Charge-Transfer Process in Surface-Enhanced Raman Scattering Based on Energy Level Locations of Rare-Earth Nd3+-Doped TiO2 Nanoparticles
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Nd-TiO2 NPs
2.3. Characterization
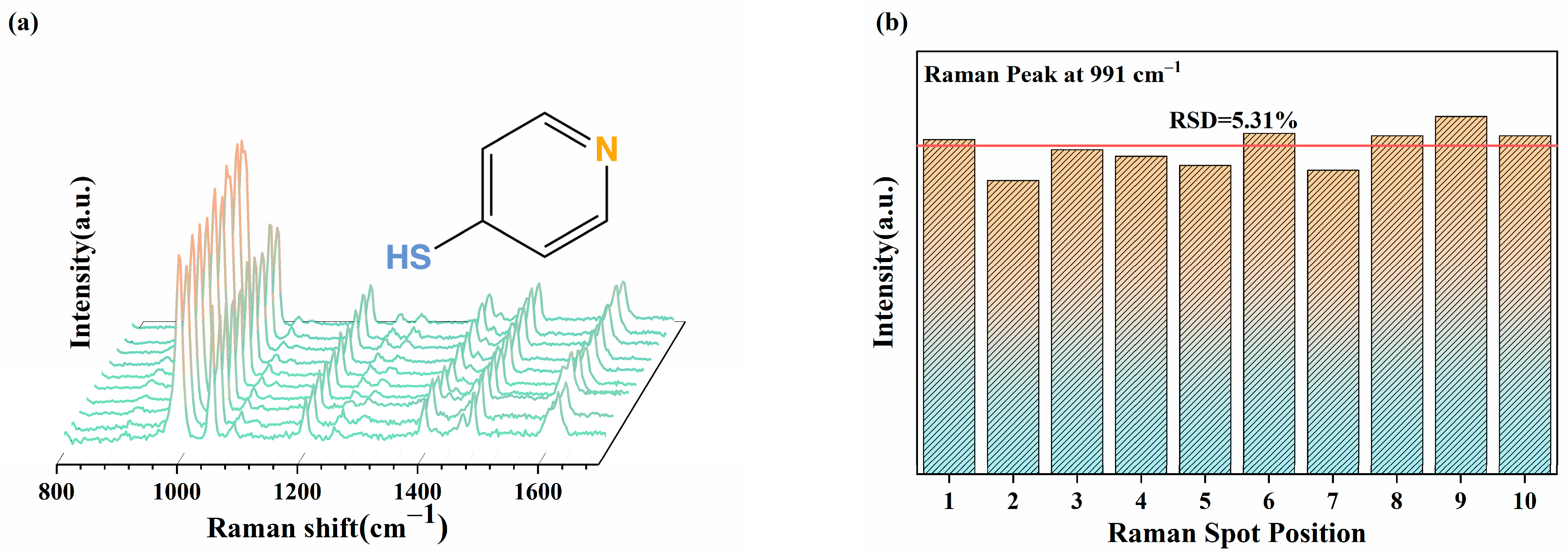
2.4. SERS Analysis
3. Results and Discussion
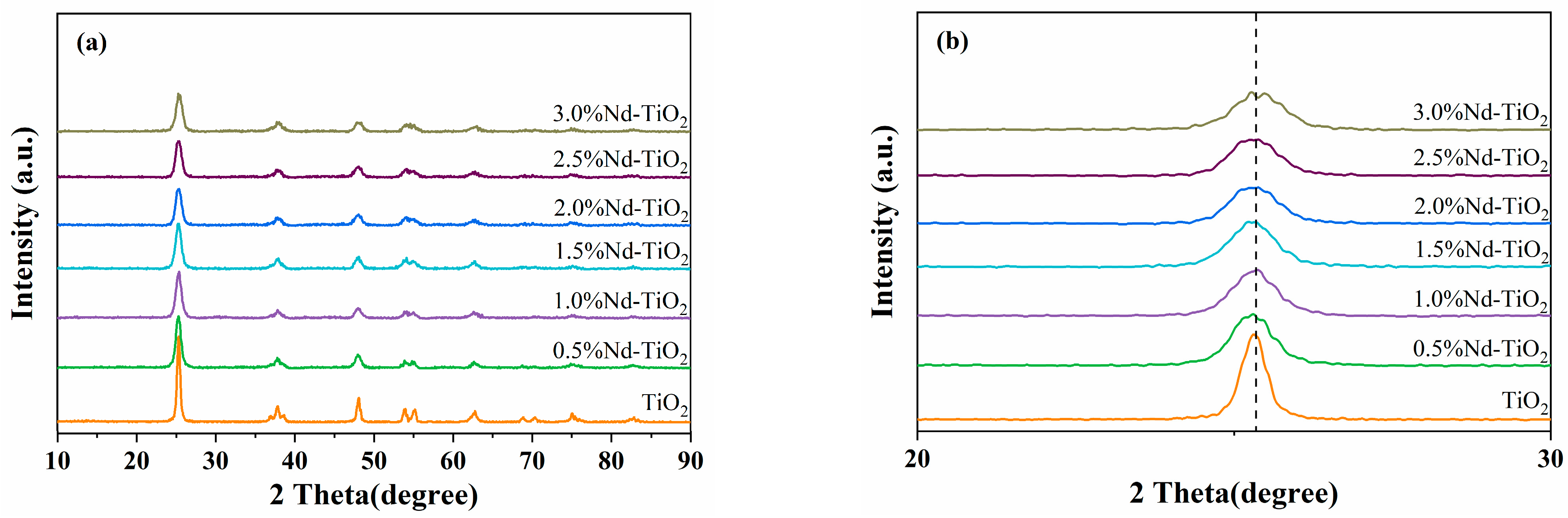
3.1. XRD Analysis
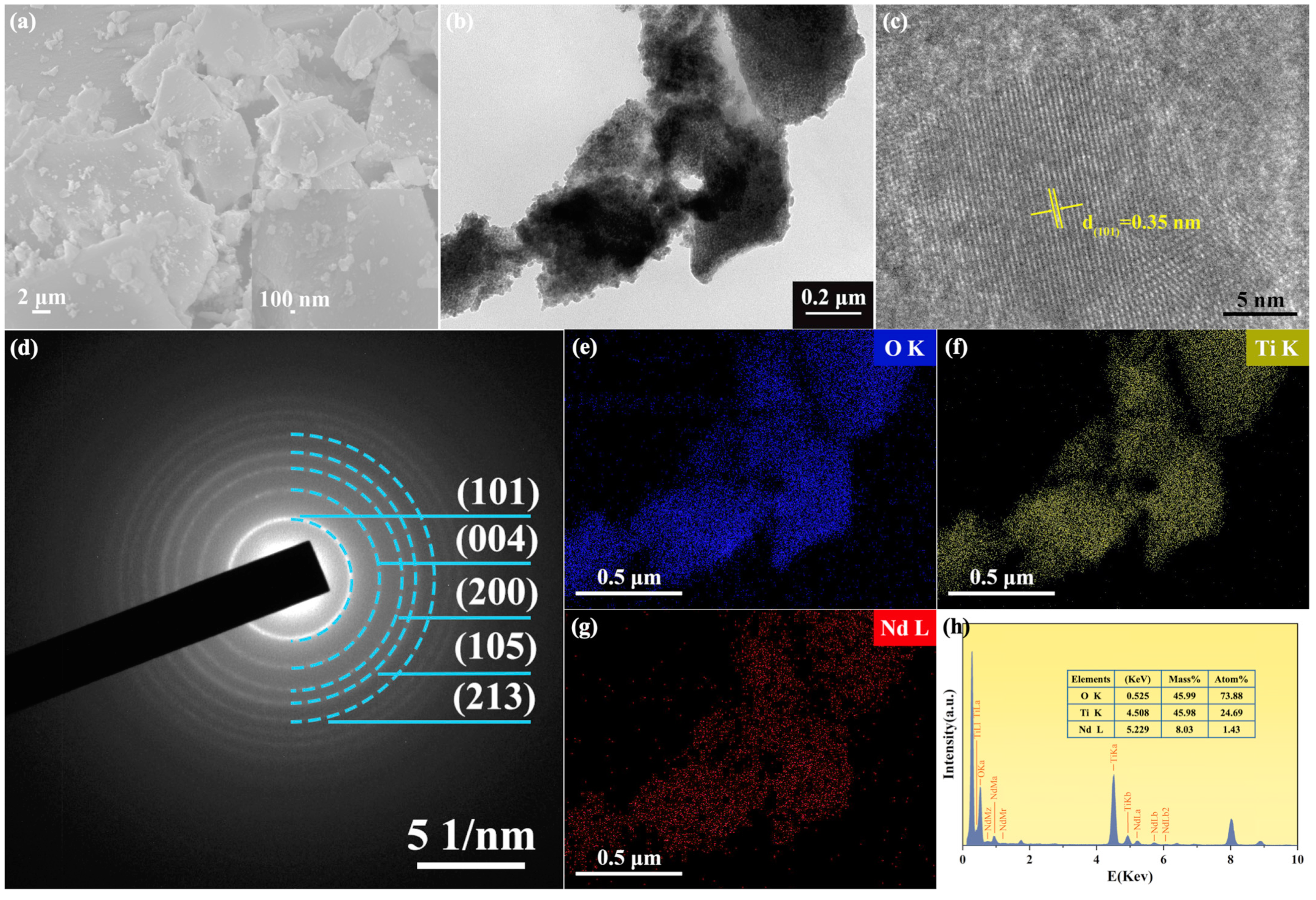
3.2. SEM and TEM Analysis
3.3. XPS Analysis
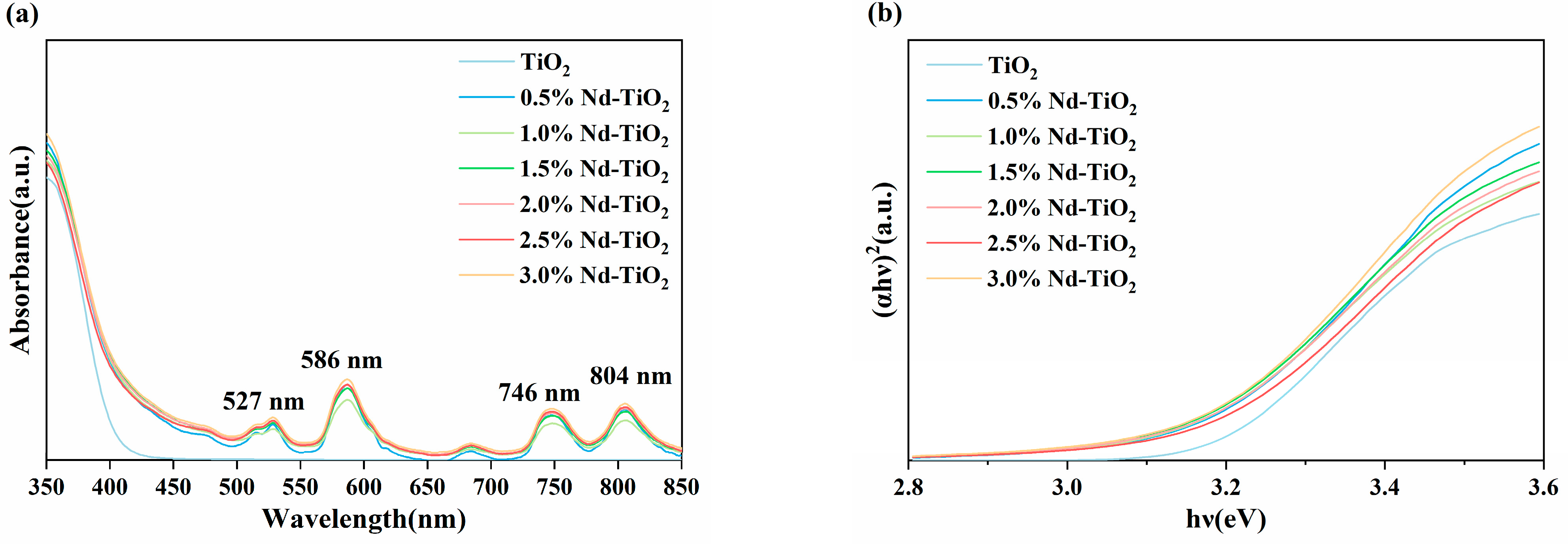
3.4. UV–Vis DRS Analysis
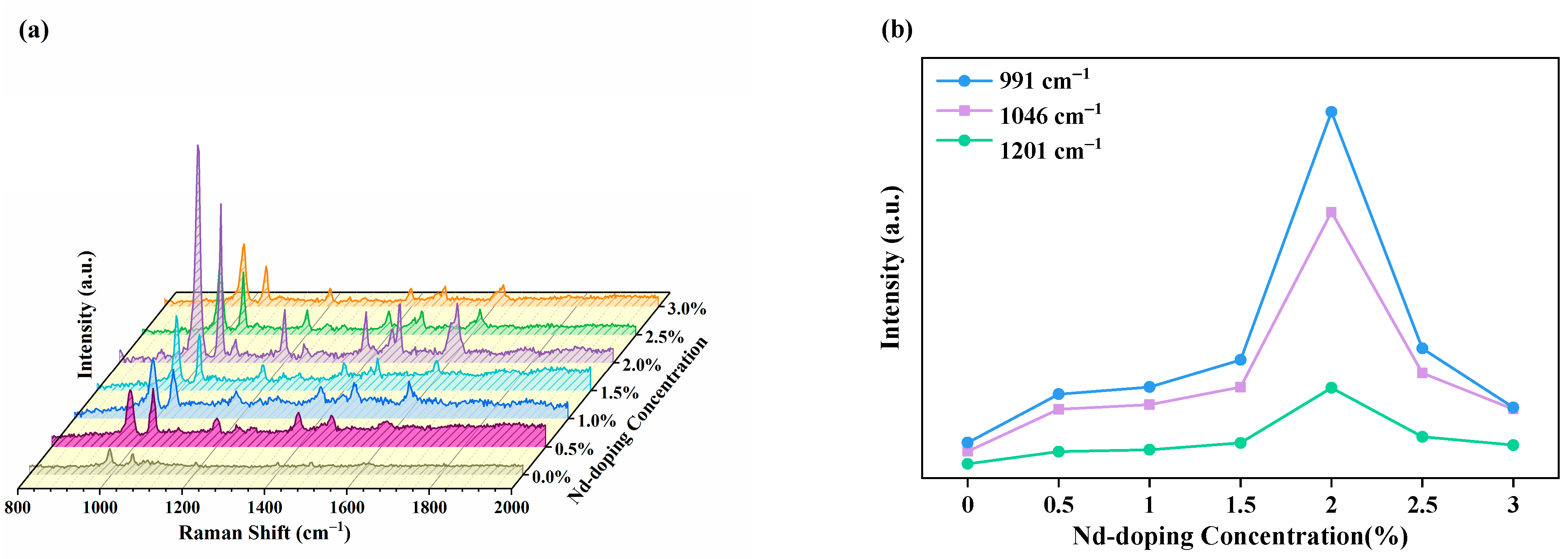
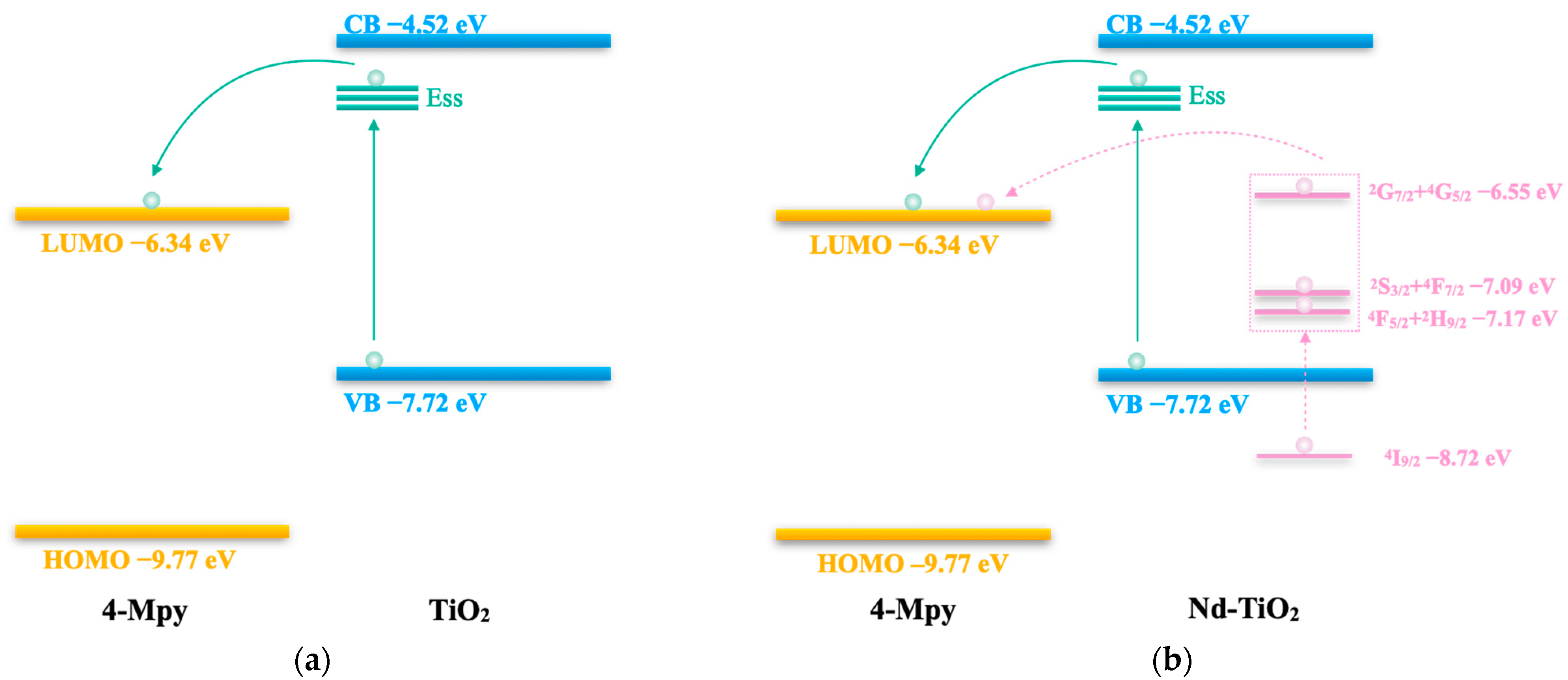
3.5. SERS Activity and Mechanism
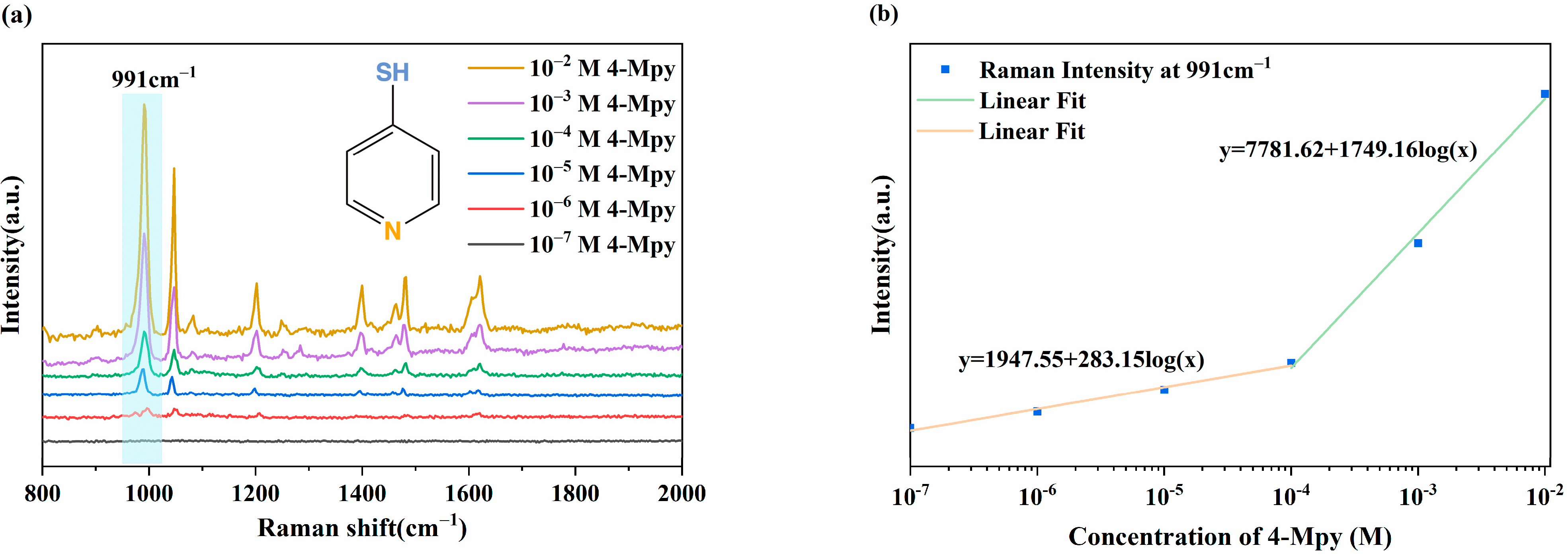
3.6. SERS Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.-X.; Zhu, W.-W.; Wu, Y.-Y.; Chen, Y.-Y.; Du, F.-K.; Yan, J.; Tan, X.-C.; Wang, Q. Sensitive surface-enhanced Raman scattering for the quantitative detection of formaldehyde in foods using gold nanorod substrate. Microchem. J. 2021, 160, 105727. [Google Scholar] [CrossRef]
- Zhou, J.; Sheth, S.; Zhou, H.; Song, Q. Highly selective detection of l-Phenylalanine by molecularly imprinted polymers coated Au nanoparticles via surface-enhanced Raman scattering. Talanta 2020, 211, 120745. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Cong, S.; Gong, W.; Xuan, J.; Li, G.; Lu, W.; Geng, F.; Zhao, Z. Semiconductor SERS enhancement enabled by oxygen incorporation. Nat. Commun. 2017, 8, 1993. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Nam, J.-M.; Jeon, K.-S.; Lim, D.-K.; Kim, H.; Kwon, S.; Lee, H.; Suh, Y.D. Tuning and Maximizing the Single-Molecule Surface-Enhanced Raman Scattering from DNA-Tethered Nanodumbbells. ACS Nano 2012, 6, 9574–9584. [Google Scholar] [CrossRef]
- Su, D.; Jiang, S.; Yu, M.; Zhang, G.; Liu, H.; Li, M.-Y. Facile fabrication of configuration controllable self-assembled Al nanostructures as UV SERS substrates. Nanoscale 2018, 10, 22737–22744. [Google Scholar] [CrossRef]
- Li, X.; Choy, W.C.H.; Ren, X.; Zhang, D.; Lu, H. Highly intensified surface enhanced Raman scattering by using monolayer graphene as the nanospacer of metal film-metal nanoparticle coupling system. Adv. Funct Mater. 2014, 24, 3114–3122. [Google Scholar] [CrossRef]
- Kong, X.-K.; Chen, Q.-W.; Sun, Z.-Y. Enhanced SERS of the complex substrate using Au supported on graphene with pyridine and R6G as the probe molecules. Chem. Phys. Lett. 2013, 564, 54–59. [Google Scholar] [CrossRef]
- Wu, H.Y.; Choi, C.J.; Cunningham, B.T. Plasmonic nanogap-enhanced raman scattering using a resonant nanodome array. Small 2012, 8, 2878–2885. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, H.B.; Yoon, J.K.; Shin, D.; Shin, K.S. Ag nanoparticle-mediated Raman scattering of 4-aminobenzenethiol on a Pt substrate. J. Phys. Chem. C 2010, 114, 13589–13595. [Google Scholar] [CrossRef]
- McMahon, J.M.; Henry, A.I.; Wustholz, K.L.; Natan, M.J.; Freeman, R.G.; Van Duyne, R.P.; Schatz, G.C. Gold nanoparticle dimer plasmonics: Finite element method calculations of the electromagnetic enhancement to surface-enhanced Raman spectroscopy. Anal. Bioanal. Chem. 2009, 394, 1819–1825. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, F.; Deng, X.Y.; Xue, X.; Wang, L.; Sun, Y.; Feng, J.D.; Zhang, Y.; Wang, Y.; Jung, Y.M. SERS study of surface plasmon resonance induced carrier movement in Au@Cu2O core-shell nanoparticles. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2018, 189, 608–612. [Google Scholar] [CrossRef]
- Morton, S.M.; Jensen, L. Understanding the molecule-surface chemical coupling in SERS. J. Am. Chem. Soc. 2009, 131, 4090–4098. [Google Scholar] [CrossRef]
- Chen, R.; Jensen, L. Interpreting the chemical mechanism in SERS using a Raman bond model. J. Chem. Phys. 2020, 152, 024126. [Google Scholar] [CrossRef] [Green Version]
- Park, W.H.; Kim, Z.H. Charge transfer enhancement in the SERS of a single molecule. Nano Lett. 2010, 10, 4040–4048. [Google Scholar] [CrossRef]
- Moore, J.E.; Morton, S.M.; Jensen, L. Importance of correctly describing charge-transfer excitations for understanding the chemical effect in SERS. J. Phys. Chem. Lett. 2012, 3, 2470–2475. [Google Scholar] [CrossRef]
- Kneipp, K. Chemical Contribution to SERS Enhancement: An Experimental Study on a Series of Polymethine Dyes on Silver Nanoaggregates. J. Phys. Chem. C 2016, 120, 21076–21081. [Google Scholar] [CrossRef]
- Mao, Z.; Song, W.; Xue, X.; Ji, W.; Li, Z.; Chen, L.; Mao, H.; Lv, H.; Wang, X.; Lombardi, J.R.; et al. Interfacial charge-transfer effects in semiconductor-molecule-metal structures: Influence of contact variation. J. Phys. Chem. C 2012, 116, 14701–14710. [Google Scholar] [CrossRef]
- Kudelski, A.; Bukowska, J. The chemical effect in surface enhanced Raman scattering (SERS) for piperidine adsorbed on a silver electrode. Surf. Sci. 1996, 368, 396–400. [Google Scholar] [CrossRef]
- Zheng, X.; Ren, F.; Zhang, S.; Zhang, X.; Wu, H.; Zhang, X.; Xing, Z.; Qin, W.; Liu, Y.; Jiang, C. A General Method for Large-Scale Fabrication of Semiconducting Oxides with High SERS Sensitivity. ACS Appl. Mater. Interfaces 2017, 9, 14534–14544. [Google Scholar] [CrossRef]
- Wang, X.T.; Shi, W.S.; She, G.W.; Mu, L.X. Surface-Enhanced Raman Scattering (SERS) on transition metal and semiconductor nanostructures. Phys. Chem. Chem. Phys. 2012, 14, 5891–5901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, H.; Chen, X.; Zhou, H.; Xiong, L.; Chen, W.; Chen, Z.; Bao, Z.; Wu, Y. The influences of graphene oxide (GO) and plasmonic Ag nanoparticles modification on the SERS sensing performance of TiO2 nanosheet arrays. J. Alloy. Compd. 2021, 864, 158189. [Google Scholar] [CrossRef]
- Qiu, H.; Wang, M.; Cao, M.; Zhang, L.; Ji, S.; Dou, J.; Ji, Y.; Kou, S.; Guo, J.; Yang, Z. Self-cleaning SERS membrane for reusable and ultrasensitive molecular detection via integrating graphitic-carbon-nitride nanosheets and Ag nanospheres into hierarchical graphene layers that covered with graphitic-carbon-nitride quantum-dots. Appl. Surf. Sci. 2019, 489, 1010–1018. [Google Scholar] [CrossRef]
- Ghopry, S.A.; Alamri, M.A.; Goul, R.; Sakidja, R.; Wu, J.Z. Extraordinary Sensitivity of Surface-Enhanced Raman Spectroscopy of Molecules on MoS2 (WS2) Nanodomes/Graphene van der Waals Heterostructure Substrates. Adv. Opt. Mater. 2019, 7, 1801249. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Zhang, Q.J.; Ma, L.P.; Song, P.; Xia, L.X. A P/N type silicon semiconductor loaded with silver nanoparticles used as a SERS substrate to selectively drive the coupling reaction induced by surface plasmons. Nanoscale Adv. 2020, 2, 3460–3466. [Google Scholar] [CrossRef]
- Yamada, H.; Yamamoto, Y.; Tani, N. Surface-enhanced raman scattering (SERS) of adsorbed molecules on smooth surfaces of metals and a metal oxide. Chem. Phys. Lett. 1982, 86, 397–400. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, X.; Ruan, W.; Zhao, B.; Xu, W.; Lombardi, J.R. Observation of Enhanced Raman Scattering for Molecules Adsorbed on TiO2 Nanoparticles: Charge-Transfer Contribution. J. Phys. Chem. C 2008, 112, 20095–20098. [Google Scholar] [CrossRef]
- Xue, X.; Ji, W.; Mao, Z.; Mao, H.; Wang, Y.; Wang, X.; Ruan, W.; Zhao, B.; Lombardi, J.R. Raman Investigation of Nanosized TiO2: Effect of Crystallite Size and Quantum Confinement. J. Phys. Chem. C 2012, 116, 8792–8797. [Google Scholar] [CrossRef]
- Wang, Y.; Ruan, W.; Zhang, J.; Bai, Y.; Lombardi, J.R. Direct observation of surface-enhanced Raman scattering in ZnO nanocrystals. J. Raman Spectrosc. 2010, 40, 1072–1077. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, J.; Lai, W.; Yang, X.; Meng, J.; Su, L.; Gu, C.; Jiang, T.; Pun, E.Y.B.; Shao, L.; et al. Irreversible accumulated SERS behavior of the molecule-linked silver and silver-doped titanium dioxide hybrid system. Nat. Commun. 2020, 11, 1785. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Wei, H.; Xu, Y.; Sun, Q.; Man, B.; Liu, M. Influence of sers activity of SnSe2 nanosheets doped with sulfur. Nanomaterials 2020, 10, 1910. [Google Scholar] [CrossRef]
- Yang, L.; Peng, Y.; Yang, Y.; Liu, J.; Huang, H.; Yu, B.; Zhao, J.; Lu, Y.; Huang, Z.; Li, Z.; et al. A Novel Ultra-Sensitive Semiconductor SERS Substrate Boosted by the Coupled Resonance Effect. Adv. Sci. 2019, 6, 1900310. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Wang, X.; Zhang, X.; Zhang, L.; Yang, X.; Zhao, B. Investigation of the charge-transfer between Ga-doped ZnO nanoparticles and molecules using surface-enhanced Raman scattering: Doping induced band-gap shrinkage. Front. Chem. 2019, 7, 144. [Google Scholar] [CrossRef] [Green Version]
- Dorenbos, P. The Electronic Structure of Lanthanide Impurities in TiO2, ZnO, SnO2, and Related Compounds. ECS J. Solid State Sci. Technol. 2013, 3, R19–R24. [Google Scholar] [CrossRef] [Green Version]
- Serga, V.; Burve, R.; Krumina, A.; Romanova, M.; Kotomin, E.A.; Popov, A.I. Extraction–Pyrolytic Method for TiO2 Polymorphs Production. Crystals 2021, 11, 431. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Pankratova, V.; Popov, A.I.; Pankratov, V. Study of phase composition, photocatalytic activity, and photoluminescence of TiO2 with Eu additive produced by the extraction-pyrolytic method. J. Mater. Res. Technol. 2021, 13, 2350–2360. [Google Scholar] [CrossRef]
- Gupta, S.K.; Desai, R.; Jha, P.K.; Sahoo, S.; Kirin, D. Titanium dioxide synthesized using titanium chloride: Size effect study using Raman spectroscopy and photoluminescence. J. Raman Spectrosc. 2010, 41, 350–355. [Google Scholar] [CrossRef]
- Wu, X.; Yin, S.; Dong, Q.; Guo, C.; Kimura, T.; Matsushita, J.-i.; Sato, T. Photocatalytic Properties of Nd and C Codoped TiO2 with the Whole Range of Visible Light Absorption. J. Phys. Chem. C 2013, 117, 8345–8352. [Google Scholar] [CrossRef]
- Hewer, T.; Souza, E.; Martins, T.; Muccillo, E.; Freire, R. Influence of neodymium ions on photocatalytic activity of TiO2 synthesized by sol–gel and precipitation methods. J. Mol. Catal. A Chem. 2011, 336, 58–63. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Bao, R.; Yu, Y.; Chen, H.; Wang, W.; Xia, J.; Li, H. Effects of Rare Earth Elements and Nitrogen Co-Doped on the Photocatalytic Performance of TiO2. Cryst. Res. Technol. 2018, 53, 1700138. [Google Scholar] [CrossRef]
- Yuan, M.; Zhang, J.; Yan, S.; Luo, G.; Xu, Q.; Wang, X.; Li, C. Effect of Nd2O3 addition on the surface phase of TiO2 and photocatalytic activity studied by UV Raman spectroscopy. J. Alloy. Compd. 2011, 509, 6227–6235. [Google Scholar] [CrossRef]
- Jing, L.; Xin, B.; Yuan, F.; Xue, L.; Wang, B.; Fu, H. Effects of Surface Oxygen Vacancies on Photophysical and Photochemical Processes of Zn-Doped TiO2 Nanoparticles and Their Relationships. J. Phys. Chem. B 2006, 110, 17860–17865. [Google Scholar] [CrossRef]
- Borgarello, E.; Kiwi, J.; Graetzel, M.; Pelizzetti, E.; Visca, M. Visible light induced water cleavage in colloidal solutions of chromium-doped titanium dioxide particles. J. Am. Chem. Soc. 1982, 104, 2996–3002. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Nithyaa, N.; Victor Jaya, N. Effect of Nd on structural, optical and magnetic behaviour of TiO2 nanoparticles. Appl. Phys. A 2021, 127, 69. [Google Scholar] [CrossRef]
- Song, W.; Wang, Y.; Zhao, B. Surface-enhanced Raman scattering of 4-mercaptopyridine on the surface of TiO2 nanofibers coated with Ag nanoparticles. J. Phys. Chem. C 2007, 111, 12786–12791. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Z.; Hu, H.; Jing, S.; Zhao, B.; Xu, W.; Zhao, C.; Lombardi, J.R. Raman scattering study of molecules adsorbed on ZnS nanocrystals. J. Raman Spectrosc. 2007, 38, 34–38. [Google Scholar] [CrossRef]
- Baldwin, J.; Schühler, N.; Butler, I.S.; Andrews, M.P. Integrated optics evanescent wave surface enhanced Raman scattering (IO-EWSERS) of mercaptopyridines on a planar optical chemical bench: Binding of hydrogen and copper ion. Langmuir 1996, 12, 6389–6398. [Google Scholar] [CrossRef]
- Kittel, C.; McEuen, P.; McEuen, P. Introduction to Solid State Physics; Wiley New York: New York, NY, USA, 1996; Volume 8. [Google Scholar]
- Yang, S.; Yao, J.; Quan, Y.; Hu, M.; Su, R.; Gao, M.; Han, D.; Yang, J. Monitoring the charge-transfer process in a Nd-doped semiconductor based on photoluminescence and SERS technology. Light Sci. Appl. 2020, 9, 117. [Google Scholar] [CrossRef]
- Boschloo, G.; Fitzmaurice, D. Spectroelectrochemical investigation of surface states in nanostructured TiO2 electrodes. J. Phys. Chem. B 1999, 103, 2228–2231. [Google Scholar] [CrossRef]
- Dorenbos, P. Valence stability of lanthanide ions in inorganic compounds. Chem. Mater. 2005, 17, 6452–6456. [Google Scholar] [CrossRef]
- Dorenbos, P. Charge transfer bands in optical materials and related defect level location. Opt. Mater. 2017, 69, 8–22. [Google Scholar] [CrossRef]
- Xue, X.; Ruan, W.; Yang, L.; Ji, W.; Xie, Y.; Chen, L.; Song, W.; Zhao, B.; Lombardi, J.R. Surface-enhanced Raman scattering of molecules adsorbed on Co-doped ZnO nanoparticles. J. Raman Spectrosc. 2012, 43, 61–64. [Google Scholar] [CrossRef]
- Chen, J.; Qin, G.; Shen, W.; Li, Y.; Das, B. Fabrication of long-range ordered, broccoli-like SERS arrays and application in detecting endocrine disrupting chemicals. J. Mater. Chem. C 2015, 3, 1309–1318. [Google Scholar] [CrossRef]
- Cheng, H.-W.; Huan, S.-Y.; Wu, H.-L.; Shen, G.-L.; Yu, R.-Q. Surface-enhanced Raman spectroscopic detection of a bacteria biomarker using gold nanoparticle immobilized substrates. Anal. Chem. 2009, 81, 9902–9912. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Lu, L.; Wang, L.; Zhang, J. Improved SERS sensitivity on plasmon-free TiO2 photonic microarray by enhancing light-matter coupling. J. Am. Chem. Soc. 2014, 136, 9886–9889. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Zhao, X.; Zhang, M.; Sun, X. Charge-Transfer Process in Surface-Enhanced Raman Scattering Based on Energy Level Locations of Rare-Earth Nd3+-Doped TiO2 Nanoparticles. Nanomaterials 2021, 11, 2063. https://doi.org/10.3390/nano11082063
Zhao Z, Zhao X, Zhang M, Sun X. Charge-Transfer Process in Surface-Enhanced Raman Scattering Based on Energy Level Locations of Rare-Earth Nd3+-Doped TiO2 Nanoparticles. Nanomaterials. 2021; 11(8):2063. https://doi.org/10.3390/nano11082063
Chicago/Turabian StyleZhao, Zihao, Xiang Zhao, Mu Zhang, and Xudong Sun. 2021. "Charge-Transfer Process in Surface-Enhanced Raman Scattering Based on Energy Level Locations of Rare-Earth Nd3+-Doped TiO2 Nanoparticles" Nanomaterials 11, no. 8: 2063. https://doi.org/10.3390/nano11082063
APA StyleZhao, Z., Zhao, X., Zhang, M., & Sun, X. (2021). Charge-Transfer Process in Surface-Enhanced Raman Scattering Based on Energy Level Locations of Rare-Earth Nd3+-Doped TiO2 Nanoparticles. Nanomaterials, 11(8), 2063. https://doi.org/10.3390/nano11082063




