Synthesis and Characterization of Tungsten Suboxide WnO3n−1 Nanotiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. X-ray Diffraction
2.3. Raman Spectroscopy
2.4. Scanning Electron Microscopy
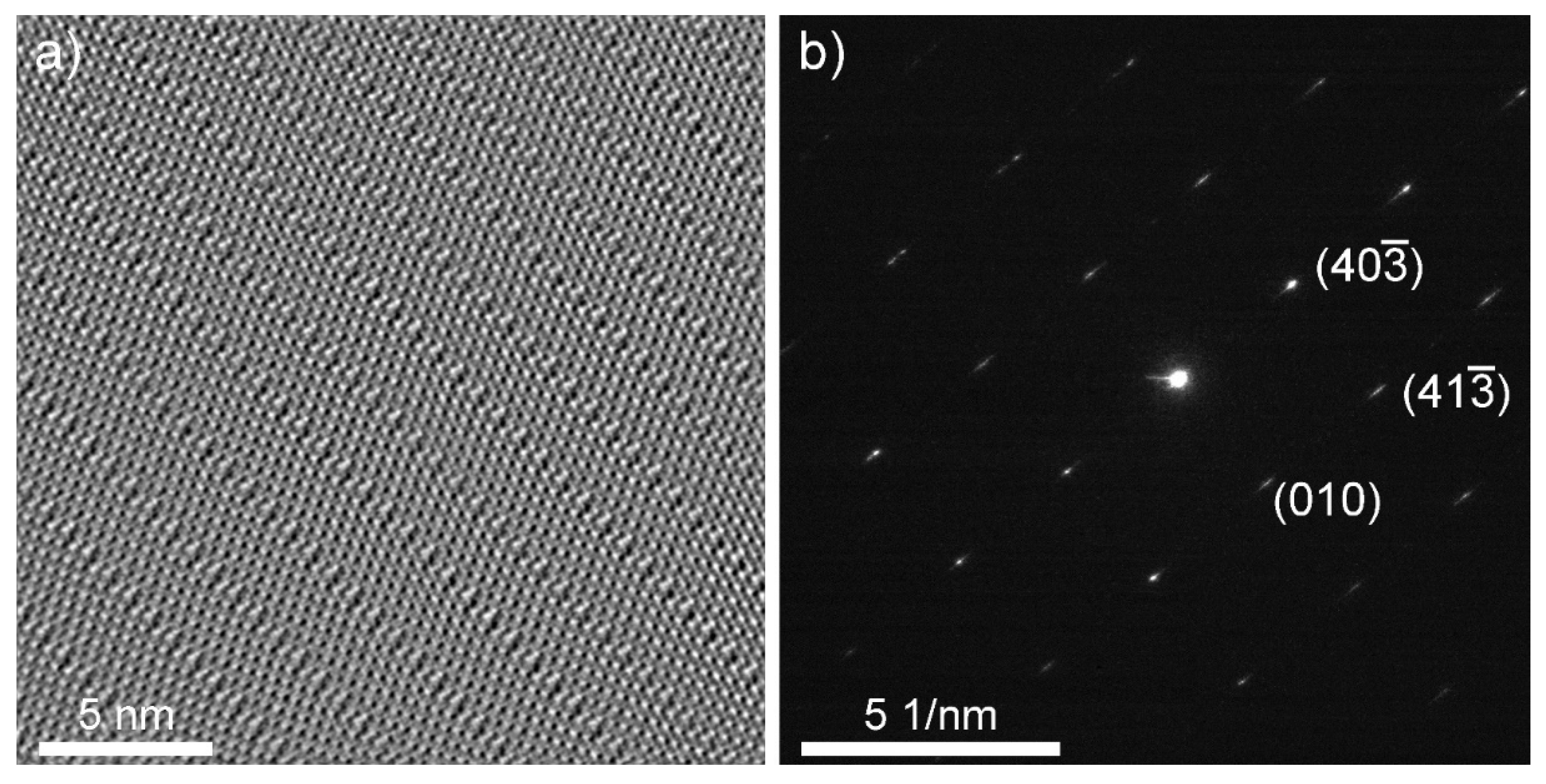
2.5. High-Resolution Transmission Electron Microscopy and Electron Diffraction
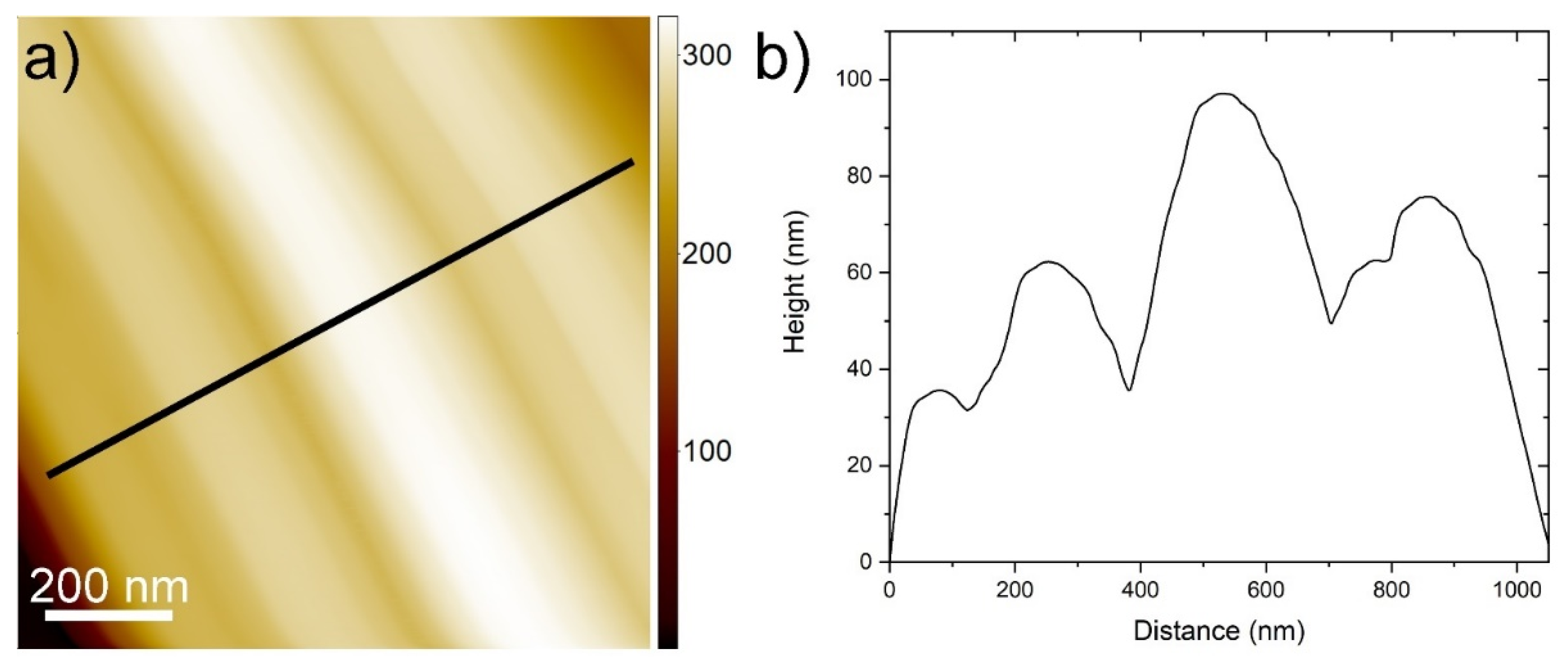
2.6. Atomic Force Microscopy
2.7. X-ray Photoelectron Spectroscopy
3. Results
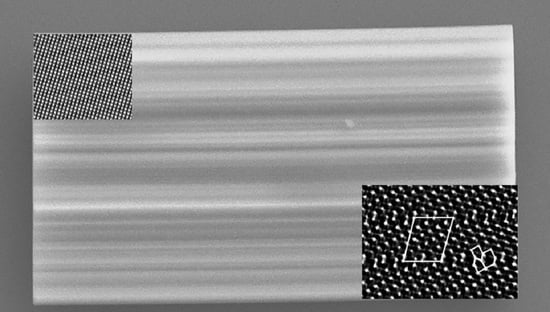
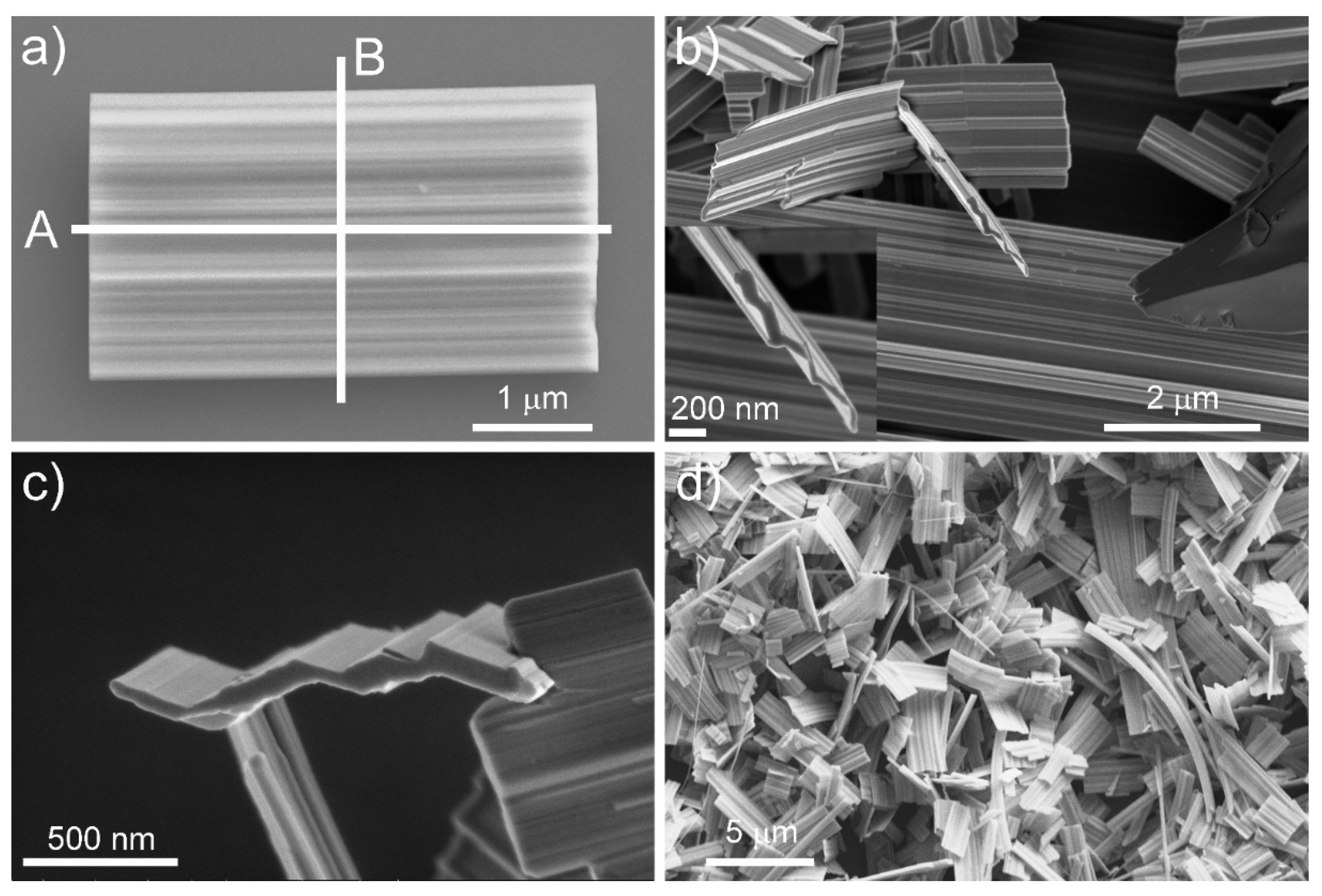
3.1. Electron Microscopy
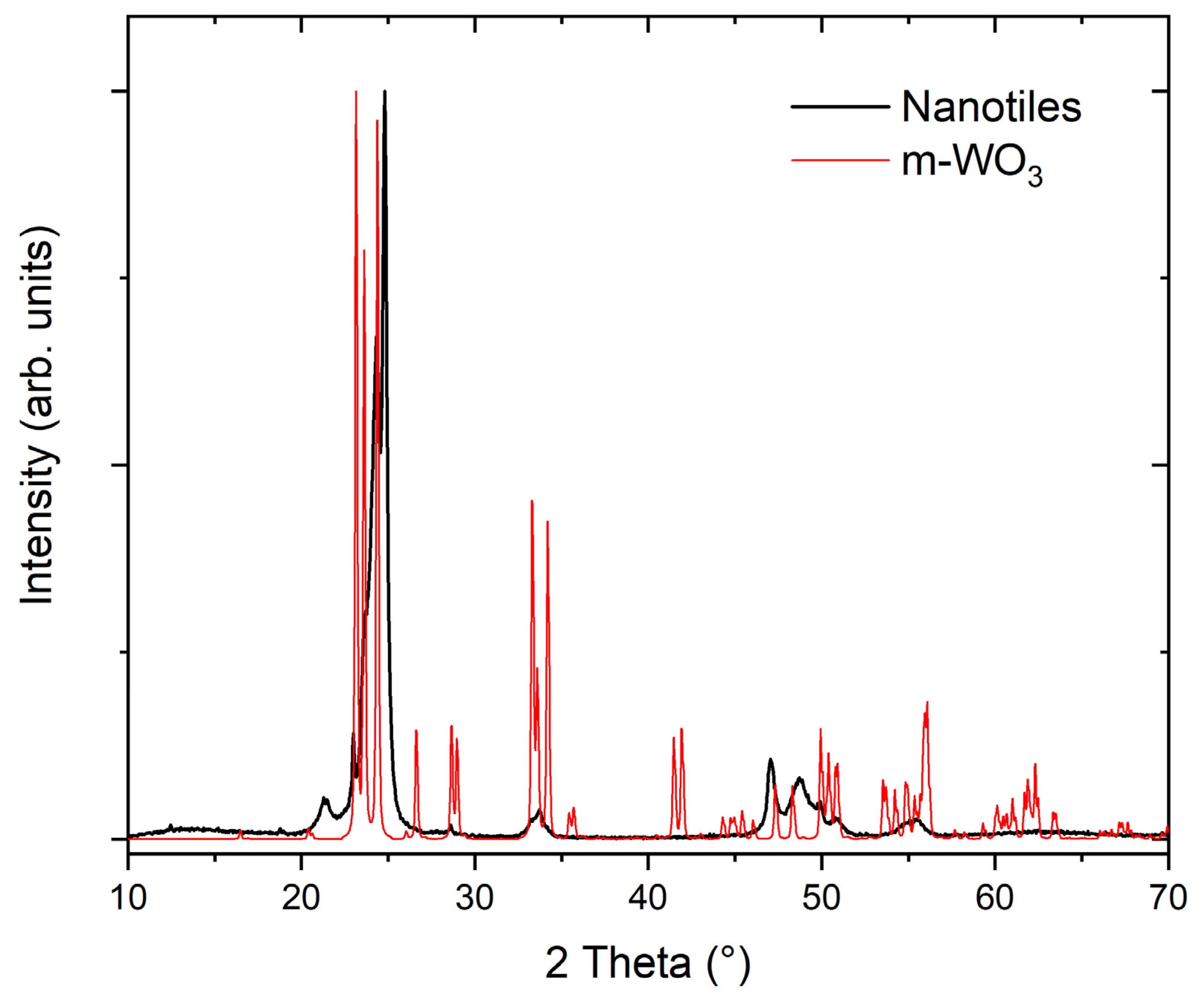
3.2. X-ray Diffraction
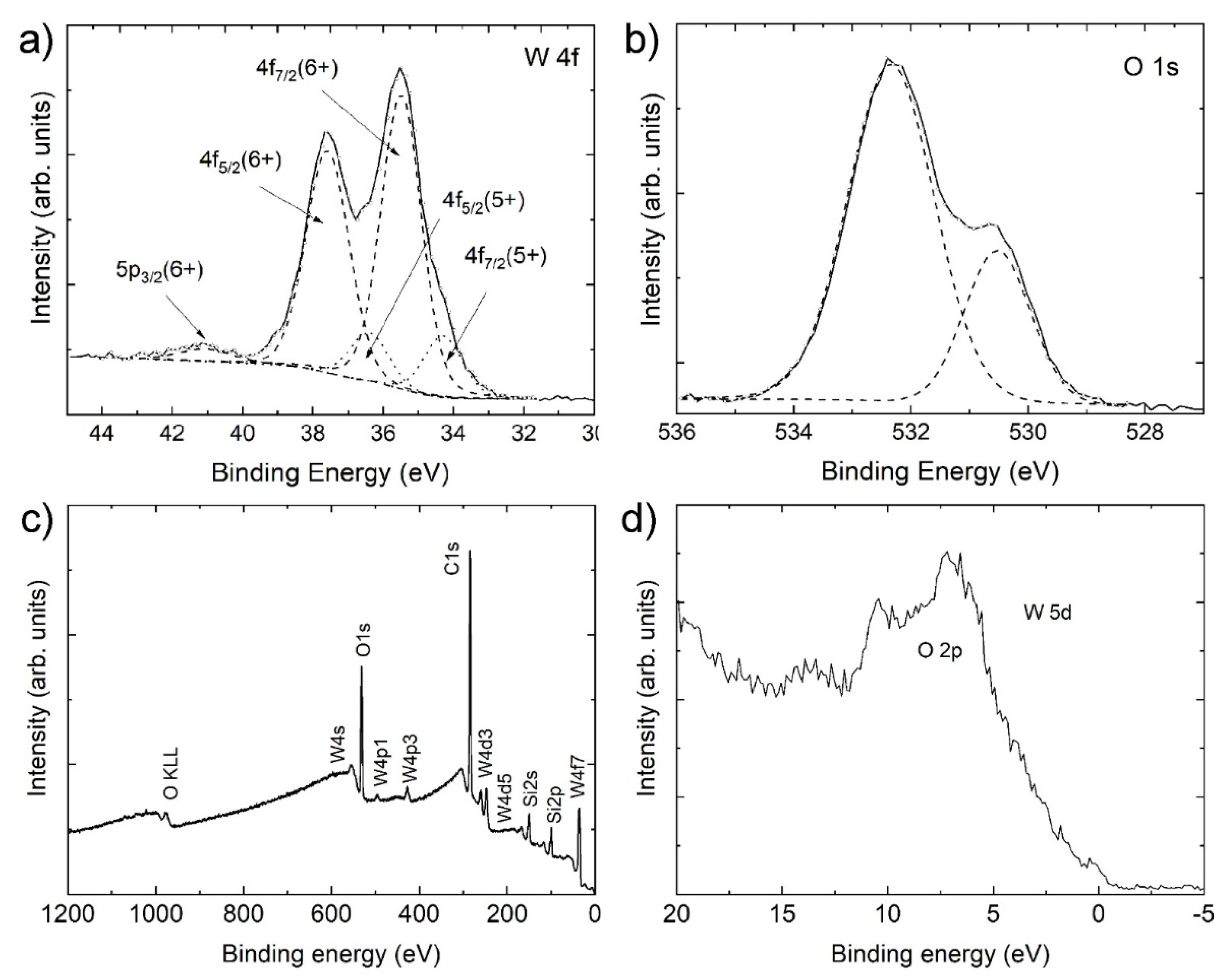
3.3. X-ray Photoelectron Spectroscopy
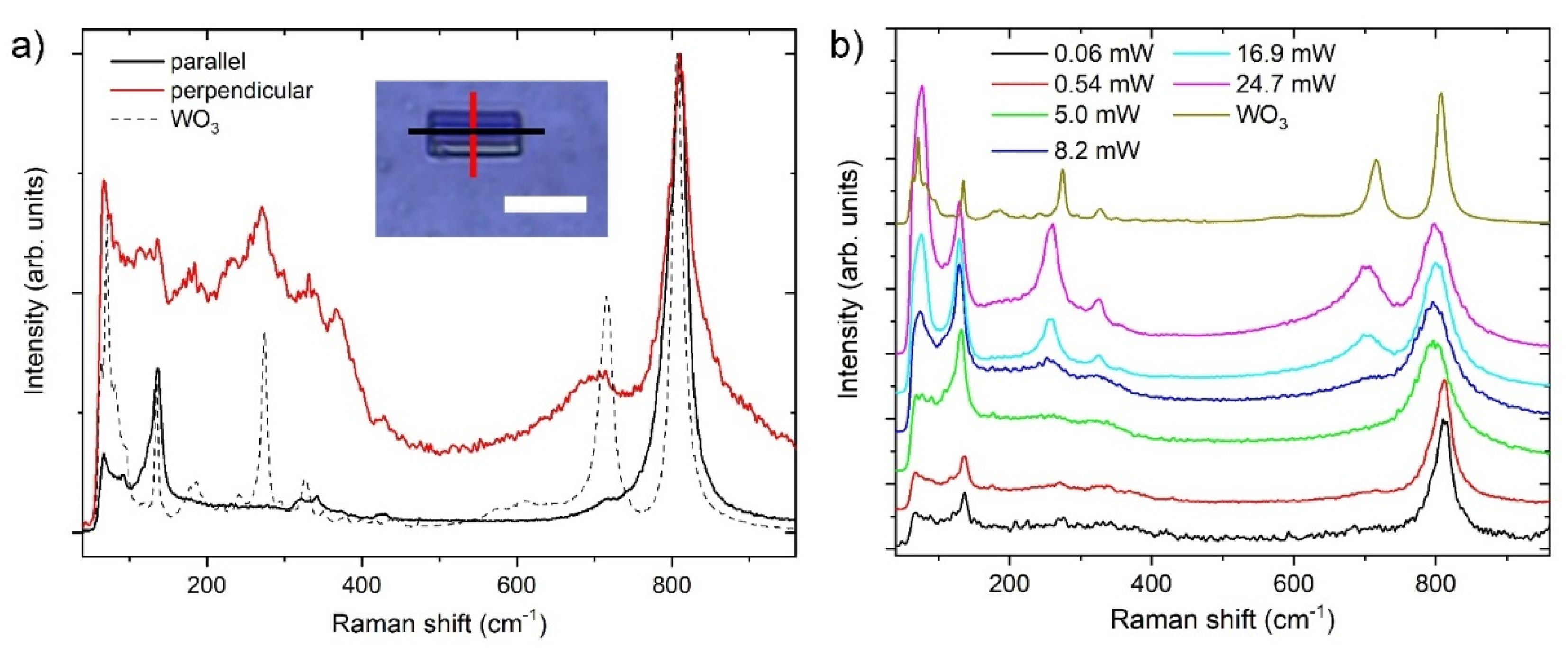
3.4. Raman Spectroscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sayama, K.; Kazuaki, M.; Ryu, A.; Yoshimoto, A.; Hironori, A. Stoichiometric water splitting into H2 and O2 using a mixture of two different photocatalysts and an IO3−/I− shuttle redox mediator under visible light irradiation. Chem. Comm. 2001, 23, 2416–2417. [Google Scholar] [CrossRef]
- Ho, J. Novel nitrogen monoxides (NO) gas sensors integrated with tungsten trioxide (WO3)/pin structure for room temperature operation. Solid-State Electron. 2003, 47, 827–830. [Google Scholar] [CrossRef]
- Reyes, L.F.; Hoel, A.; Saukko, S.; Heszler, P.; Lantto, V.; Granqvist, C.G. Gas sensor response of pure and activated WO3 nanoparticle films made by advanced reactive gas deposition. Sens. Actuators B Chem. 2006, 117, 128–134. [Google Scholar] [CrossRef]
- Hai, Z.; Wei, Z.; Xue, C.; Xu, H.; Verpoort, F. Nanostructured tungsten oxide thin film devices: From optoelectronics and ionics to iontronics. J. Mater. Chem. 2019, 7, 12968–12990. [Google Scholar] [CrossRef]
- Thind, S.S.; Chang, X.; Wentzell, J.S.; Chen, A. High-performance based supercapacitor on tantalum iridium oxides supported on tungsten oxide nanoplatelets. Electrochem. Commun. 2016, 67, 1–5. [Google Scholar] [CrossRef]
- Cazzanelli, E.; Vinegoni, C.; Mariotto, G.; Kuzmin, A.; Purans, J. Low-Temperature Polymorphism in Tungsten Trioxide Powders and Its Dependence on Mechanical Treatments. J. Solid State Chem. 1999, 143, 24–32. [Google Scholar] [CrossRef]
- Daniel, M.F.; Desbat, B.; Lassegues, J.C.; Gerand, B.; Figlarz, M. Infrared and raman study of WO3 tungsten trioxides and WO3, xH2O tungsten trioxide tydrates. J. Solid State Chem. 1987, 67, 235–247. [Google Scholar] [CrossRef]
- Filipescu, M.; Ion, V.; Colceag, D.; Ossi, P.M.; Dinescu, M. Growth and characterizations of nanostructured tungsten oxides. Rom. Rep. Phys. 2012, 64, 1213–1225. [Google Scholar]
- Salje, E. Lattice Dynamics of WO3. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1975, 31, 360–363. [Google Scholar] [CrossRef]
- Li, X.L.; Liu, J.F.; Li, Y.D. Large-scale synthesis of tungsten oxide nanowires with high aspect ratio. Inorg. Chem. 2003, 42, 921–924. [Google Scholar] [CrossRef]
- Hariharan, V.; Gnanavel, B.; Sathiyapriya, R.; Aroulmoji, V. A review on tungsten oxide (WO3) and their derivatives for sensor applications. Int. J. Adv. Sci. Eng 2019, 5, 1163–1168. [Google Scholar] [CrossRef]
- Magnéli, A. Structures of the ReO3-type with recurrent dislocations of atoms:homologous series’ of molybdenum and tungsten oxides. Acta Crystallogr. 1953, 6, 495–500. [Google Scholar] [CrossRef]
- Pickering, R.; Tilley, R.J.D. An electron microscpe study of tungsten oxides in the composition range WO2.90–WO2.72. J. Solid State Chem. 1976, 16, 247–255. [Google Scholar] [CrossRef]
- Lundberg, M.; Sundberg, M.; Magnéli, A. The “Pentagonal Column” as a building unit in crystal and defect structures of some groups of transition metal compounds. J. Solid State Chem. 1982, 44, 32–40. [Google Scholar] [CrossRef]
- Migas, D.B.; Shaposhnikov, V.L.; Rodin, V.N.; Borisenko, V.E. Tungsten oxides. I. Effects of oxygen vacancies and doping on electronic and optical properties of different phases of WO3. J. Appl. Phys. 2010, 108, 093713. [Google Scholar] [CrossRef]
- Frey, G.L.; Rothschild, A.; Sloan, J.; Rosentsveig, R.; Popovitz-Biro, R.; Tenne, R. Investigations of nonstoichiometric tungsten oxide nanoparticles. J. Solid State Chem. 2001, 162, 300–314. [Google Scholar] [CrossRef]
- Migas, D.B.; Shaposhnikov, V.L.; Borisenko, V.E. Tungsten oxides. II The metallic nature of Magnéli phases. J. Appl. Phys. 2010, 108, 093714. [Google Scholar] [CrossRef]
- Kilaas, R. Optimal and near-optimal filters in high-resolution electron microscopy. J. Microsc. 1998, 190, 45–51. [Google Scholar] [CrossRef]
- Bursill, L.A.; Hyde, B.G. CS Families drived from the ReO3 structure type: An electron microscope study of reduced WO3 and related pseudobinary systems. J. Solid State Chem. 1972, 14, 430–446. [Google Scholar] [CrossRef]
- Pirker, L.; Višić, B.; Škapin, S.D.; Dražić, G.; Kovač, J.; Remškar, M. Multi-stoichiometric quasi-two-dimensional WnO3n−1 tungsten oxides. Nanoscale 2020, 212, 15102–15114. [Google Scholar] [CrossRef]
- Leftheriotis, G.; Papaefthimiou, S.; Yianoulis, P.; Siokou, A.; Kefalas, D. Structural and electrochemical properties of opaque sol–gel deposited WO3 layers. Appl. Surf. Sci. 2003, 218, 276–281. [Google Scholar] [CrossRef]
- Katoh, M.; Takeda, Y. Chemical state analysis of tungsten and tungsten oxides using an electron probe microanalyzer. Jpn. J. Appl. Phys. 2004, 43, 7292. [Google Scholar] [CrossRef]
- Zhang, C.; Boudiba, A.; Navio, C.; Bittencourt, C.; Olivier, M.G.; Snyders, R.; Debliquy, M. Highly sensitive hydrogen sensors based on co-sputtered platinum-activated tungsten oxide films. Int. J. Hydrogen Energy 2011, 36, 1107–1114. [Google Scholar] [CrossRef]
- Lu, D.Y.; Chen, J.; Zhou, J.; Deng, S.Z.; Xu, N.S.; Xu, J.B. Raman spectroscopic study of oxidation and phase transition in W18O49 Nanowires. J. Raman Spectrosc. 2007, 38, 176–180. [Google Scholar] [CrossRef]
- Remškar, M.; Kovac, J.; Viršek, M.; Mrak, M.; Jesih, A.; Seabaugh, A. W5O14 nanowires. Adv. Funct. Mater. 2007, 17, 1974–1978. [Google Scholar] [CrossRef]
- Trapatseli, M.; Vernardou, D.; Tzanetakis, P.; Spanakis, E. Field emission properties of low-temperature, hydrothermally grown tungsten oxide. ACS Appl. Mater. Interfaces 2011, 3, 2726–2731. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, F.D.; Wachs, I.E. Determination of the molecular structures of tungstates by raman spectroscopy. J. Raman Spectrosc. 1995, 26, 397–405. [Google Scholar] [CrossRef]
- Gonzalez-Calbet, J.M.; Rosique-Perez, C.; Vallet-Regi, M.; Alario-Franco, M.A.; Rodríguez-Carvajal, J. Lithium insertion in reduced tungsten oxides. Solid State Ionics 1989, 32, 162–166. [Google Scholar] [CrossRef]
- Woodward, P.M.; Sleight, A.W.; Vogt, T. Structure refinement of triclinic tungsten trioxide. J.Phys. Chem. Solids 1995, 56, 1305–1315. [Google Scholar] [CrossRef]
- Thummavichai, K.; Wang, N.; Xu, F.; Rance, G.; Xia, Y.; Zhu, Y. In situ investigations of the phase change behaviour of tungsten oxide nanostructures. R. Soc. Open Sci. 2018, 5, 171932. [Google Scholar] [CrossRef]
- Saqib, M.; Jelenc, J.; Pirker, L.; Škapin, S.D.; De Pietro, L.; Ramsperger, U.; Knápek, A.; Müllerová, I.; Remškar, M. Field emission properties of single crystalline W5O14 and W18O49 nanowires. J. Electron. Spectros. Relat. Phenom. 2020, 241, 146837. [Google Scholar] [CrossRef]
- Zhang, Z.; Sheng, L.; Chen, L.; Zhang, Z.; Wang, Y. Atomic-scale observation of pressure-dependent reduction dynamics of W18O49 nanowires in an environmental TEM. Phys. Chem. Chem.Phys. 2017, 19, 16307. [Google Scholar] [CrossRef] [PubMed]
- Merchan-Merchan, W.; Farahani, M.F.; Moorhead-Rosenberg, Z. Electron beam induced formation of tungsten sub-oxide nanorods from flame-formed fragments. Micron 2014, 57, 23–30. [Google Scholar] [CrossRef]
- Khyzhun, O.Y. XPS, XES and XAS studies of the electronic structure of tungsten oxides. J. Alloys Compd. 2000, 305, 1–6. [Google Scholar] [CrossRef]
- Khan, H.; Zavabeti, A.; Wang, Y.; Harrison, C.J.; Carey, B.J.; Mohiuddin, M.; Chrimes, A.F.; De Castro, I.A.; Zhang, B.Y.; Sabri, Y.M.; et al. Quasi physisorptive two dimensional tungsten oxide nanosheets with extraordinary sensitivity and selectivity to NO2. Nanoscale 2017, 9, 19162–19175. [Google Scholar] [CrossRef]
- Korshunov, M.M.; Nekrasov, I.A.; Pavlov, N.S.; Slobodchikov, A.A. Band structure of tungsten oxide W20O58 with ideal octahedra. JETP Lett. 2021, 113, 57–60. [Google Scholar] [CrossRef]
- Chen, J.; Lu, D.; Zhang, W.; Xie, F.; Zhou, J.; Gong, L.; Liu, X.; Deng, S.; Xu, N. Synthesis and raman spectroscopic study of W20O58 nanowires. J. Phys. D 2008, 41, 115305. [Google Scholar] [CrossRef]
- Huang, P.; Kalyar, M.M.A.; Webster, R.F.; Cherns, D.; Ashfold, M.N.R. Tungsten oxide nanorod growth by pulsed laser deposition: Influence of substrate and process conditions. Nanoscale 2014, 6, 13586–13597. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, Y.-J.; Lee, T.; Soon, A. Phase stability diagrams of group 6 Magnéli oxides and their implications for photon-assisted applications. Chem. Mater. 2019, 31, 4282–4290. [Google Scholar] [CrossRef]
- Zhao, Z.; Bai, Y.; Ning, W.; Fan, J.; Gu, Z.; Chang, H.; Yin, S. Effect of surfactants on the performance of 3D morphology W18O49 by solvothermal synthesis. Appl. Surf. Sci. 2019, 471, 537–544. [Google Scholar] [CrossRef]
- Li, Y.; Chang, K.; Tang, H.; Li, B.; Qin, Y.; Hou, Y.; Chang, Z. Preparation of oxygen-deficient WO3−x nanosheets and their characterization as anode materials for high-performance Li-ion batteries. Electrochim. Acta 2019, 298, 640–649. [Google Scholar] [CrossRef]
- Zhuiykov, S. Material characterisation and transistor function of quasi two dimensional sub-stoichiometric WO3−x nanoflakes. Mater. Lett. 2016, 165, 173–177. [Google Scholar] [CrossRef]
- Pan, K.; Shan, K.; Wei, S.; Li, K.; Zhu, J.; Siyal, S.H.; Wu, H.-H. Enhanced photocatalytic performance of WO3−x with oxygen vacancies via heterostructuring. Compos. Commun. 2019, 16, 106–110. [Google Scholar] [CrossRef]
- Salje, E.K.H. Polaronic states and superconductivity in WO3−x. Condens. Matter 2020, 5, 32. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, Y.; Xie, W.; Wang, Y.; Hu, Z.; Zhang, W.; Zhao, H. Facile strategy for synthesizing non-stoichiometric monoclinic structured tungsten trioxide (WO3−x) with plasma resonance absorption and enhanced photocatalytic activity. Nanomaterials 2018, 8, 553. [Google Scholar] [CrossRef] [PubMed]
- Parthibavarman, M.; Karthik, M.; Prabhakaran, S. Facile and one step synthesis of WO3 nanorods and nanosheets as an efficient photocatalyst and humidity sensing material. Vaccum 2018, 155, 224–232. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Amer, A.W.; AlQaradawi, S.Y.; Allam, N.K. On the nature of defect states in tungstate nanoflake arrays as promising photoanodes in solar fuel cells. Phys. Chem. Chem. Phys. 2016, 18, 22217–22223. [Google Scholar] [CrossRef] [PubMed]

| Measured | Calculated | |||||||
|---|---|---|---|---|---|---|---|---|
| Structure | a (Å) | b (Å) | c (Å) | β (°) | a (Å) | b (Å) | c (Å) | β (°) |
| W11O32 | 17.3 | 3.83 | 18.9 | 79.3 | 17.1 | >3.83 | 19.2 | 72.2 |
| W12O35 | 17.3 | 3.83 | 20.2 | 89.6 | 17.1 | >3.83 | 20.0 | 87.9 |
| W13O38 | 17.3 | 3.83 | 22.5 | 103.7 | 17.1 | >3.83 | 22.1 | 101.5 |
| W14O41 | 17.3 | 3.83 | 24.5 | 74.2 | 17.1 | >3.83 | 24.6 | 72.0 |
| W15O43 | 17.3 | 3.83 | 25.6 | 88.0 | 17.1 | >3.83 | 25.2 | 84.4 |
| W16O47 | 17.3 | 3.83 | 27.5 | 98.3 | 17.1 | >3.83 | 26.9 | 95.7 |
| Measured | Theoretical W14O41 | |||
|---|---|---|---|---|
| 2 Theta (°) | d (Å) | Rel. Int. | Assigned Index (hkl) | d (Å) |
| 12.5 | 7.08 | 0.02 | 7.04 | |
| 18.8 | 4.70 | 0.01 | (005) | 4.68 |
| 21.5 | 4.13 | 0.05 | (403) | 4.17 |
| 23.2 | 3.84 | 0.14 | (010) | 3.83 |
| 23.9 | 3.73 | 0.30 | (110) | 3.73 |
| 24.5 | 3.63 | 0.66 | (112) | 3.63 |
| 25.0 | 3.56 | 1.00 | 3.52 | |
| 28.9 | 3.09 | 0.02 | (407) | 3.09 |
| 34.2 | 2.62 | 0.04 | 2.62 | |
| 48.5 | 1.88 | 0.10 | 1.88 | |
| 50.3 | 1.81 | 0.08 | (322) | 1.81 |
| 52.7 | 1.74 | 0.03 | 1.74 | |
| 57.6 | 1.60 | 0.02 | 1.60 | |
| Modes | Parallel | Perpendicular | WO3 Powder | |||
|---|---|---|---|---|---|---|
| Raman Shift | Int | Raman Shift | Int | Raman Shift | Int | |
| 71 | 0.65 | |||||
| Lattice modes | 136.5 | 0.26 | 136 | 0.14 | 135 | 0.29 |
| 180 | 0.09 | 186.5 | 0.06 | |||
| 232.5 | Sh | 241 | 0.03 | |||
| W-O bending | 271.5 | 0.19 | 273 | 0.41 | ||
| 322.5 | 0.02 | 331.5 | 0.12 | 327 | 0.07 | |
| 341.5 | 0.03 | 367 | 0.05 | 350 | 0.02 | |
| 417 | 0.01 | |||||
| 426.5 | 0.01 | 428 | 0.02 | 437 | 0.01 | |
| W-O stretching | 722 | sh | 702 | 0.17 | 716 | 0.49 |
| 810 | 1.00 | 810 | 1.00 | 807 | 1.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirker, L.; Višić, B.; Kovač, J.; Škapin, S.D.; Remškar, M. Synthesis and Characterization of Tungsten Suboxide WnO3n−1 Nanotiles. Nanomaterials 2021, 11, 1985. https://doi.org/10.3390/nano11081985
Pirker L, Višić B, Kovač J, Škapin SD, Remškar M. Synthesis and Characterization of Tungsten Suboxide WnO3n−1 Nanotiles. Nanomaterials. 2021; 11(8):1985. https://doi.org/10.3390/nano11081985
Chicago/Turabian StylePirker, Luka, Bojana Višić, Janez Kovač, Srečo D. Škapin, and Maja Remškar. 2021. "Synthesis and Characterization of Tungsten Suboxide WnO3n−1 Nanotiles" Nanomaterials 11, no. 8: 1985. https://doi.org/10.3390/nano11081985
APA StylePirker, L., Višić, B., Kovač, J., Škapin, S. D., & Remškar, M. (2021). Synthesis and Characterization of Tungsten Suboxide WnO3n−1 Nanotiles. Nanomaterials, 11(8), 1985. https://doi.org/10.3390/nano11081985