Pressure-Dependent Clustering in Ionic-Liquid-Poly (Vinylidene Fluoride) Mixtures: An Infrared Spectroscopic Study
Abstract
:1. Introduction
2. Materials and Methods
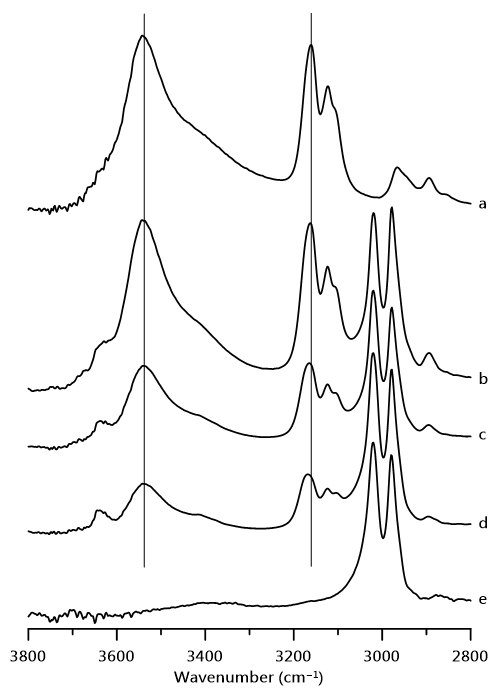
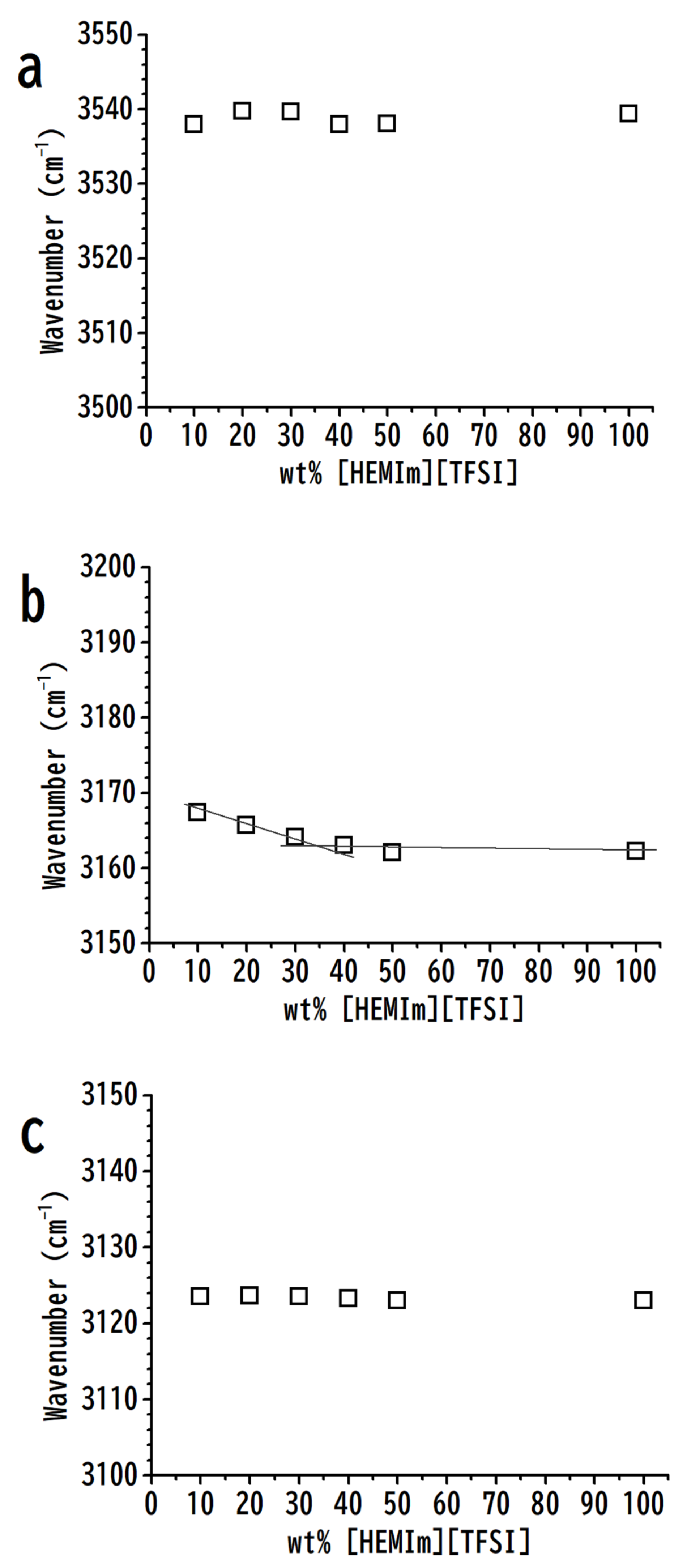
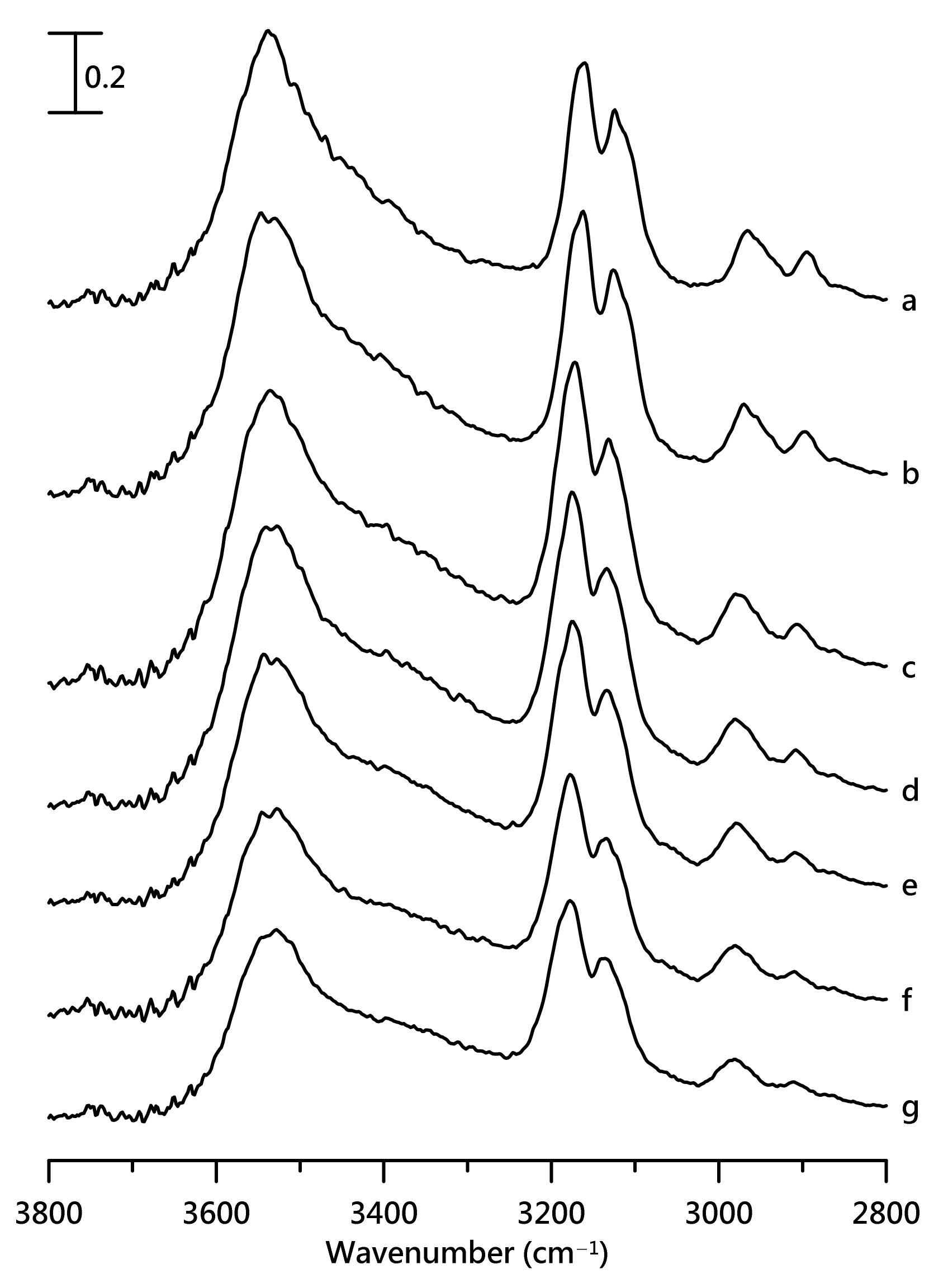
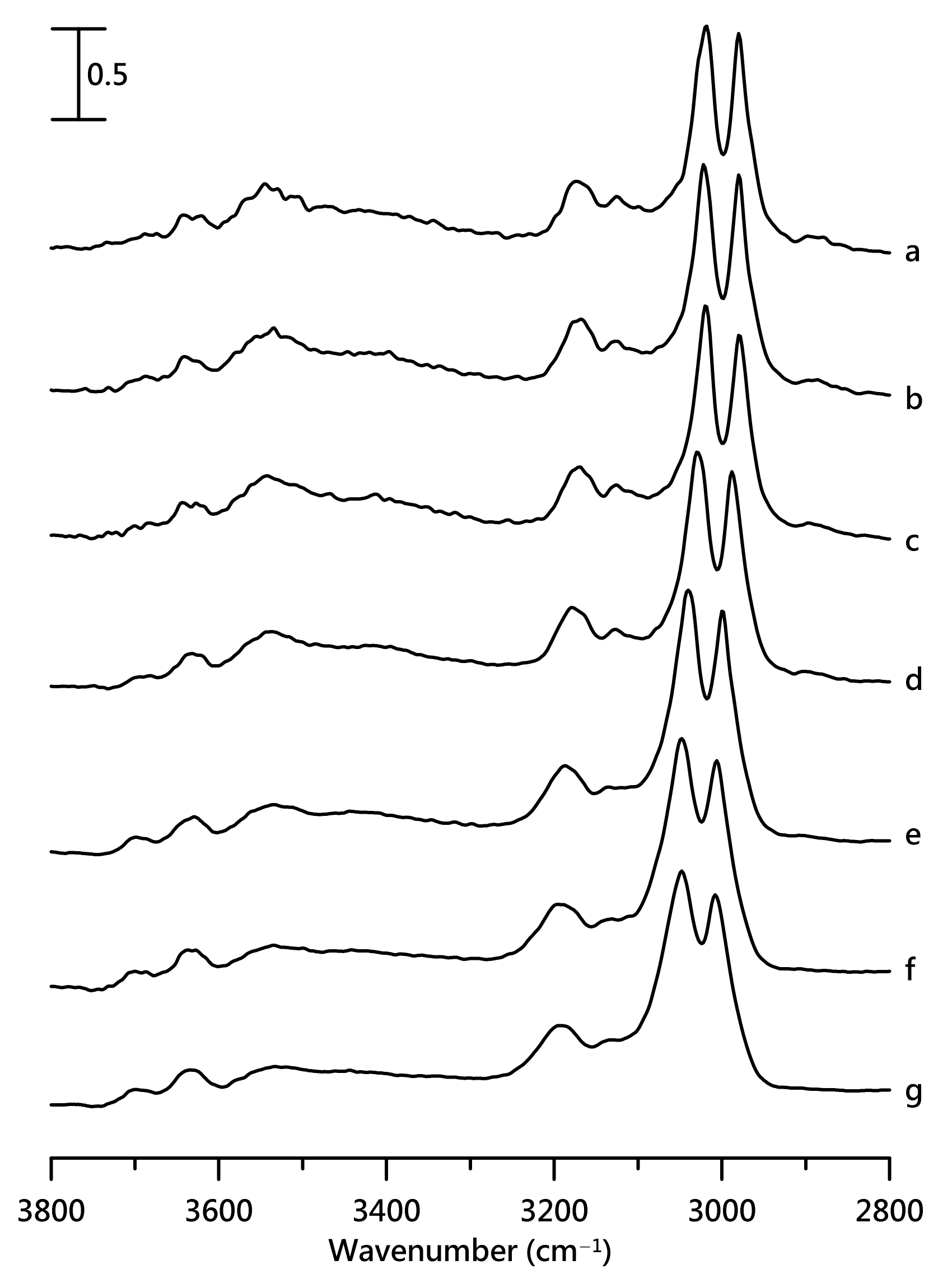
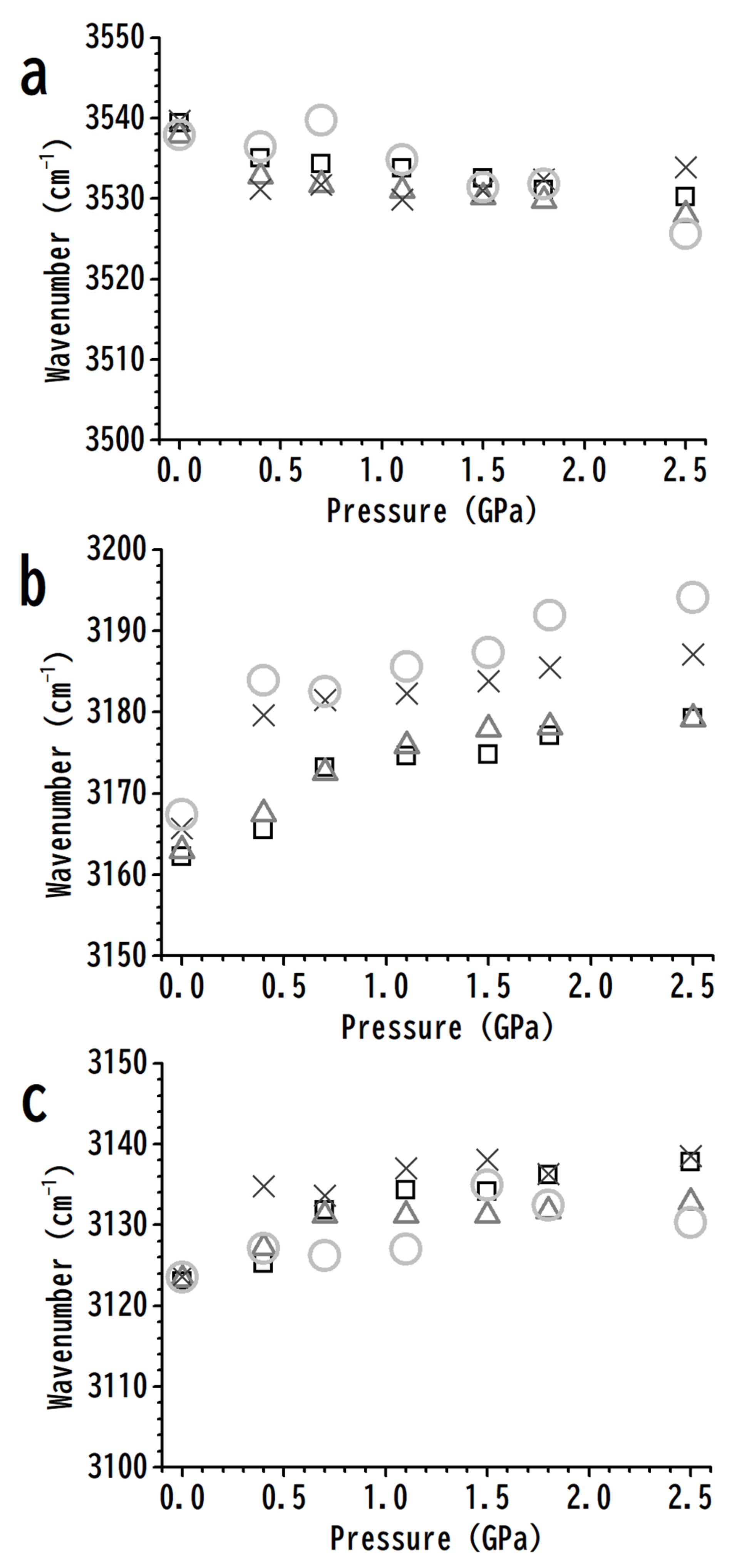
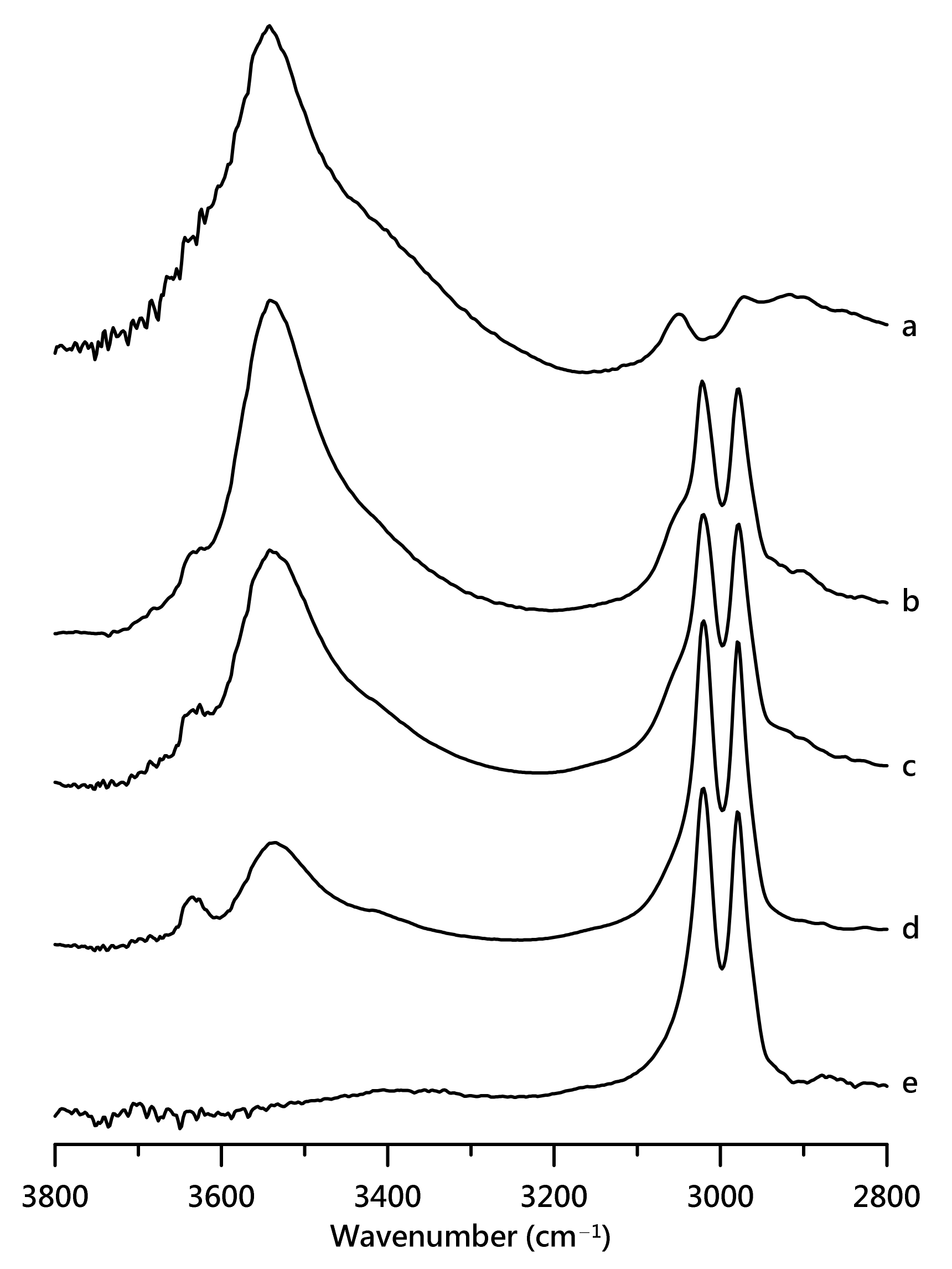
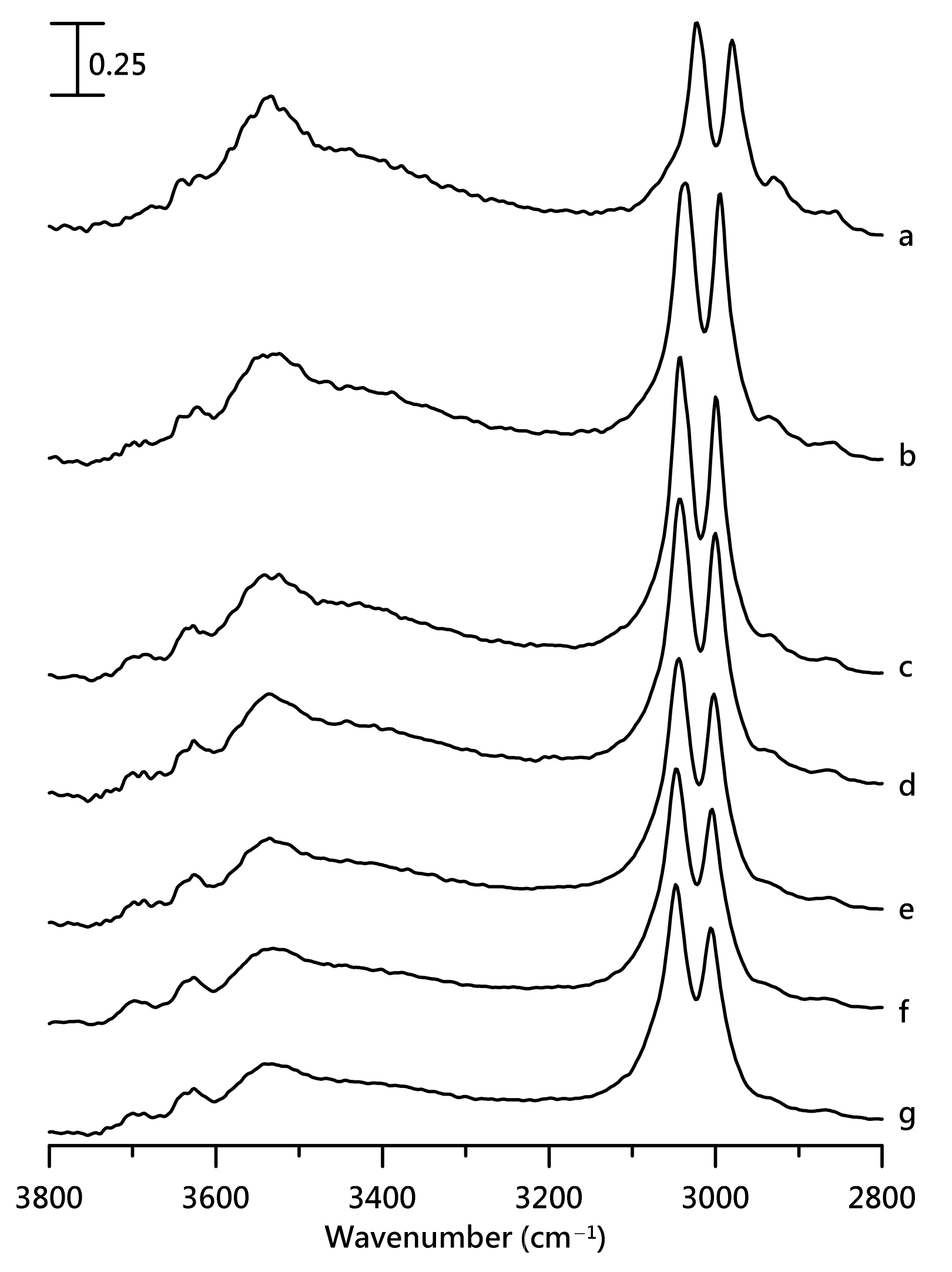
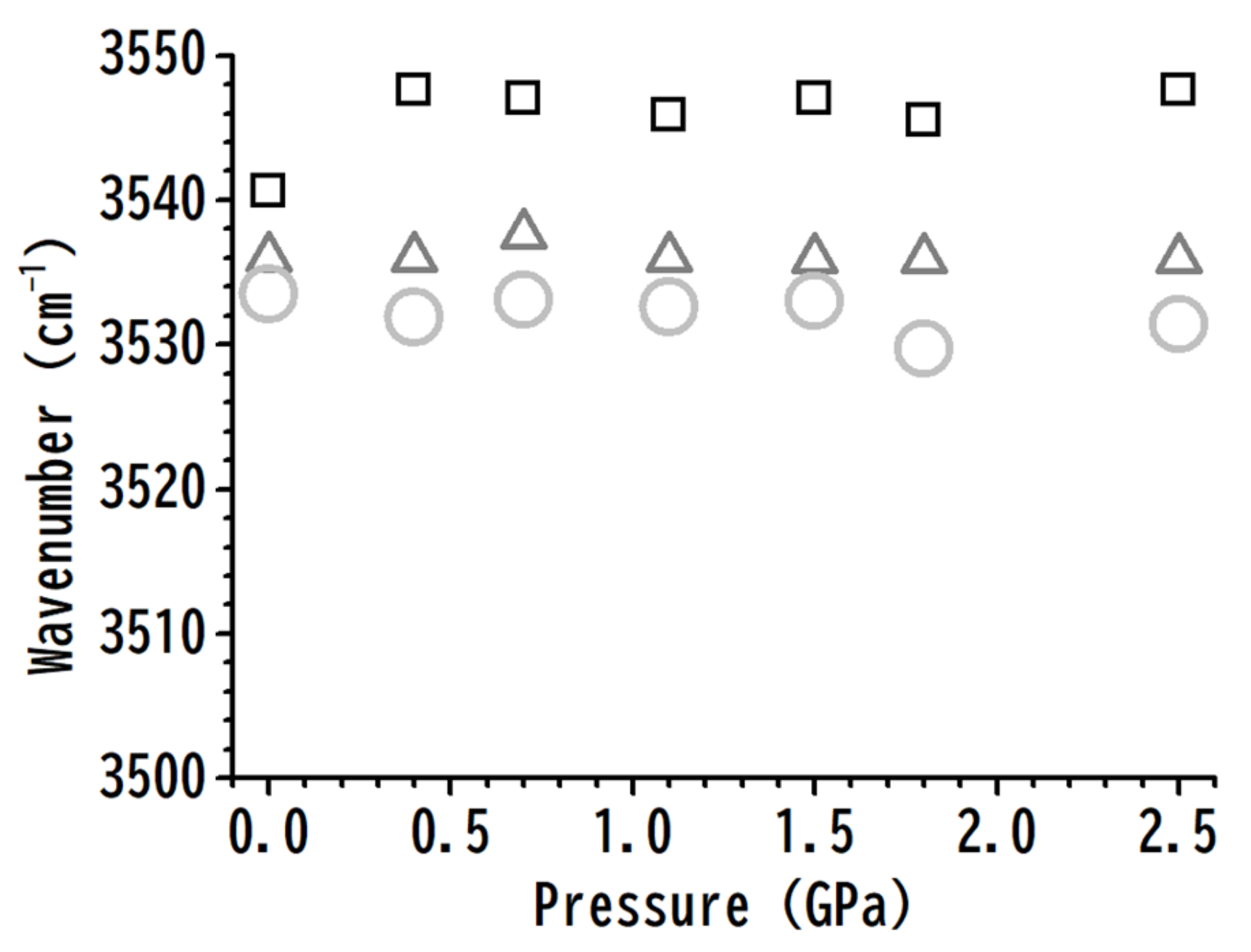
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abu Alhasssan, Z.; Burezq, Y.S.; Nair, R.; Shehata, N. Polyvinylidene Difluoride piezoelectric electrospun nanofibers: Review in synthesis, fabrication, characterizations, and applications. J. Nanomater. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Francis, C.F.J.; Kyratzis, I.L.; Best, A.S. Lithium-ion battery separators for ionic-liquid electrolytes: A review. Adv. Mater. 2020, 32, 1904205. [Google Scholar] [CrossRef]
- Friess, K.; Izák, P.; Kárászová, M.; Pasichnyk, M.; Lanč, M.; Nikolaeva, D.; Luis, P.; Jansen, J.C. A review on ionic liquid gas separation membranes. Membranes 2021, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Duan, X.; Xie, M.; Aw, K.; Xue, Q. Composites, fabrication and application of polyvinylidene fluoride for flexible electromechanical devices: A review. Micromachines 2020, 11, 1076. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Muensit, S. Review of some lesser-known applications of piezoelectric and pyroelectric polymers. Appl. Phys. A 2006, 85, 125–134. [Google Scholar] [CrossRef]
- Wang, X.; Sun, F.; Yin, G.; Wang, Y.; Liu, B.; Dong, M. Tactile-sensing based on flexible PVDF nanofibers via electrospinning: A review. Sensors 2018, 18, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chae, I.; Ahmed, S.; Ben Atitallah, H.; Luo, J.; Wang, Q.; Ounaies, Z.; Kim, S.H. Vibrational Sum frequency generation (SFG) analysis of ferroelectric response of PVDF-based copolymer and terpolymer. Macromolecules 2017, 50, 2838–2844. [Google Scholar] [CrossRef]
- Harstad, S.; D’Souza, N.; Soin, N.; El Gendy, A.; Gupta, S.; Pecharsky, V.K.; Shah, T.; Siores, E.; Hadimani, R.L. Enhancement of β-phase in PVDF films embedded with ferromagnetic Gd5Si4 nanoparticles for piezoelectric energy harvesting. AIP Adv. 2017, 7, 056411. [Google Scholar] [CrossRef]
- Li, H.; Tian, C.; Deng, Z. Energy harvesting from low frequency applications using piezoelectric materials. Appl. Phys. Rev. 2014, 1, 041301. [Google Scholar] [CrossRef] [Green Version]
- Xing, C.; Zhao, L.; You, J.; Dong, W.; Cao, X.; Li, Y. Impact of ionic liquid-modified multiwalled carbon nanotubes on the crystallization behavior of poly(vinylidene fluoride). J. Phys. Chem. B 2012, 116, 8312–8320. [Google Scholar] [CrossRef]
- Xing, C.; Zhao, M.; Zhao, L.; You, J.; Cao, X.; Li, Y. Ionic liquid modified poly(vinylidene fluoride): Crystalline structures, miscibility, and physical properties. Polym. Chem. 2013, 4, 5726–5734. [Google Scholar] [CrossRef]
- Bai, H.; Wang, X.; Zhou, Y.; Zhang, L. Preparation and characterization of poly(vinylidene fluoride) composite membranes blended with nano-crystalline cellulose. Prog. Nat. Sci. 2012, 22, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Silva, W.; Zanatta, M.; Ferreira, A.S.; Corvo, M.C.; Cabrita, E.J. Revisiting ionic liquid structure-property relationship: A critical analysis. Int. J. Mol. Sci. 2020, 21, 7745. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, S.; Liu, X.; Wang, J.; Wang, J.; Dong, K.; Sun, J.; Xu, B. Ionic liquid clusters: Structure, formation mechanism, and effect on the behavior of ionic liquids. Phys. Chem. Chem. Phys. 2014, 16, 5893–5906. [Google Scholar] [CrossRef]
- Hogan, C.J., Jr.; Fernandez de la Mora, J. Ion-pair evaporation from ionic liquid clusters. J. Am. Soc. Mass Spectrom. 2010, 21, 1382–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koßmann, S.; Thar, J.; Kirchner, B.; Hunt, P.A.; Welton, T. Cooperativity in ionic liquids. J. Chem. Phys. 2006, 124, 174506. [Google Scholar] [CrossRef] [Green Version]
- Lei, Z.; Chen, B.; Koo, Y.-M.; MacFarlane, D. Introduction: Ionic liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [Green Version]
- Osada, I.; De Vries, H.; Scrosati, B.; Passerini, S. Ionic-liquid-based polymer electrolytes for battery applications. Angew. Chem. Int. Ed. 2016, 55, 500–513. [Google Scholar] [CrossRef]
- López-Pastor, M.; Ayora-Cañada, M.J.; Valcárcel, M.; Lendl, B. Association of methanol and water in ionic liquids elucidated by infrared spectroscopy using two-dimensional correlation and multivariate curve resolution. J. Phys. Chem. B 2006, 110, 10896–10902. [Google Scholar] [CrossRef]
- Matthews, R.P.; Welton, T.; Hunt, P.A. Hydrogen bonding and pi-pi interactions in imidazolium-chloride ionic liquid clusters. Phys. Chem. Chem. Phys. 2015, 17, 14437–14453. [Google Scholar] [CrossRef]
- Na, S.; Chaurasia, S.K.; Singh, R.K.; Chandra, S. Thermal stability, complexing behavior, and ionic transport of polymeric gel membranes based on polymer PVdF-HFP and ionic liquid, [BMIM][BF4]. J. Phys. Chem. B 2013, 117, 897–906. [Google Scholar]
- Umebayashi, Y.; Jiang, J.-C.; Shan, Y.-L.; Lin, K.-H.; Fujii, K.; Seki, S.; Ishiguro, S.-I.; Lin, S.H.; Chang, H.-C. Structural change of ionic association in ionic liquid/water mixtures: A high-pressure infrared spectroscopic study. J. Chem. Phys. 2009, 130, 124503. [Google Scholar] [CrossRef] [Green Version]
- Yokozeki, A.; Kasprzak, D.J.; Shiflett, M.B. Thermal effect on C–H stretching vibrations of the imidazolium ring in ionic liquids. Phys. Chem. Chem. Phys. 2007, 9, 5018–5026. [Google Scholar] [CrossRef] [PubMed]
- Calandra, P.; Szerb, E.; Lombardo, D.; Algieri, V.; De Nino, A.; Maiuolo, L. A presentation of ionic liquids as lubricants: Some critical comments. Appl. Sci. 2021, 11, 5677. [Google Scholar] [CrossRef]
- Niemann, T.; Zaitsau, D.; Strate, A.; Villinger, A.; Ludwig, R. Cationic clustering influences the phase behaviour of ionic liquids. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, V.; Litvinchuk, A.; Todorov, N.; Abrashev, M.; Marinova, V. Infrared response of α-and β-phases of LiFe5O8. Phys. Rev. B 2011, 84, 094111. [Google Scholar] [CrossRef]
- Ivanov, V.G.; Hadjichristov, G.; Faulques, E. Characterization of chemical bonding in ion-implanted polymers by means of mid-infrared reflectivity. Appl. Spectrosc. 2009, 63, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Jemmis, E. Red-, blue-, or no-shift in hydrogen bonds: A unified explanation. J. Am. Chem. Soc. 2007, 129, 4620–4632. [Google Scholar] [CrossRef]
- Qian, W.; Krimm, S. Limitations of the molecular multipole expansion treatment of electrostatic interactions for C−H⊙⊙⊙ O and O−H⊙⊙⊙ O hydrogen bonds and application of a general charge density approach. J. Phys. Chem. A 2005, 109, 5608–5618. [Google Scholar] [CrossRef]
- Gu, Y.; Kar, T.; Scheiner, S. Fundamental properties of the CH⊙⊙⊙ O interaction: Is it a true hydrogen bond? J. Am. Chem. Soc. 1999, 121, 9411–9422. [Google Scholar] [CrossRef]
- Masunov, A.; Dannenberg, J.J.; Contreras, R.H. C−H bond-shortening upon hydrogen bond formation: Influence of an electric field. J. Phys. Chem. A 2001, 105, 4737–4740. [Google Scholar] [CrossRef]
- Wang, T.-H.; Hsu, L.-W.; Chang, H.-C. Structural reorganization of imidazolium ionic liquids induced by pressure-enhanced ionic liquid—Polyethylene oxide interactions. Int. J. Mol. Sci. 2021, 22, 981. [Google Scholar] [CrossRef]
- Wang, T.H.; Shen, M.H.; Chang, H.C. Pressure-dependent stability of imidazolium-based ionic liquid/DNA materials investigated by high-pressure infrared spectroscopy. Materials 2019, 12, 4202. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-H.; Chang, H.-C.; Fu, Y.-P. Utilizing infrared spectroscopy to analyze the interfacial structures of ionic liquids/Al2O3 and ionic liquids/mica mixtures under high pressures. Nanomaterials 2019, 9, 373. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.-H.; Lin, E.-Y.; Chang, H.-C. Pressure-dependent confinement effect of ionic liquids in porous silica. Nanomaterials 2019, 9, 620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.-H.; Wu, M.-S.; Chang, H.-C. Characterization of local structures of confined imidazolium ionic liquids in PVdF-co-HFP matrices by high pressure infrared spectroscopy. Nanomaterials 2020, 10, 1973. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.T.T.; Moffatt, D.J. The uncoupled O-H or O-D stretch in water as an internal pressure gauge for high-pressure infrared spectroscopy of aqueous systems. Appl. Spectrosc. 1987, 41, 1070–1072. [Google Scholar] [CrossRef]
- Wong, P.T.T.; Moffatt, D.J.; Baudais, F.L. Crystalline quartz as an internal pressure calibrant for high-pressure infrared spectroscopy. Appl. Spectrosc. 1985, 39, 733–735. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Tokuda, H.; Hayamizu, K.; Watanabe, M. Magnitude and directionality of interaction in ion pairs of ionic liquids: Relationship with ionic conductivity. J. Phys. Chem. B 2005, 109, 16474–16481. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.-H.; Wang, W.-X.; Chang, H.-C. Pressure-Dependent Clustering in Ionic-Liquid-Poly (Vinylidene Fluoride) Mixtures: An Infrared Spectroscopic Study. Nanomaterials 2021, 11, 2099. https://doi.org/10.3390/nano11082099
Wang T-H, Wang W-X, Chang H-C. Pressure-Dependent Clustering in Ionic-Liquid-Poly (Vinylidene Fluoride) Mixtures: An Infrared Spectroscopic Study. Nanomaterials. 2021; 11(8):2099. https://doi.org/10.3390/nano11082099
Chicago/Turabian StyleWang, Teng-Hui, Wei-Xiang Wang, and Hai-Chou Chang. 2021. "Pressure-Dependent Clustering in Ionic-Liquid-Poly (Vinylidene Fluoride) Mixtures: An Infrared Spectroscopic Study" Nanomaterials 11, no. 8: 2099. https://doi.org/10.3390/nano11082099
APA StyleWang, T.-H., Wang, W.-X., & Chang, H.-C. (2021). Pressure-Dependent Clustering in Ionic-Liquid-Poly (Vinylidene Fluoride) Mixtures: An Infrared Spectroscopic Study. Nanomaterials, 11(8), 2099. https://doi.org/10.3390/nano11082099






