Review of ZnO Binary and Ternary Composite Anodes for Lithium-Ion Batteries
Abstract
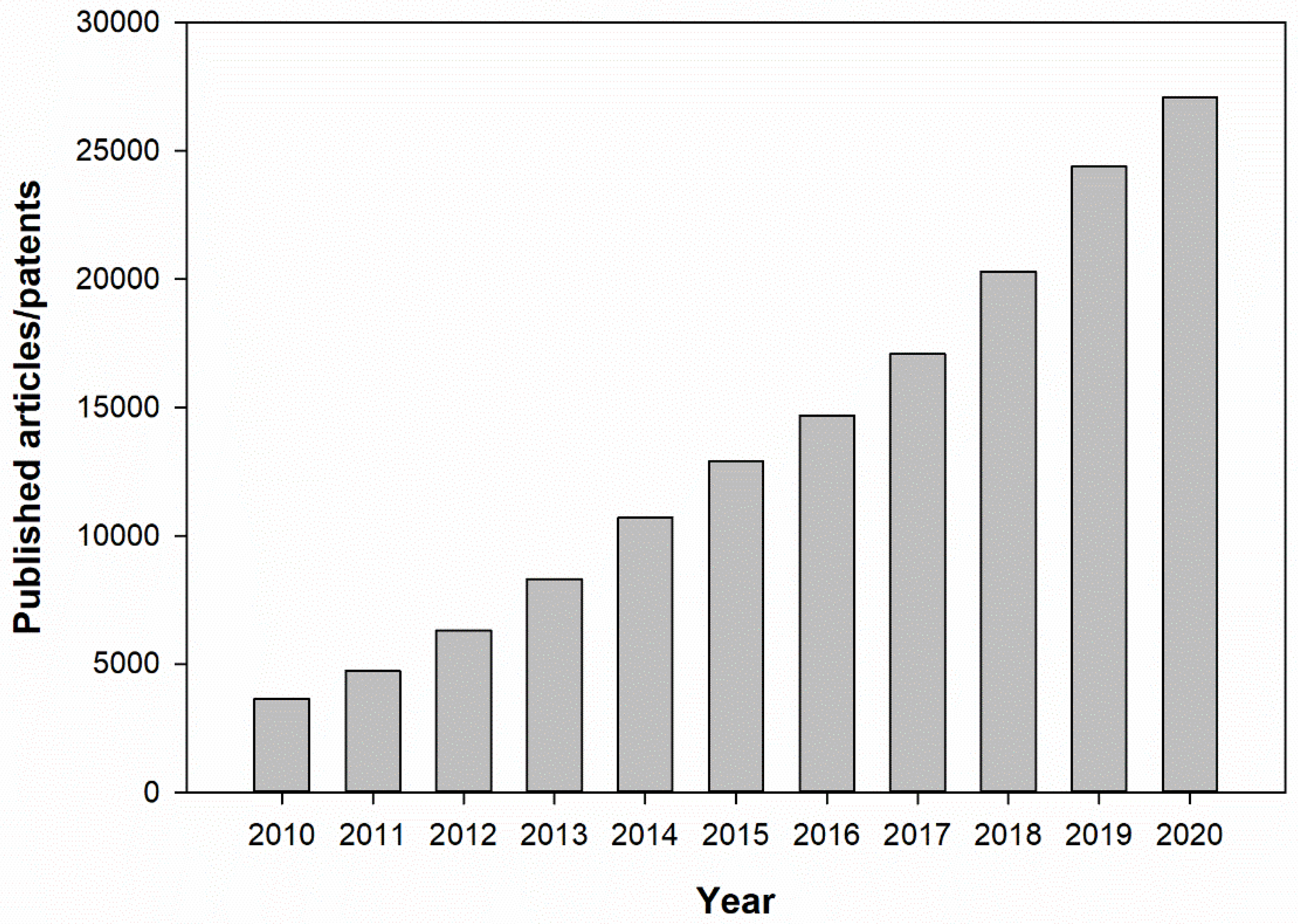
1. Introduction
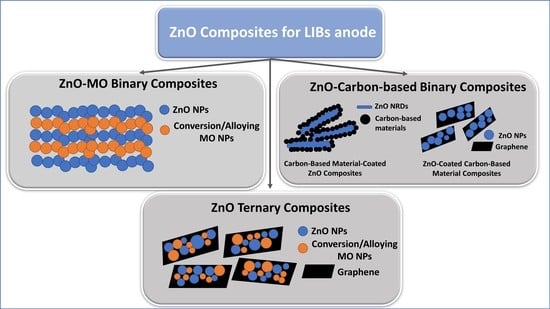
2. ZnO Binary Composites
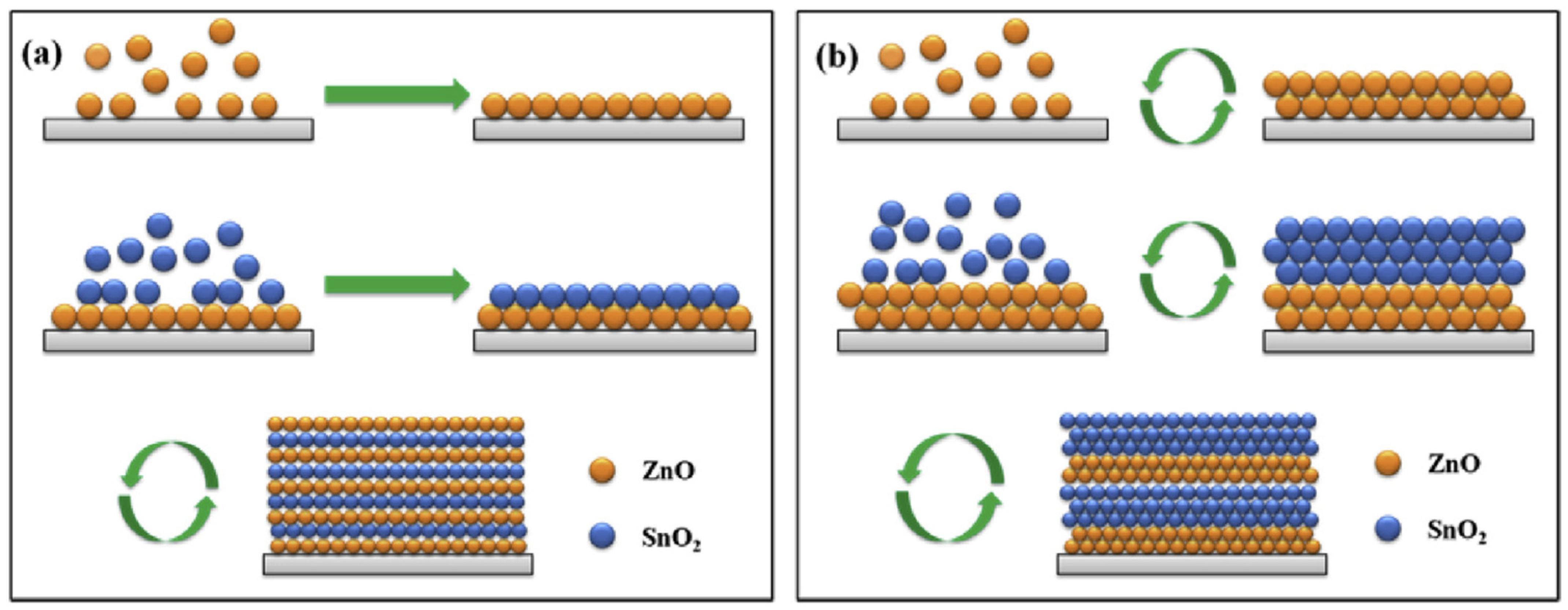
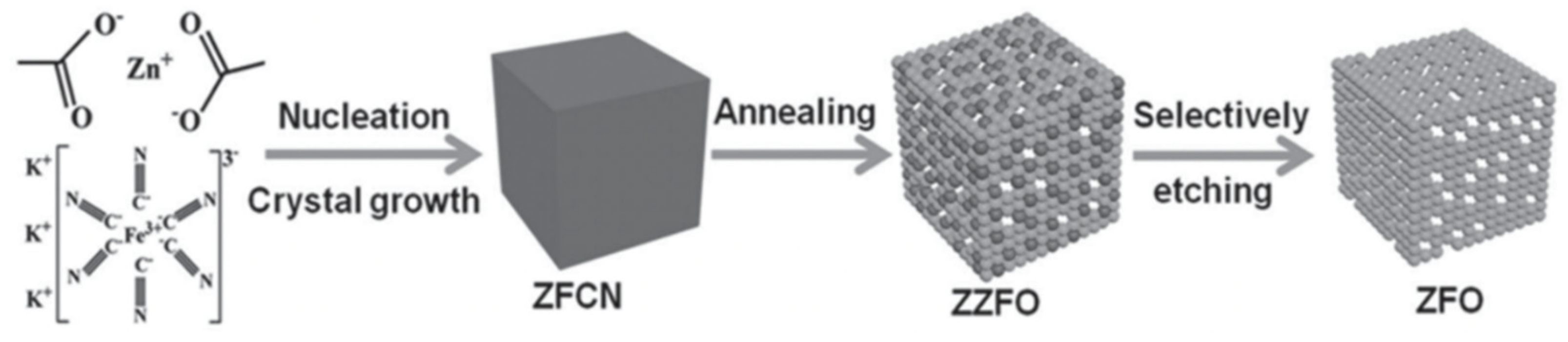
2.1. ZnO–MO Composites
2.2. ZnO–Carbon-Based Composites
2.2.1. ZnO-Coated Carbon-Based Material Composites
2.2.2. Carbon-Based Material-Coated ZnO Composites
3. ZnO Ternary Composites
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, S.; Li, Y.; Wu, M.; Cai, M.; Shen, P.K. Synthesis of hierarchically flower-like FeWO4 as high performance anode materials for Li-ion batteries by a simple hydrothermal process. Int. J. Hydrog. Energy 2014, 39, 16081–16087. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Phung, V.D.; Kidanu, W.G.; Ahn, Y.N.; Nguyen, T.L.; Kim, I.T. Carbon-free Cu/SbxOy/Sb nanocomposites with yolk-shell and hollow structures as high-performance anodes for lithium-ion storage. J. Alloys Compd. 2021, 878, 160447. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, G.; Gu, S.; Lu, B. SnO2 nanorods on ZnO nanofibers: A new class of hierarchical nanostructures enabled by electrospinning as anode material for high-performance lithium-ion batteries. Electrochim. Acta 2014, 150, 308–313. [Google Scholar] [CrossRef]
- Li, H.; Wei, Y.; Zhang, Y.; Yin, F.; Zhang, C.; Wang, G.; Bakenov, Z. Synthesis and electrochemical investigation of highly dispersed ZnO nanoparticles as anode material for lithium-ion batteries. Ionics 2016, 22, 1387–1393. [Google Scholar] [CrossRef]
- Shen, X.; Mu, D.; Chen, S.; Wu, B.; Wu, F. Enhanced electrochemical performance of ZnO-loaded/porous carbon composite as anode materials for lithium ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 3118–3125. [Google Scholar] [CrossRef]
- Zhao, B.; Mattelaer, F.; Kint, J.; Werbrouck, A.; Henderick, L.; Minjauw, M.; Dendooven, J.; Detavernier, C. Atomic layer deposition of ZnO–SnO2 composite thin film: The influence of structure, composition and crystallinity on lithium-ion battery performance. Electrochim. Acta 2019, 320, 134604. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, P.; Xu, J.; Xue, H.; Pang, H. High performance of electrochemical lithium storage batteries: ZnO-based nanomaterials for lithium-ion and lithium-sulfur batteries. Nanoscale 2016, 8, 18578–18595. [Google Scholar] [CrossRef] [PubMed]
- Aravindan, V.; Jinesh, K.B.; Prabhakar, R.R.; Kale, V.S.; Madhavi, S. Atomic layer deposited (ALD) SnO2 anodes with exceptional cycleability for Li-ion batteries. Nano Energy 2013, 2, 720–725. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Zhang, Y. A Comprehensive Review on Metal-Oxide Nanocomposites for High-Performance Lithium-Ion Battery Anodes. Energy Fuels 2021, 35, 6420–6442. [Google Scholar] [CrossRef]
- Yuan, G.; Wang, G.; Wang, H.; Bai, J. Synthesis and electrochemical investigation of radial ZnO microparticles as anode materials for lithium-ion batteries. Ionics 2015, 21, 365–371. [Google Scholar] [CrossRef]
- Fang, S.; Bresser, D.; Passerini, S. Transition Metal Oxide Anodes for Electrochemical Energy Storage in Lithium- and Sodium-Ion Batteries. Adv. Energy Mater. 2020, 10, 1902485. [Google Scholar] [CrossRef]
- Liu, X.; Si, W.; Zhang, J.; Sun, X.; Deng, J.; Baunack, S.; Oswald, S.; Liu, L.; Yan, C.; Schmidt, O.G. Free-standing Fe2O3 nanomembranes enabling ultra-long cycling life and high rate capability for Li-ion batteries. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hwang, J.; Lee, S.; Thirumalraj, B.; Kim, J.H.; Jenei, P.; Gubicza, J.; Choe, H. Synthesis of a High-Capacity NiO/Ni Foam Anode for Advanced Lithium-Ion Batteries. Adv. Energy Mater. 2020, 22, 2000351. [Google Scholar] [CrossRef]
- Hassan, A.S.; Moyer, K.; Ramachandran, B.R.; Wick, C.D. Comparison of Storage Mechanisms in RuO2, SnO2, and SnS2 for Lithium-Ion Battery Anode Materials. J. Phys. Chem. C 2016, 120, 2036–2046. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, I.T. Self-assembled few-layered MoS2 on SnO2 anode for enhancing lithium-ion storage. Nanomaterials 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Gan, Q.; Wang, H.; Xu, G.L.; Zhang, X.; Huang, D.; Fu, F.; Tang, Y.; Amine, K.; Shao, M. Structure-dependent performance of TiO2/C as anode material for Na-ion batteries. Nano Energy 2018, 44, 217–227. [Google Scholar] [CrossRef]
- Ai, C.; Yin, M.; Wang, C.; Sun, J. Synthesis and characterization of spinel type ZnCo2O4 as a novel anode material for lithium ion batteries. J. Mater. Sci. 2004, 39, 1077–1079. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Y.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S.; Liu, P. A first principles study of spinel ZnFe2O4 for electrode materials in lithium-ion batteries. Phys. Chem. Chem. Phys. 2017, 19, 26322–26329. [Google Scholar] [CrossRef]
- Yao, W.; Xu, Z.; Xu, X.; Xie, Y.; Qiu, W.; Xu, J.; Zhang, D. Two-dimensional holey ZnFe2O4 nanosheet/reduced graphene oxide hybrids by self-link of nanoparticles for high-rate lithium storage. Electrochim. Acta 2018, 292, 390–398. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Lin, C.Y.; Chen, Y.F.; Lin, J.S. Synthesis of ZnO@Graphene composites as anode materials for lithium ion batteries. Electrochim. Acta 2013, 111, 359–365. [Google Scholar] [CrossRef]
- Lu, S.; Wang, H.; Zhou, J.; Wu, X.; Qin, W. Atomic layer deposition of ZnO on carbon black as nanostructured anode materials for high-performance lithium-ion batteries. Nanoscale 2017, 9, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Lu, Z.; Zhao, J.; McDowell, M.T.; Lee, H.W.; Zhao, W.; Cui, Y. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanotechnol. 2014, 9, 187–192. [Google Scholar] [CrossRef]
- Gao, H.; Zhou, W.; Jang, J.H.; Goodenough, J.B. Cross-Linked Chitosan as a Polymer Network Binder for an Antimony Anode in Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1502130. [Google Scholar] [CrossRef]
- Li, F.; Yang, L.; Xu, G.; Xiaoqiang, H.; Yang, X.; Wei, X.; Ren, Z.; Shen, G.; Han, G. Hydrothermal self-assembly of hierarchical flower-like ZnO nanospheres with nanosheets and their application in Li-ion batteries. J. Alloys Compd. 2013, 577, 663–668. [Google Scholar] [CrossRef]
- Park, K.T.; Xia, F.; Kim, S.W.; Kim, S.B.; Song, T.; Paik, U.; Park, W.I. Facile synthesis of ultrathin ZnO nanotubes with well-organized hexagonal nanowalls and sealed layouts: Applications for lithium ion battery anodes. J. Phys. Chem. C 2013, 117, 1037–1043. [Google Scholar] [CrossRef]
- Huang, X.H.; Guo, R.Q.; Wu, J.B.; Zhang, P. Mesoporous ZnO nanosheets for lithium ion batteries. Mater. Lett. 2014, 122, 82–85. [Google Scholar] [CrossRef]
- Zhang, G.; Hou, S.; Zhang, H.; Zeng, W.; Yan, F.; Li, C.C.; Duan, H. High-performance and ultra-stable lithium-ion batteries based on MOF-derived ZnO@ZnO quantum dots/C core-shell nanorod arrays on a carbon cloth anode. Adv. Mater. 2015, 27, 2400–2405. [Google Scholar] [CrossRef]
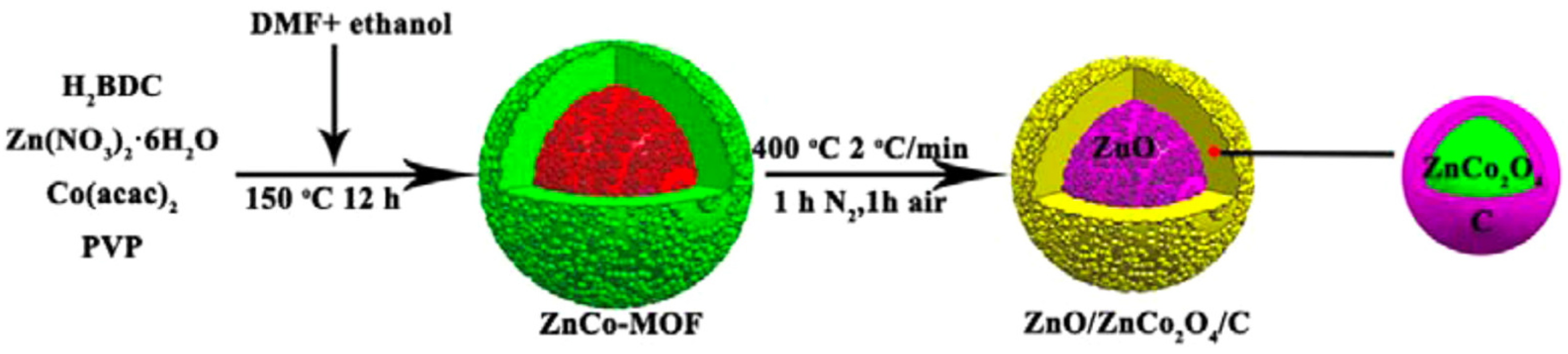
- Ge, X.; Li, Z.; Wang, C.; Yin, L. Metal-organic frameworks derived porous core/shell structured ZnO/ZnCo2O4/C hybrids as anodes for high-performance lithium-ion battery. ACS Appl. Mater. Interfaces 2015, 7, 26633–26642. [Google Scholar] [CrossRef]
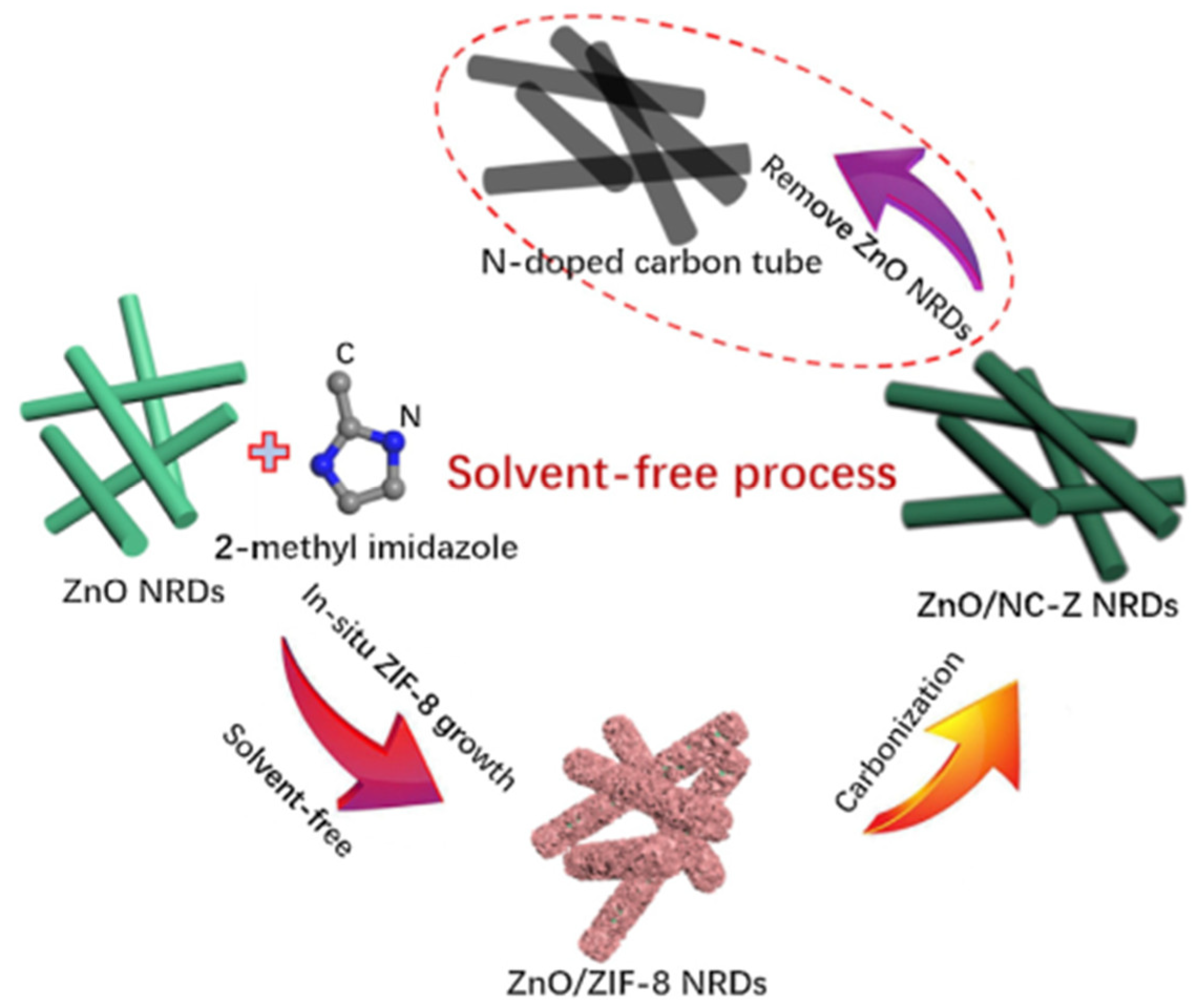
- Gan, Q.; Zhao, K.; Liu, S.; He, Z. Solvent-free synthesis of N-doped carbon coated ZnO nanorods composite anode via a ZnO support-induced ZIF-8 in-situ growth strategy. Electrochim. Acta 2017, 250, 292–301. [Google Scholar] [CrossRef]
- Bresser, D.; Mueller, F.; Fiedler, M.; Krueger, S.; Kloepsch, R.; Baither, D.; Winter, M.; Paillard, E.; Passerini, S. Transition-metal-doped zinc oxide nanoparticles as a new lithium-ion anode material. Chem. Mater. 2013, 25, 4977–4985. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, X.; Yao, K.; Zhang, J.; Liu, Z. A SnO2/graphene composite as a high stability electrode for lithium ion batteries. Carbon 2011, 49, 133–139. [Google Scholar] [CrossRef]
- Guo, W.; Sun, W.; Wang, Y. Multilayer CuO@NiO Hollow Spheres: Microwave-Assisted Metal-Organic-Framework Derivation and Highly Reversible Structure-Matched Stepwise Lithium Storage. ACS Nano 2015, 9, 11462–11471. [Google Scholar] [CrossRef] [PubMed]
- Belliard, F.; Irvine, J.T.S. Electrochemical performance of ball-milled ZnO-SnO2 systems as anodes in lithium-ion battery. J. Power Sources 2001, 97–98, 219–222. [Google Scholar] [CrossRef]
- Franken, R.H.; Van Der Werf, C.H.M.; Löffler, J.; Rath, J.K.; Schropp, R.E.I. Beneficial effects of sputtered ZnO:Al protection layer on SnO2:F for high-deposition rate hot-wire CVD p-i-n solar cells. Thin Solid Films 2006, 501, 47–50. [Google Scholar] [CrossRef]
- Wang, J.; Du, N.; Zhang, H.; Yu, J.; Yang, D. Layer-by-layer assembly synthesis of ZnO/SnO2 composite nanowire arrays as high-performance anode for lithium-ion batteries. Mater. Res. Bull. 2011, 46, 2378–2384. [Google Scholar] [CrossRef]
- Ahmad, M.; Yingying, S.; Sun, H.; Shen, W.; Zhu, J. SnO2/ZnO composite structure for the lithium-ion battery electrode. J. Solid State Chem. 2012, 196, 326–331. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Lu, L.; Fang, J.; Ni, H.; Ji, Z. Preparation and characterization of ZnO/SnO2 composite thin films as high-capacity anode for lithium-ion batteries. Appl. Phys. A 2015, 120, 519–524. [Google Scholar] [CrossRef]
- Xie, Q.; Ma, Y.; Zeng, D.; Wang, L.; Yue, G.; Peng, D.L. Facile fabrication of various zinc-nickel citrate microspheres and their transformation to ZnO-NiO hybrid microspheres with excellent lithium storage properties. Sci. Rep. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Pan, Q.; Zheng, F.; Ou, X.; Yang, C.; Xiong, X.; Liu, M. MoS2 encapsulated SnO2-SnS/C nanosheets as a high performance anode material for lithium ion batteries. Chem. Eng. J. 2017, 316, 393–400. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, X.; Noonan, O.; Zhou, L.; Yu, C. Tailored Yolk–Shell Sn@C Nanoboxes for High-Performance Lithium Storage. Adv. Funct. Mater. 2017, 27, 1606023. [Google Scholar] [CrossRef]
- Wu, Z.S.; Ren, W.; Wen, L.; Gao, L.; Zhao, J.; Chen, Z.; Zhou, G.; Li, F.; Cheng, H.M. Graphene anchored with Co3O4 nanoparticles as anode of lithium ion batteries with enhanced reversible capacity and cyclic performance. ACS Nano 2010, 4, 3187–3194. [Google Scholar] [CrossRef]
- Hu, Y.; Yan, C.; Chen, D.; Lv, C.; Jiao, Y.; Chen, G. One-dimensional Co3O4 nanonet with enhanced rate performance for lithium ion batteries: Carbonyl-Β-cyclodextrin inducing and kinetic analysis. Chem. Eng. J. 2017, 321, 31–39. [Google Scholar] [CrossRef]
- Yang, J.; Ouyang, Y.; Zhang, H.; Xu, H.; Zhang, Y.; Wang, Y. Novel Fe2P/graphitized carbon yolk/shell octahedra for high-efficiency hydrogen production and lithium storage. J. Mater. Chem. A 2016, 4, 9923–9930. [Google Scholar] [CrossRef]
- Yang, J.; Wu, Q.; Yang, X.; He, S.; Khan, J.; Meng, Y.; Zhu, X.; Tong, S.; Wu, M. Chestnut-Like TiO2@α-Fe2O3 Core-Shell Nanostructures with Abundant Interfaces for Efficient and Ultralong Life Lithium-Ion Storage. ACS Appl. Mater. Interfaces 2017, 9, 354–361. [Google Scholar] [CrossRef]
- Li, J.; Yan, D.; Lu, T.; Yao, Y.; Pan, L. An advanced CoSe embedded within porous carbon polyhedra hybrid for high performance lithium-ion and sodium-ion batteries. Chem. Eng. J. 2017, 325, 14–24. [Google Scholar] [CrossRef]
- Lin, D.; Lu, Z.; Hsu, P.C.; Lee, H.R.; Liu, N.; Zhao, J.; Wang, H.; Liu, C.; Cui, Y. A high tap density secondary silicon particle anode fabricated by scalable mechanical pressing for lithium-ion batteries. Energy Environ. Sci. 2015, 8, 2371–2376. [Google Scholar] [CrossRef]
- Wu, C.; Maier, J.; Yu, Y. Sn-Based Nanoparticles Encapsulated in a Porous 3D Graphene Network: Advanced Anodes for High-Rate and Long Life Li-Ion Batteries. Adv. Funct. Mater. 2015, 25, 3488–3496. [Google Scholar] [CrossRef]
- Seng, K.H.; Li, L.; Chen, D.P.; Chen, Z.X.; Wang, X.L.; Liu, H.K.; Guo, Z.P. The effects of FEC (fluoroethylene carbonate) electrolyte additive on the lithium storage properties of NiO (nickel oxide) nanocuboids. Energy 2013, 58, 707–713. [Google Scholar] [CrossRef][Green Version]
- Wu, L.; Buchholz, D.; Bresser, D.; Gomes Chagas, L.; Passerini, S. Anatase TiO2 nanoparticles for high power sodium-ion anodes. J. Power Sources 2014, 251, 379–385. [Google Scholar] [CrossRef]
- Zou, F.; Chen, Y.M.; Liu, K.; Yu, Z.; Liang, W.; Bhaway, S.M.; Gao, M.; Zhu, Y. Metal organic frameworks derived hierarchical hollow NiO/Ni/graphene composites for lithium and sodium storage. ACS Nano 2016, 10, 377–386. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, J.; Guan, B.; Lou, X.W.D. Unusual Formation of CoSe@carbon Nanoboxes, which have an Inhomogeneous Shell, for Efficient Lithium Storage. Angew. Chem. Int. Ed. 2016, 55, 9514–9518. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Luo, W.; Huang, Y. Self-assembled hierarchical MoO2/graphene nanoarchitectures and their application as a high-performance anode material for lithium-ion batteries. ACS Nano 2011, 5, 7100–7107. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, Q.; Wang, C.; Zachariah, M.R. Interdispersed amorphous MnOx-carbon nanocomposites with superior electrochemical performance as lithium-storage material. Adv. Funct. Mater. 2012, 22, 803–811. [Google Scholar] [CrossRef]
- Jamnik, J.; Maier, J. Nanocrystallinity effects in lithium battery materials: Aspects of nano-ionics. Part IV. Phys. Chem. Chem. Phys. 2003, 5, 5215–5220. [Google Scholar] [CrossRef]
- Xie, Q.; Li, F.; Guo, H.; Wang, L.; Chen, Y.; Yue, G.; Peng, D.L. Template-free synthesis of amorphous double-shelled zinc-cobalt citrate hollow microspheres and their transformation to crystalline ZnCo2O4 microspheres. ACS Appl. Mater. Interfaces 2013, 5, 5508–5517. [Google Scholar] [CrossRef]
- Xie, Q.; Zeng, D.; Ma, Y.; Lin, L.; Wang, L.; Peng, D.L. Synthesis of ZnO-ZnCo2O4 hybrid hollow microspheres with excellent lithium storage properties. Electrochim. Acta 2015, 169, 283–290. [Google Scholar] [CrossRef]
- Chao, D.; Zhu, C.; Yang, P.; Xia, X.; Liu, J.; Wang, J.; Fan, X.; Savilov, S.V.; Lin, J.; Fan, H.J.; et al. Array of nanosheets render ultrafast and high-capacity Na-ion storage by tunable pseudocapacitance. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, R.; Wang, H.; Jiang, Y.; Jin, L.; Guo, Y.; Song, Y.; Fang, F.; Sun, D. Construction of hybrid hollow architectures by in-situ rooting ultrafine ZnS nanorods within porous carbon polyhedra for enhanced lithium storage properties. Chem. Eng. J. 2017, 326, 680–690. [Google Scholar] [CrossRef]
- Li, J.; Yan, D.; Hou, S.; Lu, T.; Yao, Y.; Chua, D.H.C.; Pan, L. Metal-organic frameworks derived yolk-shell ZnO/NiO microspheres as high-performance anode materials for lithium-ion batteries. Chem. Eng. J. 2018, 335, 579–589. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Zhou, L.; Liu, Y.; Zhang, W.; Zhao, Z.; Hozzein, W.N.; Alharbi, H.M.S.; Li, W.; Zhao, D. Mesoporous carbon matrix confinement synthesis of ultrasmall WO3 nanocrystals for lithium ion batteries. J. Mater. Chem. A 2018, 6, 21550–21557. [Google Scholar] [CrossRef]
- Wu, X.; Yao, S. Flexible electrode materials based on WO3 nanotube bundles for high performance energy storage devices. Nano Energy 2017, 42, 143–150. [Google Scholar] [CrossRef]
- Tu, C.; Zhang, Z.; Shao, A.; Qi, X.; Zhu, C.; Li, C.; Yang, Z. Constructing a directional ion acceleration layer at WO3/ZnO heterointerface to enhance Li-ion transfer and storage. Compos. Part B Eng. 2021, 205, 108511. [Google Scholar] [CrossRef]
- Su, D.; Kim, H.S.; Kim, W.S.; Wang, G. Synthesis of tuneable porous hematites (α-Fe2O3) for gas sensing and lithium storage in lithium ion batteries. Microporous Mesoporous Mater. 2012, 149, 36–45. [Google Scholar] [CrossRef]
- Karunakaran, G.; Kundu, M.; Kumari, S.; Kolesnikov, E.; Gorshenkov, M.V.; Maduraiveeran, G.; Sasidharan, M.; Kuznetsov, D. ZnO/Cu2MgO3 hollow porous nanocage: A new class of hybrid anode material for advanced lithium-ion batteries. J. Alloys Compd. 2018, 763, 94–101. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Yin, L.; Qi, Y. Prussion blue-supported annealing chemical reaction route synthesized double-shelled Fe2O3/Co3O4 hollow microcubes as anode materials for Lithium-Ion battery. ACS Appl. Mater. Interfaces 2014, 6, 8098–8107. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.B.; Madhavi, S.; Hng, H.H.; Lou, X.W. Formation of Fe2O3 microboxes with hierarchical shell structures from metal-organic frameworks and their lithium storage properties. J. Am. Chem. Soc. 2012, 134, 17388–17391. [Google Scholar] [CrossRef]
- Yan, N.; Hu, L.; Li, Y.; Wang, Y.; Zhong, H.; Hu, X.; Kong, X.; Chen, Q. Co3O4 nanocages for high-performance anode material in lithium-ion batteries. J. Phys. Chem. C 2012, 116, 7227–7235. [Google Scholar] [CrossRef]
- Nie, P.; Shen, L.; Luo, H.; Ding, B.; Xu, G.; Wang, J.; Zhang, X. Prussian blue analogues: A new class of anode materials for lithium ion batteries. J. Mater. Chem. A 2014, 2, 5852–5857. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, F.; Zhang, L.; Du, X.; Wang, J.; Wang, L. Hierarchical NiFe2O4/Fe2O3 nanotubes derived from metal organic frameworks for superior lithium ion battery anodes. J. Mater. Chem. A 2014, 2, 8048–8053. [Google Scholar] [CrossRef]
- Zou, L.; Li, F.; Xiang, X.; Evans, D.G.; Duan, X. Self-generated template pathway to high-surface-area zinc aluminate spinel with mesopore network from a single-source inorganic precursor. Chem. Mater. 2006, 18, 5852–5859. [Google Scholar] [CrossRef]
- Hou, L.; Lian, L.; Zhang, L.; Pang, G.; Yuan, C.; Zhang, X. Self-sacrifice template fabrication of hierarchical mesoporous bi-component-active ZnO/ZnFe2O4 sub-microcubes as superior anode towards high-performance lithium-ion battery. Adv. Funct. Mater. 2015, 25, 238–246. [Google Scholar] [CrossRef]
- Yang, S.J.; Nam, S.; Kim, T.; Im, J.H.; Jung, H.; Kang, J.H.; Wi, S.; Park, B.; Park, C.R. Preparation and exceptional lithium anodic performance of porous carbon-coated ZnO quantum dots derived from a metal-organic framework. J. Am. Chem. Soc. 2013, 135, 7394–7397. [Google Scholar] [CrossRef]
- Zou, F.; Hu, X.; Li, Z.; Qie, L.; Hu, C.; Zeng, R.; Jiang, Y.; Huang, Y. MOF-derived porous ZnO/ZnFe2O4/C octahedra with hollow interiors for high-rate lithium-ion batteries. Adv. Mater. 2014, 26, 6622–6628. [Google Scholar] [CrossRef] [PubMed]
- Bresser, D.; Paillard, E.; Kloepsch, R.; Krueger, S.; Fiedler, M.; Schmitz, R.; Baither, D.; Winter, M.; Passerini, S. Carbon coated ZnFe2O4 nanoparticles for advanced lithium-ion anodes. Adv. Energy Mater. 2013, 3, 513–523. [Google Scholar] [CrossRef]
- Zheng, F.; He, M.; Yang, Y.; Chen, Q. Nano electrochemical reactors of Fe2O3 nanoparticles embedded in shells of nitrogen-doped hollow carbon spheres as high-performance anodes for lithium-ion batteries. Nanoscale 2015, 7, 3410–3417. [Google Scholar] [CrossRef]
- Wu, Z.S.; Ren, W.; Xu, L.; Li, F.; Cheng, H.M. Doped graphene sheets as anode materials with superhigh rate and large capacity for lithium ion batteries. ACS Nano 2011, 5, 5463–5471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, G.; Li, Y.; Edy, R.; Gao, P.; Tang, H.; Bao, Z.; Mei, Y. Three-dimensional carbon/ZnO nanomembrane foam as an anode for lithium-ion battery with long-life and high areal capacity. J. Mater. Chem. A 2018, 6, 7227–7235. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Lin, J.Y.; Mo, C.Y. Improved storage capacity and rate capability of Fe3O4-graphene anodes for lithium-ion batteries. Electrochim. Acta 2011, 58, 119–124. [Google Scholar] [CrossRef]
- Zhang, M.; Lei, D.; Yin, X.; Chen, L.; Li, Q.; Wang, Y.; Wang, T. Magnetite/graphene composites: Microwave irradiation synthesis and enhanced cycling and rate performances for lithium ion batteries. J. Mater. Chem. 2010, 20, 5538–5543. [Google Scholar] [CrossRef]
- Yu, Z.; Zhu, S.; Li, Y.; Liu, Q.; Feng, C.; Zhang, D. Synthesis of SnO2 nanoparticles inside mesoporous carbon via a sonochemical method for highly reversible lithium batteries. Mater. Lett. 2011, 65, 3072–3075. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Liu, F.; Liu, C.; Wang, J.; Pan, Y.; Xue, D. One-pot synthesis of mesoporous interconnected carbon-encapsulated Fe3O4 nanospheres as superior anodes for Li-ion batteries. RSC Adv. 2012, 2, 2262–2265. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Liu, B.; Wang, Q.; Tan, D.; Song, W.; Hou, X.; Chen, D.; Shen, G. Advanced rechargeable lithium-ion batteries based on bendable ZnCo2O4-urchins-on-carbon-fibers electrodes. Nano Res. 2013, 6, 525–534. [Google Scholar] [CrossRef]
- Han, Q.; Li, X.; Wang, F.; Han, Z.; Geng, D.; Zhang, W.; Li, Y.; Deng, Y.; Zhang, J.; Niu, S.; et al. Carbon fiber@ pore-ZnO composite as anode materials for structural lithium-ion batteries. J. Electroanal. Chem. 2019, 833, 39–46. [Google Scholar] [CrossRef]
- Nardecchia, S.; Carriazo, D.; Ferrer, M.L.; Gutiérrez, M.C.; Monte, F.D. Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene: Synthesis and applications. Chem. Soc. Rev. 2013, 42, 794–830. [Google Scholar] [CrossRef]
- Son, D.I.; Kwon, B.W.; Park, D.H.; Seo, W.S.; Yi, Y.; Angadi, B.; Lee, C.L.; Choi, W.K. Emissive ZnO-graphene quantum dots for white-light-emitting diodes. Nat. Nanotechnol. 2012, 7, 465–471. [Google Scholar] [CrossRef]
- Dou, Y.; Xu, J.; Ruan, B.; Liu, Q.; Pan, Y.; Sun, Z.; Dou, S.X. Atomic Layer-by-Layer Co3O4/Graphene Composite for High Performance Lithium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1501835. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, Y.; Zhang, Q.; Wu, X.; Cheng, J.; Zhang, N.; Feng, Y.; Sun, K. Graphene Aerogels with Anchored Sub-Micrometer Mulberry-Like ZnO Particles for High-Rate and Long-Cycle Anode Materials in Lithium Ion Batteries. Small 2016, 12, 5208–5216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, Y.; Li, H.; Zhao, Y.; Yin, F.; Wang, X. Simple fabrication of free-standing ZnO/graphene/carbon nanotube composite anode for lithium-ion batteries. Mater. Lett. 2016, 184, 235–238. [Google Scholar] [CrossRef]
- Sun, H.; Mei, L.; Liang, J.; Zhao, Z.; Lee, C.; Fei, H.; Ding, M.; Lau, J.; Li, M.; Wang, C.; et al. Three-dimensional holey-graphene/niobia composite architectures for ultrahigh-rate energy storage. Science 2017, 356, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Zheng, J.; Li, Q.; Xie, X.; Ferrara, S.; Nie, Z.; Mehdi, L.B.; Browning, N.D.; Zhang, J.G.; Graff, G.L.; et al. High Energy Density Lithium-Sulfur Batteries: Challenges of Thick Sulfur Cathodes. Adv. Energy Mater. 2015, 5, 1402290. [Google Scholar] [CrossRef]
- Gallagher, K.G.; Trask, S.E.; Bauer, C.; Woehrle, T.; Lux, S.F.; Tschech, M.; Lamp, P.; Polzin, B.J.; Ha, S.; Long, B.; et al. Optimizing Areal Capacities through Understanding the Limitations of Lithium-Ion Electrodes. J. Electrochem. Soc. 2016, 163, A138–A149. [Google Scholar] [CrossRef]
- Peng, H.J.; Huang, J.Q.; Cheng, X.B.; Zhang, Q. Review on High-Loading and High-Energy Lithium–Sulfur Batteries. Adv. Energy Mater. 2017, 7, 1700260. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.W. Two-dimensional nanoarchitectures for lithium storage. Adv. Mater. 2012, 24, 4097–4111. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Mönch, I.; Yan, C.; Deng, J.; Li, S.; Lin, G.; Han, L.; Mei, Y.; Schmidt, O.G. A single rolled-up Si tube battery for the study of electrochemical kinetics, electrical conductivity, and structural integrity. Adv. Mater. 2014, 26, 7973–7978. [Google Scholar] [CrossRef]
- Zhou, G.; Li, L.; Ma, C.; Wang, S.; Shi, Y.; Koratkar, N.; Ren, W.; Li, F.; Cheng, H.M. A graphene foam electrode with high sulfur loading for flexible and high energy Li-S batteries. Nano Energy 2015, 11, 356–365. [Google Scholar] [CrossRef]
- Fang, R.; Zhao, S.; Hou, P.; Cheng, M.; Wang, S.; Cheng, H.M.; Liu, C.; Li, F. 3D Interconnected Electrode Materials with Ultrahigh Areal Sulfur Loading for Li-S Batteries. Adv. Mater. 2016, 28, 3374–3382. [Google Scholar] [CrossRef] [PubMed]
- Afanasov, I.M.; Lebedev, O.I.; Kolozhvary, B.A.; Smirnov, A.V.; van Tendeloo, G. Nickel/Carbon composite materials based on expanded graphite. New Carbon Mater. 2011, 26, 335–340. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Y.; Guo, C.; Zhu, X.; Liang, J.; Qian, Y. Ferric chloride-graphite intercalation compounds as anode materials for Li-ion batteries. ChemSusChem 2014, 7, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Ma, C.; Wang, J.; Wang, H.; Shi, J.; Song, Y.; Guo, Q.; Liu, L. Exfoliated graphite as a flexible and conductive support for Si-based Li-ion battery anodes. Carbon 2014, 72, 38–46. [Google Scholar] [CrossRef]
- Jiang, B.; Tian, C.; Zhou, W.; Wang, J.; Xie, Y.; Pan, Q.; Ren, Z.; Dong, Y.; Fu, D.; Han, J.; et al. In situ growth of TiO2 in interlayers of expanded graphite for the fabrication of TiO2-graphene with enhanced photocatalytic activity. Chem. Eur. J. 2011, 17, 8379–8387. [Google Scholar] [CrossRef]
- Zhang, W.; Wan, W.; Zhou, H.; Chen, J.; Wang, X.; Zhang, X. In-situ synthesis of magnetite/expanded graphite composite material as high rate negative electrode for rechargeable lithium batteries. J. Power Sources 2013, 223, 119–124. [Google Scholar] [CrossRef]
- Naderi, H.R.; Mortaheb, H.R.; Zolfaghari, A. Supercapacitive properties of nanostructured MnO2/exfoliated graphite synthesized by ultrasonic vibration. J. Electroanal. Chem. 2014, 719, 98–105. [Google Scholar] [CrossRef]
- Huang, Y.G.; Lin, X.L.; Zhang, X.H.; Pan, Q.C.; Yan, Z.X.; Wang, H.Q.; Chen, J.J.; Li, Q.Y. Fe3C@carbon nanocapsules/expanded graphite as anode materials for lithium ion batteries. Electrochim. Acta 2015, 178, 468–475. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, X.; Pan, Q.; Li, Q.; Zhang, X.; Yan, Z.; Wu, X.; He, Z.; Wang, H. Al@C/Expanded Graphite Composite as Anode Material for Lithium Ion Batteries. Electrochim. Acta 2016, 193, 253–260. [Google Scholar] [CrossRef]
- Hu, S.; Song, Y.; Yuan, S.; Liu, H.; Xu, Q.; Wang, Y.; Wang, C.X.; Xia, Y.Y. A hierarchical structure of carbon-coated Li3VO4 nanoparticles embedded in expanded graphite for high performance lithium ion battery. J. Power Sources 2016, 303, 333–339. [Google Scholar] [CrossRef]
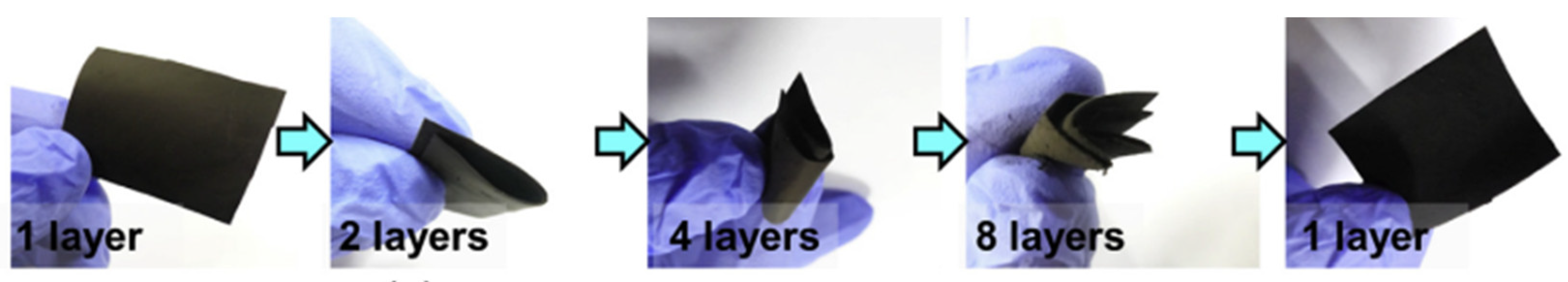
- Li, Y.; Zhao, Y.; Huang, G.; Xu, B.; Wang, B.; Pan, R.; Men, C.; Mei, Y. ZnO Nanomembrane/Expanded Graphite Composite Synthesized by Atomic Layer Deposition as Binder-Free Anode for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 38522–38529. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, W.; Li, M.; Cao, L.; Lyu, F.; Yang, M.; Wang, Z.; Shi, Y.; Nan, B.; Yu, S.; et al. Highly durable organic electrode for sodium-ion batteries via a stabilized α-C radical intermediate. Nat. Commun. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, Y.; Huo, Y.; Luo, Y.; Zhang, L.; Wan, Y.; Nan, B.; Cao, L.; Wang, Z.; Li, M.; et al. Bimetallic organic frameworks derived CuNi/carbon nanocomposites as efficient electrocatalysts for oxygen reduction reaction. Sci. China Mater. 2017, 60, 654–663. [Google Scholar] [CrossRef]
- Duan, Z.Q.; Liu, Y.T.; Xie, X.M.; Ye, X.Y.; Zhu, X.D. H-BN Nanosheets as 2D Substrates to Load 0D Fe3O4 Nanoparticles: A Hybrid Anode Material for Lithium-Ion Batteries. Chem. Asian J. 2016, 11, 828–833. [Google Scholar] [CrossRef]
- Quartarone, E.; Dall’asta, V.; Resmini, A.; Tealdi, C.; Tredici, I.G.; Tamburini, U.A.; Mustarelli, P. Graphite-coated ZnO nanosheets as high-capacity, highly stable, and binder-free anodes for lithium-ion batteries. J. Power Sources 2016, 320, 314–321. [Google Scholar] [CrossRef]
- Li, Z.; Yin, L. Sandwich-like reduced graphene oxide wrapped MOF-derived ZnCo2O4-ZnO-C on nickel foam as anodes for high performance lithium ion batteries. J. Mater. Chem. A 2015, 3, 21569–21577. [Google Scholar] [CrossRef]
- Gan, Q.; Liu, S.; Zhao, K.; Wu, Y.; He, Z.; Zhou, Z. Graphene supported nitrogen-doped porous carbon nanosheets derived from zeolitic imidazolate framework for high performance supercapacitors. RSC Adv. 2016, 6, 78947–78953. [Google Scholar] [CrossRef]
- Li, C.; Hu, Q.; Li, Y.; Zhou, H.; Lv, Z.; Yang, X.; Liu, L.; Guo, H. Hierarchical hollow Fe2O3 @MIL-101(Fe)/C derived from metal-organic frameworks for superior sodium storage. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liu, J.; Xu, X.; Zhang, L.; Hu, R.; Liu, J.; Yang, L.; Zhu, M. Metal-organic framework-derived NiSb alloy embedded in carbon hollow spheres as superior lithium-ion battery anodes. ACS Appl. Mater. Interfaces 2017, 9, 2516–2525. [Google Scholar] [CrossRef] [PubMed]
- Thauer, E.; Zakharova, G.S.; Andreikov, E.I.; Adam, V.; Wegener, S.A.; Nölke, J.H.; Singer, L.; Ottmann, A.; Asyuda, A.; Zharnikov, M.; et al. Novel synthesis and electrochemical investigations of ZnO/C composites for lithium-ion batteries. J. Mater. Sci. 2021, 56, 13227–13242. [Google Scholar] [CrossRef]
- Eisenmann, T.; Birrozzi, A.; Mullaliu, A.; Giuli, G.; Trapananti, A.; Passerini, S.; Bresser, D. Effect of Applying a Carbon Coating on the Crystal Structure and De-/Lithiation Mechanism of Mn-Doped ZnO Lithium-Ion Anodes. J. Electrochem. Soc. 2021, 168, 030503. [Google Scholar] [CrossRef]
- Köse, H.; Dombaycıoğlu, Ş.; Aydın, A.O.; Akbulut, H. Production and characterization of free-standing ZnO/SnO2/MWCNT ternary nanocomposite Li-ion battery anode. Int. J. Hydrog. Energy 2016, 41, 9924–9932. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Cao, C.; Butt, F.K. Microwave-assisted and large-scale synthesis of SnO2/carbon-nanotube hybrids with high lithium storage capacity. RSC Adv. 2015, 5, 58568–58573. [Google Scholar] [CrossRef]
- Guler, M.O.; Cetinkaya, T.; Tocoglu, U.; Akbulut, H. Electrochemical performance of MWCNT reinforced ZnO anodes for Li-ion batteries. Microelectron. Eng. 2014, 118, 54–60. [Google Scholar] [CrossRef]
- Kuwabata, S.; Masui, S.; Yoneyama, H. Charge–discharge properties of composites of LiMn2O4 and polypyrrole as positive electrode materials for 4 V class of rechargeable Li batteries. Electrochim. Acta 1999, 44, 4593–4600. [Google Scholar] [CrossRef]
- Tang, W.; Liu, L.; Zhu, Y.; Sun, H.; Wu, Y.; Zhu, K. An aqueous rechargeable lithium battery of excellent rate capability based on a nanocomposite of MoO3 coated with PPy and LiMn2O4. Energy Environ. Sci. 2012, 5, 6909–6913. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, S.; Liu, W.; Du, Z.; Fang, H. Fe3O4/PPy composite nanospheres as anode for lithium-ion batteries with superior cycling performance. Electrochim. Acta 2014, 121, 428–433. [Google Scholar] [CrossRef]
- Zhong, X.B.; Wang, H.Y.; Yang, Z.Z.; Jin, B.; Jiang, Q.C. Facile synthesis of mesoporous ZnCo2O4 coated with polypyrrole as an anode material for lithium-ion batteries. J. Power Sources 2015, 296, 298–304. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Chen, J.; He, N.; Yu, K.; Liang, C. Controllable SnO2/ZnO@PPy hollow nanotubes prepared by electrospinning technology used as anode for lithium ion battery. J. Phys. Chem. Solids 2021, 150, 109861. [Google Scholar] [CrossRef]
- Köse, H.; Aydin, A.O.; Akbulut, H. Free-standing SnO2/MWCNT nanocomposite anodes produced by different rate spin coatings for Li-ion batteries. Int. J. Hydrog. Energy 2014, 39, 21435–21446. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Dong, L.; Yan, B.; Shan, H.; Li, D.; Sun, X. Electrospun SnO2-ZnO nanofibers with improved electrochemical performance as anode materials for lithium-ion batteries. Int. J. Hydrog. Energy 2015, 40, 14338–14344. [Google Scholar] [CrossRef]
- Joshi, B.N.; An, S.; Jo, H.S.; Song, K.Y.; Park, H.G.; Hwang, S.; Al-Deyab, S.S.; Yoon, W.Y.; Yoon, S.S. Flexible, Freestanding, and Binder-free SnOx-ZnO/Carbon Nanofiber Composites for Lithium Ion Battery Anodes. ACS Appl. Mater. Interfaces 2016, 8, 9446–9453. [Google Scholar] [CrossRef]
- Qiao, L.; Wang, X.; Sun, X.; Li, X.; Zheng, Y.; He, D. Single electrospun porous NiO-ZnO hybrid nanofibers as anode materials for advanced lithium-ion batteries. Nanoscale 2013, 5, 3037–3042. [Google Scholar] [CrossRef]
- Ma, L.; Pei, X.Y.; Mo, D.C.; Lyu, S.S.; Fu, Y.X. Fabrication of NiO-ZnO/RGO composite as an anode material for lithium-ion batteries. Ceram. Int. 2018, 44, 22664–22670. [Google Scholar] [CrossRef]
- Wang, X.L.; Han, W.Q.; Chen, H.; Bai, J.; Tyson, T.A.; Yu, X.Q.; Wang, X.J.; Yang, X.Q. Amorphous hierarchical porous GeOx as high-capacity anodes for Li ion batteries with very long cycling life. J. Am. Chem. Soc. 2011, 133, 20692–20695. [Google Scholar] [CrossRef]
- Jin, S.; Li, N.; Cui, H.; Wang, C. Growth of the vertically aligned graphene@ amorphous GeOx sandwich nanoflakes and excellent Li storage properties. Nano Energy 2013, 2, 1128–1136. [Google Scholar] [CrossRef]
- Medvedev, A.G.; Mikhaylov, A.A.; Grishanov, D.A.; Yu, D.Y.W.; Gun, J.; Sladkevich, S.; Lev, O.; Prikhodchenko, P.V. GeO2 Thin Film Deposition on Graphene Oxide by the Hydrogen Peroxide Route: Evaluation for Lithium-Ion Battery Anode. ACS Appl. Mater. Interfaces 2017, 9, 9152–9160. [Google Scholar] [CrossRef]
- He, X.; Hu, Y.; Chen, R.; Shen, Z.; Wu, K.; Cheng, Z.; Pan, P. Foldable uniform GeOx/ZnO/C composite nanofibers as a high-capacity anode material for flexible lithium ion batteries. Chem. Eng. J. 2019, 360, 1020–1029. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Wang, Y.; Xie, Y.L.; Cheng, T.; Lai, W.Y.; Pang, H.; Huang, W. Porous hollow Co3O4 with rhombic dodecahedral structures for high-performance supercapacitors. Nanoscale 2014, 6, 14354–14359. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, J.; Wang, X.; Chen, G.; Chen, D.; Zhou, C.; Shen, G. Hierarchical three-dimensional ZnCo2O4 nanowire arrays/carbon cloth anodes for a novel class of high-performance flexible lithium-ion batteries. Nano Lett. 2012, 12, 3005–3011. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Xu, Y.; Zhang, H.; Yu, J.; Zhai, C.; Yang, D. Porous ZnCo2O4 nanowires synthesis via sacrificial templates: High-performance anode materials of li-ion batteries. Inorg. Chem. 2011, 50, 3320–3324. [Google Scholar] [CrossRef]
- Luo, W.; Hu, X.; Sun, Y.; Huang, Y. Electrospun porous ZnCo2O4 nanotubes as a high-performance anode material for lithium-ion batteries. J. Mater. Chem. 2012, 22, 8916–8921. [Google Scholar] [CrossRef]
- Giri, A.K.; Pal, P.; Ananthakumar, R.; Jayachandran, M.; Mahanty, S.; Panda, A.B. 3D hierarchically assembled porous wrinkled-paper-like structure of ZnCo2O4 and Co-ZnO@C as anode materials for lithium-ion batteries. Cryst. Growth Des. 2014, 14, 3352–3359. [Google Scholar] [CrossRef]
- Zhong, J.; Cao, C.; Liu, Y.; Li, Y.; Khan, W.S. Hollow core-shell η-Fe2O3 microspheres with excellent lithium-storage and gas-sensing properties. Chem. Commun. 2010, 46, 3869–3871. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, H.; Zhang, H.; Yang, J.; Hartono, S.B.; Qian, K.; Zou, J.; Yu, C. Cheap and scalable synthesis of α-Fe2O3 multi-shelled hollow spheres as high-performance anode materials for lithium ion batteries. Chem. Commun. 2013, 49, 8695–8697. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.B.; Rong, H.B.; Huang, W.Z.; Liao, Y.H.; Xing, L.D.; Xu, M.Q.; Li, X.P.; Li, W.S. Triple-shelled Mn2O3 hollow nanocubes: Force-induced synthesis and excellent performance as the anode in lithium-ion batteries. J. Mater. Chem. A 2014, 2, 14189–14194. [Google Scholar] [CrossRef]
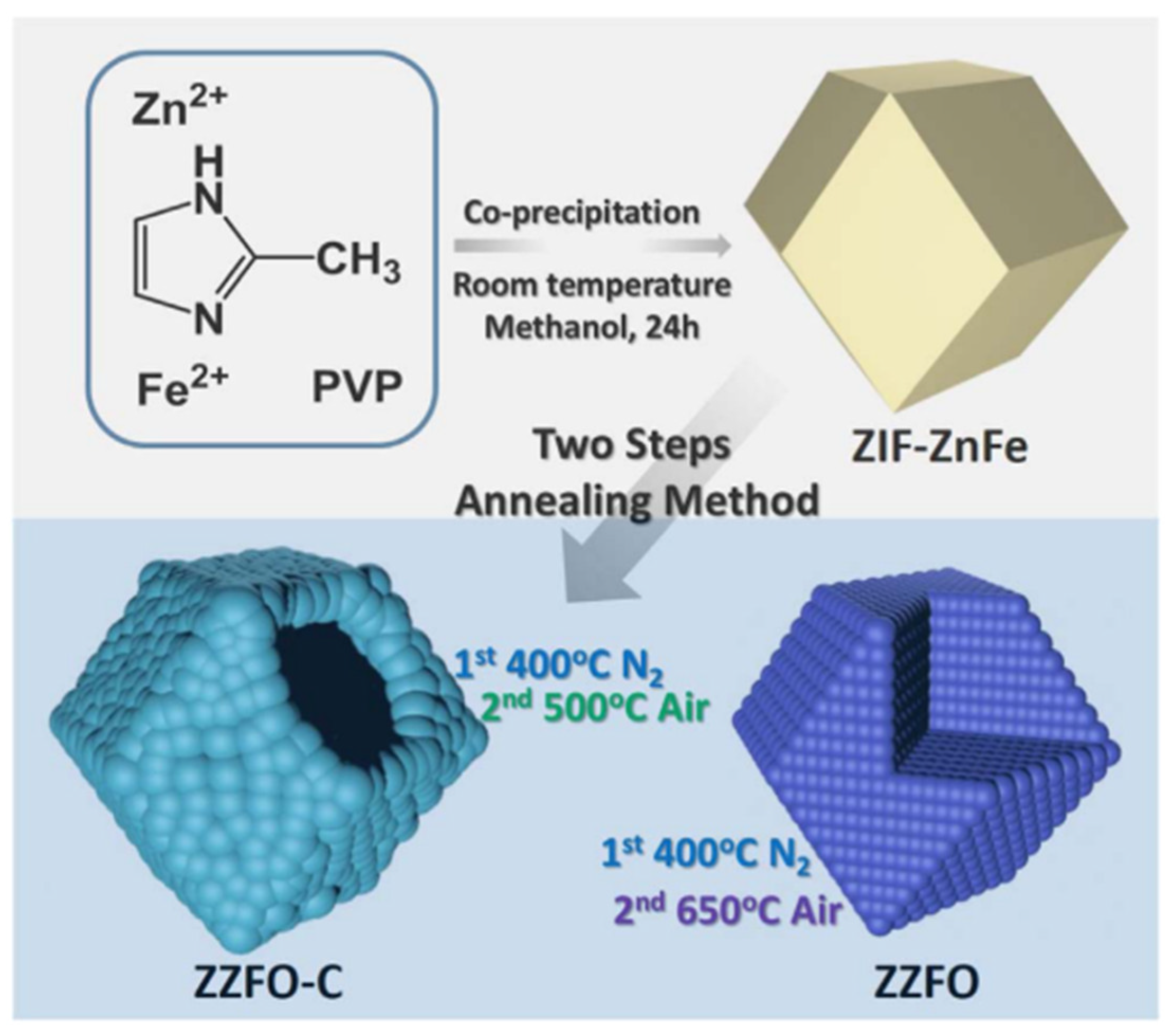
- Ma, Y.; Ma, Y.; Geiger, D.; Kaiser, U.; Zhang, H.; Kim, G.T.; Diemant, T.; Behm, R.J.; Varzi, A.; Passerini, S. ZnO/ZnFe2O4/N-doped C micro-polyhedrons with hierarchical hollow structure as high-performance anodes for lithium-ion batteries. Nano Energy 2017, 42, 341–352. [Google Scholar] [CrossRef]
| Metal Oxide | Lithium Intercalation Method | Theoretical Capacity (mAh g−1) | References |
|---|---|---|---|
| Co3O4 | Conversion | 890 | [9,11] |
| CoO | Conversion | 716 | [11] |
| CuO | Conversion | 674 | [11] |
| Fe2O3 | Conversion | 1006 | [12] |
| NiO | Conversion | 718 | [9,13] |
| RuO2 | Conversion | 806 | [11,14] |
| SnO2 | Alloying | 740 | [15] |
| TiO2 | Intercalation | 335 | [9,16] |
| ZnCo2O4 | Conversion | 903 | [9,17] |
| ZnFe2O4 | Conversion | 1000 | [18,19] |
| ZnO | Alloying | 978 | [5,7,10] |
| Anode | Synthesis Method | Electrochemical Performance | Reference | |||
|---|---|---|---|---|---|---|
| Initial Discharge Capacity (mAh g−1) | Initial CE (%) | Cycling Performance (mAh g−1/Cycles) | Current Density | |||
| Intermixed ZnO–SnO2 | ALD | 2667 | 80.2 | 1752/50 | 0.5 A g−1 | [6] |
| Nanolaminated ZnO–SnO2 | ALD | 2471 | 71.4 | ~1250/50 | 0.5 A g−1 | [6] |
| ZnO–NiO microspheres | Controlled calcination treatment | 1221.7 | 62.9 | 1008.6/200 | 0.1 A g−1 | [59] |
| WO3/ZnO film | Hydrothermal and thermal treatment | ~1500 | 79.9 | ~1100/300 at 1 C | 0.28 C | [62] |
| ZnO/Cu2MgO3 | One-step cost-effective ultrasonic spray pyrolysis | 990.75 | 66.98 | 528/400 | 0.3 A g−1 | [64] |
| ZnO/ZnFe2O4 SMCs | Thermal treatment | 1892 | ~70 | 837/200 cycles | 1 A g−1 | [71] |
| ZnO@GNs | High-performance homogenizing | 850 | 82 | Capacity decay of ~8% after 50 cycles at 1 C | 0.1 C | [20] |
| ZnO/PC | Solvothermal | 2017.4 | 52.68 | 653.7/100 | 0.1 A g−1 | [5] |
| CF@pore-ZnO | Thermal treatment | 955 | 55.81 | 510/300 | 0.1 A g−1 | [83] |
| C/ZnO NMs | Pyrolysis and immersion coating | ~1100 | 59 | 260/700 at 2 A g−1 | 0.08 A g−1 | [77] |
| ZnO/EG | ALD | ~1000 | ~60 | 438/500 | 0.2 A g−1 | [106] |
| ZnO@GAs | Solvothermal | 1001 | 71.2 | 445/500 | 1.6 A g−1 | [87] |
| Graphene-coated ZnO nanosheet | Hydrothermal and thermal evaporation process | 1470 | 65.8 | 600/100 | 1 A g−1 | [110] |
| ZnO/NC-Z NRDs | Thermal treatment | 1439 | 76 | 1011/200 | 0.2 A g−1 | [29] |
| ZnO/C | Thermal treatment | 1061 | 63.24 | 212/100 | 0.1 A g−1 | [115] |
| Carbon-coated Mn-doped ZnO | Thermal treatment | ~1200 | ~61.6% | ~740/80 | 0.1 C | [116] |
| Anode | Synthesis Method | Electrochemical Performance | Reference | |||
|---|---|---|---|---|---|---|
| Initial Discharge Capacity (mAh g−1) | Initial CE (%) | Cycling Performance (mAh g−1/Cycles) | Current Density | |||
| ZnO/SnO2/MWCNT | Sol–gel coating | 1584 | ~57 | 487/100 | 0.2 C | [117] |
| SnO2/ZnO@PPy | Electrospinning and thermal treatment | 1861 | 61.12 | 626.1/100 | 0.2 C | [124] |
| SnOx–ZnO/CNF fiber | Electrospinning and thermal treatment | 1910 | 73.3 | 963/55 | 0.1 A g−1 | [127] |
| NiO–ZnO/RGO | Ultrasonic, freeze drying, and thermal treatment | 1393 | 66.3 | 1017/200 | 0.1 A g−1 | [129] |
| GeOx/ZnO/C | Electrospinning and thermal treatment | 1000 | 66.9 | 617/200 | 0.2 A g−1 | [133] |
| ZnO/ZnCo2O4/C | Thermal treatment | 1278 | 76.2 | 669/250 | 0.5 A g−1 | [28] |
| 3D ZnO/graphene/CNTs | Sol–gel technique following by vacuum-assisted filtration | 1503 | 60 | 620/100 cycles | 0.1 A g−1 | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, V.K.H.; Pham, T.N.; Hur, J.; Lee, Y.-C. Review of ZnO Binary and Ternary Composite Anodes for Lithium-Ion Batteries. Nanomaterials 2021, 11, 2001. https://doi.org/10.3390/nano11082001
Bui VKH, Pham TN, Hur J, Lee Y-C. Review of ZnO Binary and Ternary Composite Anodes for Lithium-Ion Batteries. Nanomaterials. 2021; 11(8):2001. https://doi.org/10.3390/nano11082001
Chicago/Turabian StyleBui, Vu Khac Hoang, Tuyet Nhung Pham, Jaehyun Hur, and Young-Chul Lee. 2021. "Review of ZnO Binary and Ternary Composite Anodes for Lithium-Ion Batteries" Nanomaterials 11, no. 8: 2001. https://doi.org/10.3390/nano11082001
APA StyleBui, V. K. H., Pham, T. N., Hur, J., & Lee, Y.-C. (2021). Review of ZnO Binary and Ternary Composite Anodes for Lithium-Ion Batteries. Nanomaterials, 11(8), 2001. https://doi.org/10.3390/nano11082001