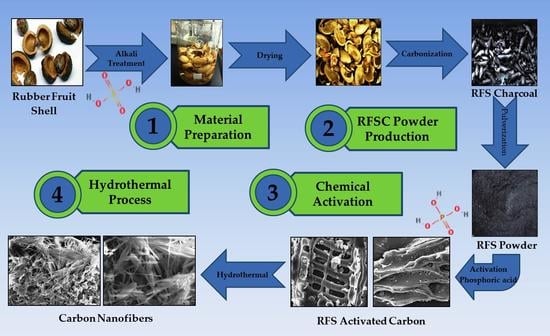
The Production of Carbon Nanofiber on Rubber Fruit Shell-Derived Activated Carbon by Chemical Activation and Hydrothermal Process with Low Temperature
Abstract
:1. Introduction
2. Materials and Methods
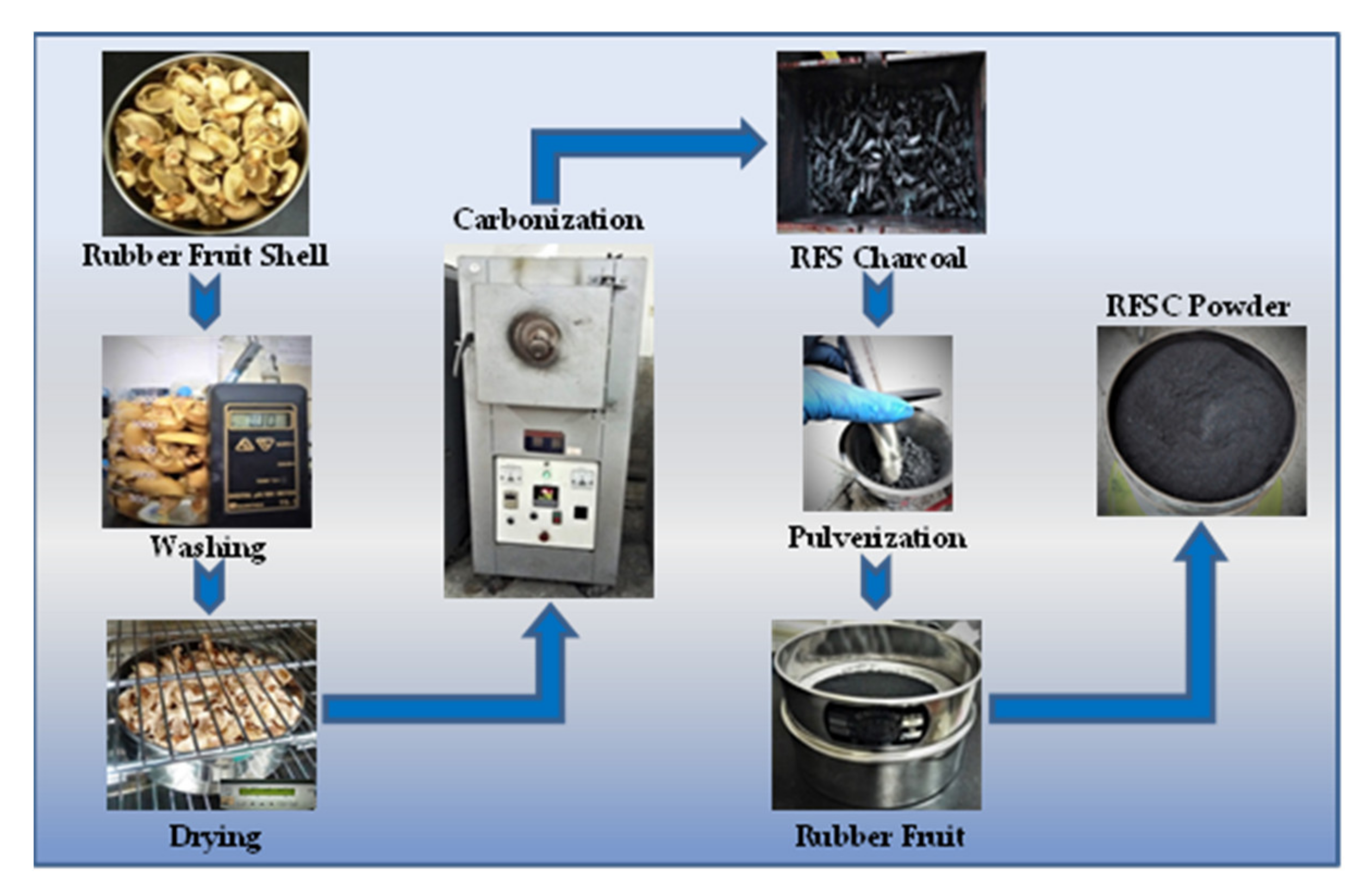
2.1. Material Preparation
2.2. Characterization
3. Results
3.1. Thermogravimetric Analisys of RFSAC and Nanofibers
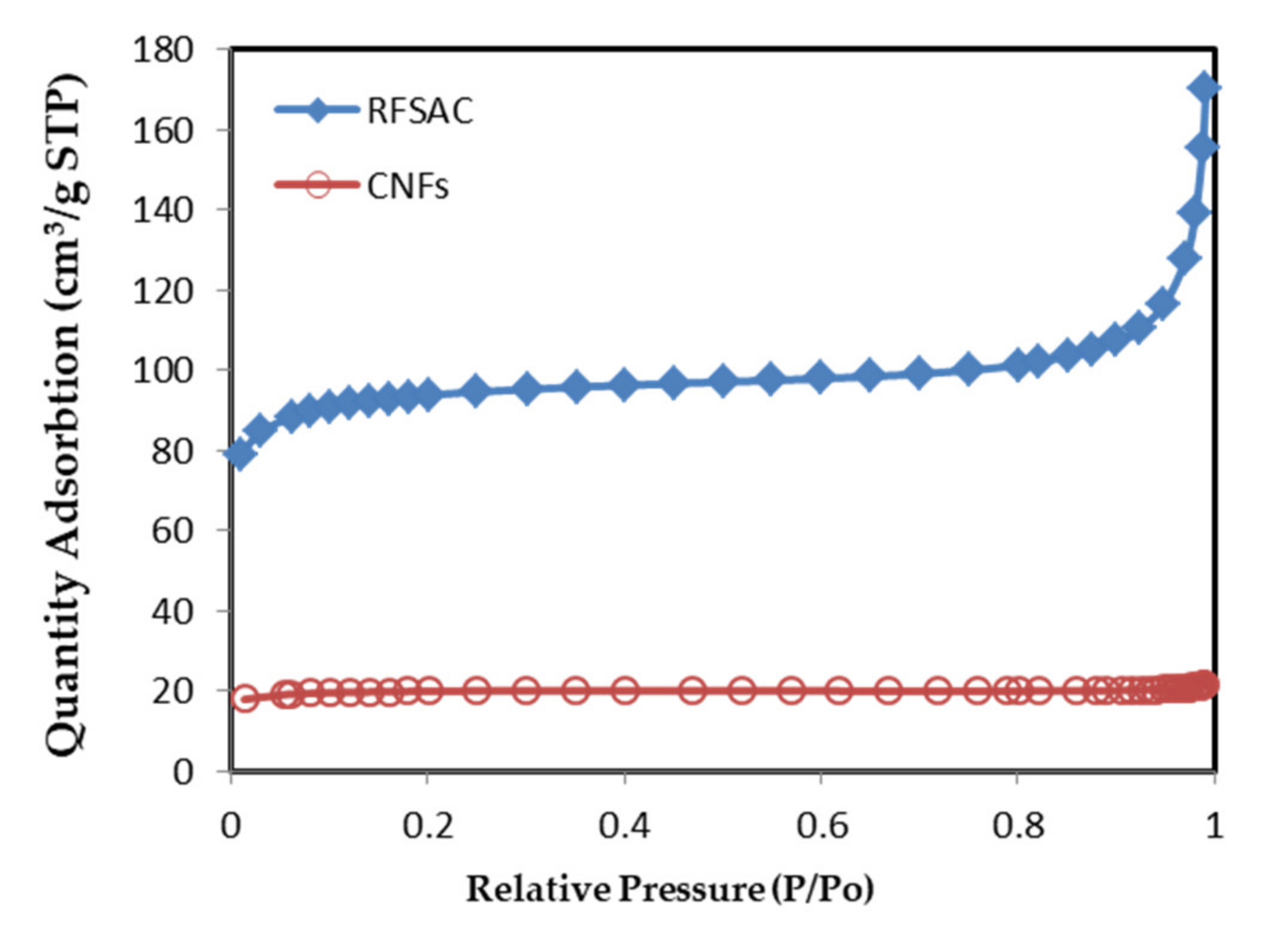
3.2. BET Specific Surface Area and Porosity of RFSAC and Carbon Nanofibers
3.3. The Element Content of Carbon Nanofibers Grown on RFSAC
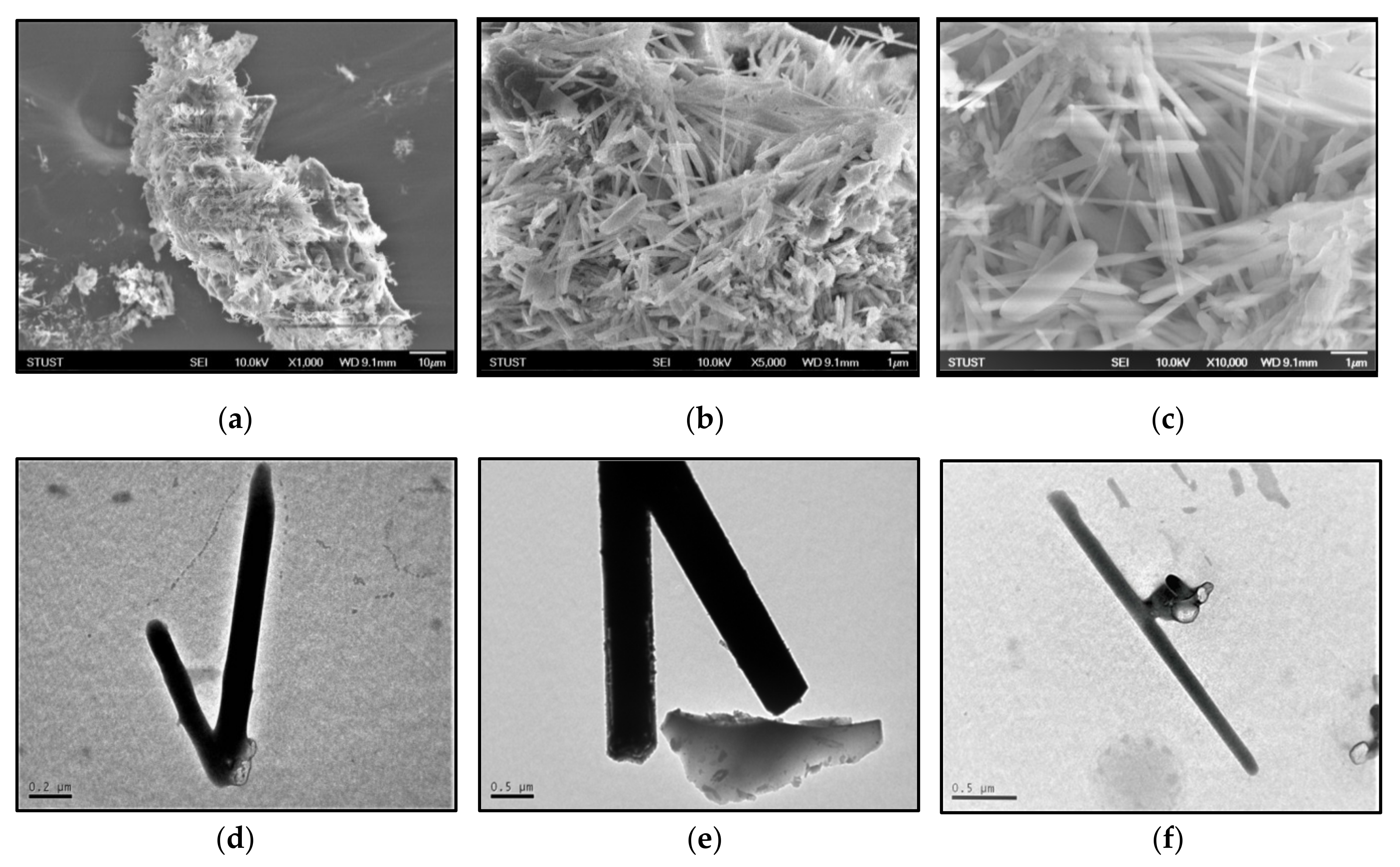
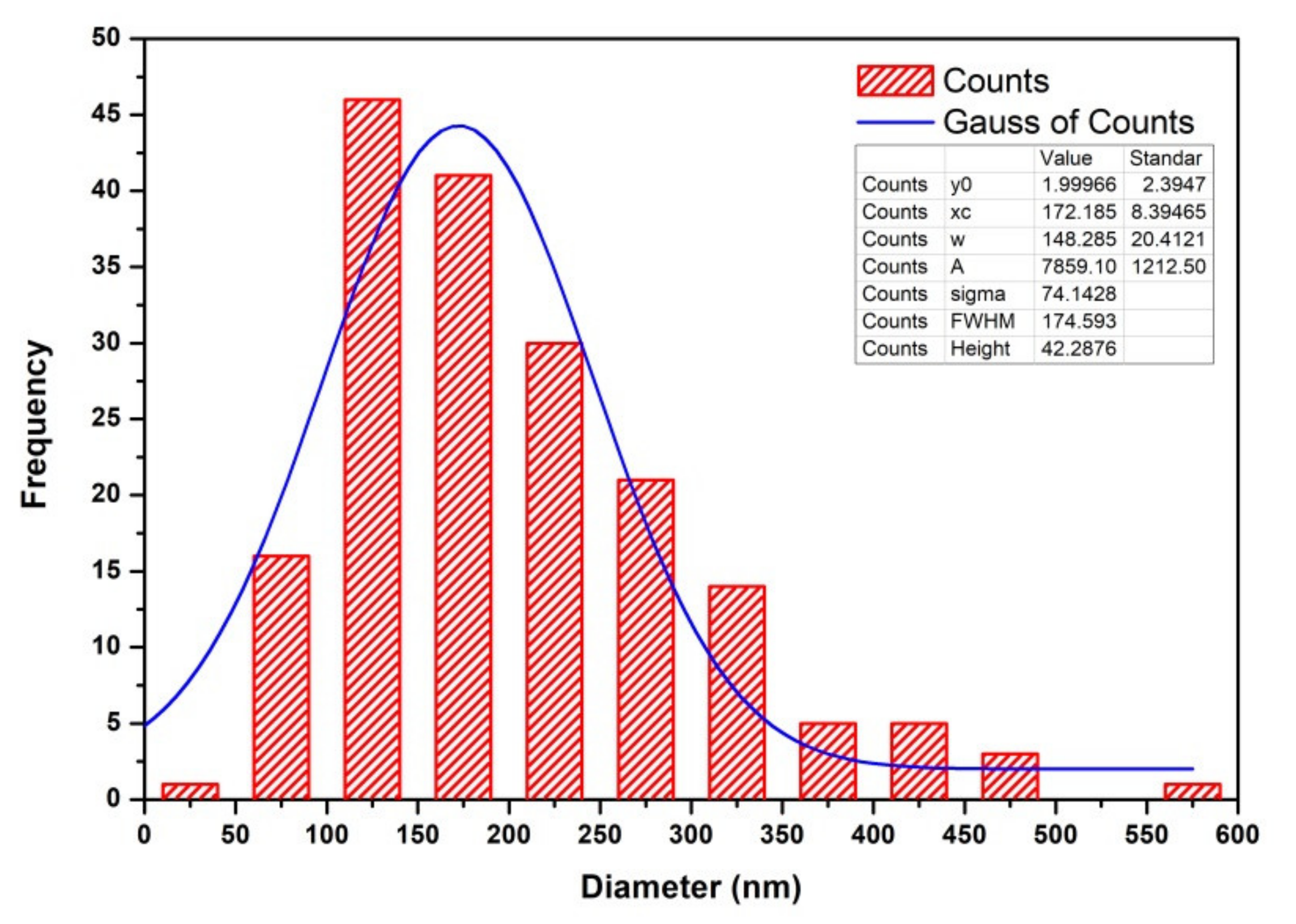
3.4. Morphology and Microstructure of CNTs
3.5. Performance of Current CNFs Grown on RFSFAC Surface in Comparison to Other CNFs Produced from Biomass-Activated Carbon
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; An, Y.; Feng, J.; Zhu, X.; Li, F. Preparation and Properties of Carbon Nanofiber Modified Emulsified Asphalt Based on Ultrasonication and Surfactant and the Impact of SBR and NH4Cl. Front. Mater. 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, S.; Wang, J.; Yu, A.; Wei, G. Carbon nanofiber-based functional nanomaterials for sensor applications. Nanomaterials 2019, 9, 1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azwar, E.; Mahari, W.A.W.; Chuah, J.H.; Vo, D.-V.N.; Ma, N.L.; Lam, W.-H.; Lam, S.S. Transformation of biomass into carbon nanofiber for supercapacitor application—A review. Int. J. Hydrogen Energy 2018, 43, 20811–20821. [Google Scholar] [CrossRef]
- Desmaris, V.; Saleem, M.A.; Shafiee, S. Examining carbon nanofibers: Properties, growth, and applications. IEEE Nanotechnol. Mag. 2015, 9, 33–38. [Google Scholar] [CrossRef]
- Kumar, M.; Hietala, M.; Oksman, K. Lignin-based electrospun carbon nanofibers. Front. Mater. 2019, 6, 1–6. [Google Scholar] [CrossRef]
- Pandia, S.; Sinaga, M.S.; Masyithah, Z.; Husin, A.; Nurfadilla, S.; Fitriani; Sipahutar, B.K.S. Rubber Fruit Shell (Hevea brasiliensis) as bio sorbent to remove FFA (Free Fatty Acid) content in CPO (Crude Palm Oil). IOP Conf. Ser. Mater. Sci. Eng. 2018, 309, 012085. [Google Scholar] [CrossRef]
- Sengupta, D.; Chen, S.H.; Michael, A.; Kwok, C.Y.; Lim, S.; Pei, Y.; Kottapalli, A.G.P. Single and bundled carbon nanofibers as ultralightweight and flexible piezoresistive sensors. Npj Flex. Electron. 2020, 4, 1–11. [Google Scholar] [CrossRef]
- Feng, L.; Xie, N.; Zhong, J. Carbon nanofibers and their composites: A review of synthesizing, properties and applications. Materials 2014, 7, 3919–3945. [Google Scholar] [CrossRef]
- Banitaba, S.N.; Ehrmann, A. Application of electrospun nanofibers for fabrication of versatile and highly efficient electrochemical devices: A review. Polymers 2021, 13, 1741. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, B.; Chen, Y.; Guo, L.; Wei, G. Carbon nanofiber-based three-dimensional nanomaterials for energy and environmental applications. Mater. Adv. 2020, 1, 2163–2181. [Google Scholar] [CrossRef]
- Kretzschmar, A.; Selmert, V.; Weinrich, H.; Kungl, H.; Tempel, H.; Eichel, R. Tailored Gas Adsorption Properties of Electrospun Carbon Nanofibers for Gas Separation and Storage. ChemSusChem 2020, 13, 3180–3191. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Adnan, A.S.; Yahya, E.B.; Olaiya, N.G.; Safrida, S.; Hossain, M.S.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.K.; Oyekanmi, A.A.; et al. A review on plant cellulose nanofibre-based aerogels for biomedical applications. Polymers 2020, 12, 1759. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, M.; Verma, N.; Khan, S. Copper/zinc bimetal nanoparticles-dispersed carbon nanofibers: A novel potential antibiotic material. Mater. Sci. Eng. C 2016, 59, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Lan, J.; Jia, X.; Cai, Q.; Yang, X. Improving interfacial adhesion with epoxy matrix using hybridized carbon nanofibers containing calcium phosphate nanoparticles for bone repairing. Mater. Sci. Eng. C 2016, 61, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Huang, Y.; Zheng, Y.; Yang, X.; Liu, C.; Di, M.; Larpkiattaworn, S.; Nimlos, M.R.; Zheng, Z. Porous carbon nanofiber derived from a waste biomass as anode material in lithium-ion batteries. J. Taiwan Inst. Chem. Eng. 2019, 95, 217–226. [Google Scholar] [CrossRef]
- Yang, C.; Chen, C.; Pan, Y.; Li, S.; Wang, F.; Li, J.; Li, N.; Li, X.; Zhang, Y.; Li, D. Flexible highly specific capacitance aerogel electrodes based on cellulose nanofibers, carbon nanotubes and polyaniline. Electrochim. Acta 2015, 182, 264–271. [Google Scholar] [CrossRef]
- Henrique, M.A.; Neto, W.P.F.; Silvério, H.A.; Martins, D.F.; Gurgel, L.V.A.; da Silva Barud, H.; de Morais, L.C.; Pasquini, D. Kinetic study of the thermal decomposition of cellulose nanocrystals with different polymorphs, cellulose I and II, extracted from different sources and using different types of acids. Ind. Crops Prod. 2015, 76, 128–140. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-J.; Wang, X.-M.; Cui, W.-J.; Dou, Y.-Q.; Zhao, D.-Y.; Xia, Y.-Y. Highly ordered mesoporous carbon nanofiber arrays from a crab shell biological template and its application in supercapacitors and fuel cells. J. Mater. Chem. 2010, 20, 4223–4230. [Google Scholar] [CrossRef]
- Deng, L.; Zhong, W.; Wang, J.; Zhang, P.; Fang, H.; Yao, L.; Liu, X.; Ren, X.; Li, Y. The enhancement of electrochemical capacitance of biomass-carbon by pyrolysis of extracted nanofibers. Electrochim. Acta 2017, 228, 398–406. [Google Scholar] [CrossRef] [Green Version]
- Mugadza, K.; Stark, A.; Ndungu, P.G.; Nyamori, V.O. Synthesis of Carbon Nanomaterials from Biomass Utilizing Ionic Liquids for Potential Application in Solar Energy Conversion and Storage. Materials 2020, 13, 3945. [Google Scholar] [CrossRef]
- Lee, B.-S.; Yu, W.-R. Electrospun carbon nanofibers as a functional composite platform: A review of highly tunable microstructures and morphologies for versatile applications. Funct. Compos. Struct. 2020, 2, 012001. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, M.; Yang, Y.; Kang, F. Carbon nanofibers prepared via electrospinning. Adv. Mater. 2012, 24, 2547–2566. [Google Scholar] [CrossRef]
- Zhang, J.; Tahmasebi, A.; Omoriyekomwan, J.E.; Yu, J. Direct synthesis of hollow carbon nanofibers on bio-char during microwave pyrolysis of pine nut shell. J. Anal. Appl. Pyrolysis 2018, 130, 142–148. [Google Scholar] [CrossRef]
- Pizzi, A.; Duca, D.; Rossini, G.; Fabrizi, S.; Toscano, G. Biofuel, Bioenergy and Feed Valorization of By-Products and Residues from Hevea brasiliensis Cultivation to Enhance Sustainability. Resources 2020, 9, 114. [Google Scholar] [CrossRef]
- Oluodo, L.A.; Huda, N.; Komilus, C.F. Potential utilization of rubber seed meal as feed and food potential utilization of rubber seed meal as feed and food. Int. J. Eng. Technol. 2018, 4, 66–71. [Google Scholar]
- Sun, K.; Jiang, J.C. Preparation and characterization of activated carbon from rubber-seed shell by physical activation with steam. Biomass Bioenergy 2010, 34, 539–544. [Google Scholar] [CrossRef]
- Yan, K.Z.; Zaini, M.A.A.; Arsad, A.; Nasri, N.S. Rubber seed shell based activated carbon by physical activation for phenol removal. Chem. Eng. Trans. 2019, 72, 151–156. [Google Scholar]
- Borhan, A.; Yusup, S.; Mun, Y.S. Surface modification of rubber seed shell activated carbon with malic acid for high CO2 adsorption. In Proceedings of the IOP Conference Series: Earth and Environmental Science, West Java, Indonesia, 19–20 August 2019. [Google Scholar]
- Borhan, A.; Yusuf, S. Activation of rubber-seed shell waste by malic acid as potential CO2 removal: Isotherm and kinetics studies. Materials 2020, 13, 4970. [Google Scholar] [CrossRef] [PubMed]
- Ekebafe, L.O.; Igbonazobi, L.C.; Anakhu, E.A. Advances in natural rubber seed shell utilization in polymer technology. J. Adv. Sci. Eng. 2020, 3, 106–112. [Google Scholar] [CrossRef]
- Naswir, M.; Yasdi; Rahima, S.; Wibowo, Y.G. Rubber fruit shell: Agricultural waste material as a potential sustainable production for wastewater treatment. IOP Conf. Ser. Mater. Sci. Eng. 2020, 801, 012080. [Google Scholar] [CrossRef]
- Suhdi; Wang, S.-C. Fine Activated Carbon from Rubber Fruit Shell Prepared by Using ZnCl2 and KOH Activation. Appl. Sci. 2021, 11, 3994. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Natural Rubber: Top 10 Producers. 2019. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 10 January 2021).
- Indonesia, Direktorat Jenderal Perkebunan Kementerian Pertanian RI. Pembangunan Perkebunan. 2020. Available online: http://ditjenbun.pertanian.go.id/info-publik/laporan-tahunan/ (accessed on 1 July 2021).
- Julian, R.T. Pemanfaatan Limbah Cangkang Biji Karet Menjadi Briket Sebagai Bahan Bakar Alternatif dengan Bahan Perekat Amilum; Politeknik Negeri Sriwijaya: Palembang, Indonesia, 2017. [Google Scholar]
- Ucar, N.; Cavdar, Z.; Karatepe, N.; Altay, P.; Kizildag, N. SO2 Adsorption capability of activated carbon nanofibers produced by different activation process parameters. Text. Appar. 2016, 26, 407–413. [Google Scholar]
- Gopiraman, M.; Kim, I.S. Preparation, Characterization, and Applications of Electrospun Carbon Nanofibers and Its Composites. In Electrospinning Electrospraying Techniques and Applications; IntechOpen: London, UK, 2019. [Google Scholar]
- Kojima, Y.; Takayasu, M.; Toma, M.; Koda, S. Degradation of cellulose in NaOH and NaOH/urea aqueous solutions by ultrasonic irradiation. Ultrason. Sonochem. 2019, 51, 419–423. [Google Scholar] [CrossRef]
- Gan, Y.X.; Jayatissa, A.H.; Yu, Z.; Chen, X.; Li, M. Hydrothermal Synthesis of Nanomaterials. J. Nanomater. 2020, 2020. [Google Scholar] [CrossRef]
- Wen, Y.; Jiang, M.; Kitchens, C.L.; Chumanov, G. Synthesis of carbon nanofibers via hydrothermal conversion of cellulose nanocrystals. Cellulose 2017, 24, 4599–4604. [Google Scholar] [CrossRef]
- Barin, G.B.; De Fátima Gimenez, I.; Da Costa, L.P.; Filho, A.G.S.; Barreto, L.S. Hollow carbon nanostructures obtained from hydrothermal carbonization of lignocellulosic biomass. J. Mater. Sci. 2014, 49, 665–672. [Google Scholar] [CrossRef]
- Manafi, S.; Rahaei, M.B.; Elli, Y.; Joughehdoust, S. High-yield synthesis of multi-walled carbon nanotube by hydrothermal method. Can. J. Chem. Eng. 2010, 88, 283–286. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Rameshwarichanu, M.; Gurumayum, S.; Shijagurumayum, C.; Student, B.E. A review on carbon nanotube. Int. J. Eng. Technol. Manag. Appl. Sci. 2017, 5, 340–348. [Google Scholar]
- Chen, X.W.; Timpe, O.; Hamid, S.B.A.; Schlögl, R.; Su, D.S. Direct synthesis of carbon nanofibers on modified biomass-derived activated carbon. Carbon 2009, 47, 340–343. [Google Scholar] [CrossRef]
- Yakout, S.M.; El-Deen, G.S. Characterization of activated carbon prepared by phosphoric acid activation of olive stones. Arab. J. Chem. 2016, 9, S1155–S1162. [Google Scholar] [CrossRef] [Green Version]
- Arami-Niya, A.; Daud, W.M.A.W.; Mjalli, F.S. Comparative study of the textural characteristics of oil palm shell activated carbon produced by chemical and physical activation for methane adsorption. Chem. Eng. Res. Des. 2011, 89, 657–664. [Google Scholar] [CrossRef]
- Hsu, L.-Y.; Teng, H. Influence of different chemical reagents on the preparation of activated carbons from bituminous coal. Fuel Process. Technol. 2000, 64, 155–166. [Google Scholar] [CrossRef]
- Su, D.S.; Chen, X.; Weinberg, G.; Klein-Hofmann, A.; Timpe, O.; Hamid, S.B.A.; Schlögl, R. Hierarchically structured carbon: Synthesis of carbon nanofibers nested inside or immobilized onto modified activated carbon. Angew. Chem. Int. Ed. 2005, 44, 5488–5492. [Google Scholar] [CrossRef]
- Shenggao, W.; Wen, H.; Mingchen, Z.; Yuan, G.; Quanrong, D.; Yangwu, M.; Qinfang, X.; Geming, W. Synergistic effects of microstructures and active nitrogen content on the oxygen reduction reaction performance of nitrogen-doped carbon nanofibers via KOH activation heat treatment. J. Mater. Sci. 2020, 55, 10725–10739. [Google Scholar] [CrossRef]
- Zhao, X.; Zou, X.; Yan, X.; Brown, C.L.; Chen, Z.-G.; Zhu, G.; Yao, X. Defect-driven oxygen reduction reaction (ORR) of carbon without any element doping. Inorg. Chem. Front. 2016, 3, 417–421. [Google Scholar] [CrossRef]
- Liu, W.J.; Tian, K.; He, Y.-R.; Jiang, H.; Yu, H.-Q. High-yield harvest of nanofibers/mesoporous carbon composite by pyrolysis of waste biomass and its application for high durability electrochemical energy storage. Environ. Sci. Technol. 2014, 48, 13951–13959. [Google Scholar] [CrossRef]
- Deng, L.; Young, R.J.; Kinloch, I.A.; Abdelkader, A.M.; Holmes, S.M.; De Haro-Del Rio, D.A.; Eichhorn, S.J. Supercapacitance from Cellulose and Carbon Nanotube Nanocomposite Fibers. ACS Appl. Mater. Interfaces 2013, 5, 9983–9990. [Google Scholar] [CrossRef]
- Cao, Q.; Zhu, M.; Chen, J.; Song, Y.; Li, Y.; Zhou, J. Novel Lignin-Cellulose-Based Carbon Nanofibers as High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2020, 12, 1210–1221. [Google Scholar] [CrossRef]
- Miniach, E.; Śliwak, A.; Moyseowicz, A.; Gryglewicz, G. Growth of carbon nanofibers from methane on a hydroxyapatite-supported nickel catalyst. J. Mater. Sci. 2016, 51, 5367–5376. [Google Scholar] [CrossRef] [Green Version]
- Shi, K.; Yan, J.; Lester, E.; Wu, T. Catalyst-free synthesis of multiwalled carbon nanotubes via microwave-induced processing of biomass. Ind. Eng. Chem. Res. 2014, 53, 15012–15019. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Wu, C.; Gu, S. State-of-the-art on the production and application of carbon nanomaterials from biomass. Green Chem. 2018, 20, 5031–5057. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Jia, J.; Kwong, F.L.; Ng, D.H.L.; Tjong, S.C. Synthesis of multiwalled carbon nanotubes from bamboo charcoal and the roles of minerals on their growth. Biomass Bioenergy 2012, 36, 12–19. [Google Scholar] [CrossRef]
- Meilianti, M. Karakteristik Karbon Aktif Dari Cangkang Buah Karet Menggunakan Aktivator H3PO4. J. Distilasi 2018, 2, 1–9. [Google Scholar] [CrossRef]


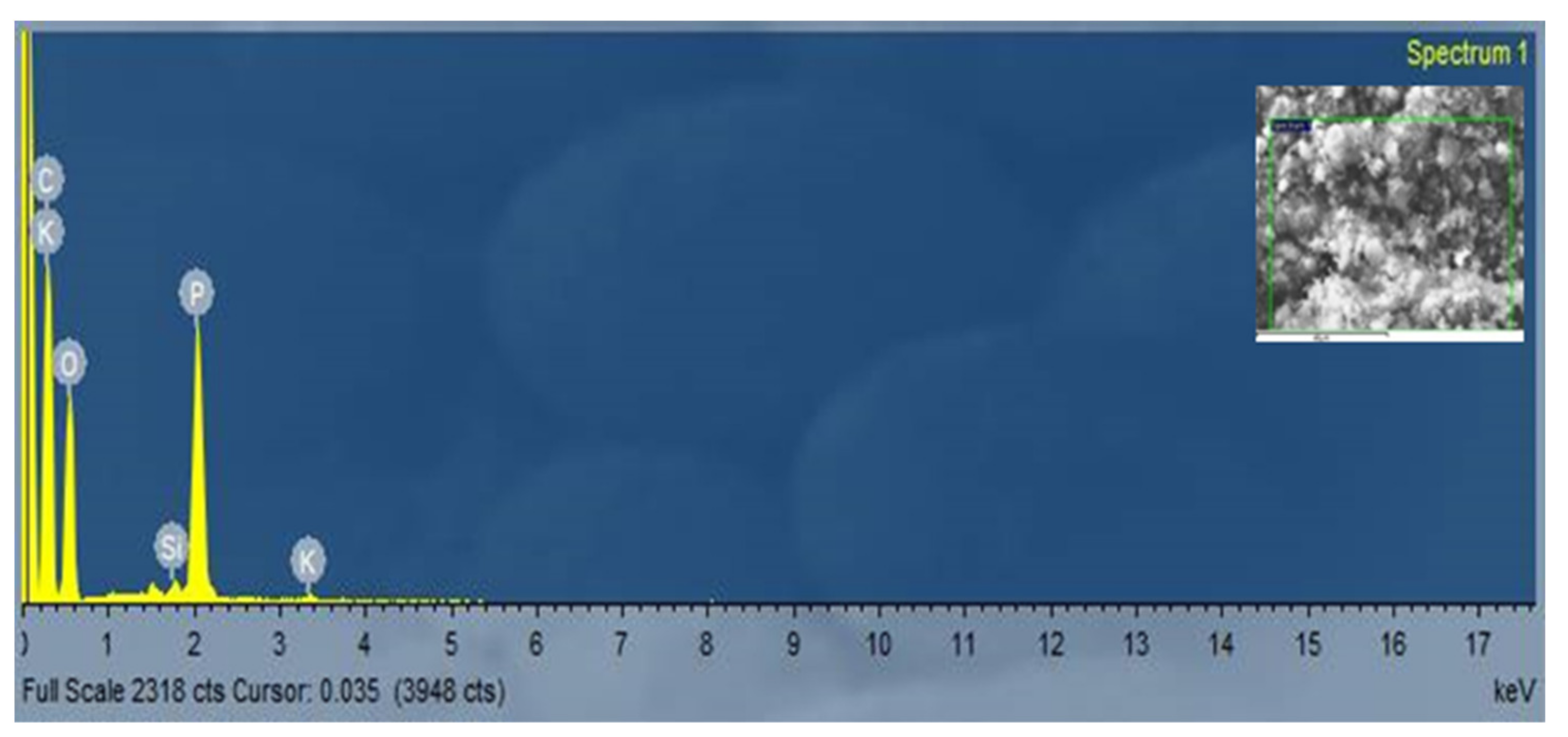

| Element | Line | Intensity Corrn. | Atomic% |
|---|---|---|---|
| C | K_SERIES | 0.6678 | 62.48 |
| O | K_SERIES | 0.5744 | 32.83 |
| Si | K_SERIES | 0.9733 | 0.22 |
| P | K_SERIES | 1.3567 | 4.38 |
| K | K_SERIES | 1.0071 | 0.09 |
| Total | 100 |
| Biomass | Synthesis Method | Catalyst | BET (m2/g) | CNF Diameter (nm) | Intensity Ratio (ID/IG) | Ref. |
|---|---|---|---|---|---|---|
| Bamboo (cellulose fibers) | Pyrolysis | - | na | 10–30 | 0.84 | [16] |
| Sawdust | Pyrolysis | Fe | 360–421 | na | 0.9–1.4 | [52] |
| Natural fungus | Hydrothermal technique at 150 °C | - | 895–1280 | 620 | 1.4–1.55 | [19] |
| Cellulose | Ultrasonication | 865 | 200 | 1.21 | [53] | |
| Poplar lignin powders | Electrospinning | 221–837 | 80–370 | 0.73–0.87 | [54] | |
| RFSAC | Hydrothermal low temperature at 90 °C | - | 63 | 38–554 (172 average) | 0.98 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhdi, S.; Wang, S.-C. The Production of Carbon Nanofiber on Rubber Fruit Shell-Derived Activated Carbon by Chemical Activation and Hydrothermal Process with Low Temperature. Nanomaterials 2021, 11, 2038. https://doi.org/10.3390/nano11082038
Suhdi S, Wang S-C. The Production of Carbon Nanofiber on Rubber Fruit Shell-Derived Activated Carbon by Chemical Activation and Hydrothermal Process with Low Temperature. Nanomaterials. 2021; 11(8):2038. https://doi.org/10.3390/nano11082038
Chicago/Turabian StyleSuhdi, Suhdi, and Sheng-Chang Wang. 2021. "The Production of Carbon Nanofiber on Rubber Fruit Shell-Derived Activated Carbon by Chemical Activation and Hydrothermal Process with Low Temperature" Nanomaterials 11, no. 8: 2038. https://doi.org/10.3390/nano11082038
APA StyleSuhdi, S., & Wang, S.-C. (2021). The Production of Carbon Nanofiber on Rubber Fruit Shell-Derived Activated Carbon by Chemical Activation and Hydrothermal Process with Low Temperature. Nanomaterials, 11(8), 2038. https://doi.org/10.3390/nano11082038