 2014, 49, 741–752. [Google Scholar] [CrossRef]
- Suarez-Diez, M.; Porras, S.; Teno, F.L.; Schaap, P.; Tamayo-Ramos, J.A. Toxicological response of the model fungus Saccharomyces cerevisiae to different concentrations of commercial graphene nanoplatelets. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Zhu, S.; Luo, F.; Li, J.; Zhu, B.; Wang, G.-X. Biocompatibility assessment of single-walled carbon nanotubes using Saccharomyces cerevisiae as a model organism. J. Nanobiotechnol. 2018, 16, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, C.L.; Yah, C.S.; Iyuke, S.E.; Rumbold, K.; Pillay, V. The cellular response of Saccharomyces cerevisiae to multi-walled carbon nanotubes (MWCNTs). J. Saudi Chem. Soc. 2015, 19, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Zhu, B.; Huang, A.; Hu, Y.; Wang, G.; Ling, F. Toxicological effects of multi-walled carbon nanotubes on Saccharomyces cerevisiae: The uptake kinetics and mechanisms and the toxic responses. J. Hazard. Mater. 2016, 318, 650–662. [Google Scholar] [CrossRef]
- Lee, D.-K.; Jeon, S.; Jeong, J.; Yu, I.J.; Song, K.S.; Kang, A.; Yun, W.S.; Kim, J.S.; Cho, W.-S. Potential Role of Soluble Metal Impurities in the Acute Lung Inflammogenicity of Multi-Walled Carbon Nanotubes. Nanomaterials 2020, 10, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pensabene, V.; Vittorio, O.; Raffa, V.; Ziaei, A.; Menciassi, A.; Dario, P. Neuroblastoma Cells Displacement by Magnetic Carbon Nanotubes. IEEE Trans. Nano Biosci. 2008, 7, 105–110. [Google Scholar] [CrossRef]
- Emmenegger, C.; Bonard, J.-M.; Mauron, P.; Sudan, P.; Lepora, A.; Grobety, B.; Züttel, A.; Schlapbach, L. Synthesis of carbon nanotubes over Fe catalyst on aluminium and suggested growth mechanism. Carbon 2003, 41, 539–547. [Google Scholar] [CrossRef]
- Moon, S.Y.; Kang, I.J.; Kim, S.M.; Kim, W.S. Influence of the Sulfur Content Catalyst on the Packing Density of Carbon Nanotube Forests. Nanomaterials 2019, 9, 889. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Zheng, C.; Hou, X.; Wang, S. Photochemical vapor generation for removing nickel impurities from carbon nanotubes and its real-time monitoring by atomic fluorescence spectrometry. Microchem. J. 2014, 117, 83–88. [Google Scholar] [CrossRef]
- Vittorio, O.; Raffa, V.; Cuschieri, A. Influence of purity and surface oxidation on cytotoxicity of multiwalled carbon nanotubes with human neuroblastoma cells. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 424–431. [Google Scholar] [CrossRef]
- Jorio, A.; Pimenta, M.A.; Filho, A.G.S.; Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Characterizing carbon nanotube samples with resonance Raman scattering. New J. Phys. 2003, 5, 139. [Google Scholar] [CrossRef]
- Dileo, R.A.; Landi, B.J.; Raffaelle, R.P. Purity assessment of multiwalled carbon nanotubes by Raman spectroscopy. J. Appl. Phys. 2007, 101, 064307. [Google Scholar] [CrossRef] [Green Version]
- Requardt, H.; Braun, A.; Steinberg, P.; Hampel, S.; Hansen, T. Surface defects reduce Carbon Nanotube toxicity in vitro. Toxicol. In Vitro 2019, 60, 12–18. [Google Scholar] [CrossRef]
- Zhao, X.; Ando, Y.; Qin, L.-C.; Kataura, H.; Maniwa, Y.; Saito, R. Multiple splitting of G-band modes from individual multiwalled carbon nanotubes. Appl. Phys. Lett. 2002, 81, 2550–2552. [Google Scholar] [CrossRef]
- Murphy, H.; Papakonstantinou, P.; Okpalugo, T.I.T. Raman study of multiwalled carbon nanotubes functionalized with oxygen groups. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2006, 24, 715. [Google Scholar] [CrossRef] [Green Version]
- Malard, L.; Pimenta, M.; Dresselhaus, G.; Dresselhaus, M. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Badri, M.A.S.; Salleh, M.M.; Noor, N.F.M.; Rahman, M.Y.A.; Umar, A.A. Green synthesis of few-layered graphene from aqueous processed graphite exfoliation for graphene thin film preparation. Mater. Chem. Phys. 2017, 193, 212–219. [Google Scholar] [CrossRef]
- Wroblewska, A.; Dużyńska, A.; Judek, J.; Stobinski, L.; Żerańska-Chudek, K.; Gertych, A.; Zdrojek, M. Statistical analysis of the reduction process of graphene oxide probed by Raman spectroscopy mapping. J. Phys. Condens. Matter 2017, 29, 475201. [Google Scholar] [CrossRef] [PubMed]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holden, P.A.; Gardea-Torresdey, J.L.; Klaessig, F.; Turco, R.F.; Mortimer, M.; Hund-Rinke, K.; Cohen Hubal, E.A.; Avery, D.; Barceló, D.; Behra, R.; et al. Considerations of Environmentally Relevant Test Conditions for Improved Evaluation of Eco-logical Hazards of Engineered Nanomaterials. Environ. Sci. Technol. 2016, 50, 6124–6145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domi, B.; Rumbo, C.; García-Tojal, J.; Sima, L.E.; Negroiu, G.; Tamayo-Ramos, J.A. Interaction Analysis of Commercial Graphene Oxide Nanoparticles with Unicellular Systems and Biomolecules. Int. J. Mol. Sci. 2019, 21, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domi, B.; Bhorkar, K.; Rumbo, C.; Sygellou, L.; Yannopoulos, S.N.; Barros, R.; Quesada, R.; Tamayo-Ramos, J.A. Assessment of Physico-Chemical and Toxicological Properties of Commercial 2D Boron Nitride Nanopowder and Nanoplatelets. Int. J. Mol. Sci. 2021, 22, 567. [Google Scholar] [CrossRef]
- Laguna-Teno, F.; Suarez-Diez, M.; Tamayo-Ramos, J.A. Commonalities and Differences in the Transcriptional Response of the Model Fungus Saccharomyces cerevisiae to Different Commercial Graphene Oxide Materials. Front. Microbiol. 2020, 11, 1943. [Google Scholar] [CrossRef] [PubMed]
- Domi, B.; Bhorkar, K.; Rumbo, C.; Sygellou, L.; Martin, S.M.; Quesada, R.; Yannopoulos, S.N.; Tamayo-Ramos, J.A. Toxicological assessment of commercial monolayer tungsten disulfide nanomaterials aqueous suspensions using human A549 cells and the model fungus Saccharomyces cerevisiae. Chemosphere 2021, 272, 129603. [Google Scholar] [CrossRef] [PubMed]
- Domi, B.; Bhorkar, K.; Rumbo, C.; Sygellou, L.; Yannopoulos, S.N.; Quesada, R.; Tamayo-Ramos, J.A. Fate assessment of commercial 2D MoS2 aqueous dispersions at physicochemical and toxicological level. Nanotechnology 2020, 31, 445101. [Google Scholar] [CrossRef] [PubMed]
- Olivi, M.; Zanni, E.; De Bellis, G.; Talora, C.; Sarto, M.S.; Palleschi, C.; Flahaut, E.; Monthioux, M.; Rapino, S.; Uccelletti, D.; et al. Inhibition of microbial growth by carbon nanotube networks. Nanoscale 2013, 5, 9023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Park, I.S.; Lee, S.J.; Bae, T.S.; Watari, F.; Uo, M.; Lee, M.H. Aqueous dispersion of surfactant-modified multiwalled carbon nanotubes and their application as an antibacterial agent. Carbon 2011, 49, 3663–3671. [Google Scholar] [CrossRef]
- Jackson, P.; Jacobsen, N.R.; Baun, A.; Birkedal, R.; Kühnel, D.; Jensen, K.A.; Vogel, U.; Wallin, H. Bioaccumulation and ecotoxicity of carbon nanotubes. Chem. Centr. J. 2013, 7, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Schurch, N.J.; Schofield, P.; Gierliński, M.; Cole, C.; Sherstnev, A.; Singh, V.; Wrobel, N.; Gharbi, K.; Simpson, G.G.; Owen-Hughes, T.; et al. How many biological replicates are needed in an RNA-seq experiment and which differential expression tool should you use? RNA 2016, 22, 839–851. [Google Scholar] [CrossRef] [Green Version]
- I Love, M.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Zhang, B.; Li, J.; Du, T.; Yi, X.; Li, M.; Chen, W.; Alvarez, P.J.J. Graphene oxide significantly inhibits cell growth at sublethal concentrations by causing extracellular iron deficiency. Nanotoxicology 2017, 11, 1102–1114. [Google Scholar] [CrossRef]
- Gross, C.; Kelleher, M.; Iyer, V.R.; Brown, P.O.; Winge, D.R. Identification of the Copper Regulon in Saccharomyces cerevisiae by DNA Microarrays. J. Biol. Chem. 2000, 275, 32310–32316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozcan, S.; Johnston, M. Three different regulatory mechanisms enable yeast hexose transporter (HXT) genes to be induced by different levels of glucose. Mol. Cell. Biol. 1995, 15, 1564–1572. [Google Scholar] [CrossRef] [Green Version]
- Tkach, J.M.; Yimit, A.; Lee, A.Y.; Riffle, M.; Costanzo, M.; Jaschob, D.; Hendry, J.A.; Ou, J.; Moffat, J.; Boone, C.; et al. Dissecting DNA damage response pathways by analysing protein localization and abundance changes during DNA replication stress. Nature 2012, 14, 966–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, S.; Nevo, E.; Grishkan, I.; Idleman, U.; Weinberg, D.; Bohnert, H.J. Oxidative Stress Responses in Yeast Strains, Saccharomyces cerevisiae, from “Evolution Canyon”, Israel. Monatshefte fur Chemie 2003, 134, 1465–1480. [Google Scholar] [CrossRef]
- Yu, L.; Guo, N.; Yang, Y.; Wu, X.; Meng, R.; Fan, J.; Ge, F.; Wang, X.; Liu, J.; Deng, X. Microarray analysis of p-anisaldehyde-induced transcriptome of Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2009, 37, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Soontorngun, N. Reprogramming of nonfermentative metabolism by stress-responsive transcription factors in the yeast Saccharomyces cerevisiae. Curr. Genet. 2016, 63, 1–7. [Google Scholar] [CrossRef]
- Bereketoglu, C.; Arga, K.Y.; Eraslan, S.; Mertoglu, B. Genome reprogramming in Saccharomyces Cerevisiaeupon nonylphenol exposure. Physiol. Genom. 2017, 49, 549–566. [Google Scholar] [CrossRef] [Green Version]
- Bergkessel, M.; Whitworth, G.B.; Guthrie, C. Diverse environmental stresses elicit distinct responses at the level of pre-mRNA processing in yeast. RNA 2011, 17, 1461–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, J.; Fiji, N.; Shihabudeen, M.S.; Chattopadhyay, D.; Roy, D.; Mg, D.A.; Thirumurugan, K. A rapid method to assess reactive oxygen species in yeast using H 2 DCF-DA. Anal. Methods 2015, 7, 8572–8575. [Google Scholar] [CrossRef]
- Pérez-Rubio, P.; Lottaz, C.; Engelmann, J.C. FastqPuri: High-performance preprocessing of RNA-seq data. BMC Bioinform. 2019, 20, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2012, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2013, 30, 923–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.M.; Hong, E.L.; Amundsen, C.; Balakrishnan, R.; Binkley, G.; Chan, E.; Christie, K.; Costanzo, M.; Dwight, S.S.; Engel, S.; et al. Saccharomyces Genome Database: The genomics resource of budding yeast. Nucleic Acids Res. 2011, 40, D700–D705. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| ppm | |
|---|---|
| Al | 8494.29 ± 2523.60 |
| P | 7.23 ± 2.05 |
| S | 1330.69 ± 45.04 |
| K | 83.26 ± 16.93 |
| V | 0.04 ± 0.01 |
| Cr | 9.35 ± 2.40 |
| Mn | 4.30 ± 1.44 |
| Fe | 18,986.00 ± 5359.66 |
| Co | 0.06 ± 0.02 |
| Ni | 0.78 ± 0.09 |
| Cu | 1.64 ± 0.33 |
| Rb | <0.001 |
| Sr | <0.001 |
| Zn | 0.02 ± 0.02 |
| Nb | 0.24 ± 0.07 |
| Mo | 4.97 ± 1.38 |
| Ba | <0.001 |
| Ce | 0.0016 ± 0.0030 |
| Pr | <0.001 |
| Nd | <0.001 |
| W | <0.001 |
| Pb | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martel Martín, S.; Barros, R.; Domi, B.; Rumbo, C.; Poddighe, M.; Aparicio, S.; Suarez-Diez, M.; Tamayo-Ramos, J.A. Low Toxicological Impact of Commercial Pristine Multi-Walled Carbon Nanotubes on the Yeast Saccharomyces cerevisiae. Nanomaterials 2021, 11, 2272. https://doi.org/10.3390/nano11092272
Martel Martín S, Barros R, Domi B, Rumbo C, Poddighe M, Aparicio S, Suarez-Diez M, Tamayo-Ramos JA. Low Toxicological Impact of Commercial Pristine Multi-Walled Carbon Nanotubes on the Yeast Saccharomyces cerevisiae. Nanomaterials. 2021; 11(9):2272. https://doi.org/10.3390/nano11092272
Chicago/Turabian StyleMartel Martín, Sonia, Rocío Barros, Brixhilda Domi, Carlos Rumbo, Matteo Poddighe, Santiago Aparicio, Maria Suarez-Diez, and Juan Antonio Tamayo-Ramos. 2021. "Low Toxicological Impact of Commercial Pristine Multi-Walled Carbon Nanotubes on the Yeast Saccharomyces cerevisiae" Nanomaterials 11, no. 9: 2272. https://doi.org/10.3390/nano11092272
APA StyleMartel Martín, S., Barros, R., Domi, B., Rumbo, C., Poddighe, M., Aparicio, S., Suarez-Diez, M., & Tamayo-Ramos, J. A. (2021). Low Toxicological Impact of Commercial Pristine Multi-Walled Carbon Nanotubes on the Yeast Saccharomyces cerevisiae. Nanomaterials, 11(9), 2272. https://doi.org/10.3390/nano11092272







