Targeting Intracellular Mycobacteria Using Nanosized Niosomes Loaded with Antibacterial Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Measurement of Antibacterial Agent Concentration
2.2. Nanoniosome Preparation
2.3. Nanoniosome Size and Cholesterol Concentration
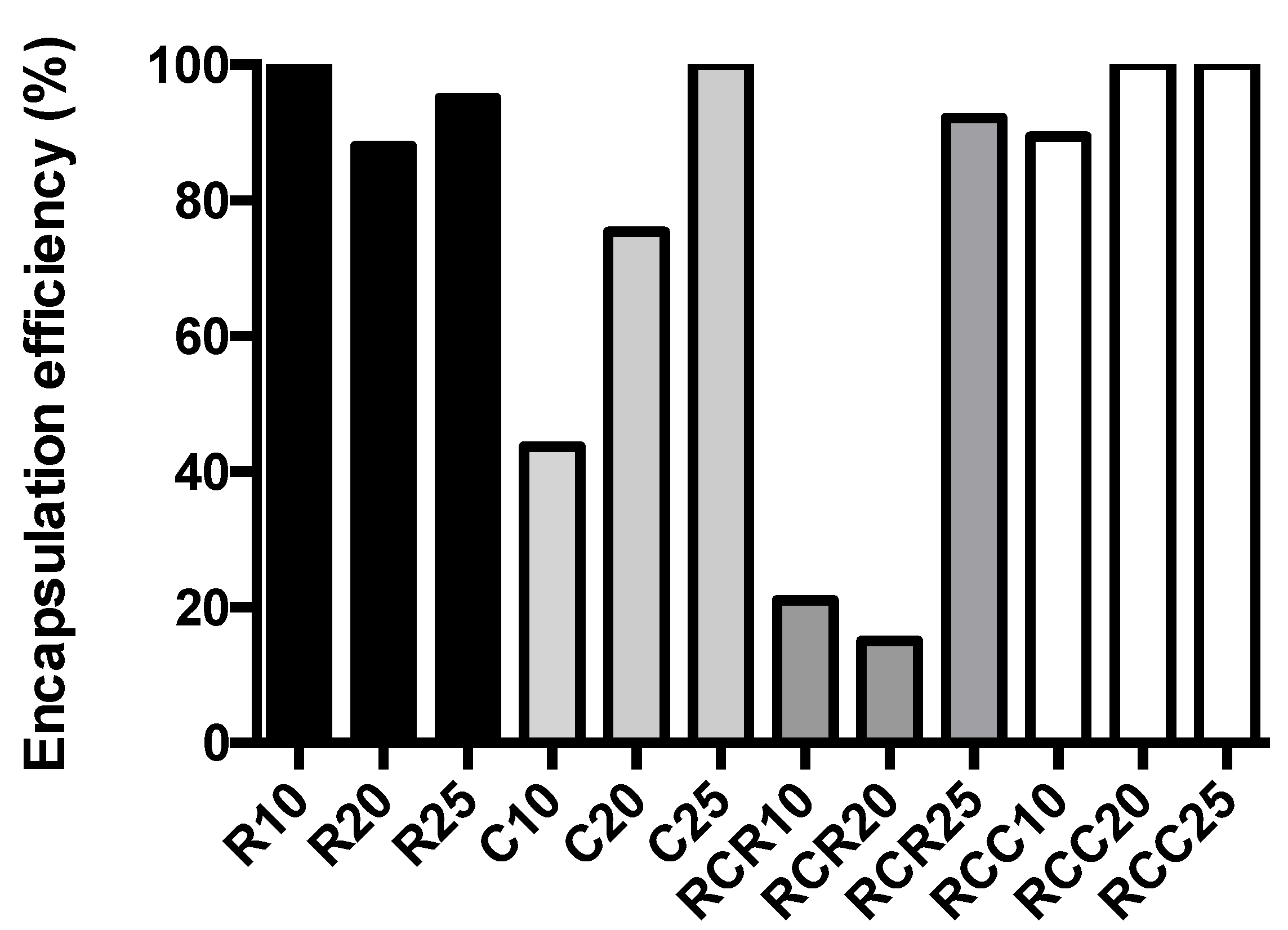
2.4. Calculation of Encapsulated Drug
2.5. Strains, Cell Line, and Culture Conditions
2.6. Minimum Inhibitory Concentration Detection
2.7. THP-1 Cell Infection
2.8. Cytotoxicity and DNA Proliferation
2.9. Immunological Response
2.10. Statistical Analysis
3. Results
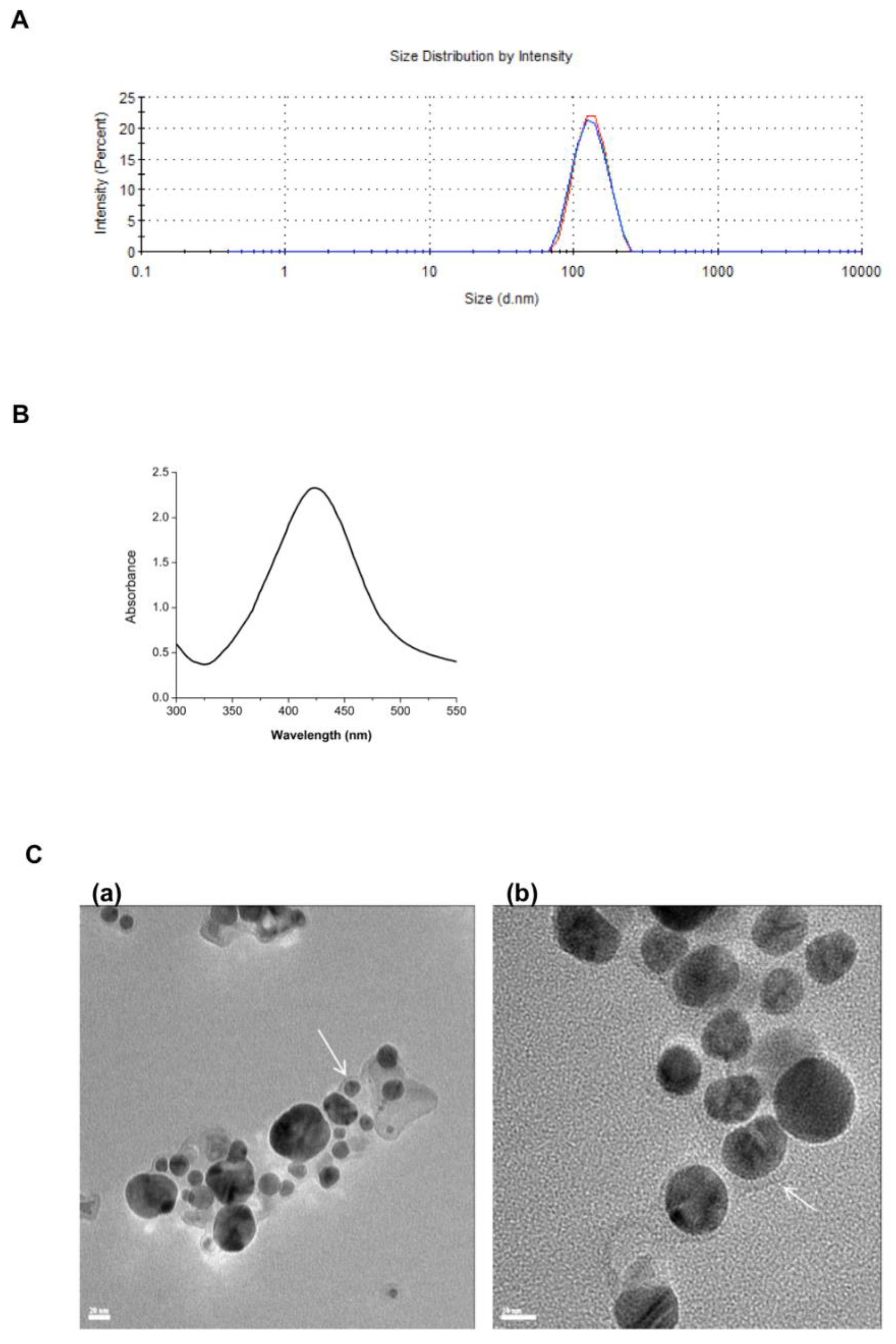
3.1. Nanoniosome Size, L-AgNP Fabrication, and Encapsulation Efficiency
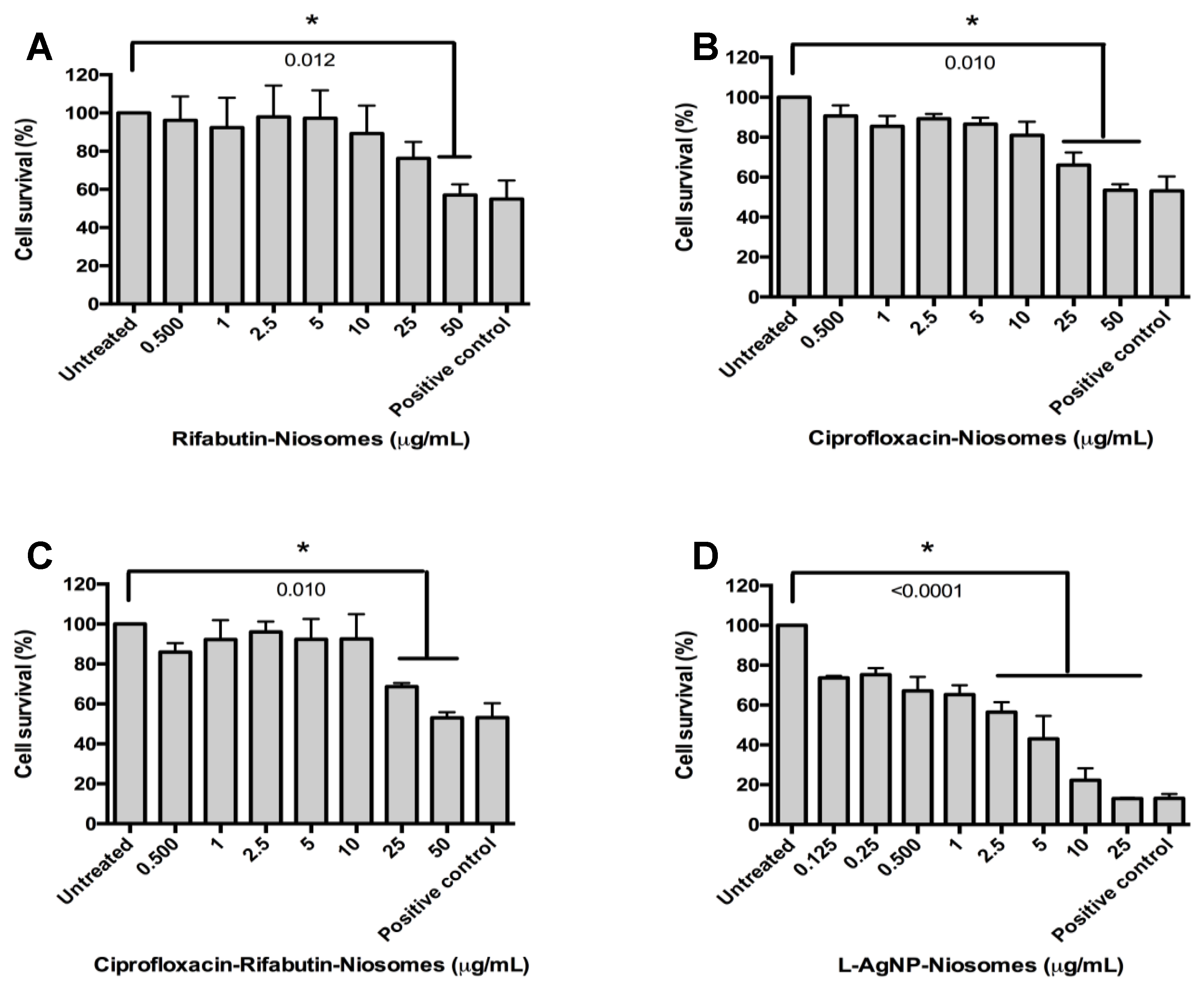
3.2. MICs and Cytotoxicity Results
3.3. Nanoniosome Effect on Infected THP-1 Cells
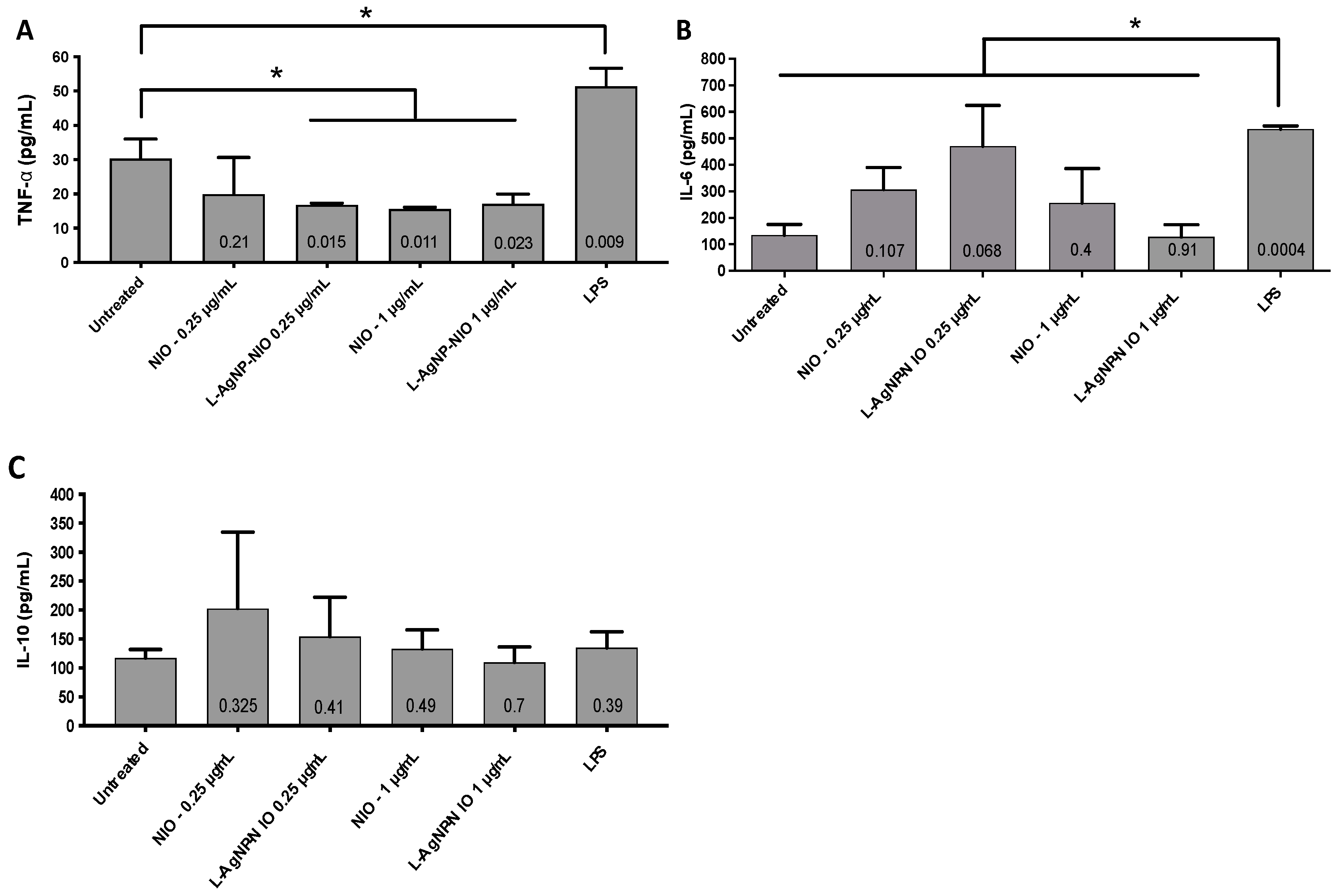
3.4. Inflammatory Response
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry: 2015 Annual Report; Cystic Fibrosis Foundation: Bethesda, MD, USA, 2015. [Google Scholar]
- Pierre-Audigier, C.; Ferroni, A.; Sermet-Gaudelus, I.; Le Bourgeois, M.; Offredo, C.; Vu-Thien, H.; Fauroux, B.; Mariani, P.; Munck, A.; Bingen, E.; et al. Age-related prevalence and distribution of nontuberculous mycobacterial species among patients with cystic fibrosis. J. Clin. Microbiol. 2005, 43, 3467–3470. [Google Scholar] [CrossRef]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef]
- Broda, A.; Jebbari, H.; Beaton, K.; Mitchell, S.; Drobniewski, F. Comparative drug resistance of Mycobacterium abscessus and chelonae from patients with and without cystic fibrosis in the UK. J. Clin. Microbiol. 2013, 51, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Daley, C.L.; Griffith, D.E. Pulmonary disease caused by rapidly growing mycobacteria. Clin. Chest Med. 2002, 23, 623–632. [Google Scholar] [CrossRef]
- Ebert, D.L.; Olivier, K.N. Nontuberculous mycobacteria in the setting of cystic fibrosis. Clin. Chest Med. 2002, 23, 655–663. [Google Scholar] [CrossRef]
- Koh, W.-J.; Jeong, B.-H.; Kim, S.-Y.; Jeon, K.; Park, K.U.; Jhun, B.W.; Lee, H.; Park, H.Y.; Kim, D.H.; Huh, H.J.; et al. Mycobacterial characteristics and treatment outcomes in Mycobacterium abscessus lung disease. Clin. Infect. Dis. 2017, 64, 309–316. [Google Scholar] [CrossRef]
- Olivier, K.N.; Weber, D.J.; Wallace, R.J., Jr.; Faiz, A.R.; Lee, J.H.; Zhang, Y.; Brown-Elliot, B.A.; Handler, A.; Wilson, R.W.; Schechter, M.S.; et al. Nontuberculous mycobacteria: I. multicenter prevalence study in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003, 167, 828–834. [Google Scholar] [CrossRef]
- Roux, A.L.; Catherinot, E.; Soismier, N.; Heym, B.; Bellis, G.; Lemonnier, L.; Chiron, R.; Fauroux, B.; Le Bourgeois, M.; Munck, A.; et al. Comparing Mycobacterium massiliense and Mycobacterium abscessus lung infections in cystic fibrosis patients. J. Cyst. Fibros. 2015, 14, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, M.; Ardito, F.; Fiscarelli, E.; La Sorda, M.; D’Argenio, P.; Ricciotti, G.; Fadda, G. Fatal pulmonary infection due to multidrug-resistant Mycobacterium abscessus in a patient with cystic fibrosis. J. Clin. Microbiol. 2001, 39, 816–819. [Google Scholar] [CrossRef]
- Wallace, R.J.; Bedsole, G.; Sumter, G.; Sanders, C.V.; Steele, L.C.; Brown, B.A.; Smith, J.; Graham, D.R. Activities of ciprofloxacin and ofloxacin against rapidly growing mycobacteria with demonstration of acquired resistance following single-drug therapy. Antimicrob. Agents Chemother. 1990, 34, 65–70. [Google Scholar] [CrossRef]
- Jeon, K.; Kwon, O.J.; Lee, N.Y.; Kim, B.-J.; Kook, Y.-H.; Lee, S.-H.; Kil Park, Y.; Kim, C.K.; Koh, W.-J. Antibiotic treatment of Mycobacterium abscessus lung disease. Am. J. Respir. Crit. Care Med. 2009, 180, 896–902. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, C.K.; Bae, I.K.; Jeong, S.H.; Yim, J.J.; Jung, J.Y.; Park, M.S.; Kim, Y.S.; Kim, S.K.; Chang, J.; et al. The drug susceptibility profile and inducible resistance to macrolides of Mycobacterium abscessus and Mycobacterium massiliense in Korea. Diagn. Microbiol. Infect. Dis. 2015, 81, 107–111. [Google Scholar] [CrossRef]
- Mor, N.; Vanderkolk, J.; Mezo, N.; Heifets, L. Effects of clarithromycin and rifabutin alone and in combination on intracellular and extracellular replication of Mycobacterium avium. Antimicrob. Agents Chemother. 1994, 38, 2738–2742. [Google Scholar] [CrossRef]
- Hernández-Garduño, E.; Rodrigues, M.; Elwood, R.K. The incidence of pulmonary non-tuberculous mycobacteria in British Columbia, Canada. Int. J. Tuberc. Lung Dis. 2009, 13, 1086–1093. [Google Scholar] [PubMed]
- Moazeni, E.; Gilani, K.; Sotoudegan, F.; Pardakhty, A.; Najafabadi, A.R.; Ghalandari, R.; Fazeli, M.R.; Jamalifar, H. Formulation and in vitro evaluation of ciprofloxacin containing niosomes for pulmonary delivery. J. Microencapsul. 2010, 27, 618–627. [Google Scholar] [CrossRef]
- D’Souza, S.A.; Ray, J.; Pandey, S.; Udupa, N. Absorption of ciprofloxacin and norfloxacin when administered as niosome-encapsulated inclusion complexes. J. Pharm. Pharmacol. 1997, 49, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.W. History of the medical use of silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gutierrez, F.; Boegli, L.; Agostinho, A.; Sánchez, E.M.; Bach, H.; Ruiz, F.; James, G. Anti-biofilm activity of silver nanoparticles against different microorganisms. Biofouling 2013, 29, 651–660. [Google Scholar] [CrossRef]
- Baral, V.; Dewar, A.; Connett, G. Colloidal silver for lung disease in cystic fibrosis. J. R. Soc. Med. 2008, 101, 51–52. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Borrego, B.; Juarez-Moreno, K.O.; García-García, M.; Mota-Morales, J.; Bogdanchikova, N.; Huerta-Saquero, A. Toxicity of silver nanoparticles in biological systems: Does the complexity of biological systems matter? Toxicol. Lett. 2017, 276, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, G.; Bassegoda, A.; Hoyo, J.; Torrent-Burgués, J.; Tzanov, T. Metal-enzyme nanoaggregates eradicate both Gram-positive and Gram-negative bacteria and their biofilms. ACS Appl. Mater. Interfaces 2018, 10, 40434–40442. [Google Scholar] [CrossRef] [PubMed]
- Francesko, A.; Fossas, M.C.; Petkova, P.; Fernandes, M.M.; Mendoza, E.; Tzanov, T. Sonochemical synthesis and stabilization of concentrated antimicrobial silver-chitosan nanoparticle dispersions. J. Appl. Polym. Sci. 2017, 134, 45136. [Google Scholar] [CrossRef]
- Francesko, A.; Ivanova, K.; Hoyo, J.; Pérez-Rafael, S.; Petkova, P.; Fernandes, M.; Heinze, T.; Mendoza, E.; Tzanov, T. Bottom-up layer-by-layer assembling of antibacterial freestanding nanobiocomposite films. Biomacromolecules 2018, 19, 3628–3636. [Google Scholar] [CrossRef] [PubMed]
- Aziz, D.; Low, J.L.; Wu, M.-L.; Gengenbacher, M.; Teo, J.W.P.; Dartois, V.; Dick, T. Rifabutin is active against Mycobacterium abscessus complex. Antimicrob. Agents Chemother. 2017, 61, e00155-17. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Ivanova, K.; Hoyo, J.; Perelshtein, I.; Owen, G.; Haegert, A.; Lin, Y.-Y.; LeBihan, S.; Gedanken, A.; Häfeli, U.O.; et al. Novel lignin-capped silver nanoparticles against multidrug-resistant bacteria. ACS Appl. Mater. Interfaces 2021, 13, 22098–22109. [Google Scholar] [CrossRef]
- Bach, H.; Sun, J.; Hmama, Z.; Av-Gay, Y. Mycobacterium avium subsp. paratuberculosis PtpA is an endogenous tyrosine phosphatase secreted during infection. Infect. Immun. 2006, 74, 6540–6546. [Google Scholar] [CrossRef][Green Version]
- Contreras Cárdenas, A.V.; Hernández, L.R.; Juárez, Z.N.; Sánchez-Arreola, E.; Bach, H. Antimicrobial, cytotoxic, and anti-inflammatory activities of Pleopeltis polylepis. J. Ethnopharmacol. 2016, 194, 981–986. [Google Scholar] [CrossRef]
- Martínez-Gutierrez, F.; Thi, E.P.; Silverman, J.M.; de Oliveira, C.C.; Svensson, S.; Hoek, A.V.; Sánchez, E.M.; Reiner, N.E.; Gaynor, E.C.; Pryzdial, E.L.; et al. Antibacterial activity, inflammatory response, coagulation and cytotoxicity effects of silver nanoparticles. Nanomedicine 2012, 8, 328–336. [Google Scholar] [CrossRef]
- Akbari, V.; Abedi, D.; Pardakhty, A.; Sadeghi-Aliabadi, H. Release studies on ciprofloxacin loaded non-ionic surfactant vesicles. Avicenna J. Med. Biotechnol. 2015, 7, 69–75. [Google Scholar]
- Akbari, V.; Abedi, D.; Pardakhty, A.; Sadeghi-Aliabadi, H. Ciprofloxacin nano-niosomes for targeting intracellular infections: An in vitro evaluation. J. Nanopart. Res. 2013, 15, 1556. [Google Scholar] [CrossRef]
- Cunha, L.; Rodrigues, S.; Da Costa, A.M.R.; Faleiro, M.L.; Buttini, F.; Grenha, A. Inhalable fucoidan microparticles combining two antitubercular drugs with potential application in pulmonary tuberculosis therapy. Polymers 2018, 10, 636. [Google Scholar] [CrossRef]
- Verma, R.K.; Kaur, J.; Kumar, K.; Yadav, A.B.; Misra, A. Intracellular time course, pharmacokinetics, and biodistribution of isoniazid and rifabutin following pulmonary delivery of inhalable microparticles to mice. Antimicrob. Agents Chemother. 2008, 52, 3195–3201. [Google Scholar] [CrossRef]
- Easmon, C.S.; Crane, J.P. Uptake of ciprofloxacin by macrophages. J. Clin. Pathol. 1985, 38, 442–444. [Google Scholar] [CrossRef]
- Byrd, T.F.; Lyons, C.R. Preliminary characterization of a Mycobacterium abscessus mutant in human and murine models of infection. Infect. Immun. 1999, 67, 4700–4707. [Google Scholar] [CrossRef]
- Wong, J.P.; Yang, H.; Blasetti, K.L.; Schnell, G.; Conley, J.; Schofield, L.N. Liposome delivery of ciprofloxacin against intracellular Francisella tularensis infection. J. Control. Release 2003, 92, 265–273. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, F.; Olive, P.L.; Banuelos, A.; Orrantia, E.; Nino, N.; Sanchez, E.M.; Ruiz, F.; Bach, H.; Av-Gay, Y. Synthesis, characterization, and evaluation of antimicrobial and cytotoxic effect of silver and titanium nanoparticles. Nanomedicine 2010, 6, 681–688. [Google Scholar] [CrossRef]
- Panáček, A.; Kvitek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Sharma, V.K.; Nevěčná, T.J.; Zbořil, R. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef]
- Samberg, M.E.; Orndorff, P.E.; Monteiro-Riviere, N.A. Antibacterial efficacy of silver nanoparticles of different sizes, surface conditions and synthesis methods. Nanotoxicology 2011, 5, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Hao, Y.-M.; Li, K. Entrapment and release difference resulting from hydrogen bonding interactions in niosome. Int. J. Pharm. 2011, 403, 245–253. [Google Scholar] [CrossRef]
- Oh, Y.K.; Nix, D.E.; Straubinger, R.M. Formulation and efficacy of liposome-encapsulated antibiotics for therapy of intracellular Mycobacterium avium infection. Antimicrob. Agents Chemother. 1995, 39, 2104–2111. [Google Scholar] [CrossRef][Green Version]
- Leitzke, S.; Bucke, W.; Borner, K.; Müller, R.; Hahn, H.; Ehlers, S. Rationale for and efficacy of prolonged-interval treatment using liposome-encapsulated amikacin in experimental Mycobacterium avium infection. Antimicrob. Agents Chemother. 1998, 42, 459–461. [Google Scholar] [CrossRef]
- Gaspar, M.M.; Cruz, A.; Penha, A.F.; Reymão, J.; Sousa, A.C.; Eleutério, C.V.; Domingues, S.A.; Fraga, A.G.; Longatto Filho, A.; Cruz, M.E.M.; et al. Rifabutin encapsulated in liposomes exhibits increased therapeutic activity in a model of disseminated tuberculosis. Int. J. Antimicrob. Agents 2008, 31, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Seral, C.; Barcia-Macay, M.; Mingeot-Leclercq, M.P.; Tulkens, P.M.; Van Bambeke, F. Comparative activity of quinolones (ciprofloxacin, levofloxacin, moxifloxacin and garenoxacin) against extracellular and intracellular infection by Listeria monocytogenes and Staphylococcus aureus in J774 macrophages. J. Antimicrob. Chemother. 2005, 55, 511–517. [Google Scholar] [CrossRef]
- Carlier, M.-B.; Scorneaux, B.; Zenebergh, A.; Desnottes, J.-F.; Tulkens, P.M. Cellular uptake, localization and activity of fluoroquinolones in uninfected and infected macrophages. J. Antimicrob. Chemother. 1990, 26, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, S.; Van Bambeke, F.; Tulkens, P.M. Activity of finafloxacin, a novel fluoroquinolone with increased activity at acid pH, towards extracellular and intracellular Staphylococcus aureus, Listeria monocytogenes and Legionella pneumophila. Int. J. Antimicrob. Agents 2011, 38, 52–59. [Google Scholar] [CrossRef]
- Grimaldo, E.R.; Tupasi, T.E.; Rivera, A.B.; Quelapio Ma, I.D.; Cardaño, R.C.; Derilo, J.O.; Belen, V.A. Increased resistance to ciprofloxacin and ofloxacin in multidrug-resistant Mycobacterium tuberculosis isolates from patients seen at a tertiary hospital in the Philippines. Int. J. Tuberc. Lung Dis. 2001, 5, 546–550. [Google Scholar]
- ISO 10993-5:2009—Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. Available online: https://www.iso.org/standard/36406.html (accessed on 14 April 2021).
- Murphy, A.; Casey, A.; Byrne, G.; Chambers, G.; Howe, O. Silver nanoparticles induce pro-inflammatory gene expression and inflammasome activation in human monocytes. J. Appl. Toxicol. 2016, 36, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Park, M.V.; Neigh, A.M.; Vermeulen, J.P.; de la Fonteyne, L.J.; Verharen, H.W.; Briedé, J.J.; van Loveren, H.; de Jong, W.H. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef]
- Leelarungrayub, J.; Manorsoi, J.; Manorsoi, A. Anti-inflammatory activity of niosomes entrapped with Plai oil (Zingiber cassumunar Roxb.) by therapeutic ultrasound in a rat model. Int. J. Nanomed. 2017, 12, 2469–2476. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.P.; Singh, R.P.; Jain, S.; Mishra, V.; Mahor, S.; Singh, P.; Gupta, P.N.; Rawat, A.; Dubey, P. Non-ionic surfactant based vesicles (niosomes) for non-invasive topical genetic immunization against hepatitis B. Int. J. Pharm. 2005, 296, 80–86. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Cytotoxicity (µg/mL) | Cell Viability (%) |
|---|---|---|
| Rifabutin | 1 | 76.29 |
| Ciprofloxacin | 500 (>250) | 68.37 |
| R-NIO | 50 | 74.87 |
| C-NIO | 25 | 77.91 |
| CR-NIO | 25 | 78.81 |
| L-AgNPs | 25 | 55.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slavin, Y.N.; Ivanova, K.; Tang, W.-l.; Tzanov, T.; Li, S.-d.; Bach, H. Targeting Intracellular Mycobacteria Using Nanosized Niosomes Loaded with Antibacterial Agents. Nanomaterials 2021, 11, 1984. https://doi.org/10.3390/nano11081984
Slavin YN, Ivanova K, Tang W-l, Tzanov T, Li S-d, Bach H. Targeting Intracellular Mycobacteria Using Nanosized Niosomes Loaded with Antibacterial Agents. Nanomaterials. 2021; 11(8):1984. https://doi.org/10.3390/nano11081984
Chicago/Turabian StyleSlavin, Yael Nicole, Kristina Ivanova, Wei-lun Tang, Tzanko Tzanov, Shyh-dar Li, and Horacio Bach. 2021. "Targeting Intracellular Mycobacteria Using Nanosized Niosomes Loaded with Antibacterial Agents" Nanomaterials 11, no. 8: 1984. https://doi.org/10.3390/nano11081984
APA StyleSlavin, Y. N., Ivanova, K., Tang, W.-l., Tzanov, T., Li, S.-d., & Bach, H. (2021). Targeting Intracellular Mycobacteria Using Nanosized Niosomes Loaded with Antibacterial Agents. Nanomaterials, 11(8), 1984. https://doi.org/10.3390/nano11081984








