Biological Potential of Silver Nanoparticles Mediated by Leucophyllum frutescens and Russelia equisetiformis Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Preparation of Aqueous Extracts for AgNPs Synthesis
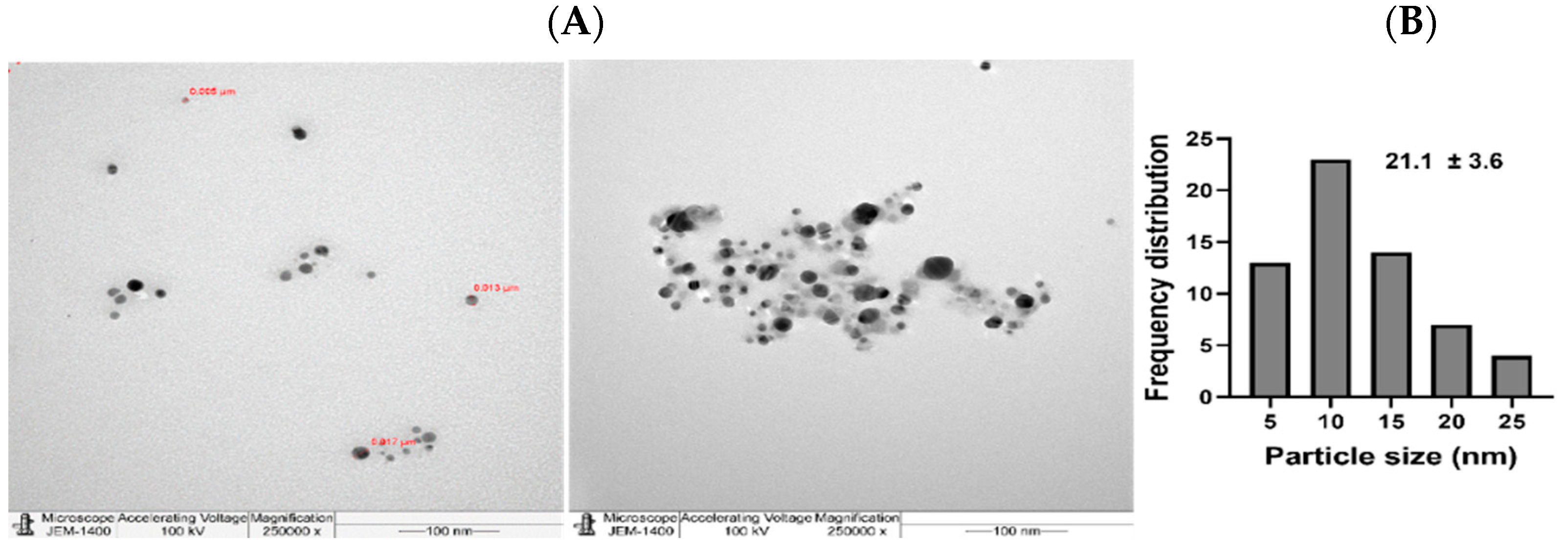
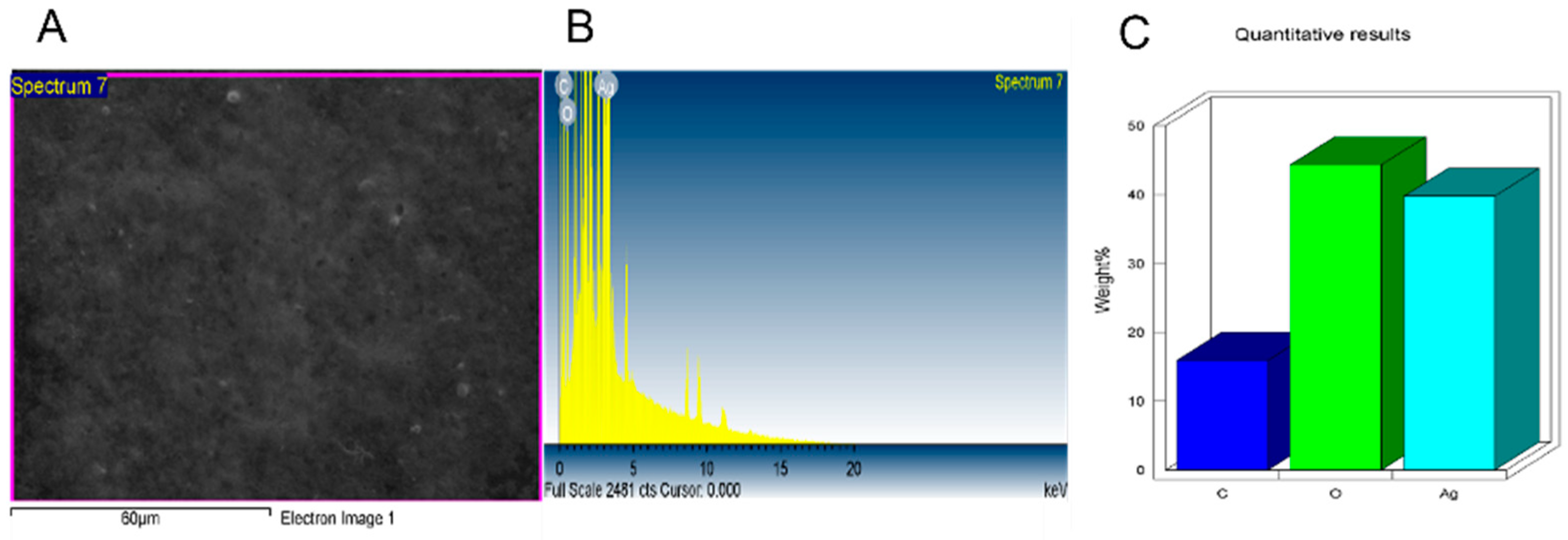
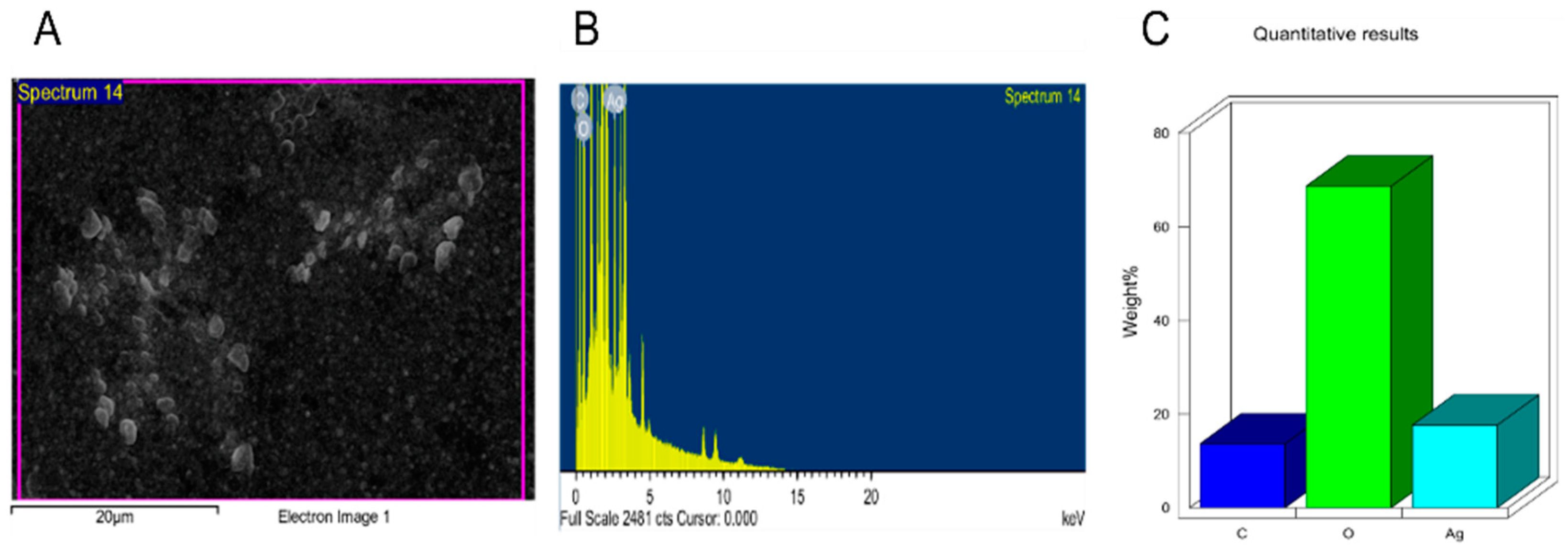
2.3. NPs Characterization
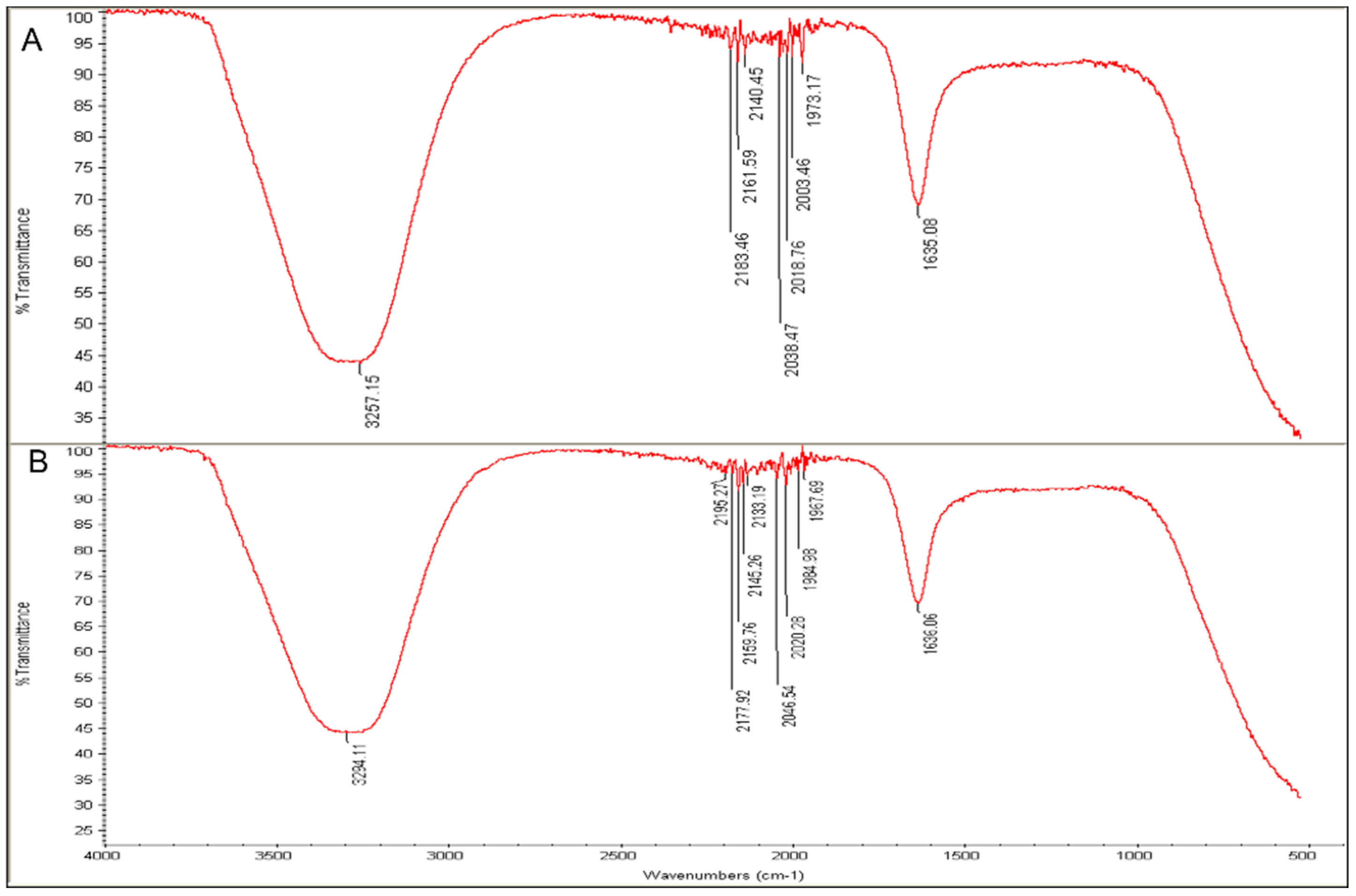
2.4. Analysis of Surface Functional Groups
2.5. Antibacterial Screening
2.6. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
2.7. Ampicillin-Conjugated NPs (Amp-NPs) Synthesis and Application
2.8. Anticancer Action Induced by Biogenic NPs
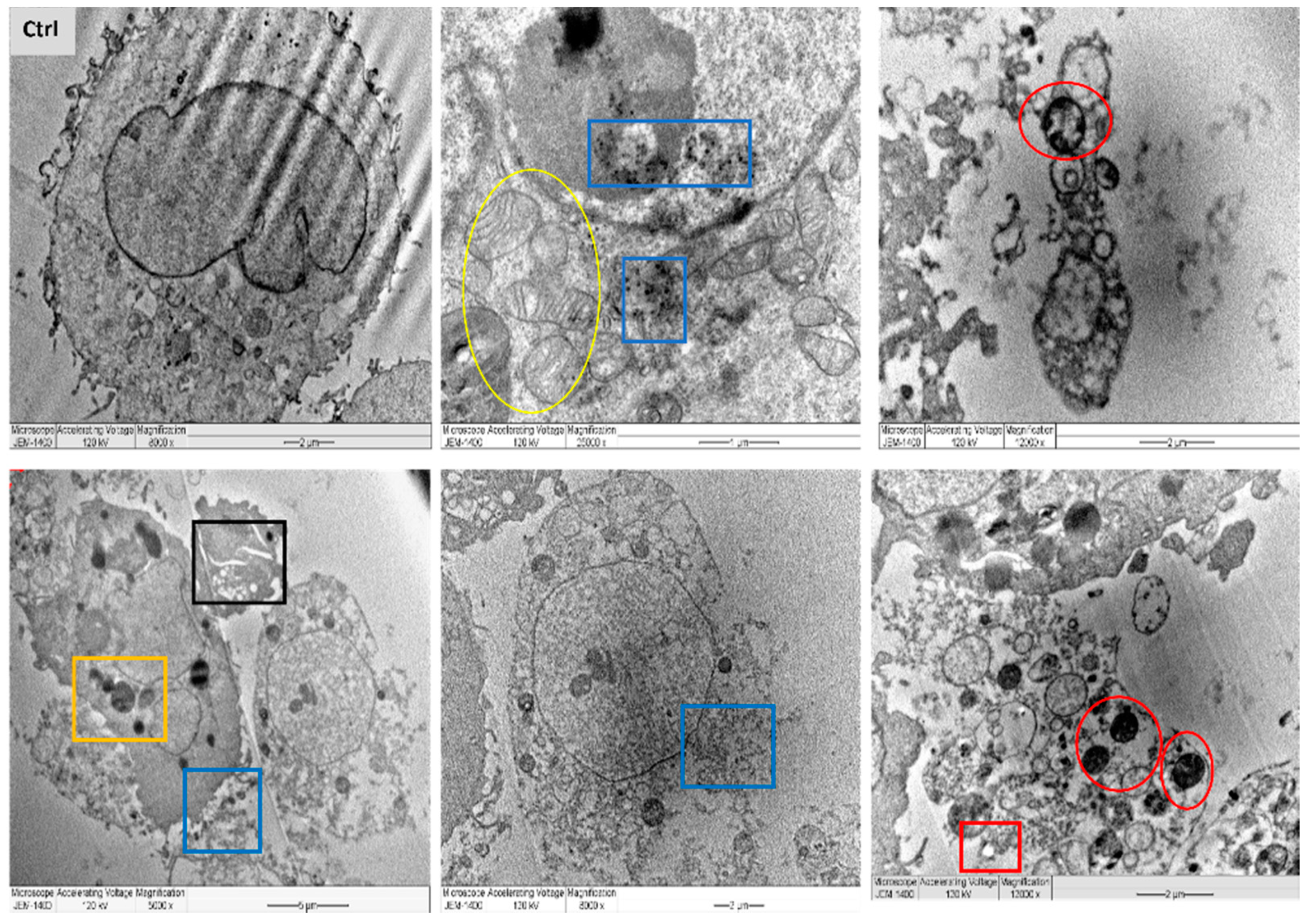
2.9. Ultrastructural Cell Changes by Transmission Electron Microscopy
2.10. Laser Scanning Microscopy (LSM)
2.11. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronflehet, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Li, S.; Liu, H.; Deng, Y.; Lin, L.; He, N. Development of a magnetic nanoparticles microarray for simultaneous and simple detection of foodborne pathogens. J. Biomed. Nanotechnol. 2013, 9, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold Nanoparticles in Biomedical Applications: Recent Advances and Perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Gil, P.R.; Zhang, F.; Zanella, M.; Parak, W.J. Biological applications of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1896–1908. [Google Scholar] [CrossRef]
- Noruzi, M.; Zare, D.; Davoodi, D. A rapid biosynthesis route for the preparation of gold nanoparticles by aqueous extract of cypress leaves at room temperature. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 94, 84–88. [Google Scholar] [CrossRef]
- Rosi, N.; Mirkin, C.A. Nanostructures in Biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.H.; Ramstroem, O.; Yan, M. Engineering nanomaterial surfaces for biomedical applications. Exp. Biol. Med. 2009, 234, 1128–1139. [Google Scholar] [CrossRef] [Green Version]
- Weir, E.; Lawlor, A.; Whelan, A.; Regan, F. The use of nanoparticles in anti-microbial materials and their characterization. Analyst 2008, 133, 835–845. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Dose the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 27, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Yang, D.; Ivleva, N.P.; Mircescu, N.E.; Niessner, R.; Haisch, C. SERS detection of bacteria in water by in situ coating with Ag nanoparticles. Anal. Chem. 2014, 83, 1525–1533. [Google Scholar] [CrossRef]
- Da Silva, B.L.; Abuçafy, M.P.; Manaia, E.B.; Junior, J.A.O.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacciet, L.A. Relationship between structure and antimicrobial activity of zinc oxide nanoparticles: An overview. Int. J. Nanomed. 2019, 14, 9395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [Green Version]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Khandel, P.; Yadaw, R.K.; Soni, D.K.; Kanwar, L.; Shahi, S.K. Biogenesis of metal nanoparticles and their pharmacological applications: Present status and application prospects. J. Nanostruct. Chem. 2018, 8, 217–254. [Google Scholar] [CrossRef] [Green Version]
- Abou El-Nour, K.M.; Eftaiha, A.A.; Al-Warthan, A.; Ammar, R.A. Synthesis and applications of silver nanoparticles. Arabian J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xu, H.; Chen, Z.S.; Chen, G. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomat. 2011, 2011, 270974. [Google Scholar] [CrossRef] [Green Version]
- Dhillon, G.S.; Brar, S.K.; Kaur, S.; Verma, M. Green approach for nanoparticle biosynthesis by fungi: Current trends and applications. Crit. Rev. Biotechnol. 2012, 32, 49–73. [Google Scholar] [CrossRef]
- Azizi, S.; Namvar, F.; Mahdavi, M.; Ahmad, M.B.; Mohamad, R. Biosynthesis of silver nanoparticles using brown marine macroalga, Sargassum muticum aqueous extract. Materials 2013, 6, 5942–5950. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; Lima, R.D. Synthesis of silver nanoparticles mediated by fungi: A review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.E.; Al-Qahtani, A.; Al-Mutairi, A.; Al-Shamrim, B.; Aabed, K. Antibacterial and cytotoxic potential of biosynthesized silver nanoparticles by some plant extracts. Nanomaterials 2018, 8, 382. [Google Scholar] [CrossRef] [Green Version]
- Aabed, K.; Mohammed, A.E. Synergistic and Antagonistic Effects of Biogenic Silver Nanoparticles in Combination with Antibiotics against Some Pathogenic Microbes. Front. Bioeng. Biotechnol. 2021, 9, 249. [Google Scholar] [CrossRef]
- Algebaly, A.S.; Mohammed, A.A.; Abutaha, N.; Elobeid, M.M. cBiogenic synthesis of silver nanoparticles: Antibacterial and cytotoxic potential. Saudi. J. Biolog. Sci. 2020, 27, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Choudhury, B.K.; Sarkar, T.; Gogoi, S.K.; Kakati, N.; Baishya, D.; Kari, Z.A.; Edinur, H.A. Green Synthesis of Silver Nanoparticles Using Diospyros malabarica Fruit Extract and Assessments of Their Antimicrobial, Anticancer and Catalytic Reduction of 4-Nitrophenol (4-NP). Nanomaterials 2021, 11, 1999. [Google Scholar] [CrossRef]
- Beer, C.; Foldbjerg, R.; Hayashi, Y.; Sutherland, D.S.; Autrup, H. Toxicity of silver nanoparticles—Nanoparticle or silver ion? Toxicol. Lett. 2012, 208, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Długosz, O.; Szostak, K.; Staroń, A.; Pulit-Prociak, J.; Banach, M. Methods for reducing the toxicity of metal and metal oxide NPs as biomedicine. Materials 2020, 13, 279. [Google Scholar] [CrossRef] [Green Version]
- Jabbar, A.H.; Hamzah, M.Q.; Mezan, S.O.; Binti Ameruddin, A.S.; Agam, M.A. Green Synthesis of Silver/Polystyrene Nano composite (Ag/PS NCs) via Plant Extracts Beginning a New Era in Drug Delivery. Indian J. Sci. Technol. 2018, 11, 22. [Google Scholar] [CrossRef]
- Alanís-Garza, B.; Salazar-Aranda, R.; Ramírez-Durón, R.; Garza-González, E.; de Torres, N.W. A new antimycobacterial furanolignan from Leucophyllum frutescens. Nat. Prod. Comm. 2012, 7, 1934578X1200700512. [Google Scholar]
- Younis, A.; Riaz, A.; Tariq, U.; Nadeem, M.; Khan, N.A.; Ahsan, M.; Adil, W.; Naseem, M.K. Drought tolerance of Leucophyllum frutescens: Physiological and morphological studies reveal the potential xerophyte. Acta Sci. Pol. Hortorum Cultus. 2017, 16, 85–94. [Google Scholar] [CrossRef]
- Beltrán-Peña, H.; Felix-Gastelum, R.; Camacho-Tapia, M.; Correia, K.C.; Herrera-Rodriguez, G.; Garcia-Estrada, R.S.; Tovar-Pedraza, J.M. First Report of Powdery Mildew on Leucophyllum frutescens Caused by Podosphaera xanthii in Mexico. Plant Dis. 2021, 105, 706. [Google Scholar] [CrossRef] [PubMed]
- Molina-Salinas, G.M.; Rivas-Galindo, V.M.; Said-Fernandez, S.; Lankin, D.C.; Muñoz, M.A.; Joseph-Nathan, P.; Pauli, G.F.; Waksman, N. Stereochemical analysis of an anti-TB active diterpene from Leucophyllum frutescens. J. Nat. Prod. 2011, 74, 1842–1850. [Google Scholar] [CrossRef] [Green Version]
- Riaz, M. Biological and Phytochemical Studies of Selected Medicinal Plants from the Family Scrophulariaceae. Ph.D. Thesis, GC University, Faisalabad, Pakistan, 2013. [Google Scholar]
- Ahmed, E.; Desoukey, S.; Fouad, M.; Kamel, M. A pharmacognostical study of Russelia equisetiformis Sch. & Cham. Int. J. Pharm. Phytochem. Res. 2016, 8, 174–192. [Google Scholar]
- Gilman, E.F. Russelia Equisetiformis. Environmental Horticultural Department, Cooperative Extension Service. Fact Sheet FPS-516; Institute of Food and Agricultural Sciences University of Florida: Gainesville, FL, USA, 1999. [Google Scholar]
- Awe, E.O.; Makinde, J.M.; Adeloye, O.A.; Banjoko, S.O. Membrane stabilizing activity of Russelia equisetiformis, Schlecht & Chan. J. Nat. Prod. 2009, 2, 3–9. [Google Scholar]
- Mie, R.; Samsudin, M.W.; Din, L.B.; Ahmad, A.; Ibrahim, N.; Adnan, S.N.A. Synthesis of silver nanoparticles with antibacterial activity using the lichen Parmotrema praesorediosum. Int. J. Nanomed. 2014, 9, 121. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.; Singh, A.R.J.; Sasikumar, C.S. Green synthesis of Bio-Silver Nanoparticles by Parmelia perlata, Ganoderma lucidum and Phellinus igniarius & Their Fields of Application. Indian J. Res. Pharm. Biotech. 2015, 5674, 100–110. [Google Scholar]
- Siddiqi, K.S.; Rashid, M.; Rahman, A.; Husen, A.; Rehman, S. Biogenic fabrication and characterization of silver nanoparticles using aqueous-ethanolic extract of lichen (Usnea longissima) and their antimicrobial activity. Biomater. Res. 2018, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pauer, A.C.; Gonzales, A.A.; Fenniri, H. Enhanced antibiotic activity of ampicillin conjugated to gold nanoparticles on PEGylated rosette nanotubes. Int. J. Nanomed. 2019, 14, 7281–7289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, R.; Al Zahrani, H.; Barhoumi, T.; Alhallaj, A.; Mashhour, A.; Alshammari, M.A.; Alshawakir, Y.A.; Baz, O.; Alanazi, A.H.; Khan, A.L.; et al. Isolation and Establishment of a Highly Proliferative, Cancer Stem Cell-Like, and Naturally Immortalized Triple-Negative Breast Cancer Cell Line, KAIMRC2. Cells 2021, 10, 1303. [Google Scholar] [CrossRef]
- Tripathy, A.; Ashok, M.; Raichur, A.; Chandrasekaran, N.; Prathna, T.C.; Amitava, M. Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachta indica (Neem) leaves. J. Nanopart. Res. 2010, 12, 237–246. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Bharath, L.V. Mechanism of plant-mediated synthesis of silver nanoparticles—A review on biomolecules involved, characterization and antibacterial activity. Chem. Biol. Interact. 2017, 273, 219–227. [Google Scholar] [CrossRef]
- Banerjee, P.; Nath, D. A phytochemical approach to synthesize silver nanoparticles for non-toxic biomedical application and study on their antibacterial efficacy. Nanosci. Technol. 2015, 2, 1–14. [Google Scholar]
- Sujitha, M.V.; Kannan, S. Green synthesis of gold nanoparticles using Citrus fruits (Citrus limon, Citrus reticulata and Citrus sinensis) aqueous extract and its characterization. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 102, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Leela, K.; Anchana, D.C. A Study on the Applications of Silver Nanoparticle Synthesized Using the Aqueous Extract and the Purified Secondary Metabolites of Lichen Parmelia perlata. Int. J. Pharmac. Sci. Invent 2017, 6, 42–59. [Google Scholar]
- Dasari, S.; Suresh, K.A.; Rajesh, M.; Reddy, S.; Samba, C.; Hemalatha, C.S.; Wudayagiri, R.; Valluru, L. Biosynthesis, characterization, antibacterial and antioxidant activity of silver nanoparticles produced by lichens. J. Bionanosci. 2013, 7, 237–244. [Google Scholar] [CrossRef]
- Khandel, P.; Kumar Shahi, S.; Kanwar, L.; Kumar Yadaw, R.; Kumar Soni, D. Biochemical profiling of microbes inhibiting Silver nanoparticles using symbiotic organisms. Int. J. Nano Dimens. 2018, 9, 273–285. [Google Scholar]
- Miller, G.P.; Bhat, W.W.; Lanier, E.R.; Johnson, S.R.; Mathieu, D.T.; Hamberger, B. The biosynthesis of the anti-microbial diterpenoid leubethanol in Leucophyllum frutescens proceeds via an all-cis prenyl intermediate. Plant J. 2020, 104, 693–705. [Google Scholar] [CrossRef]
- Din, L.B.; Mie, R.; Samsudin, M.W.; Ahmad, A.; Ibrahim, N. Biomimetic synthesis of silver nanoparticles using the lichen Ramalina dumeticola and the antibacterial activity. Malays. J. Analyt. Sci. 2015, 19, 369–376. [Google Scholar]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive oxygen species-related nanoparticle toxicity in the biomedical field. Nanoscale Res. Lett. 2020, 15, 1–14. [Google Scholar] [CrossRef]
- Balderas-Renteria, I.; Camacho-Corona, M.R.; Carranza-Rosales, P.; Lozano-Garza, H.G.; Castillo-Nava, D.; Alvarez-Mendoza, F.J.; Tamez-Cantú, E.M. Hepatoprotective effect of Leucophyllum frutescens on Wistar albino rats intoxicated with carbon tetrachloride. Ann. Hepatol. 2007, 6, 251–254. [Google Scholar] [CrossRef]
- Ojurongbe, O.; Ojo, J.A.; Adefokun, D.I.; Abiodun, O.O.; Odewale, G.; Awe, E.O. In vivo Antimalarial Activities of Russelia Equisetiformis in Plasmodium Berghei Infected Mice. Indian J. Pharm. Sci. 2015, 77, 504–510. [Google Scholar] [CrossRef] [Green Version]
- Kolawole, O.T.; Kolawole, S.O. Effects of Russelia equisetiformis methanol and aqueous extracts on hepatic function indices. Biol. Med. 2010, 2, 38–41. [Google Scholar]
- Miller, M.L.; Andringa, A.; Dixon, K.; Carty, M.P. Insights into UV-induced apoptosis: Ultrastructure, trichrome stain and spectral imaging. Micron 2002, 33, 157–166. [Google Scholar] [CrossRef]
- Vieira, L.F.D.A.; Lins, M.P.; Viana, I.M.M.N.; Dos Santos, J.E.; Smaniotto, S.; Reis, M.D.D.S. Metallic nanoparticles reduce the migration of human fibroblasts in vitro. Nanoscale Res. Lett. 2017, 12, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrader, M.; Fahimi, H.D. Mammalian peroxisomes and reactive oxygen species. Histochem. Cell Biol. 2004, 122, 383–393. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Liu, L.; Lu, T.; Wang, Y. An efficient gold nanocarrier for combined chemo-photodynamic therapy on tumour cells. RSC Adv. 2015, 5, 34831–34838. [Google Scholar] [CrossRef]
- Shafiu Kamba, A.; Ismail, M.; Tengku Ibrahim, T.A.; Zakaria, Z.A.B.; Hassan Gusau, L. In vitro ultrastructural changes of MCF-7 for metastasise bone cancer and induction of apoptosis via mitochondrial cytochrome C released by CaCO3/Dox nanocrystals. Biomed Res. Int. 2014, 2014, 391869. [Google Scholar] [CrossRef]
- Palvai, S.; Kuman, M.M.; Sengupta, P.; Basu, S. Hyaluronic acid layered chimeric nanoparticles: Targeting MAPK-PI3K signaling hub in colon cancer cells. ACS Omega 2017, 2, 7868–7880. [Google Scholar] [CrossRef]
- Mahalingaiah, P.K.; Singh, K.P. Chronic oxidative stress increases growth and tumorigenic potential of MCF-7 breast cancer cells. PLoS ONE 2014, 9, e87371192. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.G.; Piskounova, E.; Morrison, S.J. Cancer, oxidative stress, and metastasis. Cold Spring Harb. Symp. Quant. Biol. 2016, 81, 163–175. [Google Scholar] [CrossRef] [Green Version]
- Menchaca, M.D.C.V.; Morales, C.R.; Star, J.V.; Or, A.; Morales, M.E.R.; Nuntilde, M.A.; Gallardo, L.B.S. Antimicrobial activity of five plants from Northern Mexico on medically important bacteria. Afr. J. Microbiol. Res. 2013, 7, 5011–5017. [Google Scholar]
- Olorunju, A.E.; Adewale, A.; Modupe, M.J. Anti-inflammatory activity of Russelia equisetiformis Schlecht & Cham: Identification of its active constituent. J. Complement Med. Res. 2012, 1, 25–29. [Google Scholar]
- Siemer, S.; Westmeier, D.; Barz, M.; Eckrich, J.; Wunsch, D.; Seckert, C.; Thyssen, C.; Schilling, O.; Hasenberg, M.; Pang, C.; et al. Biomolecule-corona formation confers resistance of bacteria to nanoparticle-induced killing: Implications for the design of improved nanoantibiotics. Biomaterials 2019, 192, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.A.; Al Othman, M.R.; Mohammed, A.E. Bio fabrication of silver nanoparticles with antibacterial and cytotoxic abilities using lichens. Sci. Rep. 2020, 10, 16781. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [Green Version]
- Patra, P.; Mitra, S.; Debnath, N.; Pramanik, P.; Goswami, A. Ciprofloxacin conjugated zinc oxide nanoparticle: A camouflage towards multidrug resistant bacteria. Bull. Mater. Sci. 2014, 37, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Zendegani, E.; Dolatabadi, S. The efficacy of imipenem conjugated with synthesized silver nanoparticles against Acinetobacter baumannii clinical isolates, Iran. Biol. Trace Elem. Res. 2019, 197, 330–340. [Google Scholar] [CrossRef]
- Shahbandeh, M.; Eghdami, A.; Moghaddam, M.M.; Nadoushan, M.J.; Salimi, A.; Fasihi-Ramandi, M.; Mohammadi, S.; Mirzaei, M.; Mirnejad, R. Conjugation of imipenem to silver nanoparticles for enhancement of its antibacterial activity against multidrug-resistant isolates of Pseudomonas aeruginosa. J. Biosci. 2021, 46, 26. [Google Scholar] [CrossRef]
- Naqvi, S.Z.; Kiran, U.; Ali, M.I.; Jamal, A.; Hameed, A.; Ahmed, S.; Ali, N. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int. J. Nanomed. 2013, 8, 3187–3195. [Google Scholar] [CrossRef] [Green Version]
- Masri, A.; Anwar, A.; Ahmed, D.; Siddiqui, R.B.; Shah, M.R.; Khan, N.A. Silver Nanoparticle Conjugation-Enhanced Antibacterial Efficacy of Clinically Approved Drugs Cephradine and Vildagliptin. Antibiotics 2018, 7, 100. [Google Scholar] [CrossRef] [Green Version]
- Payne, J.N.; Wahwani, H.K.; Connor, M.G.; Hamilton, L.; Tockstein, S.; Moolani, H.; Chavda, F.; Badwaik, V.; Lawrenz, M.B.; Dakshinamurthy, R. Novel Synthesis of Kanamycin Conjugated Gold Nanoparticles with Potent Antibacterial Activity. Front. Microbiol. 2016, 7, 607. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef] [Green Version]


 ) and MDA-MB-231 (
) and MDA-MB-231 (  ) and a normal cell line; MCF 10A (
) and a normal cell line; MCF 10A (  ) (A). Log dose-response relationship of L-AgNPs on the normalised viability of two human cancer cell lines; HCT116 (
) (A). Log dose-response relationship of L-AgNPs on the normalised viability of two human cancer cell lines; HCT116 (  ) and MDA-MB-231 (
) and MDA-MB-231 (  ) and one normal cell line MCF 10A (
) and one normal cell line MCF 10A (  ) (B). IC50 values of AgNPs on each cell line was calculated using Log viability vs. normalised response–variable slope (four-parameter).
) (B). IC50 values of AgNPs on each cell line was calculated using Log viability vs. normalised response–variable slope (four-parameter).
 ) and MDA-MB-231 (
) and MDA-MB-231 (  ) and a normal cell line; MCF 10A (
) and a normal cell line; MCF 10A (  ) (A). Log dose-response relationship of L-AgNPs on the normalised viability of two human cancer cell lines; HCT116 (
) (A). Log dose-response relationship of L-AgNPs on the normalised viability of two human cancer cell lines; HCT116 (  ) and MDA-MB-231 (
) and MDA-MB-231 (  ) and one normal cell line MCF 10A (
) and one normal cell line MCF 10A (  ) (B). IC50 values of AgNPs on each cell line was calculated using Log viability vs. normalised response–variable slope (four-parameter).
) (B). IC50 values of AgNPs on each cell line was calculated using Log viability vs. normalised response–variable slope (four-parameter).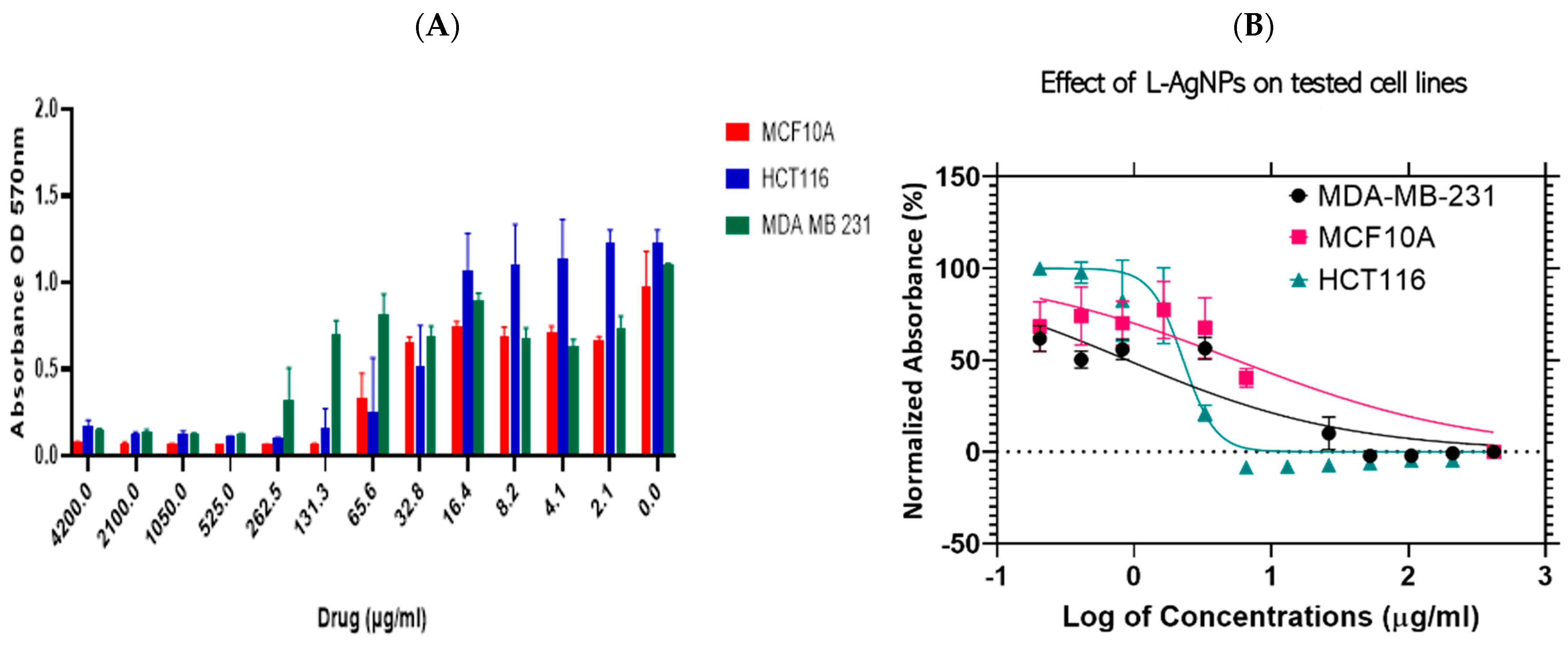
 ) and MDA-MB-231 (
) and MDA-MB-231 (  ) and a normal cell line; MCF 10A (
) and a normal cell line; MCF 10A (  ) (A). Log dose-response relationship of R-AgNPs on the normalised viability of two human cancer cell lines; HCT116 (
) (A). Log dose-response relationship of R-AgNPs on the normalised viability of two human cancer cell lines; HCT116 (  ) and MDA-MB-231 (
) and MDA-MB-231 (  ) and one normal cell line MCF 10A (
) and one normal cell line MCF 10A (  ) (B). IC50 values of AgNPs on each cell line was calculated using Log viability vs. normalised response–variable slope (four-parameter).
) (B). IC50 values of AgNPs on each cell line was calculated using Log viability vs. normalised response–variable slope (four-parameter).
 ) and MDA-MB-231 (
) and MDA-MB-231 (  ) and a normal cell line; MCF 10A (
) and a normal cell line; MCF 10A (  ) (A). Log dose-response relationship of R-AgNPs on the normalised viability of two human cancer cell lines; HCT116 (
) (A). Log dose-response relationship of R-AgNPs on the normalised viability of two human cancer cell lines; HCT116 (  ) and MDA-MB-231 (
) and MDA-MB-231 (  ) and one normal cell line MCF 10A (
) and one normal cell line MCF 10A (  ) (B). IC50 values of AgNPs on each cell line was calculated using Log viability vs. normalised response–variable slope (four-parameter).
) (B). IC50 values of AgNPs on each cell line was calculated using Log viability vs. normalised response–variable slope (four-parameter).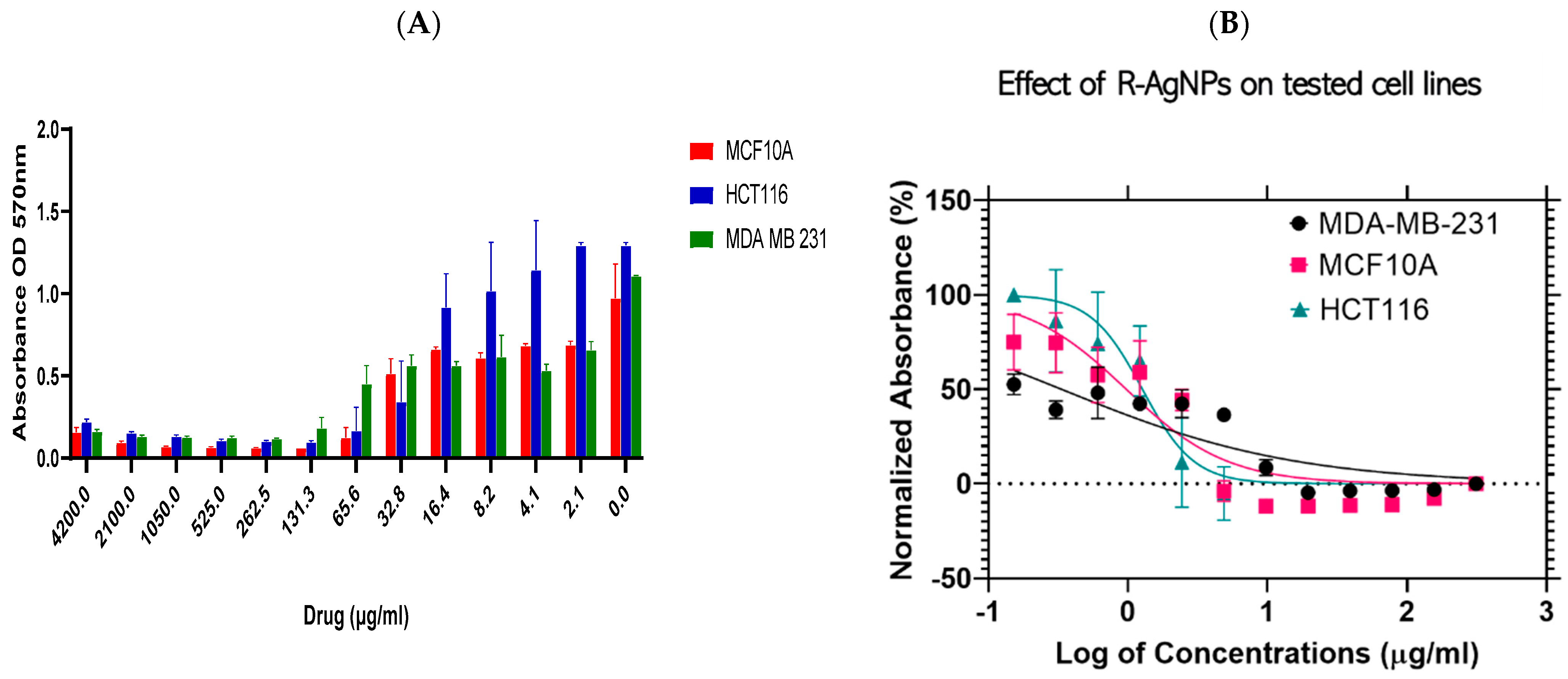



| Treatment | DLS Measurements | Cancer Cell | Normal Cell | |||
|---|---|---|---|---|---|---|
| PDI | Size (d.nm) | Zeta Potential (mV) | MDA-MB-231 | HCT116 | MCF10A | |
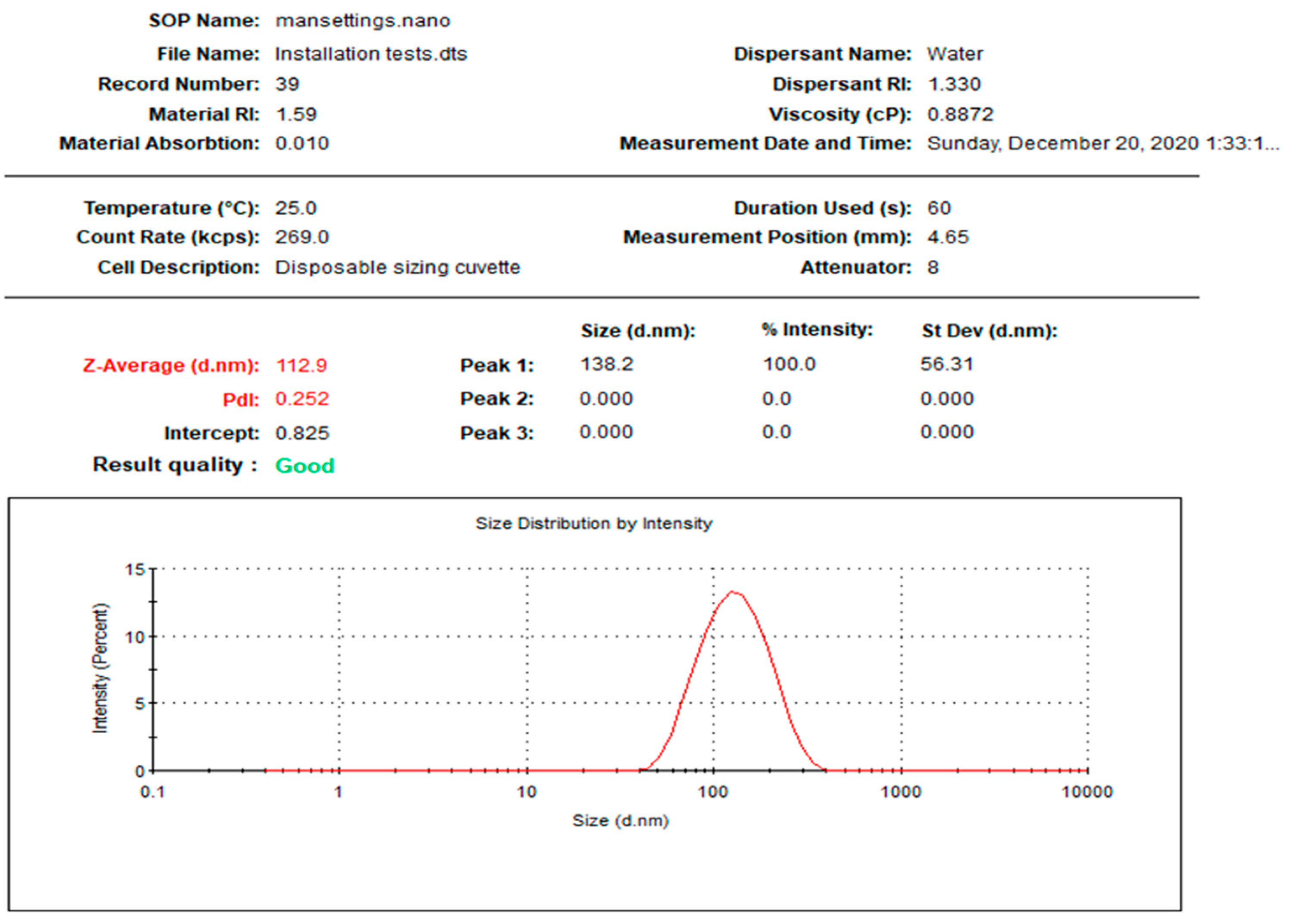
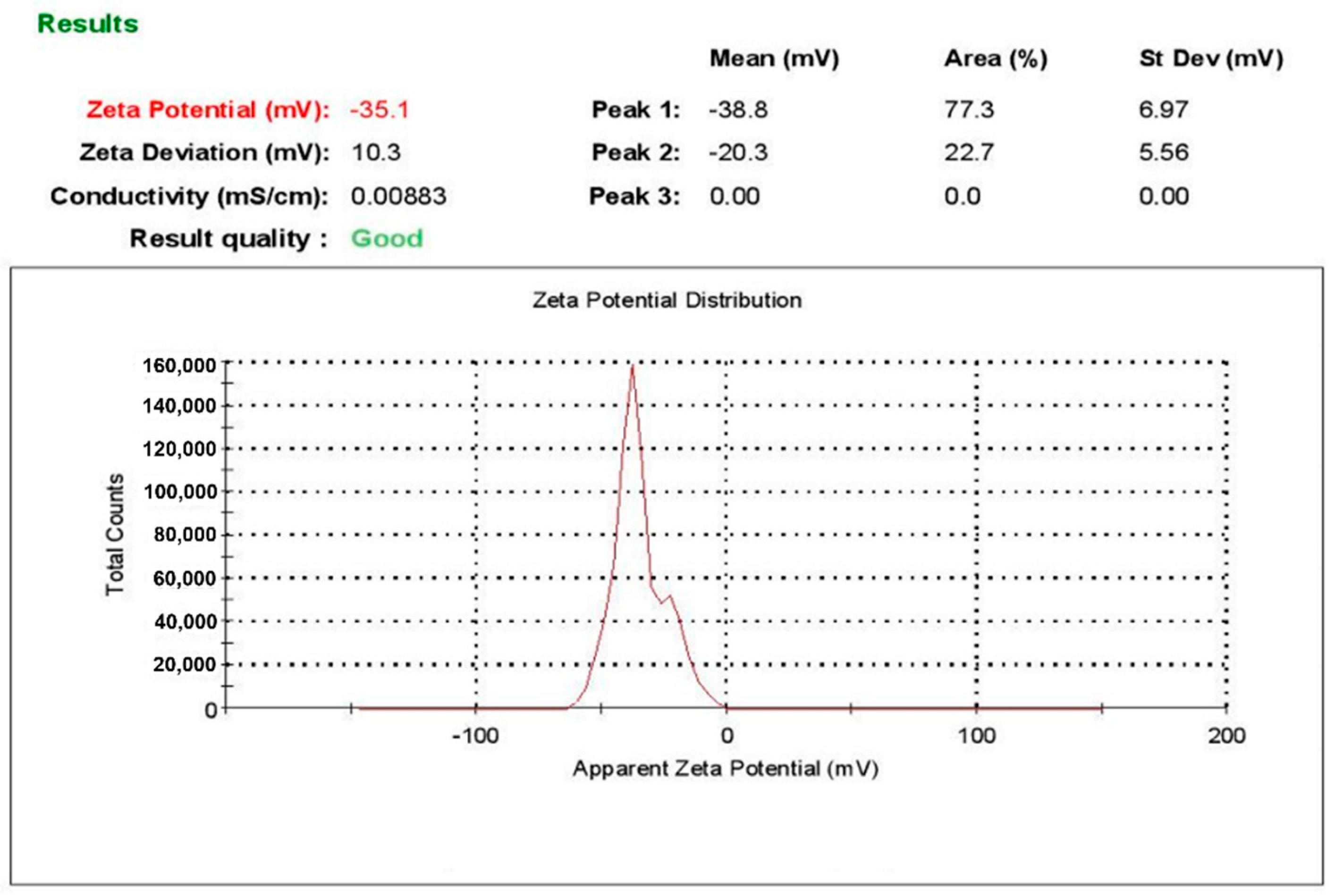
| L-AgNPs | 0.252 | 112.9 | −35.1 | 8.898 | 22.77 | 55.48 |
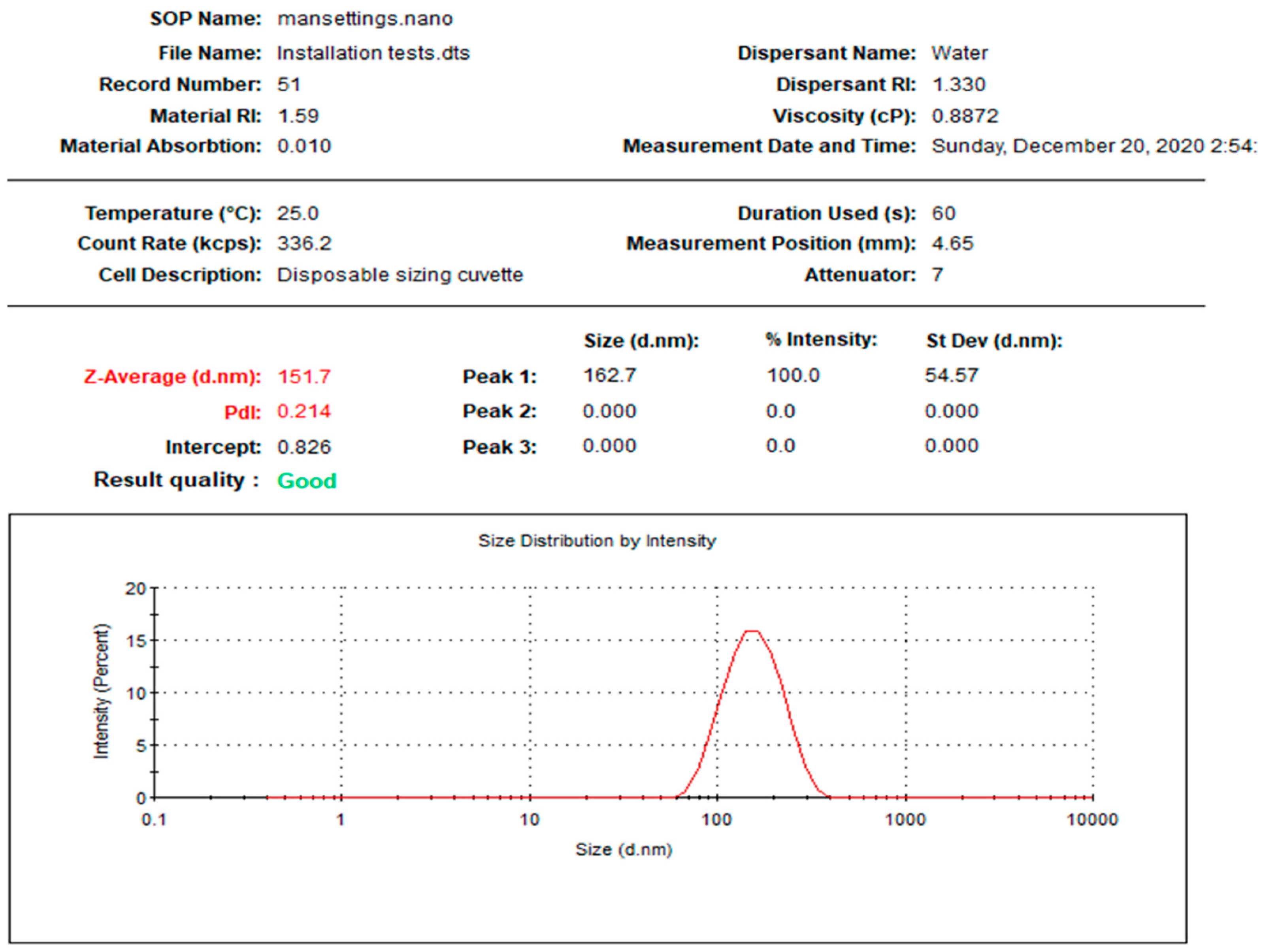
| R-AgNPs | 0.214 | 151.7 | −33.7 | 3.341 | 12.70 | 9.731 |
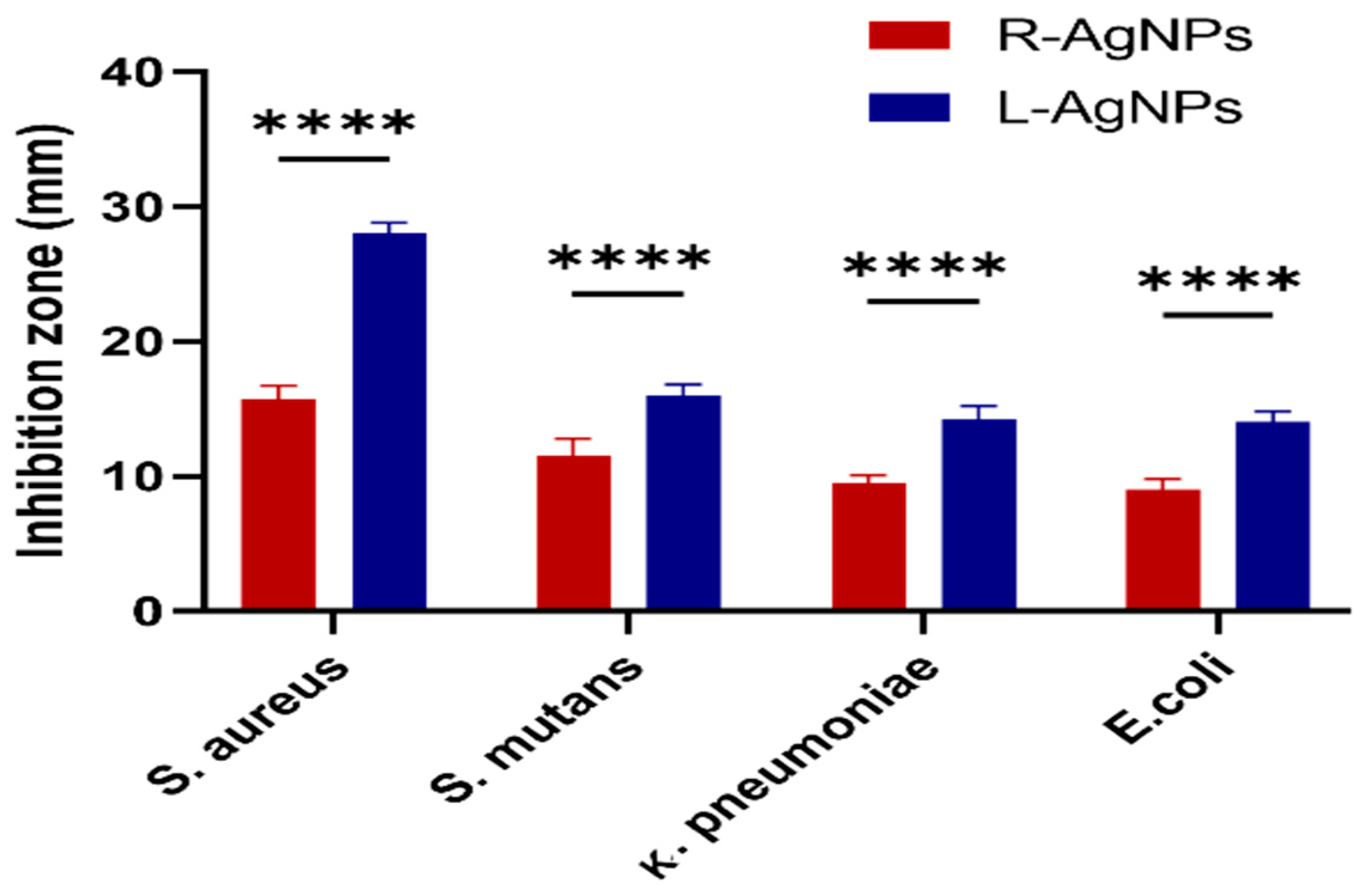
| L-AgNPs | R-AgNPs | |||||
|---|---|---|---|---|---|---|
| Microbes | MIC (mg/mL) | MBC (mg/mL) | MIC/MBC | MIC (mg/mL) | MBC (mg/mL) | MIC/MBC |
| S. aureus | 0.5 | 0.8 | 0.62 | 0.5 | 0.8 | 0.62 |
| S. mutans | 0.5 | 0.8 | 0.62 | 0.5 | 0.8 | 0.62 |
| E. coli | 0.8 | 1.1 | 0.72 | 0.8 | 1.1 | 0.72 |
| K. pneumoniae | 0.8 | 1.1 | 0.72 | 0.8 | 1.1 | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, A.E.; Al-Megrin, W.A. Biological Potential of Silver Nanoparticles Mediated by Leucophyllum frutescens and Russelia equisetiformis Extracts. Nanomaterials 2021, 11, 2098. https://doi.org/10.3390/nano11082098
Mohammed AE, Al-Megrin WA. Biological Potential of Silver Nanoparticles Mediated by Leucophyllum frutescens and Russelia equisetiformis Extracts. Nanomaterials. 2021; 11(8):2098. https://doi.org/10.3390/nano11082098
Chicago/Turabian StyleMohammed, Afrah E., and Wafa Abdullah Al-Megrin. 2021. "Biological Potential of Silver Nanoparticles Mediated by Leucophyllum frutescens and Russelia equisetiformis Extracts" Nanomaterials 11, no. 8: 2098. https://doi.org/10.3390/nano11082098
APA StyleMohammed, A. E., & Al-Megrin, W. A. (2021). Biological Potential of Silver Nanoparticles Mediated by Leucophyllum frutescens and Russelia equisetiformis Extracts. Nanomaterials, 11(8), 2098. https://doi.org/10.3390/nano11082098





