Experimental and Theoretical Study of Sorption Capacity of Hexagonal Boron Nitride Nanoparticles: Implication for Wastewater Purification from Antibiotics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of BN Nanoparticles
2.2. Structural Characterization of BNNPs
2.3. UV-Vis-Spectrophotometry
2.4. Adsorption Studies
- -
- Tetracycline: 1 tablet contains the active substance 100 mg of tetracycline hydrochloride;
- -
- Bicillin: powder for suspension for intramuscular injection contains benzathine benzylpenicillin (80%) and benzylpenicillin procaine (20%);
- -
- Ciprofloxacin: 1 tablet contains ciprofloxacin monohydrate (72%), microcrystalline cellulose, corn starch, povidone-CZO, magnesium stearate, polyvinyl alcohol, titanium dioxide, macrogol, and talc.
2.5. BNNP Purification from Adsorbed Antibiotics
- Method 1:
- Purification in acetonitrile solution (1:6 by volume), acetate buffer solution pH 4.4 (1:6 by volume) and ethanol (4:6 by volume);
- Method 2:
- Purification in pH 4.4 acetate buffer solution;
- Method 3:
- Purification in ethanol solution (1:2 by volume) and acetonitrile (1:2 by volume).
2.6. Sorption Kinetic Analysis
2.7. DFT Calculations of Antibiotic Adsorption on BN Surface
3. Results and Discussion
3.1. Characterization of BN Nanoparticles
3.2. Interaction Mechanisms of Antibiotic Molecules with BN Surface: Theoretical Insight
3.3. Kinetics of Antibiotic Adsorption on BNNPs
3.4. BNNP Purification Strategy from Adsorbed Antibiotics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kerrigan, J.F.; Sandberg, K.D.; Engstrom, D.R.; LaPara, T.M.; Arnold, W.A. Small and Large-Scale Distribution of Four Classes of Antibiotics in Sediment: Association with Metals and Antibiotic Resistance Genes. Environ. Sci. Process. Impacts 2018, 20, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- McConnell, M.M.; Truelstrup Hansen, L.; Jamieson, R.C.; Neudorf, K.D.; Yost, C.K.; Tong, A. Removal of Antibiotic Resistance Genes in Two Tertiary Level Municipal Wastewater Treatment Plants. Sci. Total Environ. 2018, 643, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Tototzintle, M.; Ferreira, I.J.; da Silva Duque, S.; Guimarães Barrocas, P.R.; Saggioro, E.M. Removal of Contaminants of Emerging Concern (CECs) and Antibiotic Resistant Bacteria in Urban Wastewater Using UVA/TiO2/H2O2 Photocatalysis. Chemosphere 2018, 210, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.T.; Moreau-Guigon, E.; Labadie, P.; Alliot, F.; Teil, M.-J.; Blanchard, M.; Chevreuil, M. Occurrence of Antibiotics in Rural Catchments. Chemosphere 2017, 168, 483–490. [Google Scholar] [CrossRef]
- Karthikeyan, K.G.; Meyer, M.T. Occurrence of Antibiotics in Wastewater Treatment Facilities in Wisconsin, USA. Sci. Total Environ. 2006, 361, 196–207. [Google Scholar] [CrossRef]
- Siedlewicz, G.; Białk-Bielińska, A.; Borecka, M.; Winogradow, A.; Stepnowski, P.; Pazdro, K. Presence, Concentrations and Risk Assessment of Selected Antibiotic Residues in Sediments and near-Bottom Waters Collected from the Polish Coastal Zone in the Southern Baltic Sea—Summary of 3years of Studies. Mar. Pollut. Bull. 2018, 129, 787–801. [Google Scholar] [CrossRef]
- Dong, D.; Zhang, L.; Liu, S.; Guo, Z.; Hua, X. Antibiotics in Water and Sediments from Liao River in Jilin Province, China: Occurrence, Distribution, and Risk Assessment. Environ. Earth Sci. 2016, 75, 1202. [Google Scholar] [CrossRef]
- Antibiotics Found in Some of the World’s Rivers Exceed ‘Safe’ Levels, Global Study Finds. Available online: https://www.york.ac.uk/news-and-events/news/2019/research/antibiotics-found-in-some-of-worlds-rivers/ (accessed on 3 March 2021).
- Yang, X.; Chen, Z.; Zhao, W.; Liu, C.; Qian, X.; Zhang, M.; Wei, G.; Khan, E.; Hau Ng, Y.; Sik Ok, Y. Recent Advances in Photodegradation of Antibiotic Residues in Water. Chem. Eng. J. 2021, 405, 126806. [Google Scholar] [CrossRef]
- Akyon, B.; McLaughlin, M.; Hernández, F.; Blotevogel, J.; Bibby, K. Characterization and Biological Removal of Organic Compounds from Hydraulic Fracturing Produced Water. Environ. Sci. Process. Impacts 2019, 21, 279–290. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, H.; Ma, J.; Sun, W.; Yang, Y.; Zhang, X. Compost-Bulking Agents Reduce the Reservoir of Antibiotics and Antibiotic Resistance Genes in Manures by Modifying Bacterial Microbiota. Sci. Total Environ. 2019, 649, 396–404. [Google Scholar] [CrossRef]
- de Souza Santos, L.V.; Meireles, A.M.; Lange, L.C. Degradation of Antibiotics Norfloxacin by Fenton, UV and UV/H2O2. J. Environ. Manag. 2015, 154, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Han, L.; Yin, X.; Li, H.; Fang, D.; Hong, G. Three Dimensional Functionalized Carbon/Tin(IV) Sulfide Biofoam for Photocatalytical Purification of Chromium(VI)-Containing Wastewater. ACS Sustain. Chem. Eng. 2018, 6, 10660–10667. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, Y.-J.; He, G.; Li, H.; Tao, J.-C. Multifunctional Photocatalytic Filter Paper Based on Ultralong Nanowires of the Calcium-Alendronate Complex for High-Performance Water Purification. ACS Appl. Mater. Interfaces 2022, 14, 9464–9479. [Google Scholar] [CrossRef] [PubMed]
- Alagha, O.; Ouerfelli, N.; Kochkar, H.; Almessiere, M.A.; Slimani, Y.; Manikandan, A.; Baykal, A.; Mostafa, A.; Zubair, M.; Barghouthi, M.H. Kinetic Modeling for Photo-Assisted Penicillin G Degradation of (Mn0.5Zn0.5)[CdxFe2-x]O4 (x ≤ 0.05) Nanospinel Ferrites. Nanomaterials 2021, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Asthana, A.; Singh, A.K.; Chakraborty, R.; Vidya, S.S.; Singh, A.; Carabineiro, S.A.C. Methionine-Functionalized Graphene Oxide/Sodium Alginate Bio-Polymer Nanocomposite Hydrogel Beads: Synthesis, Isotherm and Kinetic Studies for an Adsorptive Removal of Fluoroquinolone Antibiotics. Nanomaterials 2021, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-X.; Song, S.; Ye, M.-L.; Zhu, Y.; Zhao, Y.-G.; Lu, Y. Nanomaterials with Excellent Adsorption Characteristics for Sample Pretreatment: A Review. Nanomaterials 2022, 12, 1845. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yin, R.; Zeng, L.; Zhu, M. A Review of Graphene-Based Nanomaterials for Removal of Antibiotics from Aqueous Environments. Environ. Pollut. 2019, 253, 100–110. [Google Scholar] [CrossRef]
- Tang, H.; Li, W.; Jiang, H.; Lin, R.; Wang, Z.; Wu, J.; He, G.; Shearing, P.R.; Brett, D.J.L. ZIF-8-Derived Hollow Carbon for Efficient Adsorption of Antibiotics. Nanomaterials 2019, 9, 117. [Google Scholar] [CrossRef]
- Bao, J.; Zhu, Y.; Yuan, S.; Wang, F.; Tang, H.; Bao, Z.; Zhou, H.; Chen, Y. Adsorption of Tetracycline with Reduced Graphene Oxide Decorated with MnFe2O4 Nanoparticles. Nanoscale Res. Lett. 2018, 13, 396. [Google Scholar] [CrossRef]
- Zeidman, A.B.; Rodriguez-Narvaez, O.M.; Moon, J.; Bandala, E.R. Removal of Antibiotics in Aqueous Phase Using Silica-Based Immobilized Nanomaterials: A Review. Environ. Technol. Innov. 2020, 20, 101030. [Google Scholar] [CrossRef]
- Masoudi, F.; Kamranifar, M.; Safari, F.; Naghizadeh, A. Mechanism, Kinetics and Thermodynamic of Penicillin G Antibiotic Removal by Silica Nanoparticles from Simulated Hospital Wastewater. Desalination Water Treat. 2019, 169, 333–341. [Google Scholar] [CrossRef]
- Li, Y.; Gutiérrez Moreno, J.J.; Song, Z.; Liu, D.; Wang, M.; Ramiere, A.; Feng, Z.; Niu, Q.J.; Sasaki, T.; Cai, X. Controlled Synthesis of Perforated Oxide Nanosheets with High Density Nanopores Showing Superior Water Purification Performance. ACS Appl. Mater. Interfaces 2022, 14, 18513–18524. [Google Scholar] [CrossRef] [PubMed]
- Sturini, M.; Puscalau, C.; Guerra, G.; Maraschi, F.; Bruni, G.; Monteforte, F.; Profumo, A.; Capsoni, D. Combined Layer-by-Layer/Hydrothermal Synthesis of Fe3O4@MIL-100(Fe) for Ofloxacin Adsorption from Environmental Waters. Nanomaterials 2021, 11, 3275. [Google Scholar] [CrossRef]
- Sun, T.; Fan, R.; Zhang, J.; Qin, M.; Chen, W.; Jiang, X.; Zhu, K.; Ji, C.; Hao, S.; Yang, Y. Stimuli-Responsive Metal–Organic Framework on a Metal–Organic Framework Heterostructure for Efficient Antibiotic Detection and Anticounterfeiting. ACS Appl. Mater. Interfaces 2021, 13, 35689–35699. [Google Scholar] [CrossRef]
- Dehghan, A.; Mohammadi, A.A.; Yousefi, M.; Najafpoor, A.A.; Shams, M.; Rezania, S. Enhanced Kinetic Removal of Ciprofloxacin onto Metal-Organic Frameworks by Sonication, Process Optimization and Metal Leaching Study. Nanomaterials 2019, 9, 1422. [Google Scholar] [CrossRef]
- Tang, C.; Bando, Y.; Shen, G.; Zhi, C.; Golberg, D. Single-Source Precursor for Chemical Vapour Deposition of Collapsed Boron Nitride Nanotubes. Nanotechnology 2006, 17, 5882–5888. [Google Scholar] [CrossRef]
- Dai, P.; Xue, Y.; Wang, X.; Weng, Q.; Zhang, C.; Jiang, X.; Tang, D.; Wang, X.; Kawamoto, N.; Ide, Y.; et al. Pollutant Capturing SERS Substrate: Porous Boron Nitride Microfibers with Uniform Silver Nanoparticle Decoration. Nanoscale 2015, 7, 18992–18997. [Google Scholar] [CrossRef]
- Gudz, K.Y.; Permyakova, E.S.; Matveev, A.T.; Bondarev, A.V.; Manakhov, A.M.; Sidorenko, D.A.; Filippovich, S.Y.; Brouchkov, A.V.; Golberg, D.V.; Ignatov, S.G.; et al. Pristine and Antibiotic-Loaded Nanosheets/Nanoneedles-Based Boron Nitride Films as a Promising Platform to Suppress Bacterial and Fungal Infections. ACS Appl. Mater. Interfaces 2020, 12, 42485–42498. [Google Scholar] [CrossRef]
- Zhang, X.; Lian, G.; Zhang, S.; Cui, D.; Wang, Q. Boron Nitride Nanocarpets: Controllable Synthesis and Their Adsorption Performance to Organic Pollutants. CrystEngComm 2012, 14, 4670–4676. [Google Scholar] [CrossRef]
- Portehault, D.; Giordano, C.; Gervais, C.; Senkovska, I.; Kaskel, S.; Sanchez, C.; Antonietti, M. High-Surface-Area Nanoporous Boron Carbon Nitrides for Hydrogen Storage. Adv. Funct. Mater. 2010, 20, 1827–1833. [Google Scholar] [CrossRef]
- Xue, L.; Lu, B.; Wu, Z.-S.; Ge, C.; Wang, P.; Zhang, R.; Zhang, X.-D. Synthesis of Mesoporous Hexagonal Boron Nitride Fibers with High Surface Area for Efficient Removal of Organic Pollutants. Chem. Eng. J. 2014, 243, 494–499. [Google Scholar] [CrossRef]
- Li, Q.; Yang, T.; Yang, Q.; Wang, F.; Chou, K.-C.; Hou, X. Porous Hexagonal Boron Nitride Whiskers Fabricated at Low Temperature for Effective Removal of Organic Pollutants from Water. Ceram. Int. 2016, 42, 8754–8762. [Google Scholar] [CrossRef]
- Maiti, K.; Thanh, T.D.; Sharma, K.; Hui, D.; Kim, N.H.; Lee, J.H. Highly Efficient Adsorbent Based on Novel Cotton Flower-like Porous Boron Nitride for Organic Pollutant Removal. Compos. Part B Eng. 2017, 123, 45–54. [Google Scholar] [CrossRef]
- Wang, J.; Hao, J.; Liu, D.; Qin, S.; Chen, C.; Yang, C.; Liu, Y.; Yang, T.; Fan, Y.; Chen, Y.; et al. Flower Stamen-like Porous Boron Carbon Nitride Nanoscrolls for Water Cleaning. Nanoscale 2017, 9, 9787–9791. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Zhang, J.; Hanagata, N.; Wang, X.; Weng, Q.; Ito, A.; Bando, Y.; Golberg, D. Hollow Boron Nitride Nanospheres as Boron Reservoir for Prostate Cancer Treatment. Nat. Commun. 2017, 8, 13936. [Google Scholar] [CrossRef]
- Abramova, A.A.; Isakov, V.G.; Nepogodin, A.M.; Grakhova, E.V.; Dyagelev, M.Y. Classification of Antibiotics Contained in Urban Wastewater. IOP Conf. Ser. Earth Environ. Sci. 2020, 548, 052078. [Google Scholar] [CrossRef]
- Kovalskii, A.M.; Matveev, A.T.; Lebedev, O.I.; Sukhorukova, I.V.; Firestein, K.L.; Steinman, A.E.; Shtansky, D.V.; Golberg, D. Growth of Spherical Boron Oxynitride Nanoparticles with Smooth and Petalled Surfaces during a Chemical Vapour Deposition Process. CrystEngComm 2016, 18, 6689–6699. [Google Scholar] [CrossRef]
- Gudz, K.Y.; Antipina, L.Y.; Permyakova, E.S.; Kovalskii, A.M.; Konopatsky, A.S.; Filippovich, S.Y.; Dyatlov, I.A.; Slukin, P.V.; Ignatov, S.G.; Shtansky, D.V. Ag-Doped and Antibiotic-Loaded Hexagonal Boron Nitride Nanoparticles as Promising Carriers to Fight Different Pathogens. ACS Appl. Mater. Interfaces 2021, 13, 23452–23468. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Sheelarani, B.; Joshi, R.G.; Dash, S. Solubilization and Interaction of Ciprofloxacin with Pluronics and Their Mixed Micelles. New J. Chem. 2019, 43, 16530–16537. [Google Scholar] [CrossRef]
- Parolo, M.E.; Savini, M.C.; Vallés, J.M.; Baschini, M.T.; Avena, M.J. Tetracycline Adsorption on Montmorillonite: PH and Ionic Strength Effects. Appl. Clay Sci. 2008, 40, 179–186. [Google Scholar] [CrossRef]
- Berkani, M.; Smaali, A.; Kadmi, Y.; Almomani, F.; Vasseghian, Y.; Lakhdari, N.; Alyane, M. Photocatalytic Degradation of Penicillin G in Aqueous Solutions: Kinetic, Degradation Pathway, and Microbioassays Assessment. J. Hazard. Mater. 2022, 421, 126719. [Google Scholar] [CrossRef] [PubMed]
- Chavoshan, S.; Khodadadi, M.; Nasseh, N. Photocatalytic Degradation of Penicillin G from Simulated Wastewater Using the UV/ZnO Process: Isotherm and Kinetic Study. J. Environ. Health Sci. Eng. 2020, 18, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gong, H.; Liu, W.; Lu, B.; Ye, L. Separation and Recycling of Functional Nanoparticles Using Reversible Boronate Ester and Boroxine Bonds. Ind. Eng. Chem. Res. 2019, 58, 4695–4703. [Google Scholar] [CrossRef]
- Mohseni-Bandpi, A.; Al-Musawi, T.J.; Ghahramani, E.; Zarrabi, M.; Mohebi, S.; Vahed, S.A. Improvement of Zeolite Adsorption Capacity for Cephalexin by Coating with Magnetic Fe3O4 Nanoparticles. J. Mol. Liq. 2016, 218, 615–624. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, 864–871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, 1133–1138. [Google Scholar] [CrossRef]
- Troullier, N.; Martins, J.L. Efficient Pseudopotentials for Plane-Wave Calculations. Phys. Rev. B Condens. Matter 1991, 43, 1993–2006. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-Type Density Functional Constructed with a Long-Range Dispersion Correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Moon, O.M.; Kang, B.C.; Lee, S.B.; Boo, J.H. Temperature Effect on Structural Properties of Boron Oxide Thin Films Deposited by MOCVD Method. Thin Solid Film. 2004, 464–465, 164. [Google Scholar] [CrossRef]
- Murei, A.; Ayinde, W.B.; Gitari, M.W.; Samie, A. Functionalization and Antimicrobial Evaluation of Ampicillin, Penicillin and Vancomycin with Pyrenacantha Grandiflora Baill and Silver Nanoparticles. Sci. Rep. 2020, 10, 11596. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.K.; Patil, S.; Shettigar, H.; Bairwa, K.; Jana, S. Spectroscopic Characterization of Chloramphenicol and Tetracycline: An Impact of Biofield Treatment. Pharm. Anal. Acta 2015, 6, 395. [Google Scholar] [CrossRef]
- Sahoo, S.; Chakraborti, C.K.; Mishra, S.C. Qualitative Analysis of Controlled Release Ciprofloxacin/Carbopol 934 Mucoadhesive Suspension. J. Adv. Pharm. Technol. Res. 2011, 2, 195–204. [Google Scholar] [CrossRef]
- Rahdar, S.; Rahdar, A.; Khodadadi, M.; Ahmadi, S. Error Analysis of Adsorption Isotherm Models for Penicillin G onto Magnesium Oxide Nanoparticles. Appl. Water Sci. 2019, 9, 190. [Google Scholar] [CrossRef]
- Rakshit, S.; Sarkar, D.; Elzinga, E.J.; Punamiya, P.; Datta, R. Mechanisms of Ciprofloxacin Removal by Nano-Sized Magnetite. J. Hazard. Mater. 2013, 246–247, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.A.; Kareem, S.L. Adsorption of Tetracycline Fom Wastewater by Using Pistachio Shell Coated with ZnO Nanoparticles: Equilibrium, Kinetic and Isotherm Studies. Alex. Eng. J. 2019, 58, 917–928. [Google Scholar] [CrossRef]
- Balarak, D.; Mostafapour, F.K.; Joghataei, A. Experimental and Kinetic Studies on Penicillin G Adsorption by Lemna Minor. J. Pharm. Res. Int. 2016, 9, 1–10. [Google Scholar] [CrossRef]
- Danalıoğlu, S.T.; Bayazit, Ş.S.; Kerkez Kuyumcu, Ö.; Salam, M.A. Efficient Removal of Antibiotics by a Novel Magnetic Adsorbent: Magnetic Activated Carbon/Chitosan (MACC) Nanocomposite. J. Mol. Liq. 2017, 240, 589–596. [Google Scholar] [CrossRef]
- Soori, M.M.; Ghahramani, E.; Kazemian, H.; Al-Musawi, T.J.; Zarrabi, M. Intercalation of Tetracycline in Nano Sheet Layered Double Hydroxide: An Insight into UV/VIS Spectra Analysis. J. Taiwan Inst. Chem. Eng. 2016, 63, 271–285. [Google Scholar] [CrossRef]
- Kamranifar, M.; Allahresani, A.; Naghizadeh, A. Application of CoFe2O4@CuS Magnetic Nanocomposite as a Novel Adsorbent for Removal of Penicillin G from Aqueous Solutions: Isotherm, Kinetic and Thermodynamic Study. Desalination Water Treat. 2019, 148, 263–273. [Google Scholar] [CrossRef]
- Mao, H.; Wang, S.; Lin, J.-Y.; Wang, Z.; Ren, J. Modification of a Magnetic Carbon Composite for Ciprofloxacin Adsorption. J. Environ. Sci. 2016, 49, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, T.; Liu, J.; Li, D. Adsorption of Tetracycline Antibiotics from an Aqueous Solution onto Graphene Oxide/Calcium Alginate Composite Fibers. RSC Adv. 2018, 8, 2616–2621. [Google Scholar] [CrossRef] [PubMed]
- Masoudi; Kamranifar, M.; Naghizadeh, A. The Efficiency of Chitosan Extracted from Persian Gulf Shrimp Shell in Removal of Penicillin G Antibiotic from Aqueous Environment. Iran. J. Chem. Chem. Eng. (IJCCE) 2020, 39, 235–244. [Google Scholar] [CrossRef]
- Sharifpour, N.; Moghaddam, F.M.; Mardani, G.; Malakootian, M. Evaluation of the Activated Carbon Coated with Multiwalled Carbon Nanotubes in Removal of Ciprofloxacin from Aqueous Solutions. Appl. Water Sci. 2020, 10, 140. [Google Scholar] [CrossRef]
- Chang, J.; Shen, Z.; Hu, X.; Schulman, E.; Cui, C.; Guo, Q.; Tian, H. Adsorption of Tetracycline by Shrimp Shell Waste from Aqueous Solutions: Adsorption Isotherm, Kinetics Modeling, and Mechanism. ACS Omega 2020, 5, 3467–3477. [Google Scholar] [CrossRef]
- Peng, X.; Hu, F.; Lam, F.L.-Y.; Wang, Y.; Liu, Z.; Dai, H. Adsorption Behavior and Mechanisms of Ciprofloxacin from Aqueous Solution by Ordered Mesoporous Carbon and Bamboo-Based Carbon. J. Colloid Interface Sci. 2015, 460, 349–360. [Google Scholar] [CrossRef]
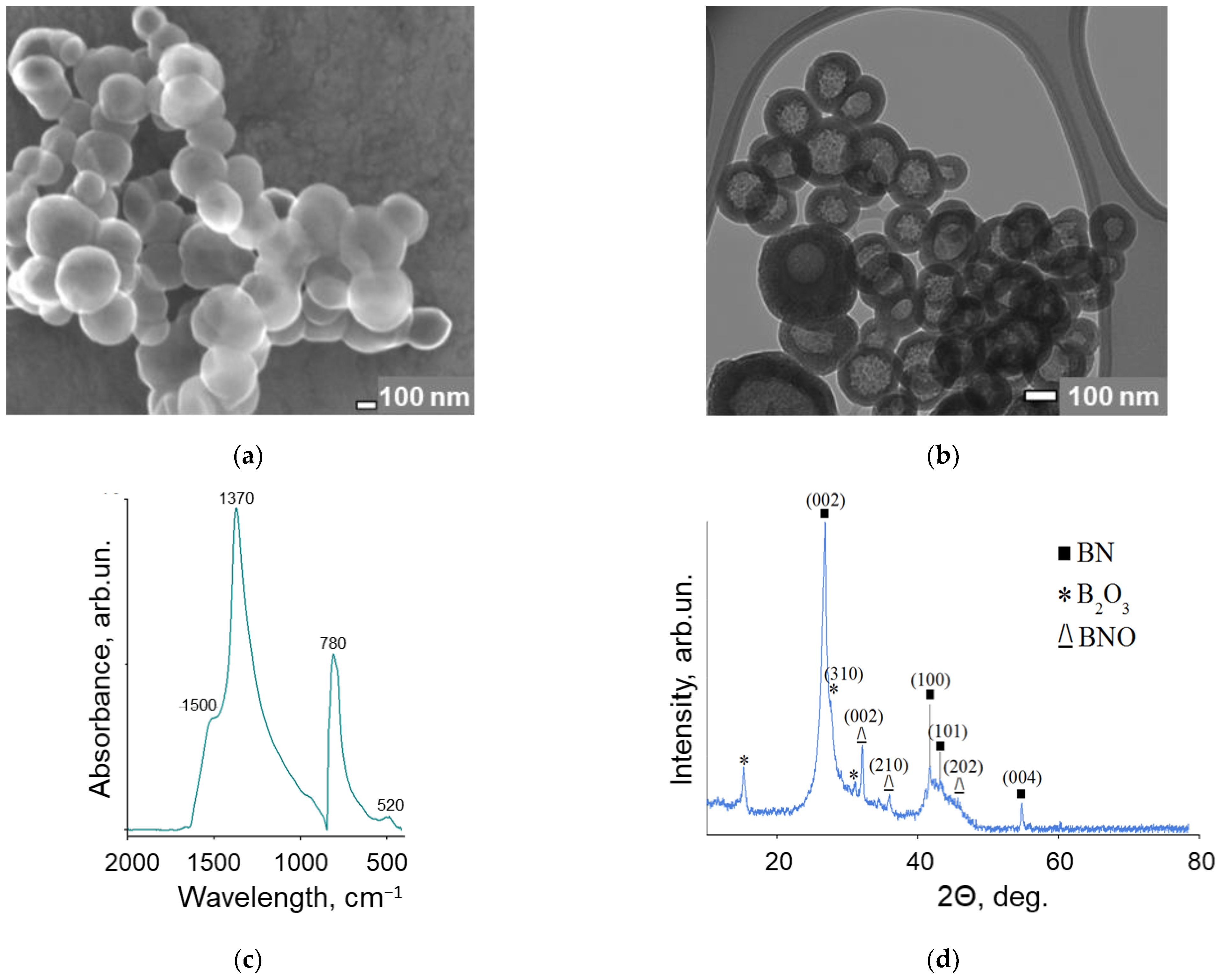

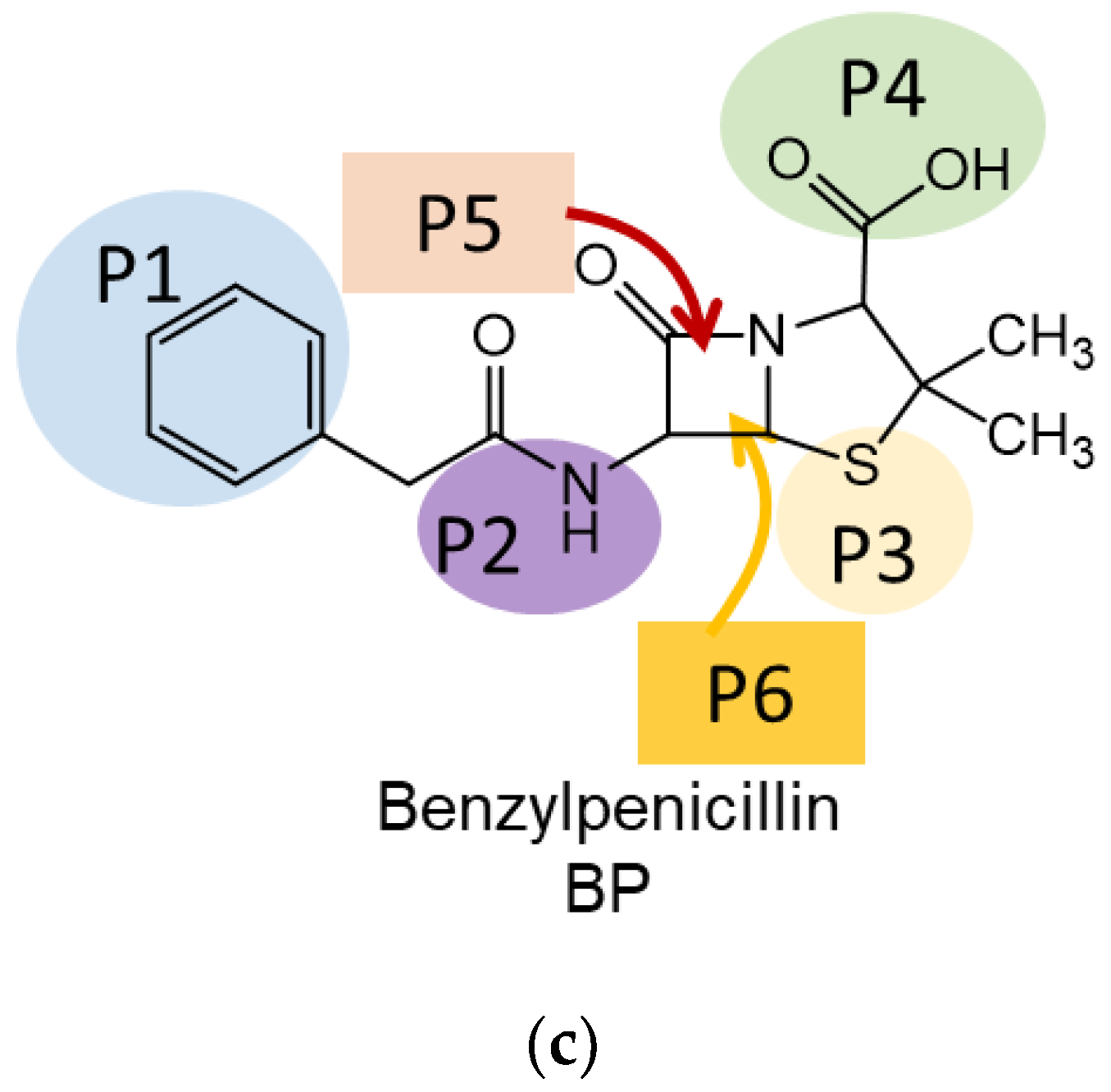
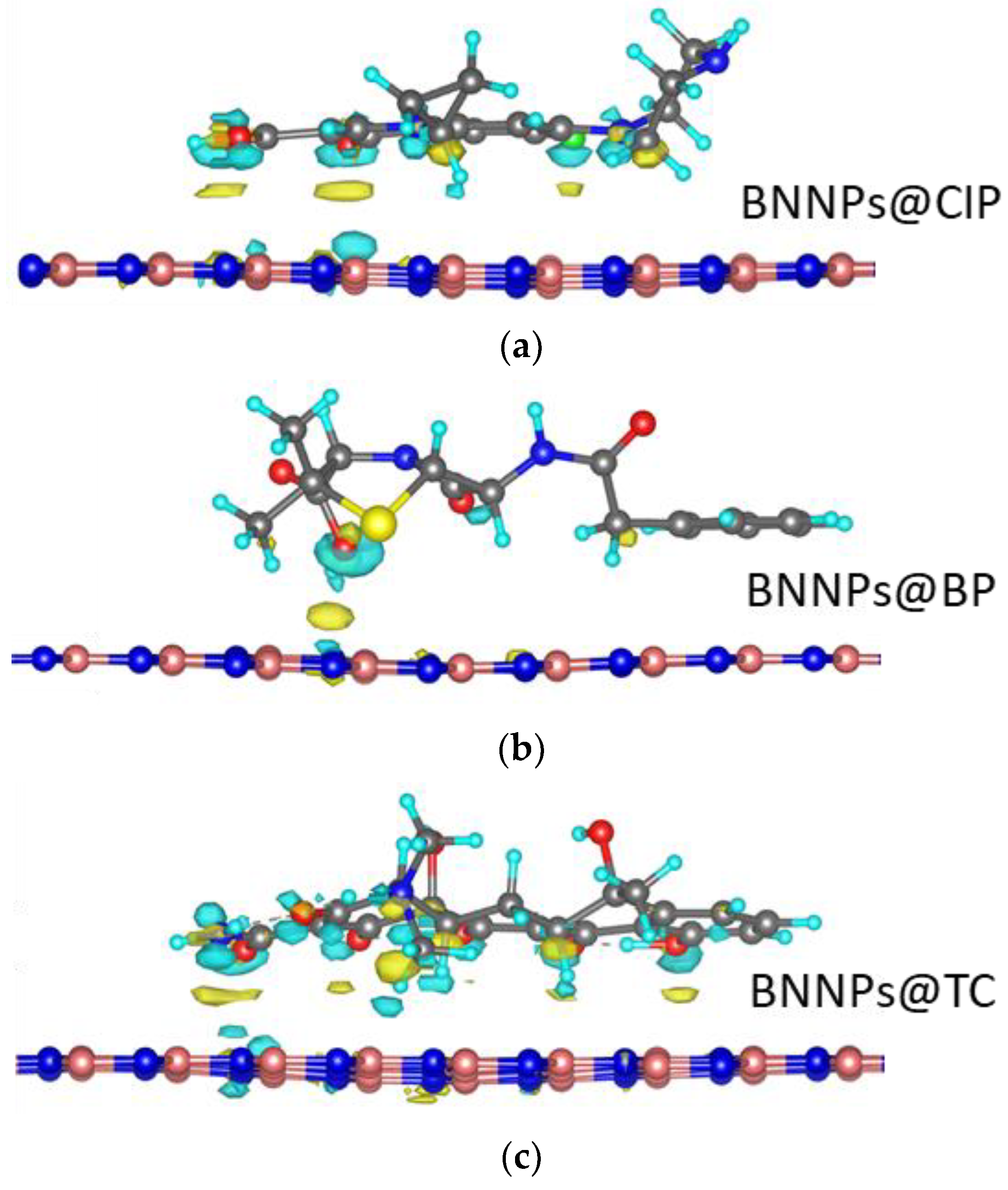
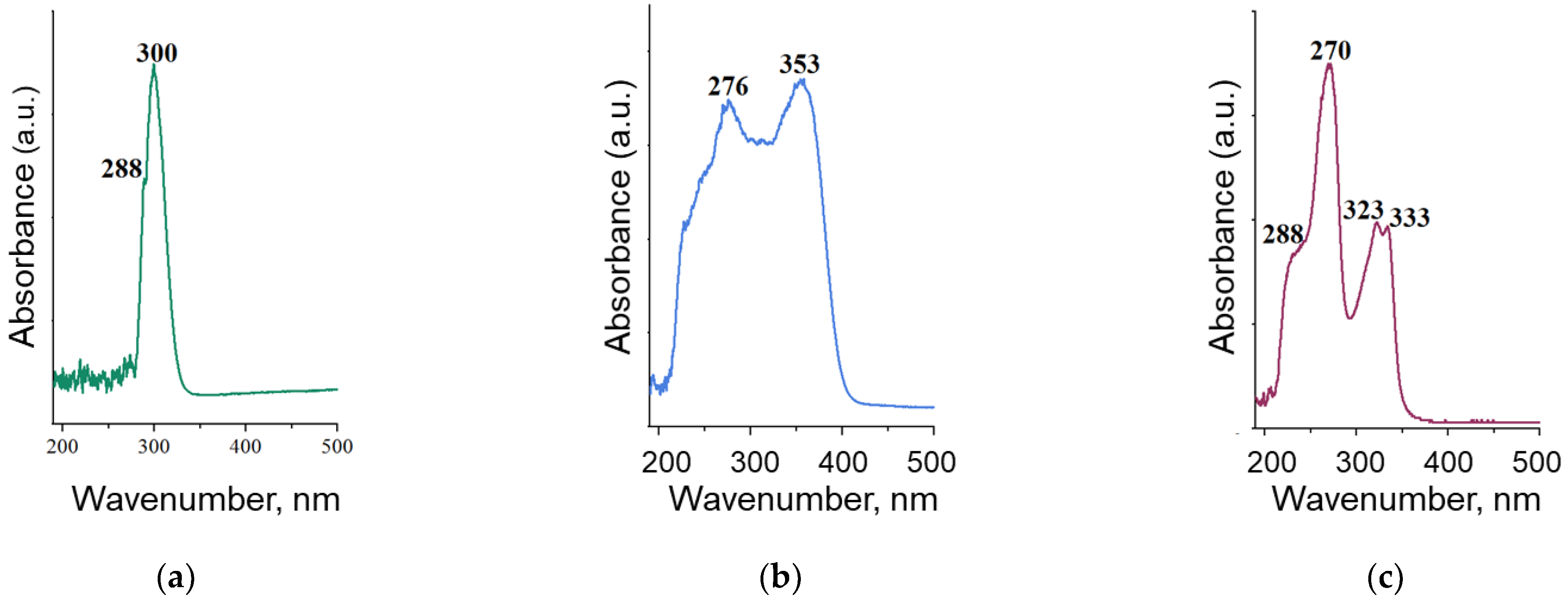


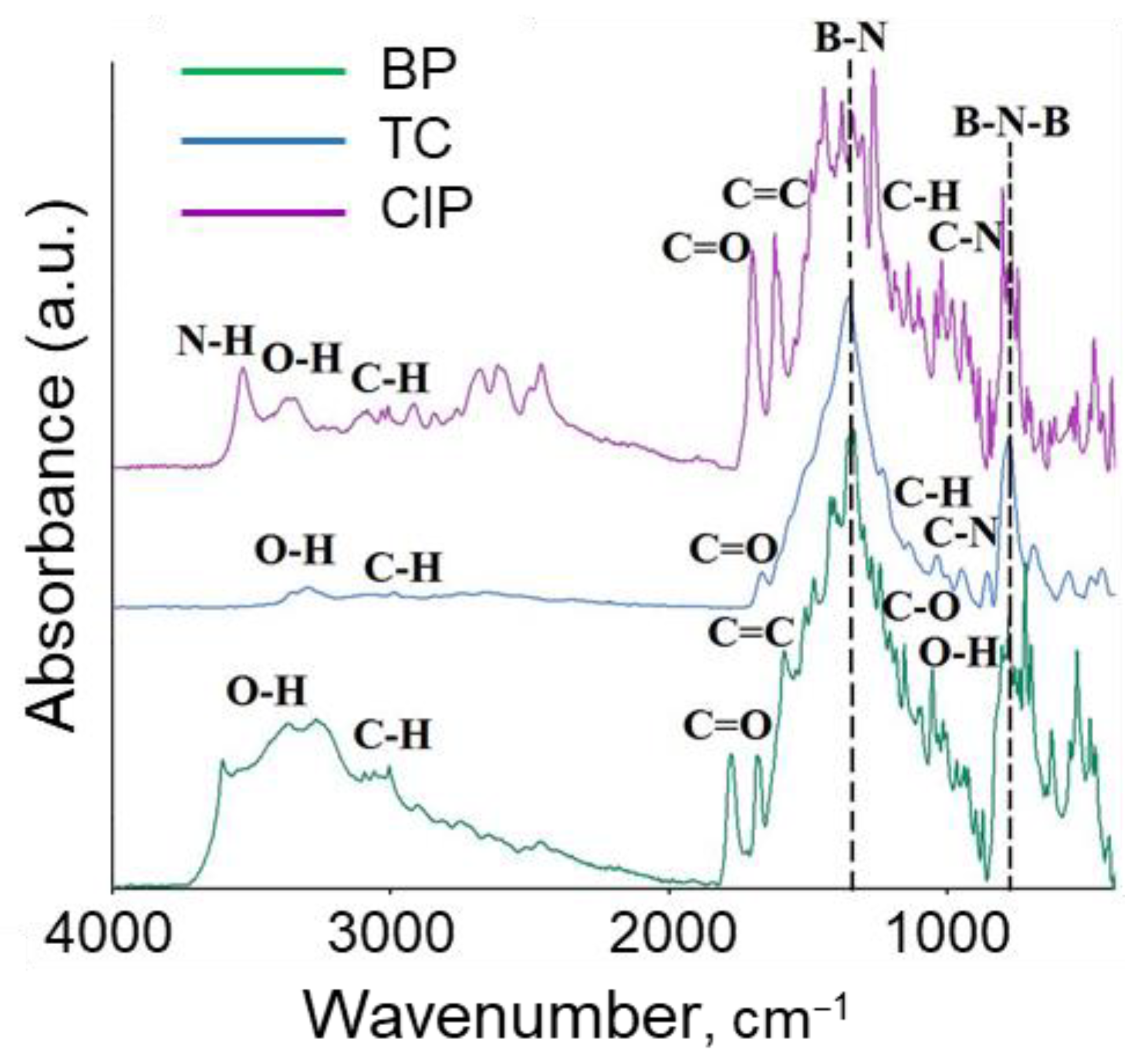
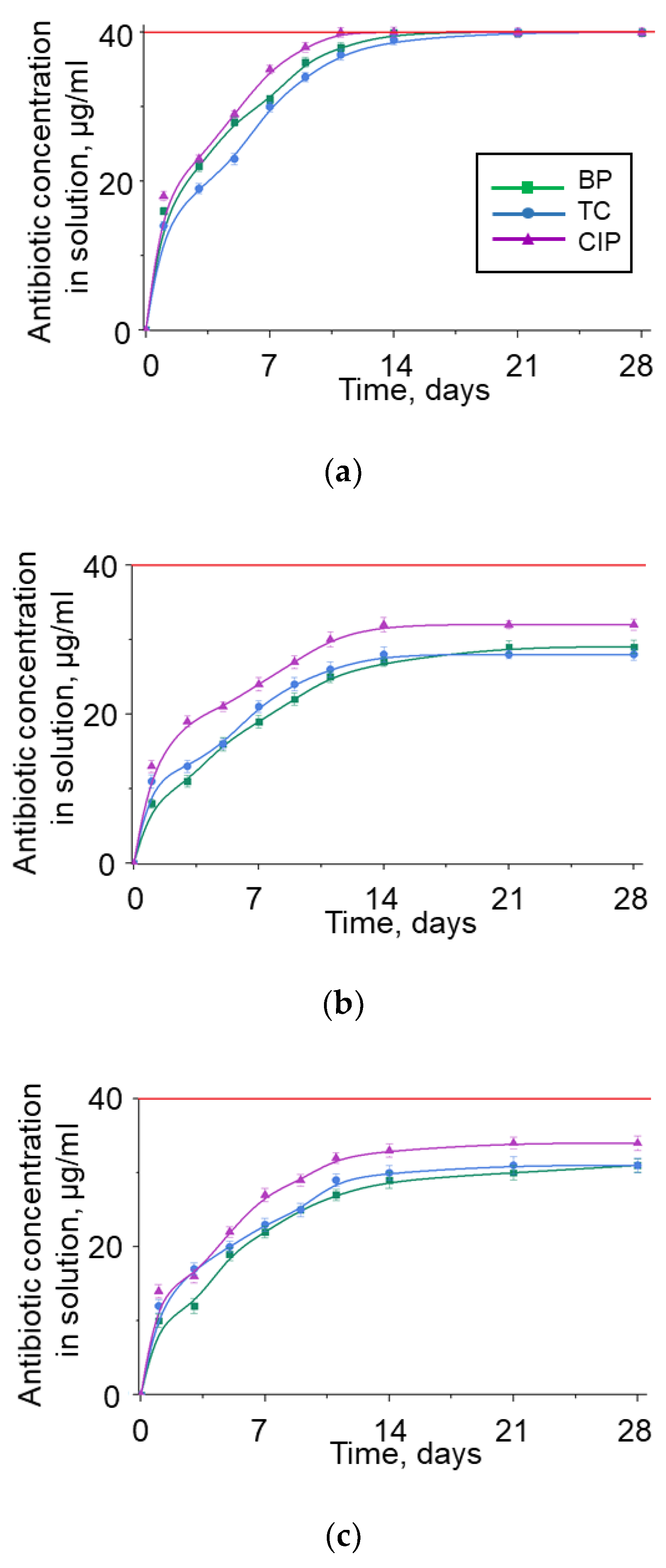
| Position | Ciprofloxacin | Tetracycline | Benzylpenicillin |
|---|---|---|---|
| P1 | Carboxylic | Dimethylamino- | Phenylacetyl- |
| P2 | Cyclopropyl- | Hydroxy- | Amino- |
| P3 | Pipyrazine ring | Amide- | Thioether- |
| P4 | Fluoro- | Peripheral region | Carboxylic |
| P5 | Oxo- | Vertical stacking (OH group is oriented from BN plane) | Vertical stacking (thio group is oriented from BN plane) |
| P6 | Vertical stacking of dihydroquinoline ring (vertical stacking) | Vertical stacking (OH group is oriented to BN plane) | Vertical stacking (thio group is oriented to BN plane) |
| Position | Eb, eV | |||||||
|---|---|---|---|---|---|---|---|---|
| CIP | TC | BP | ||||||
| −1 | 0 | +1 | −1 | 0 | +1 | −1 | 0 | |
| P1 | −3.66 | −1.28 | −0.48 | −2.91 | −2.6 | −2.95 | −0.07 | −0.63 |
| P2 | −2.62 | −2.38 | −3.06 | −2.91 | −2.59 | −2.92 | −4.14 | −3.12 |
| P3 | −1.98 | −1.81 | −2.46 | −2.98 | −2.62 | −2.01 | −4.06 | −3.31 |
| P4 | −2.58 | −2.14 | −2.51 | −4.26 | −4.69 | −3.34 | −3.37 | −2.03 |
| P5 | −4.35 | −2.07 | −1.91 | −6.55 | −6.36 | −6.44 | −4.33 | −3.14 |
| P6 | −5.44 | −4.04 | −4.78 | −6.56 | −5.54 | −5.93 | −4.05 | −3.73 |
| Adsorbent | Sorption Capacity qe, mg/g | Adsorbent | Sorption Capacity qe, mg/g | Adsorbent | Sorption Capacity qe, mg/g |
|---|---|---|---|---|---|
| TC | BP | CIP | |||
| Reduced graphene oxide decorated with MnFe2O4 NPs [20] | 41.00 | Magnesium oxide nanoparticles [56] | 25.66 | Nano-sized magnetite [57] | 12.73 |
| Pistachio shell coated with ZnO NPs [58] | 95.06 | Lemna minor [59] | 36.18 | Magnetic activated carbon/chitosan nanocomposite [60] | 90.00 |
| Nano sheet layered double hydroxide Mg/Al [61] | 98.04 | CoFe2O4@CuS magnetic nanocomposite [62] | 41.00 | Magnetic carbon composite, Fe3O4/C [63] | 90.10 |
| Graphene oxide/calcium alginate composite fibers [64] | 131.60 | Chitosan extracted from Persian gulf shrimp shell [65] | 101.44 | Activated carbon supported with multivalent carbon nanotubes [66] | 150.00 |
| Shrimp shell waste [67] | 229.98 | Silica NPs [22] | 211.35 | Ordered mesoporous carbon [68] | 233.37 |
| BNNPs * | 297.29 | BNNPs * | 254.76 | BNNPs * | 238.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antipina, L.Y.; Kotyakova, K.Y.; Tregubenko, M.V.; Shtansky, D.V. Experimental and Theoretical Study of Sorption Capacity of Hexagonal Boron Nitride Nanoparticles: Implication for Wastewater Purification from Antibiotics. Nanomaterials 2022, 12, 3157. https://doi.org/10.3390/nano12183157
Antipina LY, Kotyakova KY, Tregubenko MV, Shtansky DV. Experimental and Theoretical Study of Sorption Capacity of Hexagonal Boron Nitride Nanoparticles: Implication for Wastewater Purification from Antibiotics. Nanomaterials. 2022; 12(18):3157. https://doi.org/10.3390/nano12183157
Chicago/Turabian StyleAntipina, Liubov Yu., Kristina Yu. Kotyakova, Mariya V. Tregubenko, and Dmitry V. Shtansky. 2022. "Experimental and Theoretical Study of Sorption Capacity of Hexagonal Boron Nitride Nanoparticles: Implication for Wastewater Purification from Antibiotics" Nanomaterials 12, no. 18: 3157. https://doi.org/10.3390/nano12183157
APA StyleAntipina, L. Y., Kotyakova, K. Y., Tregubenko, M. V., & Shtansky, D. V. (2022). Experimental and Theoretical Study of Sorption Capacity of Hexagonal Boron Nitride Nanoparticles: Implication for Wastewater Purification from Antibiotics. Nanomaterials, 12(18), 3157. https://doi.org/10.3390/nano12183157








