Laser-Induced Modification of Hydrogenated Detonation Nanodiamonds in Ethanol
Abstract
:1. Introduction
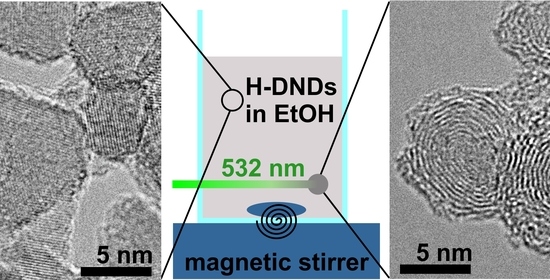
2. Materials and Methods
3. Results and Discussion
3.1. Visual Appearance Evolution
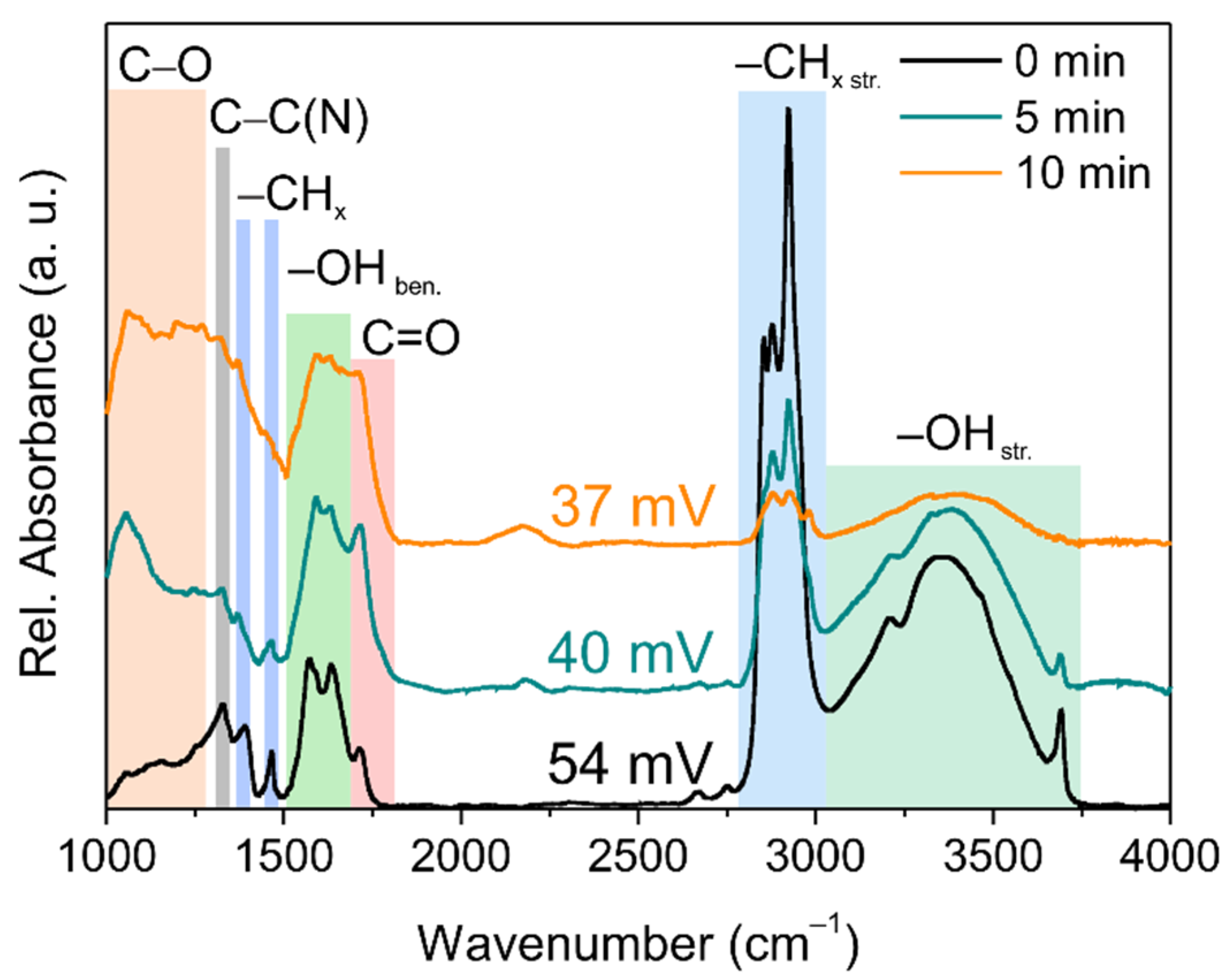
3.2. Infrared Spectroscopy
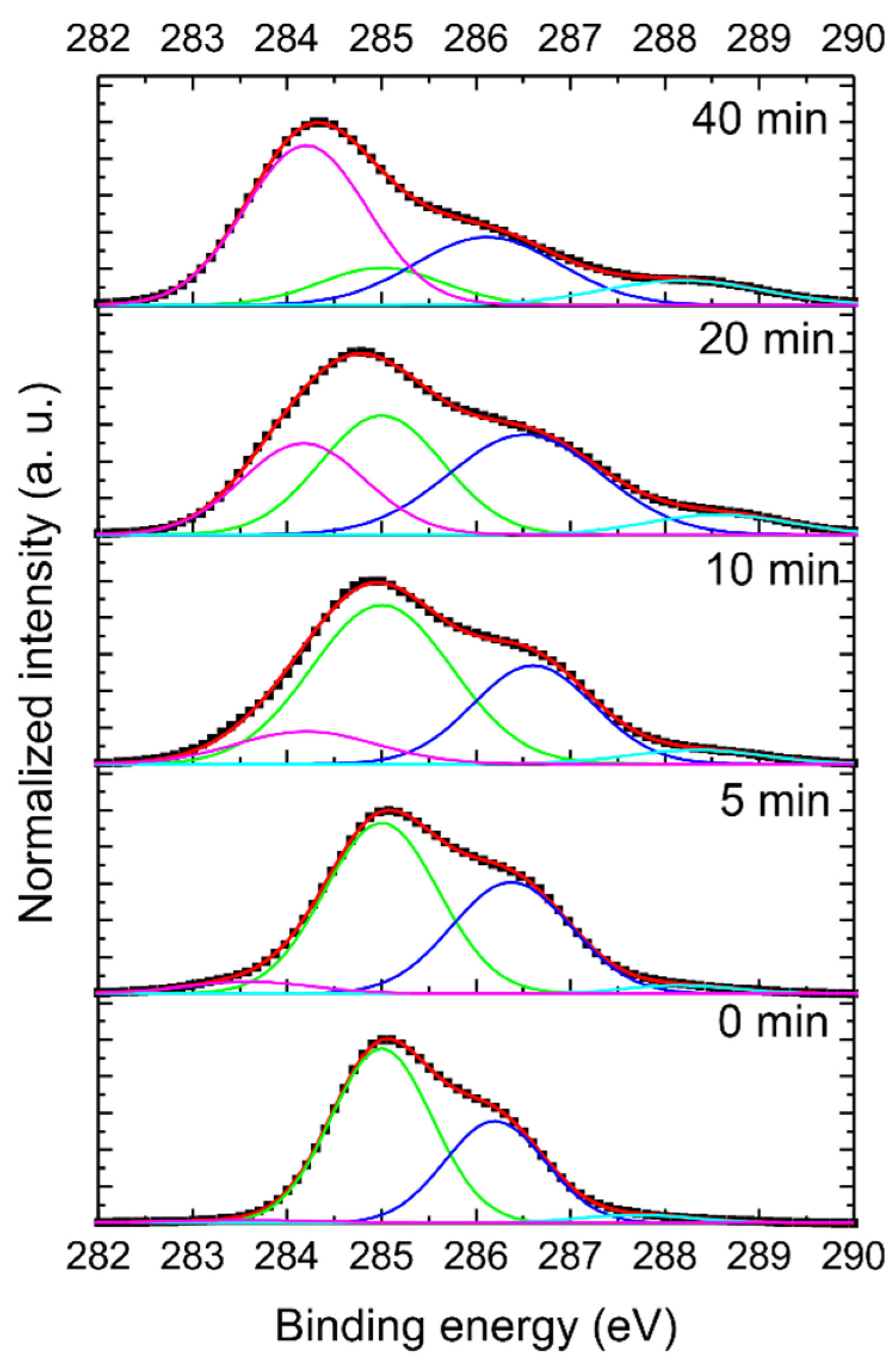
3.3. X-ray Photoelectron Spectroscopy
3.4. Electron Microscopy
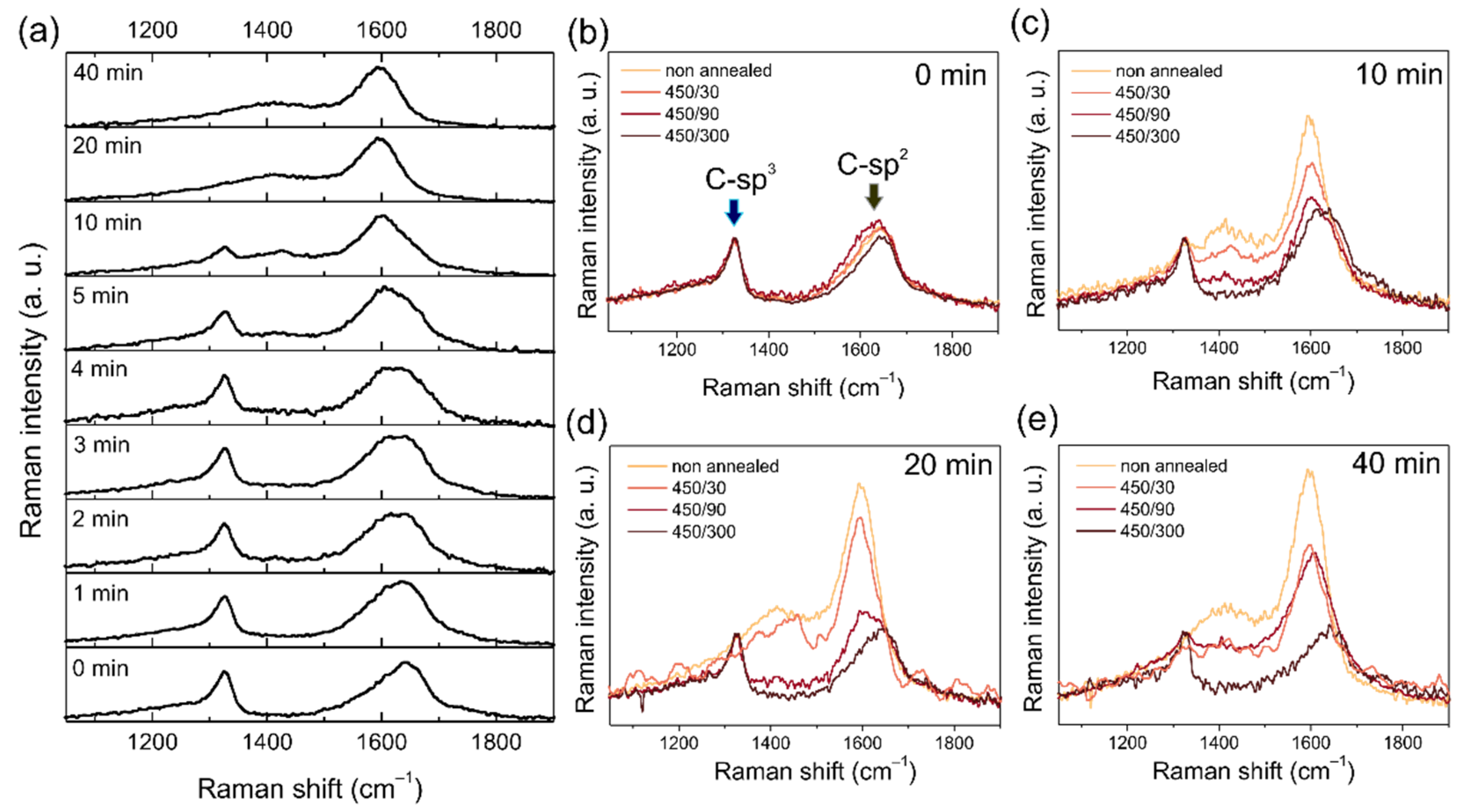
3.5. Raman Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohapatra, D.; Nemala, S.S.; Sayed, M.S.; Shim, J.-J.; Mallick, S.; Bhargava, P.; Parida, S. Carbon Nano-Onion-Powered Optically Transparent and Economical Dye-Sensitized Solar Cells. Nanoscale 2020, 12, 20621–20630. [Google Scholar] [CrossRef]
- Pech, D.; Brunet, M.; Durou, H.; Huang, P.; Mochalin, V.; Gogotsi, Y.; Taberna, P.-L.; Simon, P. Ultrahigh-Power Micrometre-Sized Supercapacitors Based on Onion-like Carbon. Nat. Nanotechnol. 2010, 5, 651–654. [Google Scholar] [CrossRef]
- Jang, D.M.; Im, H.S.; Back, S.H.; Park, K.; Lim, Y.R.; Jung, C.S.; Park, J.; Lee, M. Laser-Induced Graphitization of Colloidal Nanodiamonds for Excellent Oxygen Reduction Reaction. Phys. Chem. Chem. Phys. 2014, 16, 2411–2416. [Google Scholar] [CrossRef]
- Sonkar, S.K.; Ghosh, M.; Roy, M.; Begum, A.; Sarkar, S. Carbon Nano-Onions as Nontoxic and High-Fluorescence Bioimaging Agent in Food Chain—An In Vivo Study from Unicellular E. Coli to Multicellular C. Elegans. Mater. Express 2012, 2, 105–114. [Google Scholar] [CrossRef]
- Zeiger, M.; Jäckel, N.; Mochalin, V.N.; Presser, V. Review: Carbon Onions for Electrochemical Energy Storage. J. Mater. Chem. A 2016, 4, 3172–3196. [Google Scholar] [CrossRef] [Green Version]
- Aleksenskii, A.E.; Baidakova, M.V.; Vul, A.Y.; Davydov, V.Y.; Pevtsova, Y.A. Diamond-Graphite Phase Transition in Ultradisperse-Diamond Clusters. Phys. Solid State 1997, 39, 1007–1015. [Google Scholar] [CrossRef]
- Ahmed, A.-I.; Mandal, S.; Gines, L.; Williams, O.A.; Cheng, C.-L. Low Temperature Catalytic Reactivity of Nanodiamond in Molecular Hydrogen. Carbon 2016, 110, 438–442. [Google Scholar] [CrossRef]
- Petit, T.; Arnault, J.-C.; Girard, H.A.; Sennour, M.; Bergonzo, P. Early Stages of Surface Graphitization on Nanodiamond Probed by X-ray Photoelectron Spectroscopy. Phys. Rev. B 2011, 84, 233407. [Google Scholar] [CrossRef]
- Petit, T.; Arnault, J.-C.; Girard, H.A.; Sennour, M.; Kang, T.-Y.; Cheng, C.-L.; Bergonzo, P. Oxygen Hole Doping of Nanodiamond. Nanoscale 2012, 4, 6792. [Google Scholar] [CrossRef]
- Schüpfer, D.B.; Badaczewski, F.; Peilstöcker, J.; Guerra-Castro, J.M.; Shim, H.; Firoozabadi, S.; Beyer, A.; Volz, K.; Presser, V.; Heiliger, C.; et al. Monitoring the Thermally Induced Transition from Sp3-Hybridized into Sp2-Hybridized Carbons. Carbon 2021, 172, 214–227. [Google Scholar] [CrossRef]
- Dhand, V.; Yadav, M.; Kim, S.H.; Rhee, K.Y. A Comprehensive Review on the Prospects of Multi-Functional Carbon Nano Onions as an Effective, High- Performance Energy Storage Material. Carbon 2021, 175, 534–575. [Google Scholar] [CrossRef]
- Mykhaylyk, O.O.; Solonin, Y.M.; Batchelder, D.N.; Brydson, R. Transformation of Nanodiamond into Carbon Onions: A Comparative Study by High-Resolution Transmission Electron Microscopy, Electron Energy-Loss Spectroscopy, X-ray Diffraction, Small-Angle X-ray Scattering, and Ultraviolet Raman Spectroscopy. J. Appl. Phys. 2005, 97, 074302. [Google Scholar] [CrossRef]
- Hu, H.; Li, Q.; Li, L.; Teng, X.; Feng, Z.; Zhang, Y.; Wu, M.; Qiu, J. Laser Irradiation of Electrode Materials for Energy Storage and Conversion. Matter 2020, 3, 95–126. [Google Scholar] [CrossRef]
- De Feudis, M.; Caricato, A.P.; Taurino, A.; Ossi, P.M.; Castiglioni, C.; Brambilla, L.; Maruccio, G.; Monteduro, A.G.; Broitman, E.; Chiodini, G.; et al. Diamond Graphitization by Laser-Writing for All-Carbon Detector Applications. Diam. Relat. Mater. 2017, 75, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Cadot, G.B.J.; Thomas, K.; Best, J.P.; Taylor, A.A.; Michler, J.; Axinte, D.A.; Billingham, J. Investigation of the Microstructure Change Due to Phase Transition in Nanosecond Pulsed Laser Processing of Diamond. Carbon 2018, 127, 349–365. [Google Scholar] [CrossRef]
- Fraczyk, J.; Rosowski, A.; Kolesinska, B.; Koperkiewcz, A.; Sobczyk-Guzenda, A.; Kaminski, Z.; Dudek, M. Orthogonal Functionalization of Nanodiamond Particles after Laser Modification and Treatment with Aromatic Amine Derivatives. Nanomaterials 2018, 8, 908. [Google Scholar] [CrossRef] [Green Version]
- Jang, D.M.; Im, H.S.; Myung, Y.; Cho, Y.J.; Kim, H.S.; Back, S.H.; Park, J.; Cha, E.H.; Lee, M. Hydrogen and Carbon Monoxide Generation from Laser-Induced Graphitized Nanodiamonds in Water. Phys. Chem. Chem. Phys. 2013, 15, 7155. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Ouyang, G.; Liu, P.; Wang, C.X.; Yang, G.W. Reversible Nanodiamond-Carbon Onion Phase Transformations. Nano Lett. 2014, 14, 3645–3652. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Shiral Fernando, K.A.; Liang, W.; Seilkop, A.; Monica Veca, L.; Sun, Y.-P.; Bunker, C.E. Carbon Dots for Energy Conversion Applications. J. Appl. Phys. 2019, 125, 220903. [Google Scholar] [CrossRef] [Green Version]
- Stehlik, S.; Henych, J.; Stenclova, P.; Kral, R.; Zemenova, P.; Pangrac, J.; Vanek, O.; Kromka, A.; Rezek, B. Size and Nitrogen Inhomogeneity in Detonation and Laser Synthesized Primary Nanodiamond Particles Revealed via Salt-Assisted Deaggregation. Carbon 2021, 171, 230–239. [Google Scholar] [CrossRef]
- Stehlik, S.; Varga, M.; Stenclova, P.; Ondic, L.; Ledinsky, M.; Pangrac, J.; Vanek, O.; Lipov, J.; Kromka, A.; Rezek, B. Ultrathin Nanocrystalline Diamond Films with Silicon Vacancy Color Centers via Seeding by 2 Nm Detonation Nanodiamonds. ACS Appl. Mater. Interfaces 2017, 9, 38842–38853. [Google Scholar] [CrossRef] [PubMed]
- Nunn, N.; Shenderova, O. Toward a Golden Standard in Single Digit Detonation Nanodiamond: Toward a Golden Standard in Single Digit Detonation Nanodiamond. Phys. Status Solidi A 2016, 213, 2138–2145. [Google Scholar] [CrossRef]
- Stehlik, S.; Glatzel, T.; Pichot, V.; Pawlak, R.; Meyer, E.; Spitzer, D.; Rezek, B. Water Interaction with Hydrogenated and Oxidized Detonation Nanodiamonds—Microscopic and Spectroscopic Analyses. Diam. Relat. Mater. 2015, 63, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Petit, T.; Puskar, L.; Dolenko, T.; Choudhury, S.; Ritter, E.; Burikov, S.; Laptinskiy, K.; Brzustowski, Q.; Schade, U.; Yuzawa, H.; et al. Unusual Water Hydrogen Bond Network around Hydrogenated Nanodiamonds. J. Phys. Chem. C 2017, 121, 5185–5194. [Google Scholar] [CrossRef] [Green Version]
- Lawson, S.C.; Fisher, D.; Hunt, D.C.; Newton, M.E. On the Existence of Positively Charged Single-Substitutional Nitrogen in Diamond. J. Phys. Condens. Matter 1998, 10, 6171–6180. [Google Scholar] [CrossRef]
- Ginés, L.; Mandal, S.; Cheng, C.-L.; Sow, M.; Williams, O.A. Positive Zeta Potential of Nanodiamonds. Nanoscale 2017, 9, 12549–12555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehm, H.P. Some Aspects of the Surface Chemistry of Carbon Blacks and Other Carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Stehlik, S.; Mermoux, M.; Schummer, B.; Vanek, O.; Kolarova, K.; Stenclova, P.; Vlk, A.; Ledinsky, M.; Pfeifer, R.; Romanyuk, O.; et al. Size Effects on Surface Chemistry and Raman Spectra of Sub-5 Nm Oxidized High-Pressure High-Temperature and Detonation Nanodiamonds. J. Phys. Chem. C 2021, 125, 5647–5669. [Google Scholar] [CrossRef]
- Chang, S.L.Y.; Reineck, P.; Williams, D.; Bryant, G.; Opletal, G.; El-Demrdash, S.A.; Chiu, P.-L.; Ōsawa, E.; Barnard, A.S.; Dwyer, C. Dynamic Self-Assembly of Detonation Nanodiamond in Water. Nanoscale 2020, 12, 5363–5367. [Google Scholar] [CrossRef]
- Osswald, S.; Yushin, G.; Mochalin, V.; Kucheyev, S.O.; Gogotsi, Y. Control of Sp2/Sp3 Carbon Ratio and Surface Chemistry of Nanodiamond Powders by Selective Oxidation in Air. J. Am. Chem. Soc. 2006, 128, 11635–11642. [Google Scholar] [CrossRef] [PubMed]
- Mermoux, M.; Chang, S.; Girard, H.A.; Arnault, J.-C. Raman Spectroscopy Study of Detonation Nanodiamond. Diam. Relat. Mater. 2018, 87, 248–260. [Google Scholar] [CrossRef]
- Mermoux, M.; Crisci, A.; Petit, T.; Girard, H.A.; Arnault, J.-C. Surface Modifications of Detonation Nanodiamonds Probed by Multiwavelength Raman Spectroscopy. J. Phys. Chem. C 2014, 118, 23415–23425. [Google Scholar] [CrossRef]
- Stehlik, S.; Varga, M.; Ledinsky, M.; Miliaieva, D.; Kozak, H.; Skakalova, V.; Mangler, C.; Pennycook, T.J.; Meyer, J.C.; Kromka, A.; et al. High-Yield Fabrication and Properties of 1.4 Nm Nanodiamonds with Narrow Size Distribution. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Osswald, S.; Mochalin, V.N.; Havel, M.; Yushin, G.; Gogotsi, Y. Phonon Confinement Effects in the Raman Spectrum of Nanodiamond. Phys. Rev. B 2009, 80, 075419. [Google Scholar] [CrossRef]
- Korepanov, V.I.; Hamaguchi, H.; Osawa, E.; Ermolenkov, V.; Lednev, I.K.; Etzold, B.J.M.; Levinson, O.; Zousman, B.; Epperla, C.P.; Chang, H.-C. Carbon Structure in Nanodiamonds Elucidated from Raman Spectroscopy. Carbon 2017, 121, 322–329. [Google Scholar] [CrossRef]
- Duan, X.; Tian, W.; Zhang, H.; Sun, H.; Ao, Z.; Shao, Z.; Wang, S. Sp2/Sp3 Framework from Diamond Nanocrystals: A Key Bridge of Carbonaceous Structure to Carbocatalysis. ACS Catal. 2019, 9, 7494–7519. [Google Scholar] [CrossRef]
- Duan, X.; Ao, Z.; Li, D.; Sun, H.; Zhou, L.; Suvorova, A.; Saunders, M.; Wang, G.; Wang, S. Surface-Tailored Nanodiamonds as Excellent Metal-Free Catalysts for Organic Oxidation. Carbon 2016, 103, 404–411. [Google Scholar] [CrossRef]
- Reinert, L.; Zeiger, M.; Suárez, S.; Presser, V.; Mücklich, F. Dispersion Analysis of Carbon Nanotubes, Carbon Onions, and Nanodiamonds for Their Application as Reinforcement Phase in Nickel Metal Matrix Composites. RSC Adv. 2015, 5, 95149–95159. [Google Scholar] [CrossRef] [Green Version]



| Atomic Concentration (±0.5 at. %) | |||||
|---|---|---|---|---|---|
| Sample | C | O | N | sp2/sp3 | Cdef/sp3 |
| 0 min | 96.4 | 2.0 | 1.6 | 0.03 | 0.59 |
| 5 min | 94.6 | 3.8 | 1.6 | 0.14 | 0.77 |
| 10 min | 89.5 | 9.2 | 1.3 | 0.20 | 0.53 |
| 20 min | 84.2 | 15.0 | 0.8 | 0.77 | 1.05 |
| 40 min | 81.4 | 18.2 | 0.4 | 4.3 | 2.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bydzovska, I.; Shagieva, E.; Gordeev, I.; Romanyuk, O.; Nemeckova, Z.; Henych, J.; Ondic, L.; Kromka, A.; Stehlik, S. Laser-Induced Modification of Hydrogenated Detonation Nanodiamonds in Ethanol. Nanomaterials 2021, 11, 2251. https://doi.org/10.3390/nano11092251
Bydzovska I, Shagieva E, Gordeev I, Romanyuk O, Nemeckova Z, Henych J, Ondic L, Kromka A, Stehlik S. Laser-Induced Modification of Hydrogenated Detonation Nanodiamonds in Ethanol. Nanomaterials. 2021; 11(9):2251. https://doi.org/10.3390/nano11092251
Chicago/Turabian StyleBydzovska, Irena, Ekaterina Shagieva, Ivan Gordeev, Oleksandr Romanyuk, Zuzana Nemeckova, Jiri Henych, Lukas Ondic, Alexander Kromka, and Stepan Stehlik. 2021. "Laser-Induced Modification of Hydrogenated Detonation Nanodiamonds in Ethanol" Nanomaterials 11, no. 9: 2251. https://doi.org/10.3390/nano11092251
APA StyleBydzovska, I., Shagieva, E., Gordeev, I., Romanyuk, O., Nemeckova, Z., Henych, J., Ondic, L., Kromka, A., & Stehlik, S. (2021). Laser-Induced Modification of Hydrogenated Detonation Nanodiamonds in Ethanol. Nanomaterials, 11(9), 2251. https://doi.org/10.3390/nano11092251