Innovative Textiles Used in Face Masks: Filtration Efficiency and Self-Disinfecting Properties against Coronaviruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Filtration Efficiency Trials
2.1.1. Characterization of Mask Materials
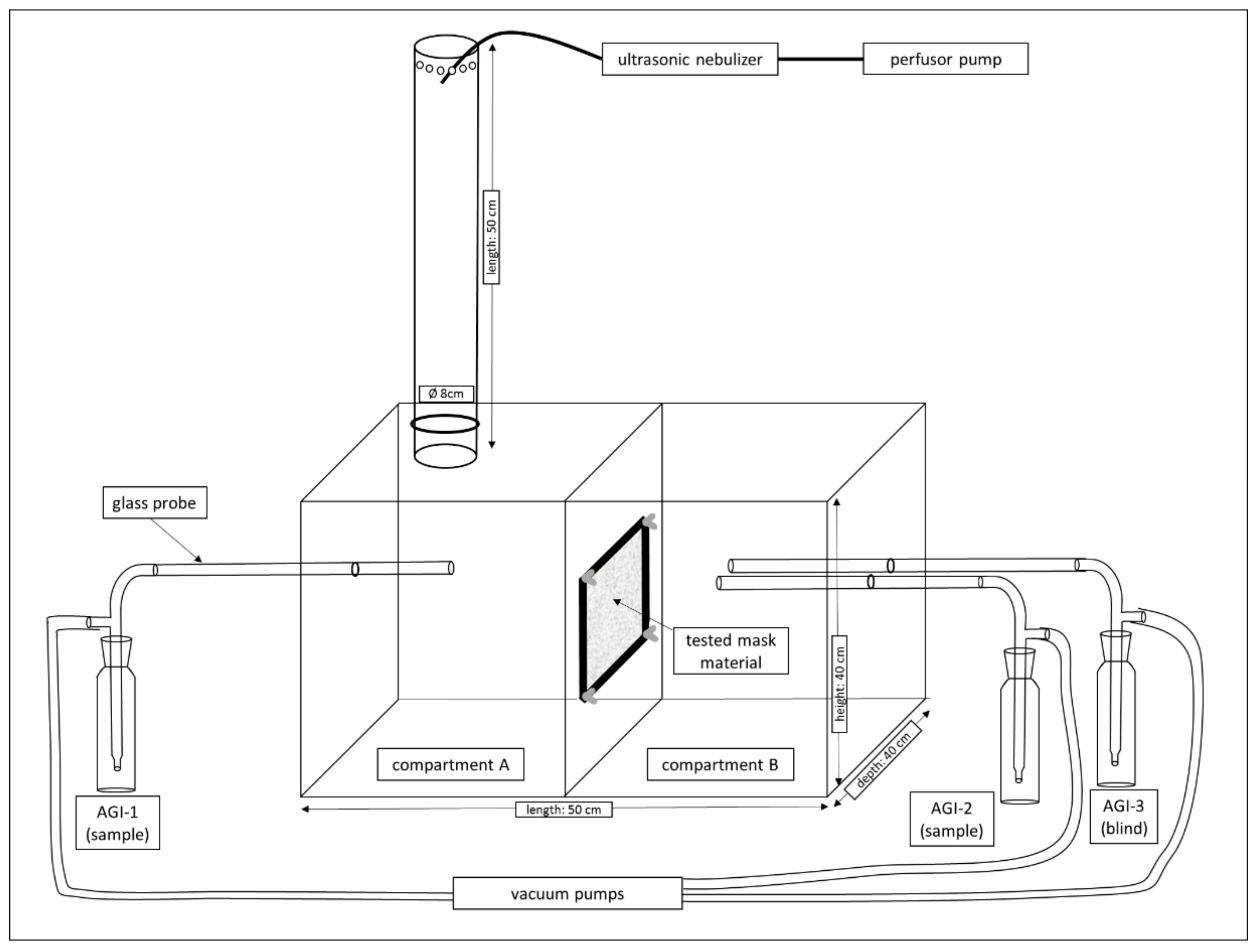
2.1.2. Experimental Setup for the Viral Filtration Efficiency Trials
2.1.3. Air Sampling
2.1.4. Preparation of Viral Suspensions
2.1.5. Quantification of the Viral Suspensions and Air Samples
2.2. Textile Surface Treatment Trials
2.2.1. Tested Textiles
2.2.2. Virus and Cell Lines
2.2.3. Antiviral Activity Test of Textiles
2.3. Statistical Analysis
3. Results
3.1. Filtration Efficiency of the Investigated Mask Materials against Aerosolized FCoV
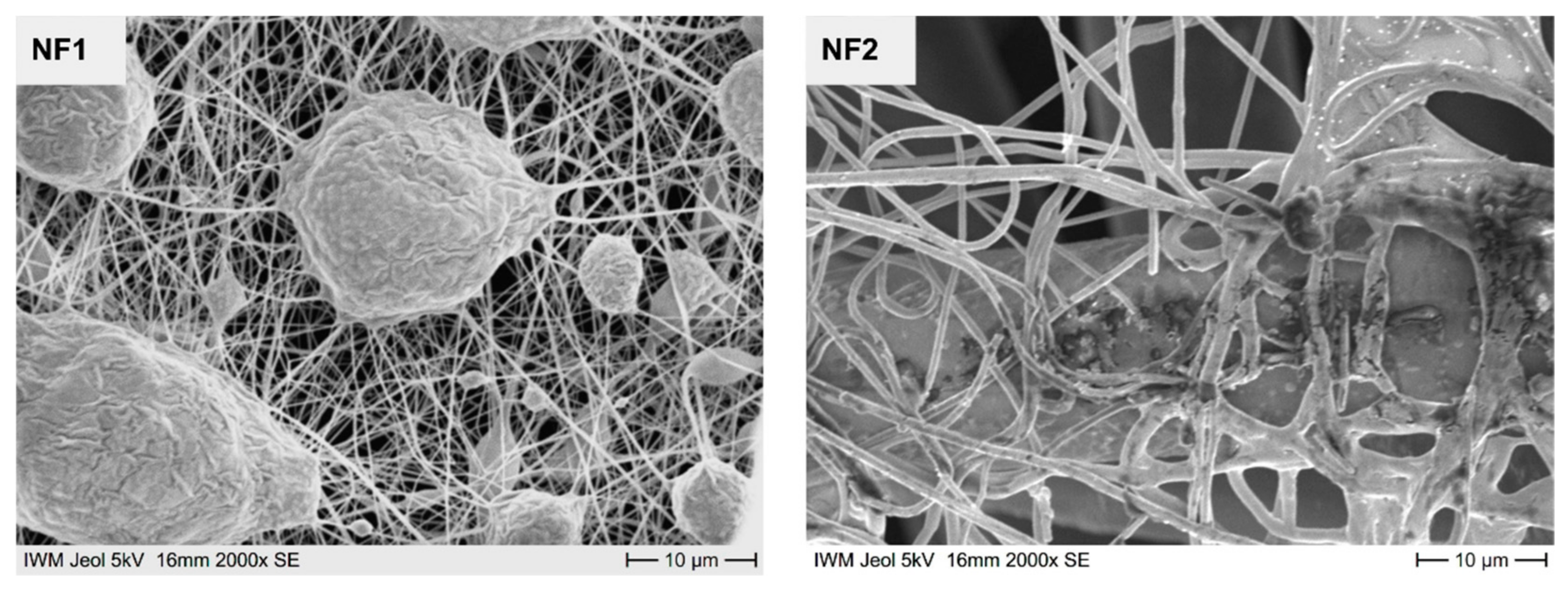
3.2. Structure of the Nanofleece Textiles after Aerosol Exposure
3.3. Antiviral Activity of Treated Textiles against SARS-CoV-2
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef]
- Gandhi, M.; Beyrer, C.; Goosby, E. Masks Do More Than Protect Others During COVID-19: Reducing the Inoculum of SARS-CoV-2 to Protect the Wearer. J. Gen. Intern. Med. 2020, 35, 3063–3066. [Google Scholar] [CrossRef]
- Wilson, N.; Corbett, S.; Tovey, E. Airborne transmission of covid-19. BMJ 2020, 370, 10–11. [Google Scholar] [CrossRef]
- Morawska, L.; Cao, J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ. Int. 2020, 139, 105730. [Google Scholar] [CrossRef]
- Leung, N.H.L.; Chu, D.K.W.; Shiu, E.Y.C.; Chan, K.H.; McDevitt, J.J.; Hau, B.J.P.; Yen, H.L.; Li, Y.; Ip, D.K.M.; Peiris, J.S.M.; et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020, 26, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Ueki, H.; Furusawa, Y.; Iwatsuki-Horimoto, K.; Imai, M.; Kabata, H.; Nishimura, H.; Kawaoka, Y. Effectiveness of Face Masks in Preventing Airborne Transmission of SARS-CoV-2. mSphere 2020, 5, 2–6. [Google Scholar] [CrossRef]
- Chua, M.H.; Cheng, W.; Goh, S.S.; Kong, J.; Li, B.; Lim, J.Y.C.; Mao, L.; Wang, S.; Xue, K.; Yang, L.; et al. Face Masks in the New COVID-19 Normal: Materials, Testing, and Perspectives. Research 2020, 2020, 7286735. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Huang, J.; Zhang, C.J.P.; He, Z.; Ming, W.K. Facemask shortage and the novel coronavirus disease (COVID-19) outbreak: Reflections on public health measures. EClinicalMedicine 2020, 21, 100329. [Google Scholar] [CrossRef] [PubMed]
- Zar, H.J.; Dawa, J.; Fischer, G.B.; Castro-Rodriguez, J.A. Challenges of COVID-19 in children in low- and middle-income countries. Paediatr. Respir. Rev. 2020, 35, 70–74. [Google Scholar] [CrossRef]
- Czubryt, M.P.; Stecy, T.; Popke, E.; Aitken, R.; Jabusch, K.; Pound, R.; Lawes, P.; Ramjiawan, B.; Pierce, G.N. N95 mask reuse in a major urban hospital: COVID-19 response process and procedure. J. Hosp. Infect. 2020, 106, 277–282. [Google Scholar] [CrossRef]
- Hossain, E.; Bhadra, S.; Jain, H.; Das, S.; Bhattacharya, A.; Ghosh, S.; Levine, D. Recharging and rejuvenation of decontaminated N95 masks. Phys. Fluids 2020, 32, 093304. [Google Scholar] [CrossRef]
- Otrisal, P.; Bungau, C.; Obsel, V.; Melicharik, Z.; Tont, G. Selected respiratory protective devices: Respirators and significance of some markings. Sustainability 2021, 13, 4988. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.; Federici, J.; Imura, Y.; Catalani, L.H. Charge generation, charge transport, and residual charge in the electrospinning of polymers: A review of issues and complications. J. Appl. Phys. 2012, 111, 044701. [Google Scholar] [CrossRef]
- Gao, H.; He, W.; Zhao, Y.-B.; Opris, D.M.; Xu, G.; Wang, J. Electret mechanisms and kinetics of electrospun nanofiber membranes and lifetime in filtration applications in comparison with corona-charged membranes. J. Memb. Sci. 2020, 600, 117879. [Google Scholar] [CrossRef]
- Ullah, S.; Ullah, A.; Lee, J.; Jeong, Y.; Hashmi, M.; Zhu, C.; Joo, K.I.; Cha, H.J.; Kim, I.S. Reusability Comparison of Melt-Blown vs Nanofiber Face Mask Filters for Use in the Coronavirus Pandemic. ACS Appl. Nano Mater. 2020, 3, 7231–7241. [Google Scholar] [CrossRef]
- Marzoli, F.; Bortolami, A.; Pezzuto, A.; Mazzetto, E.; Piro, R.; Terregino, C.; Bonfante, F.; Belluco, S. A systematic review of human coronaviruses survival on environmental surfaces. Sci. Total Environ. 2021, 778, 146191. [Google Scholar] [CrossRef]
- Chin, A.W.H.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions—Authors’ reply. Lancet Microbe 2020, 1, e146. [Google Scholar] [CrossRef]
- Ramanathan, K.; Antognini, D.; Combes, A.; Paden, M.; Zakhary, B.; Ogino, M.; Maclaren, G.; Brodie, D. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: The possible role of dry surface contamination. J. Hosp. Infect. 2020, 92, 235–250. [Google Scholar]
- Boone, S.A.; Gerba, C.P. Significance of fomites in the spread of respiratory and enteric viral disease. Appl. Environ. Microbiol. 2007, 73, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Openshaw, J.J.; Morris, W.M.; Lowry, G.V.; Nazmi, A. Reduction in bacterial contamination of hospital textiles by a novel silver-based laundry treatment. Am. J. Infect. Control 2016, 44, 1705–1708. [Google Scholar] [CrossRef][Green Version]
- Tian, Z.; Yang, Y.; Wang, L. An improved method for assessing environmental impacts caused by chemical pollutants: A case study in textiles production. Toxicol. Ind. Health 2020, 36, 228–236. [Google Scholar] [CrossRef]
- Preferred Fiber & Materials Market Report 2020. p. 103. Available online: https://textileexchange.org/wp-content/uploads/2020/06/Textile-Exchange_Preferred-Fiber-Material-Market-Report_2020.pdf (accessed on 11 August 2021).
- Lundgren, D.A.; Cooper, D.W. Effect of Humidity on Light-Scattering Methods of Measuring Particle Concentration. J. Air Pollut. Control Assoc. 1969, 19, 243–247. [Google Scholar] [CrossRef]
- Spearman, C. The method of right and wrong cases (constant stimuli) without Gauss’s formulae. Br. J. Psychol. 1908, 2, 227–242. [Google Scholar] [CrossRef]
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer. Beitrag Kollekt. Behandl. Pharmakol. Reihenversuche 1931, 162, 480–483. [Google Scholar]
- Osterrieder, N.; Bertzbach, L.D.; Dietert, K.; Abdelgawad, A.; Vladimirova, D.; Kunec, D.; Hoffmann, D.; Beer, M.; Gruber, A.D.; Trimpert, J. Age-Dependent Progression of SARS-CoV-2 Infection in Syrian Hamsters. Viruses 2020, 12, 779. [Google Scholar] [CrossRef]
- Searle, S.R.; Speed, F.M.; Milliken, G.A. Population marginal means in the linear model: An alternative to least squares means. Am. Stat. 1980, 34, 216–221. [Google Scholar]
- Zeileis, A.; Köll, S.; Graham, N. Various versatile variances: An object-oriented implementation of clustered covariances in R. J. Stat. Softw. 2020, 95, 1–36. [Google Scholar] [CrossRef]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J.; El-harakeh, A.; Bognanni, A.; Lotfi, T.; Loeb, M.; et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- Santarsiero, A.; Ciambelli, P.; Donsì, G.; Quadrini, F.; Briancesco, R.; D’Alessandro, D.; Fara, G.M. Face masks. Technical, technological and functional characteristics and hygienic-sanitary aspects related to the use of filtering mask in the community. Ann. Ig. 2020, 32, 472–520. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Dana, T.; Jungbauer, R.; Weeks, C.; McDonagh, M.S. Masks for Prevention of Respiratory Virus Infections, Including SARS-CoV-2, in Health Care and Community Settings: A Living Rapid Review. Ann. Intern. Med. 2020, 173, 542–555. [Google Scholar] [CrossRef]
- Forouzandeh, P.; O’Dowd, K.; Pillai, S.C. Face masks and respirators in the fight against the COVID-19 pandemic: An overview of the standards and testing methods. Saf. Sci. 2021, 133, 104995. [Google Scholar] [CrossRef]
- Bałazy, A.; Toivola, M.; Adhikari, A.; Sivasubramani, S.K.; Reponen, T.; Grinshpun, S.A. Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks? Am. J. Infect. Control 2006, 34, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lukula, S.; Chiossone, C.; Nims, R.W.; Suchmann, D.B.; Ijaz, M.K. Assessment of a respiratory face mask for capturing air pollutants and pathogens including human influenza and rhinoviruses. J. Thorac. Dis. 2018, 10, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Konda, A.; Prakash, A.; Moss, G.A.; Schmoldt, M.; Grant, G.D.; Guha, S. Aerosol Filtration Efficiency of Common Fabrics Used in Respiratory Cloth Masks. ACS Nano 2020, 14, 6339–6347. [Google Scholar] [CrossRef]
- Shen, H.; Zhou, Z.; Wang, H.; Zhang, M.; Han, M.; Durkin, D.P.; Shuai, D.; Shen, Y. Development of Electrospun Nanofibrous Filters for Controlling Coronavirus Aerosols. Environ. Sci. Technol. Lett. 2021, 8, 545–550. [Google Scholar] [CrossRef]
- Nayak, R.; Padhye, R. Nano Fibres by Electro spinning: Properties and Applications. J. Text. Eng. Fash. Technol. 2017, 2, 486–497. [Google Scholar] [CrossRef]
- Yao, M.; Mainelis, G. Investigation of cut-off sizes and collection efficiencies of portable microbial samplers. Aerosol. Sci. Technol. 2006, 40, 595–606. [Google Scholar] [CrossRef]
- Lindsley, W.G.; Green, B.J.; Blachere, F.M.; Stephen, B.; Martin, B.F.L.; Jensen, P.A.; Schafer, M.P. Sampling and Characterization of Bioaerosols. In NIOSH Manual Manual Methods—Sampling Characterization Bioaerosols; NIOSH: Washington, DC, USA, 2017; pp. 2–115. [Google Scholar]
- Fischer, R.J.; Morris, D.H.; Van Doremalen, N.; Sarchette, S.; Matson, M.J.; Bushmaker, T.; Yinda, C.K.; Seifert, S.N.; Gamble, A.; Williamson, B.N.; et al. Effectiveness of N95 respirator decontamination and reuse against SARS-CoV-2 Virus. Emerg. Infect. Dis. 2020, 26, 2253–2255. [Google Scholar] [CrossRef] [PubMed]
- Cherrie, J.W.; Wang, S.; Mueller, W.; Wendelboe-Nelson, C.; Loh, M. In-mask temperature and humidity can validate respirator wear-time and indicate lung health status. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 578–583. [Google Scholar] [CrossRef]
- Ahlstrom, B.; Chelminska-Bertilsson, M.; Thompson, R.A.; Edebo, L. Long-chain alkanoylcholines, a new category of soft antimicrobial agents that are enzymatically degradable. Antimicrob. Agents Chemother. 1995, 39, 50–55. [Google Scholar] [CrossRef]
- Morais, D.S.; Guedes, R.M.; Lopes, M.A. Antimicrobial approaches for textiles: From research to market. Materials 2016, 9, 498. [Google Scholar] [CrossRef]
- Camero, M.; Lanave, G.; Catella, C.; Lucente, M.S.; Decaro, N.; Martella, V.; Buonavoglia, C. Evaluation of virucidal activity of fabrics using feline coronavirus. J. Virol. Methods 2021, 295, 114214. [Google Scholar] [CrossRef]
- Stefaniak, A.B.; Duling, M.G.; Lawrence, R.B.; Thomas, T.A.; LeBouf, R.F.; Wade, E.E.; Abbas Virji, M. Dermal exposure potential from textiles that contain silver nanoparticles. Int. J. Occup. Environ. Health 2014, 20, 220–234. [Google Scholar] [CrossRef]
- Rosenberg, M.; Ilic, K.; Juganson, K.; Ivask, A.; Ahonen, M.; Vrček, I.V.; Kahru, A. Potential ecotoxicological effects of antimicrobial surface coatings: A literature survey backed up by analysis of market reports. PeerJ 2019, 7, e6315. [Google Scholar] [CrossRef]
- Shahid-Ul-Islam; Shahid, M.; Mohammad, F. Green chemistry approaches to develop antimicrobial textiles based on sustainable biopolymers—A review. Ind. Eng. Chem. Res. 2013, 52, 5245–5260. [Google Scholar] [CrossRef]
- Valdez-Salas, B.; Beltran-Partida, E.; Cheng, N.; Salvador-Carlos, J.; Valdez-Salas, E.A.; Curiel-Alvarez, M.; Ibarra-Wiley, R. Promotion of surgical masks antimicrobial activity by disinfection and impregnation with disinfectant silver nanoparticles. Int. J. Nanomed. 2021, 16, 2689–2702. [Google Scholar] [CrossRef] [PubMed]
- Peshin, R.; Hansra, B.S.; Nanda, R.; Singh, K.; Sharma, R.; Garg, L.; Bajiya, M.R.; Showkat, A.; Kumar, R.; Yangsdon, S. Pesticides Hazardous Hotspots: Empirical Evidences from North India. Environ. Manag. 2020, 66, 899–915. [Google Scholar] [CrossRef]
- Sidwell, R.W.; Dixon, G.J.; McNeil, E. Quantitative studies on fabrics as disseminators of viruses. 3. Persistence of vaccinia virus on fabrics impregnated with a virucidal agent. Appl. Microbiol. 1967, 15, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Beesoon, S.; Behary, N.; Perwuelz, A. Universal masking during COVID-19 pandemic: Can textile engineering help public health? Narrative review of the evidence. Prev. Med. 2020, 139, 106236. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, S.; Bhopal, R.; Nguyen Tien, H. Review of Infective Dose, Routes of Transmission, and Outcome of COVID-19 Caused by the SARS-CoV-2 Virus: Comparison with Other Respiratory Viruses. Preprint 2020, 20944. [Google Scholar] [CrossRef]
- Migueres, M.; Mengelle, C.; Dimeglio, C.; Didier, A.; Alvarez, M.; Delobel, P.; Mansuy, J.M.; Izopet, J. Saliva sampling for diagnosing SARS-CoV-2 infections in symptomatic patients and asymptomatic carriers. J. Clin. Virol. 2020, 130, 104580. [Google Scholar] [CrossRef]
- Jeong, H.W.; Kim, S.-M.; Kim, H.-S.; Kim, Y.-I.; Kim, J.H.; Cho, J.Y.; Kim, S.; Kang, H.; Kim, S.-G.; Park, S.; et al. Viable SARS-CoV-2 in various specimens from COVID-19 patients. Clin. Microbiol. Infect. 2020, 26, 1520–1524. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, A.L.; Fournier, J.; Casanovas-Massana, A.; Campbell, M.; Tokuyama, M.; Vijayakumar, P.; Warren, J.L.; Geng, B.; Muenker, M.C.; Moore, A.J.; et al. Saliva or Nasopharyngeal Swab Specimens for Detection of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
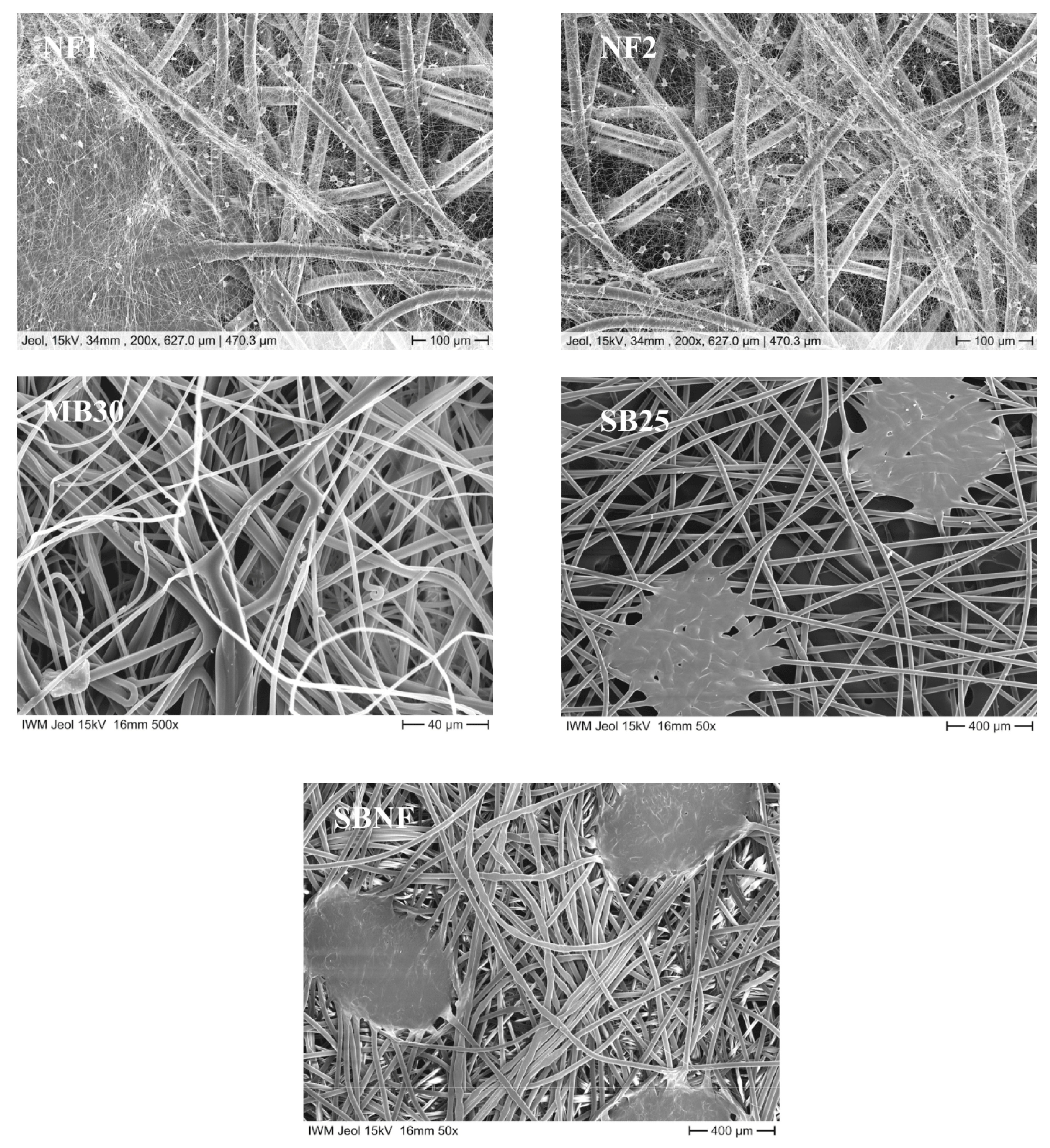
| Name | Layer 1 | Layer 2 | Layer 3 | Layer 4 | NaCl-Test (leakage %) |
|---|---|---|---|---|---|
| NFA | Spunbond (SBNF) | Electrospun (NF1) | Spunbond (SBNF) | - | 28.1 |
| NFB | Spunbond (SBNF) | Electrospun (NF2) | Spunbond (SBNF) | - | n.a. |
| NFA2 | Spunbond (SBNF) | Electrospun (NF1) | Electrospun (NF1) | Spunbond (SBNF) | 6 |
| NFB2 | Spunbond (SBNF) | Electrospun (NF2) | Electrospun (NF2) | Spunbond (SBNF) | n.a. |
| Surgical mask | Spunbond (SB25) | Meltblown (MB30) | Spunbond (SB25) | 29.8 | |
| FFP-2 mask | Spunbond (SB25) | Meltblown (MB30) | Meltblown (MB30) | Spunbond (SB25) | 11 |
| Community mask | Canvas Woven | - | - | - | 84.5 |
| Textile ID | Textile Name | Textile Treatment | Applied on Textile in % |
|---|---|---|---|
| A1 | Cotton Canvas 100 | no treatment | |
| A2 | Cotton Canvas 100 | PolyHexaMethylene Biguanide (PHMB) | 0.16 |
| C1 | Cotton Knit 180 | no treatment | |
| C2 | Cotton Knit 180 | PolyHexaMethylene Biguanide (PHMB) proprietary binder | 0.16 0.1 |
| F1 | Cotton Canvas 130 | no treatment | |
| F2 | Cotton Canvas 130 | Dimethyloctadecyl [3-(trimethoxysilyl)propyl]ammonium chloride 68–70% Methanol 30–32% | 1.22 |
| F3 | Cotton Canvas 130 | Dimethyloctadecyl[3-(trimethoxysilyl)propyl]ammonium chloride 64–66% Methanol 34–36% | 1.3 |
| F4 | Cotton Canvas 130 | Dimethyloctadecyl[3-(trimethoxysilyl)propyl]ammonium chloride 38–42% Ethylene glycol 58–62% | 2.1 |
| H1 | PES Canvas 120 | no treatment | |
| H2 | PES Canvas 120 | Dimethyloctadecyl[3-(trimethoxysilyl)propyl]ammonium chloride 30–50% Methanol 0.1%–<1% Dimethylmyristylamine >1%–<5% N-Dimethyl-3-(trimethoxysilyl)propylamine >1%–<5% | 2.0 |
| M1 | Cotton Canvas 90 | no treatment | |
| M2 | Cotton Canvas 90 | (a.) Dimethyloctadecyl[3-(trimethoxysilyl)propyl]ammonium chloride 3–5% Methanol 0.5–1.7% * | 8 |
| (b.) Polyetheramine-epichlorohydrin resin 8–21% Isotridecanol, branched, ethoxylated 2–10% Coco alkylbis(hydroxyethyl), ethoxylated, chlorides 5–6% Alcohols C9–11 ethoxylates 1.5–2.5% | 2 | ||
| N1 | PES Canvas 120 | no treatment | |
| N2 | PES Canvas 120 | See M2 | See M2 |
| Textile ID | TCID50/mL Washing out Solution in log(10) after 6 h | Antiviral Activity in log(10) after 6 h | ||
|---|---|---|---|---|
| (A) | (B) | (A) | (B) | |
| A1 | 3.85 | 4.35 | ||
| A2 | 4.1 | 4.6 | −0.25 | −0.25 |
| C1 | 4.85 | 4.475 | ||
| C2 | 2.475 | 2.1 | 2.375 | 2.375 |
| F1 | 3.725 | 3.6 | ||
| F2 | 2.35 | 2.35 | 1.375 | 1.25 |
| F3 | 1.6 | 3.225 | 2.125 | 0.375 |
| F4 | 1.85 | 2.35 | 1.875 | 1.25 |
| H1 | 5.1 | 5.225 | ||
| H2 | 2.475 | 2.1 | 2.625 | 3.125 |
| M1 | 4.725 | 4.85 | ||
| M2 | 2.975 | 3.225 | 1.75 | 1.625 |
| N1 | 4.475 | 4.725 | ||
| N2 | 2.6 | 2.35 | 1.875 | 2.375 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siller, P.; Reissner, J.; Hansen, S.; Kühl, M.; Bartel, A.; Schmelzeisen, D.; Gries, T.; Roesler, U.; Friese, A. Innovative Textiles Used in Face Masks: Filtration Efficiency and Self-Disinfecting Properties against Coronaviruses. Nanomaterials 2021, 11, 2088. https://doi.org/10.3390/nano11082088
Siller P, Reissner J, Hansen S, Kühl M, Bartel A, Schmelzeisen D, Gries T, Roesler U, Friese A. Innovative Textiles Used in Face Masks: Filtration Efficiency and Self-Disinfecting Properties against Coronaviruses. Nanomaterials. 2021; 11(8):2088. https://doi.org/10.3390/nano11082088
Chicago/Turabian StyleSiller, Paul, Janina Reissner, Sabrina Hansen, Michael Kühl, Alexander Bartel, David Schmelzeisen, Thomas Gries, Uwe Roesler, and Anika Friese. 2021. "Innovative Textiles Used in Face Masks: Filtration Efficiency and Self-Disinfecting Properties against Coronaviruses" Nanomaterials 11, no. 8: 2088. https://doi.org/10.3390/nano11082088
APA StyleSiller, P., Reissner, J., Hansen, S., Kühl, M., Bartel, A., Schmelzeisen, D., Gries, T., Roesler, U., & Friese, A. (2021). Innovative Textiles Used in Face Masks: Filtration Efficiency and Self-Disinfecting Properties against Coronaviruses. Nanomaterials, 11(8), 2088. https://doi.org/10.3390/nano11082088






