Photoinitiated Polymerization of Hydrogels by Graphene Quantum Dots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of GQDs
2.2. Characterization of GQDs
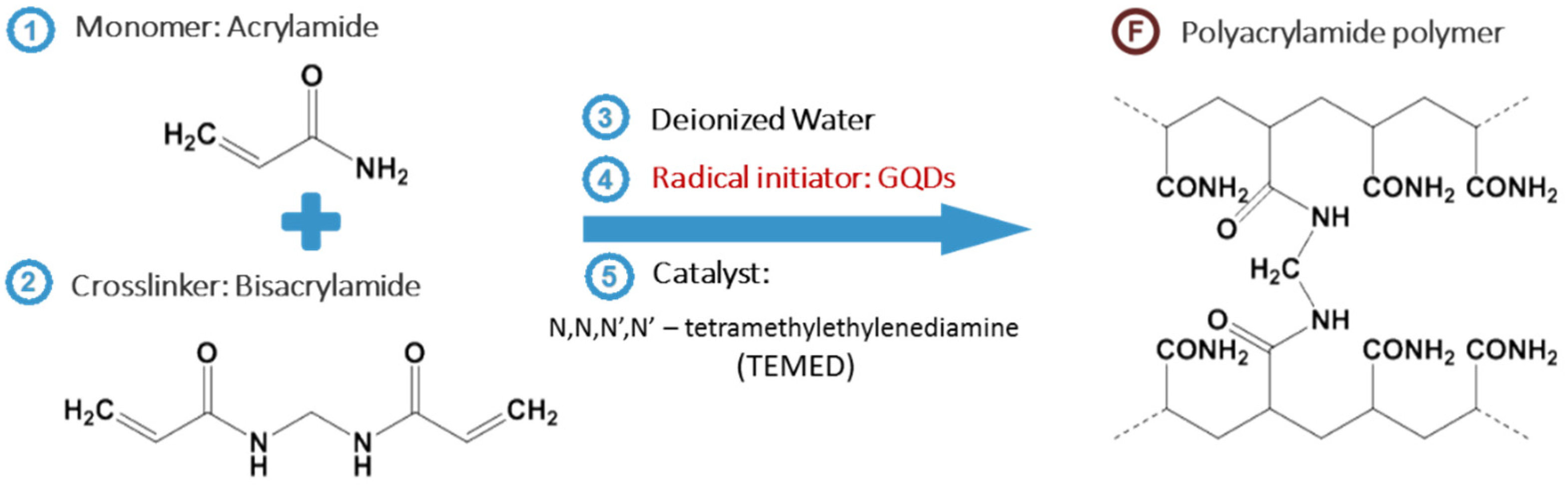
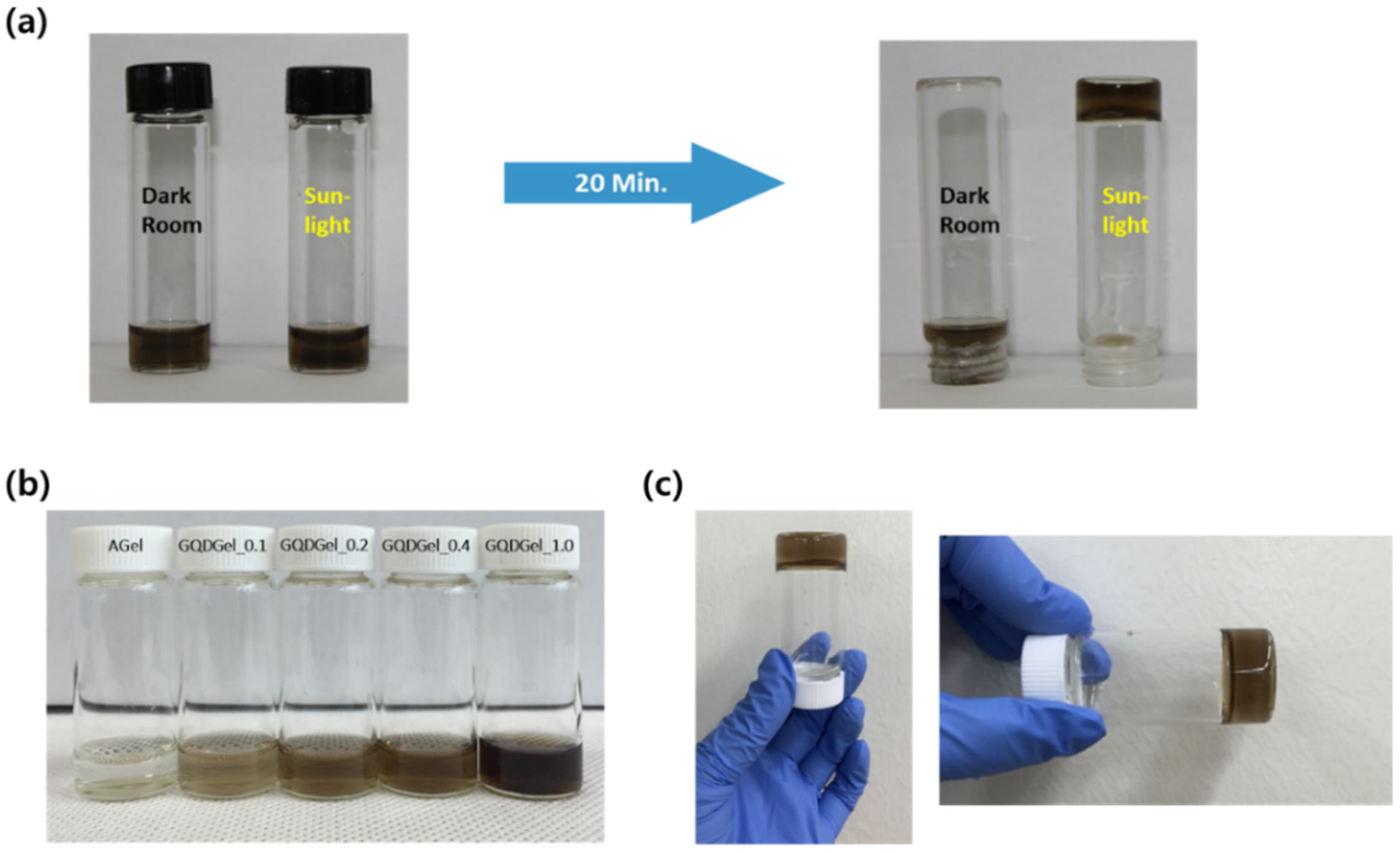
2.3. Preparation of GQDGel and AGel
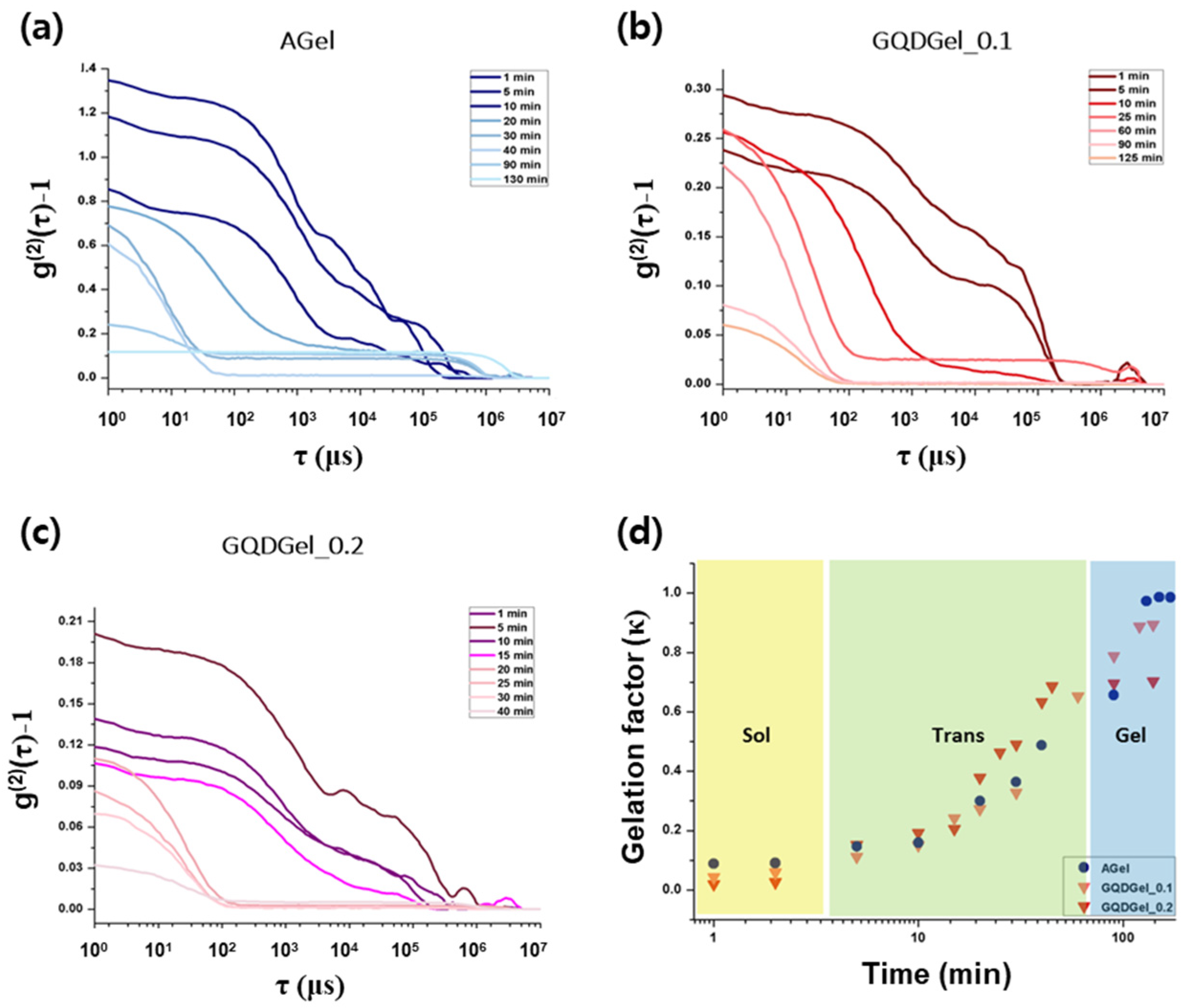
2.4. Dynamic Light Scattering (DLS) Analysis
2.5. Mechanical Characterization of Hydrogels
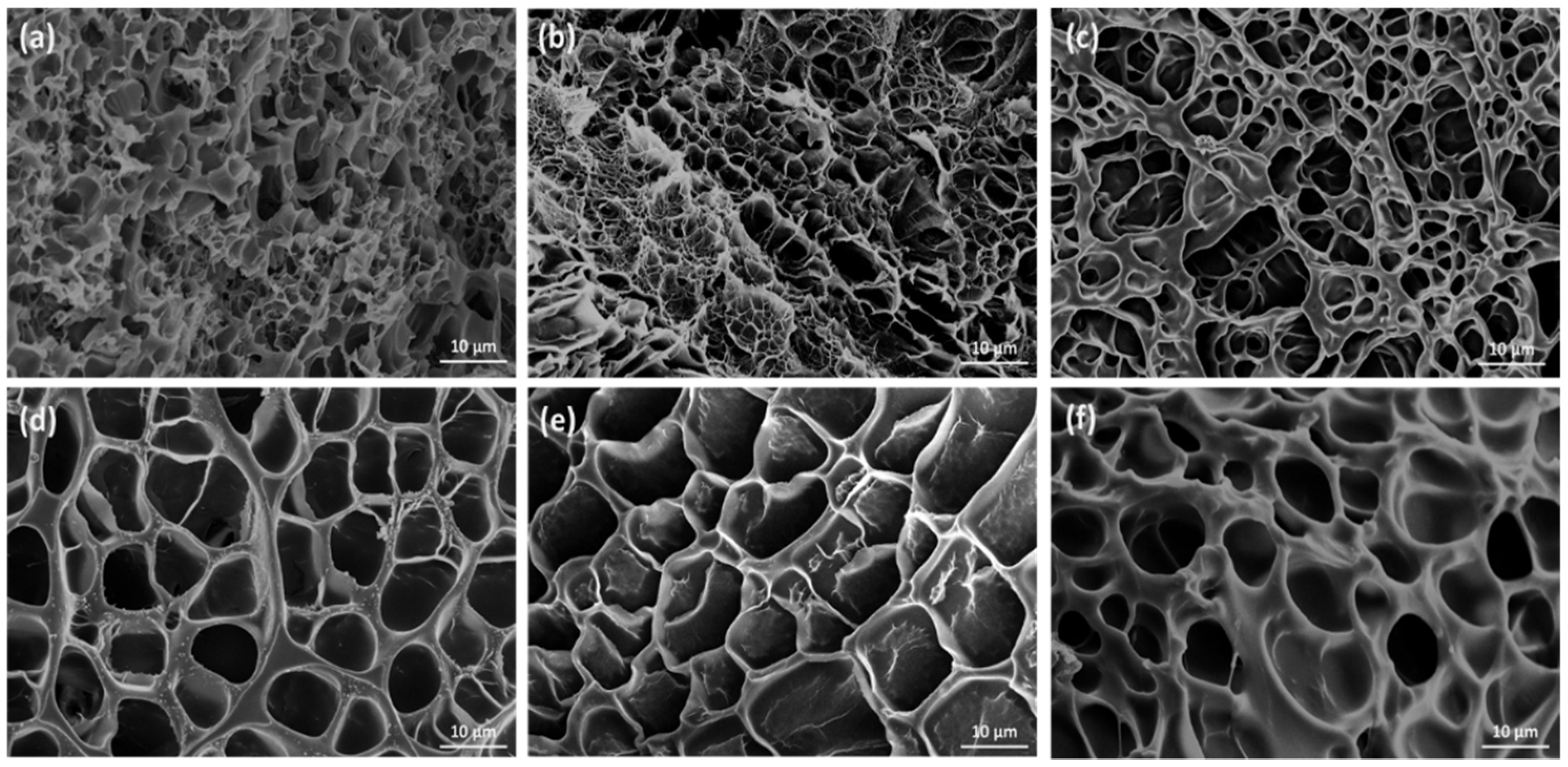
2.6. Electron Microscopy Analysis
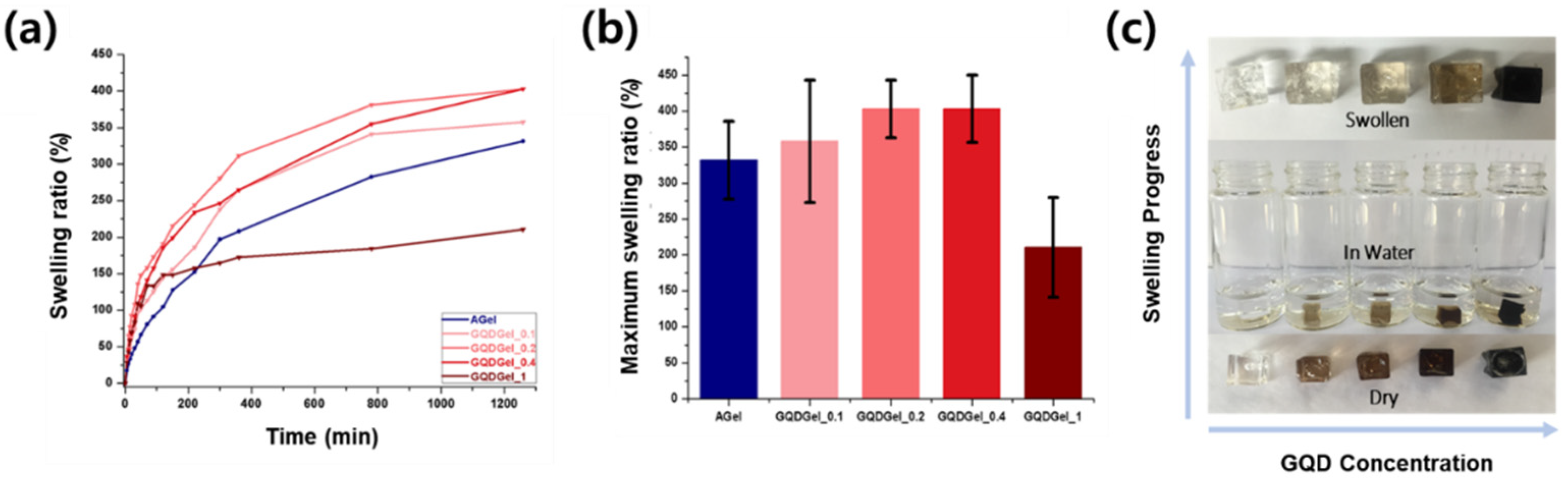
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Heilshorn, S.C. Adaptable Hydrogel Networks with Reversible Linkages for Tissue Engineering. Adv. Mater. 2015, 27, 3717–3736. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels as Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Tomczykowa, M.; Plonska-Brzezinska, M.E. Conducting Polymers, Hydrogels and their Composites: Preparation, Properties and Bioapplications. Polymers 2019, 11, 350. [Google Scholar] [CrossRef] [Green Version]
- Javadi, M.; Gu, Q.; Naficy, S.; Farajikhah, S.; Crook, J.M.; Wallace, G.G.; Beirne, S.; Moulton, S.E. Conductive Tough Hydrogel for Bioapplications. Macromol. Biosci. 2018, 18, 1700270. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel Nanoparticles in Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Hunt, J.A.; Chen, R.; van Veen, T.; Bryan, N. Hydrogels for Tissue Engineering and Regenerative Medicine. J. Mater. Chem. B 2014, 2, 5319–5338. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.; West, J.L. Photopolymerizable Hydrogels for Tissue Engineering Applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Rosiak, J.; Burczak, K.; Pekala, W. Polyacrylamide Hydrogels as Sustained-Release Drug Delivery Dressing Materials. Radiat. Phys. Chem. 1983, 22, 907–915. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A Review of Trends and Limitations in Hydrogel-Rapid Prototyping for Tissue Engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef]
- Chatterjee, S.; Upadhyay, P.; Mishra, M.; Srividya, M.; Akshara, M.R.; Kamali, N.; Zaidi, Z.S.; Iqbal, S.F.; Misra, S.K. Advances in Chemistry and Composition of Soft Materials for Drug Releasing Contact Lenses. RSC Adv. 2020, 10, 36751–36777. [Google Scholar] [CrossRef]
- Moreddu, R.; Vigolo, D.; Yetisen, A.K. Contact Lens Technology: From Fundamentals to Applications. Adv. Healthc. Mater. 2019, 8, 1900368. [Google Scholar] [CrossRef]
- Driest, P.J.; Allijn, I.E.; Dijkstra, D.J.; Stamatialis, D.; Grijpma, D.W. Poly(Ethylene Glycol)-Based Poly(Urethane Isocyanurate) Hydrogels for Contact Lens Applications. Polym. Int. 2020, 69, 131–139. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Xu, C.; Li, Y.; Gao, J.; Wang, W.; Liu, Y. High Strength Graphene Oxide/Polyvinyl Alcohol Composite Hydrogels. J. Mater. Chem. 2011, 21, 10399–10406. [Google Scholar] [CrossRef]
- Janney, M.A.; Omatete, O.O.; Walls, C.A.; Nunn, S.D.; Ogle, R.J.; Westmoreland, G. Development of Low-Toxicity Gelcasting Systems. J. Am. Ceram. Soc. 1998, 81, 581–591. [Google Scholar] [CrossRef]
- Williams, C.G.; Malik, A.N.; Kim, T.K.; Manson, P.N.; Elisseeff, J.H. Variable Cytocompatibility of Six Cell Lines with Photoinitiators Used for Polymerizing Hydrogels and Cell Encapsulation. Biomaterials 2005, 26, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Arno, M.C.; Inam, M.; Weems, A.C.; Li, Z.; Binch, A.L.; Platt, C.I.; Richardson, S.M.; Hoyland, J.A.; Dove, A.P.; O’Reilly, R.K. Exploiting the Role of Nanoparticle Shape in Enhancing Hydrogel Adhesive and Mechanical Properties. Nat. Commun. 2020, 11, 1420. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.-Y.; Barber, T.; Mills, G.; Morris, M.-B. Heterogeneous Photopolymerization of Methyl Methacrylate Initiated by Small ZnO Particles. J. Phys. Chem. 1994, 98, 12746–12752. [Google Scholar] [CrossRef]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle–Hydrogel Composites: Concept, Design, and Applications of these Promising, Multi-Functional Materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Winey, K.I. Polymer Nanocomposites Containing Carbon Nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, J.; Bao, S.; Wu, Q.; Wang, Q. Semiconductor Nanoparticle-Based Hydrogels Prepared via Self-Initiated Polymerization under Sunlight, even Visible Light. Sci. Rep. 2013, 3, 1399. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Palomero, C.; Benítez-Martínez, S.; Soriano, M.L.; Valcárcel, M. Fluorescent nanocellulosic hydrogels based on graphene quantum dots for sensing laccase. Anal. Chim. Acta 2017, 974, 93–99. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lee, M.W.; Woo, S.H. Enhanced Mechanical Strength of Chitosan Hydrogel Beads by Impregnation with Carbon Nanotubes. Carbon 2009, 47, 2933–2936. [Google Scholar] [CrossRef]
- Bai, H.; Sheng, K.; Zhang, P.; Li, C.; Shi, G. Graphene Oxide/Conducting Polymer Composite Hydrogels. J. Mater. Chem. 2011, 21, 18653–18658. [Google Scholar] [CrossRef]
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Jia, Q.; Niu, G.; Huang, X.; Zhou, H.; et al. A Graphene Quantum Dot Photodynamic Therapy Agent with High Singlet Oxygen Generation. Nat. Commun. 2014, 5, 4596. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Sun, H.; Wang, F.; Ren, J.; Qu, X. How Functional Groups Influence the ROS Generation and Cytotoxicity of Graphene Quantum Dots. Chem. Commun. 2017, 53, 10588–10591. [Google Scholar] [CrossRef] [PubMed]
- Chatani, S.; Kloxin, C.J.; Bowman, C.N. The Power of Light in Polymer Science: Photochemical Processes to Manipulate Polymer Formation, Structure, and Properties. Polym. Chem. 2014, 5, 2187–2201. [Google Scholar] [CrossRef] [Green Version]
- Tajik, S.; Dourandish, Z.; Zhang, K.; Beitollahi, H.; Van Le, Q.; Jang, H.W.; Shokouhimehr, M. Carbon and Graphene Quantum Dots: A Review on Syntheses, Characterization, Biological and Sensing Applications for Neurotransmitter Determination. RSC Adv. 2020, 10, 15406–15429. [Google Scholar] [CrossRef] [Green Version]
- Moon, B.J.; Lee, K.S.; Shim, J.; Park, S.; Kim, S.H.; Bae, S.; Park, M.; Lee, C.-L.; Choi, W.K.; Yi, Y.; et al. Enhanced Photovoltaic Performance of Inverted Polymer Solar Cells Utilizing Versatile Chemically Functionalized ZnO@ Graphene Quantum Dot Monolayer. Nano Energy 2016, 20, 221–232. [Google Scholar] [CrossRef]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene Quantum Dots Derived from Carbon Fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef]
- Konstantatos, G.; Badioli, M.; Gaudreau, L.; Osmond, J.; Bernechea, M.; De Arquer, F.P.G.; Gatti, F.; Koppens, F.H. Hybrid Graphene–Quantum Dot Phototransistors with Ultrahigh Gain. Nat. Nanotechnol. 2012, 7, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of Carbon and Graphene Quantum Dots for Sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chang, K.; Peeters, F. Tuning of Energy Levels and Optical Properties of Graphene Quantum Dots. Phys. Rev. B 2008, 77, 235411. [Google Scholar] [CrossRef] [Green Version]
- Das, P.; Ganguly, S.; Banerjee, S.; Das, N.C. Graphene based emergent nanolights: A short review on the synthesis, properties and application. Res. Chem. Intermed. 2019, 45, 3823–3853. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Itzhaki, E.; Hadad, E.; Gedanken, A.; Margel, S. Microwave-Synthesized Polysaccharide-Derived Carbon Dots as Therapeutic Cargoes and Toughening Agents for Elastomeric Gels. ACS Appl. Mater. Interfaces 2020, 12, 51940–51951. [Google Scholar] [CrossRef] [PubMed]
- Khabibullin, A.; Alizadehgiashi, M.; Khuu, N.; Prince, E.; Tebbe, M.; Kumacheva, E. Injectable Shear-Thinning Fluorescent Hydrogel Formed by Cellulose Nanocrystals and Graphene Quantum Dots. Langmuir 2017, 33, 12344–12350. [Google Scholar] [CrossRef]
- Zhang, H.; Ba, S.; Yang, Z.; Wang, T.; Lee, J.Y.; Li, T.; Shao, F. Graphene Quantum Dot-Based Nanocomposites for Diagnosing Cancer Biomarker APE1 in Living Cells. ACS Appl. Mater. Interfaces 2020, 12, 13634–13643. [Google Scholar] [CrossRef]
- Kang, I.; Yoo, J.M.; Kim, D.; Kim, J.; Cho, M.K.; Lee, S.-E.; Kim, D.J.; Lee, B.-C.; Lee, J.Y.; Kim, J.-J.; et al. Graphene Quantum Dots Alleviate Impaired Functions in Niemann-Pick Disease Type C In Vivo. Nano Lett. 2021, 21, 2339–2346. [Google Scholar] [CrossRef]
- Lee, B.-C.; Lee, J.Y.; Kim, J.; Yoo, J.M.; Kang, I.; Kim, J.-J.; Shin, N.; Kim, D.J.; Choi, S.W.; Kim, D.; et al. Graphene Quantum Dots as Anti-Inflammatory Therapy for Colitis. Sci. Adv. 2020, 6, eaaz2630. [Google Scholar] [CrossRef]
- Kim, D.; Yoo, J.M.; Hwang, H.; Lee, J.; Lee, S.H.; Yun, S.P.; Park, M.J.; Lee, M.; Choi, S.; Kwon, S.H.; et al. Graphene Quantum Dots Prevent α-Synucleinopathy in Parkinson’s Disease. Nat. Nanotechnol. 2018, 13, 812–818. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene Quantum Dots: Emergent Nanolights for Bioimaging, Sensors, Catalysis and Photovoltaic Devices. Chem. Commun. 2012, 48, 3686–3699. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-C.; Lee, J.Y.; Kim, J.; Shin, N.; Yoo, J.M.; Kang, I.; Kim, J.-J.; Lee, S.-E.; Kim, D.; Choi, S.W.; et al. Oral Administration of Microbiome-Friendly Graphene Quantum Dots as Therapy for Colitis. 2D Mater. 2021, 8, 025036. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, S.; Wang, G.; Mo, R.; He, P.; Sun, J.; Di, Z.; Kang, Z.; Yuan, N.; Ding, J.; et al. A New Mild, Clean and Highly Efficient Method for the Preparation of Graphene Quantum Dots without By-Products. J. Mater. Chem. B 2015, 3, 6871–6876. [Google Scholar] [CrossRef] [PubMed]
- Sabareesh, K.P.V.; Jena, S.S.; Tata, B.V.R. Dynamic Light Scattering Studies on Photo Polymerized and Chemically Cross-linked Polyacrylamide Hydrogels. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2006; Volume 832, pp. 307–310. [Google Scholar] [CrossRef]
- Matsunaga, T.; Shibayama, M. Gel point determination of gelatin hydrogels by dynamic light scattering and rheological measurements. Phys. Rev. E 2007, 76, 030401. [Google Scholar] [CrossRef] [PubMed]
- Asai, H.; Nishi, K.; Hiroi, T.; Fujii, K.; Sakai, T.; Shibayama, M. Gelation Process of Tetra-PEG Ion-Gel Investigated by Time-Resolved Dynamic Light Scattering. Polymer 2013, 54, 1160–1166. [Google Scholar] [CrossRef]
- Rochas, C.; Geissler, E. Measurement of Dynamic Light Scattering Intensity in Gels. Macromolecules 2014, 47, 8012–8017. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Kumar, V.; Sharma, J. Relaxations in Gelatin Hydrogels Probed by Dynamic Light Scattering. Adv. Mater. 2016, 7, 136–143. [Google Scholar] [CrossRef]
- Kong, H.J.; Wong, E.; Mooney, D.J. Independent Control of Rigidity and Toughness of Polymeric Hydrogels. Macromolecules 2003, 36, 4582–4588. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Singh, S. Analysis of Swelling Behavior of Poly(Methacrylamide-co-Methacrylic Acid) Hydrogels and Effect of Synthesis Conditions on Water Uptake. React. Funct. Polym. 2006, 66, 431–440. [Google Scholar] [CrossRef]
- Gerlach, G.; Guenther, M.; Sorber, J.; Suchaneck, G.; Arndt, K.-F.; Richter, A. Chemical and pH Sensors Based on the Swelling Behavior of Hydrogels. Sens. Actuators B Chem. 2005, 111–112, 555–561. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Y. Relationship between Water Absorbency and Reaction Conditions in Aqueous Solution Polymerization of Polyacrylate Superabsorbents. J. Appl. Polym. Sci. 2000, 75, 808–814. [Google Scholar] [CrossRef]
- Sadeghi, M.; Heidari, B. Crosslinked Graft Copolymer of Methacrylic Acid and Gelatin as a Novel Hydrogel with pH-Responsiveness Properties. Materials 2011, 4, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Hsu, S.-C.; Don, T.-M.; Chiu, W.-Y. Free Radical Degradation of Chitosan with Potassium Persulfate. Polym. Degrad. Stab. 2002, 75, 73–83. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Q.; Dai, Z.; Dai, Y.; Xia, F.; Zhang, X. Nanocomposite Adhesive Hydrogels: From Design to Application. J. Mater. Chem. B 2021, 9, 585–593. [Google Scholar] [CrossRef]
- Li, F.; Zhang, G.; Wang, Z.; Jiang, H.; Yan, S.; Zhang, L.; Li, H. Strong Wet Adhesion of Tough Transparent Nanocomposite Hydrogels for Fast Tunable Focus Lenses. ACS Appl. Mater. Interfaces 2019, 11, 15071–15078. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Wang, S.; Dong, X.; Xu, X.; Cao, X.; Chen, Y.; Zhou, X.; Ding, J.; Yuan, N. A Transparent, Tough Self-Healing Hydrogel Based on a Dual Physically and Chemically Triple Crosslinked Network. J. Mater. Chem. C 2019, 7, 14581–14587. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Song, J.; Park, S.C.; Ahn, M.; Park, M.J.; Song, S.H.; Yoo, S.-Y.; Hong, S.G.; Hong, B.H. Photoinitiated Polymerization of Hydrogels by Graphene Quantum Dots. Nanomaterials 2021, 11, 2169. https://doi.org/10.3390/nano11092169
Kim Y, Song J, Park SC, Ahn M, Park MJ, Song SH, Yoo S-Y, Hong SG, Hong BH. Photoinitiated Polymerization of Hydrogels by Graphene Quantum Dots. Nanomaterials. 2021; 11(9):2169. https://doi.org/10.3390/nano11092169
Chicago/Turabian StyleKim, Yuna, Jaekwang Song, Seong Chae Park, Minchul Ahn, Myung Jin Park, Sung Hyuk Song, Si-Youl Yoo, Seung Gweon Hong, and Byung Hee Hong. 2021. "Photoinitiated Polymerization of Hydrogels by Graphene Quantum Dots" Nanomaterials 11, no. 9: 2169. https://doi.org/10.3390/nano11092169
APA StyleKim, Y., Song, J., Park, S. C., Ahn, M., Park, M. J., Song, S. H., Yoo, S.-Y., Hong, S. G., & Hong, B. H. (2021). Photoinitiated Polymerization of Hydrogels by Graphene Quantum Dots. Nanomaterials, 11(9), 2169. https://doi.org/10.3390/nano11092169





