Solvent-Free Mechanochemical Synthesis and Characterization of Nickel Tellurides with Various Stoichiometries: NiTe, NiTe2 and Ni2Te3
Abstract
:1. Introduction
2. Materials and Methods
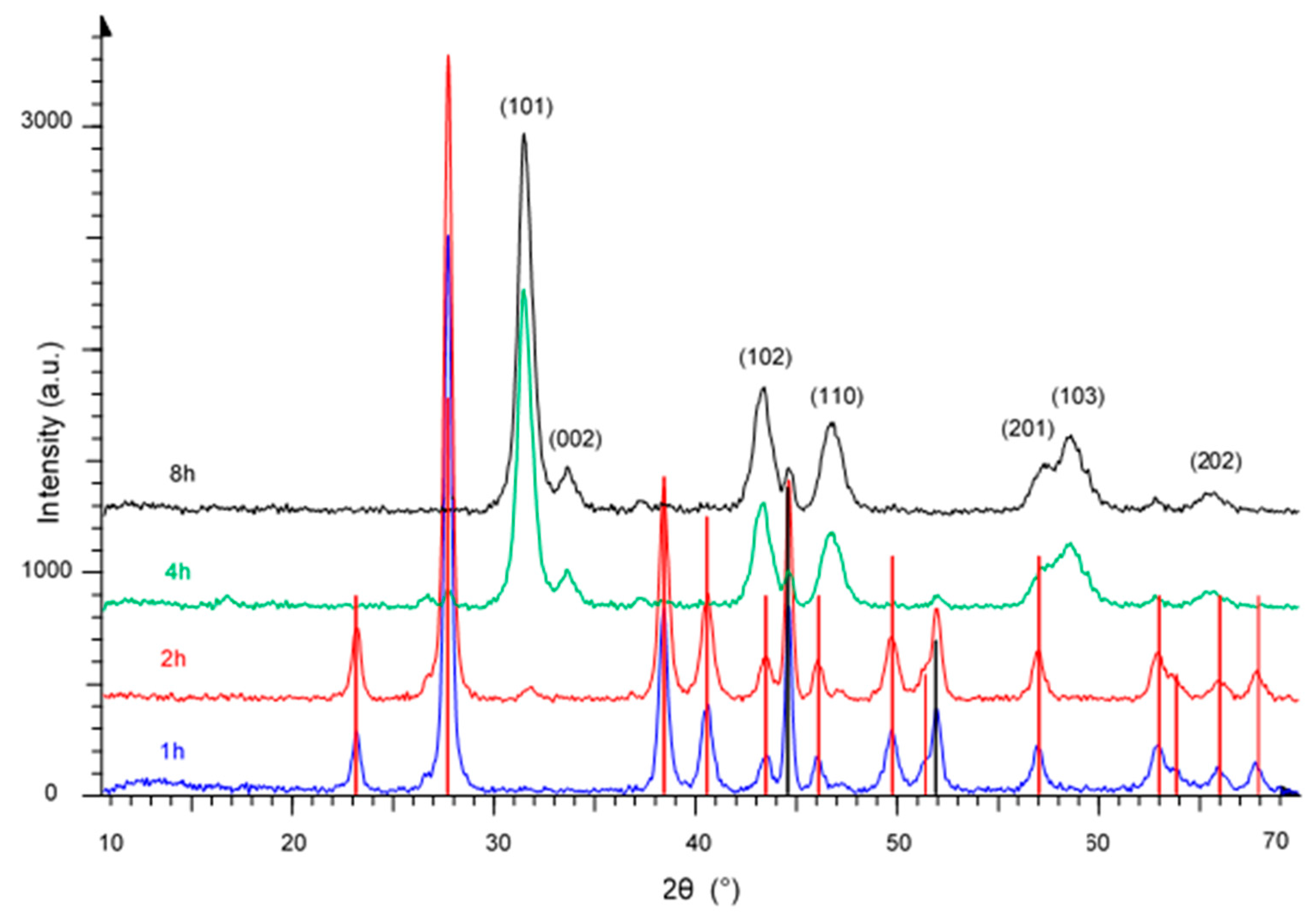
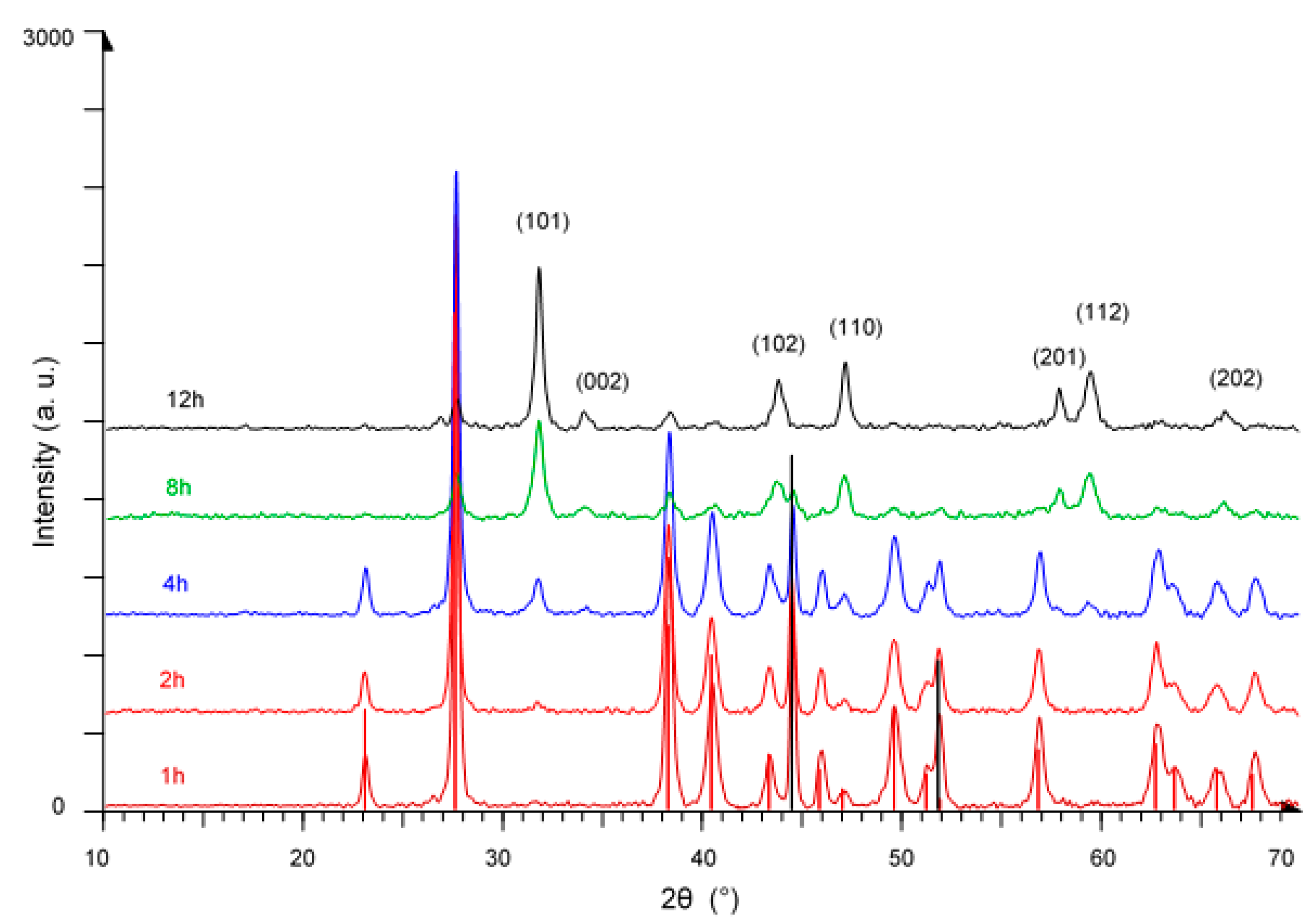
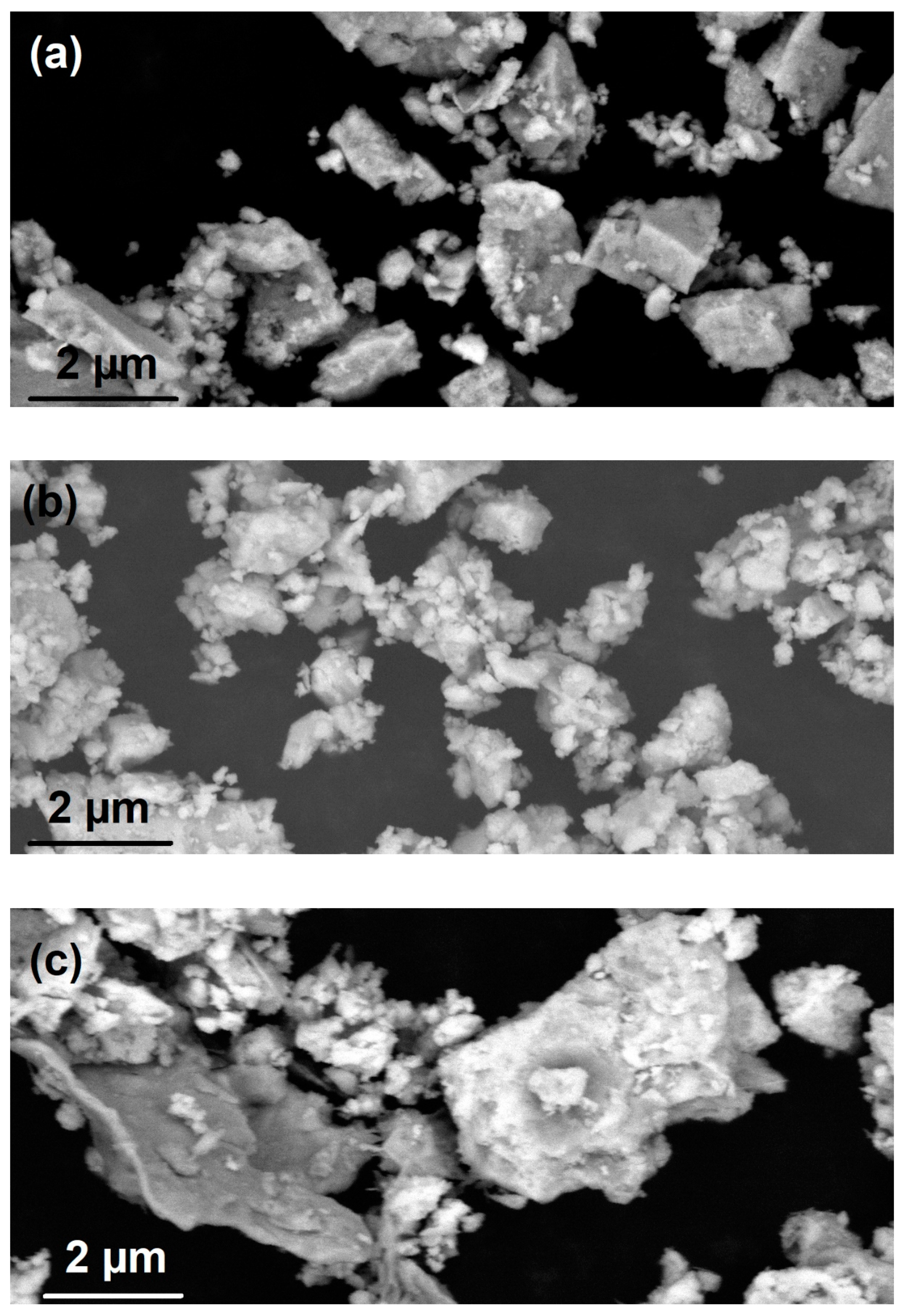
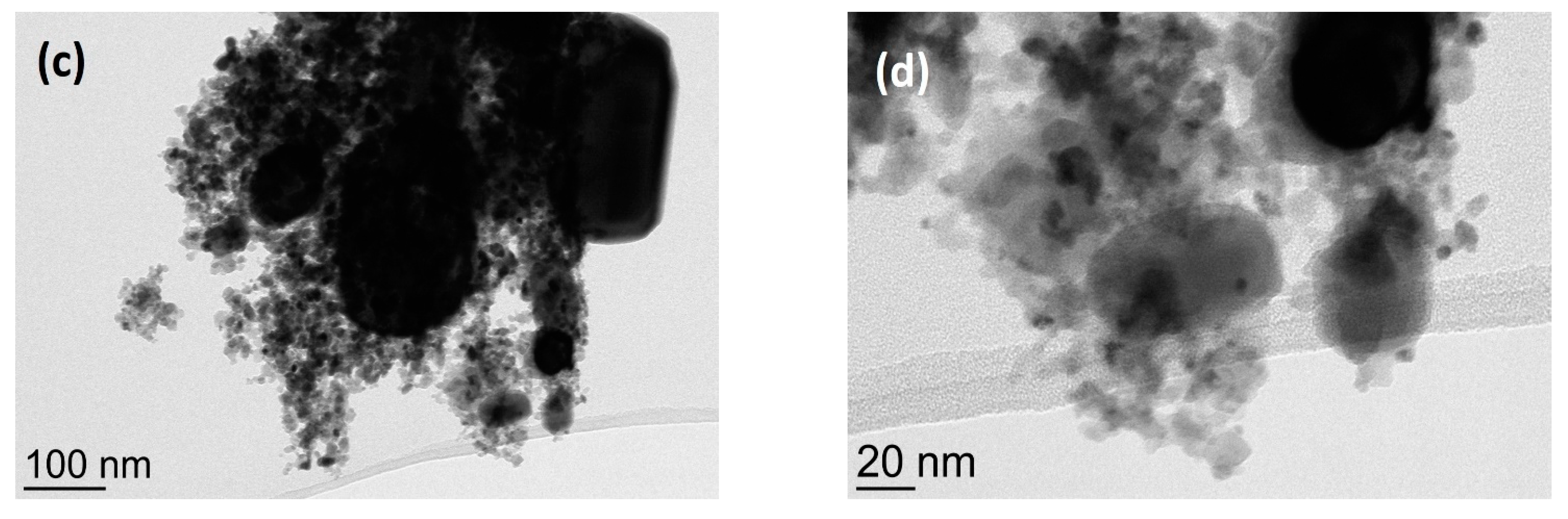
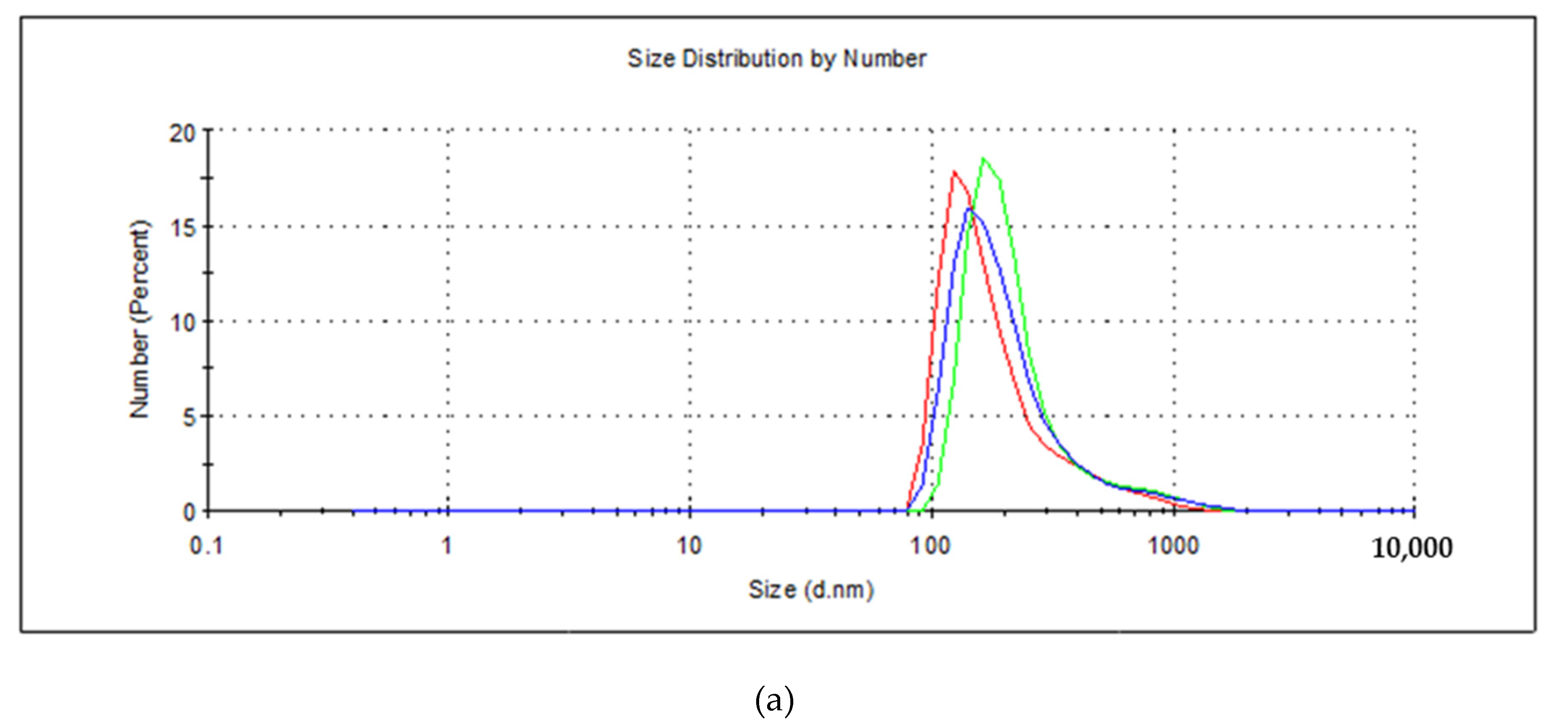
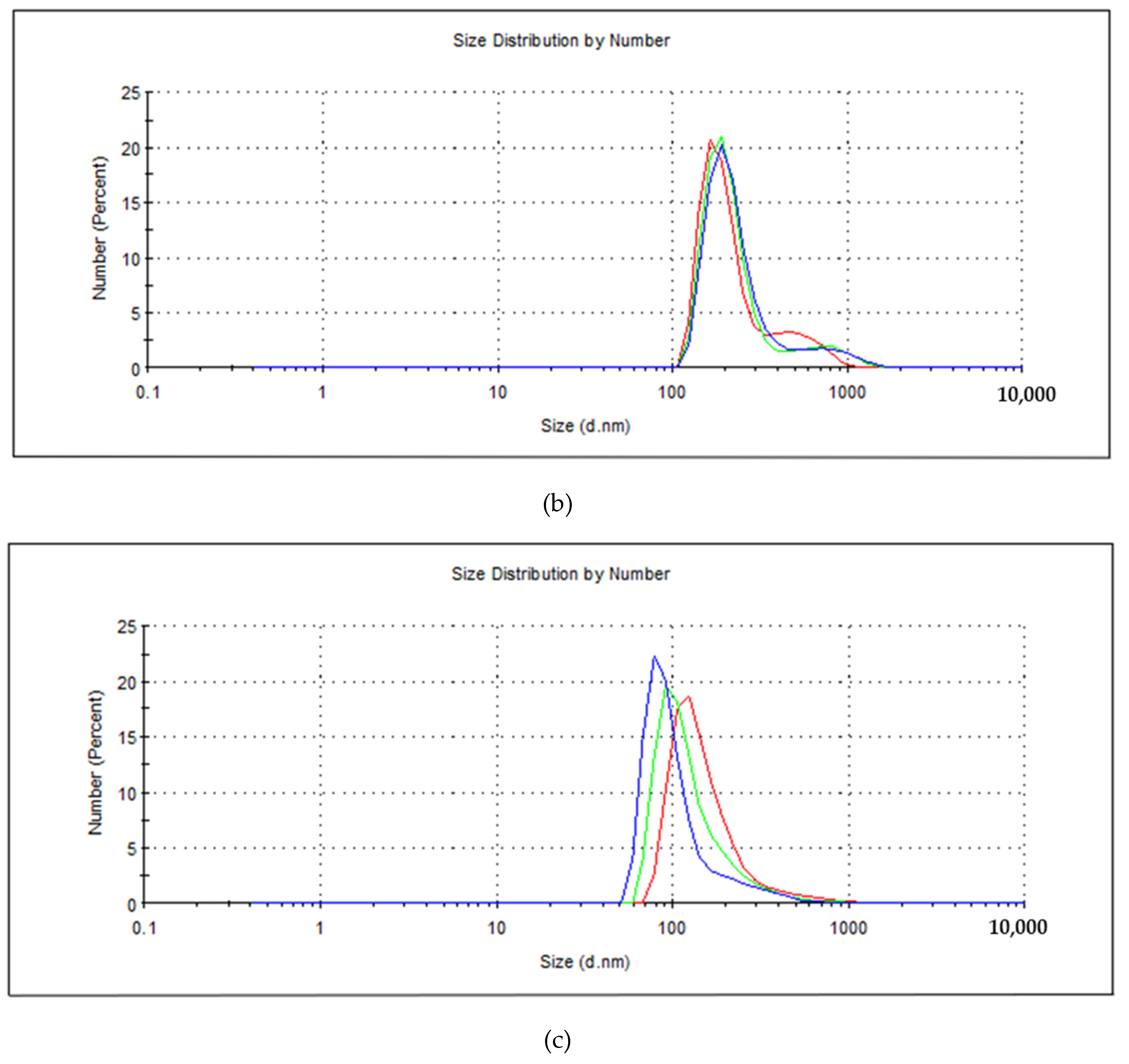
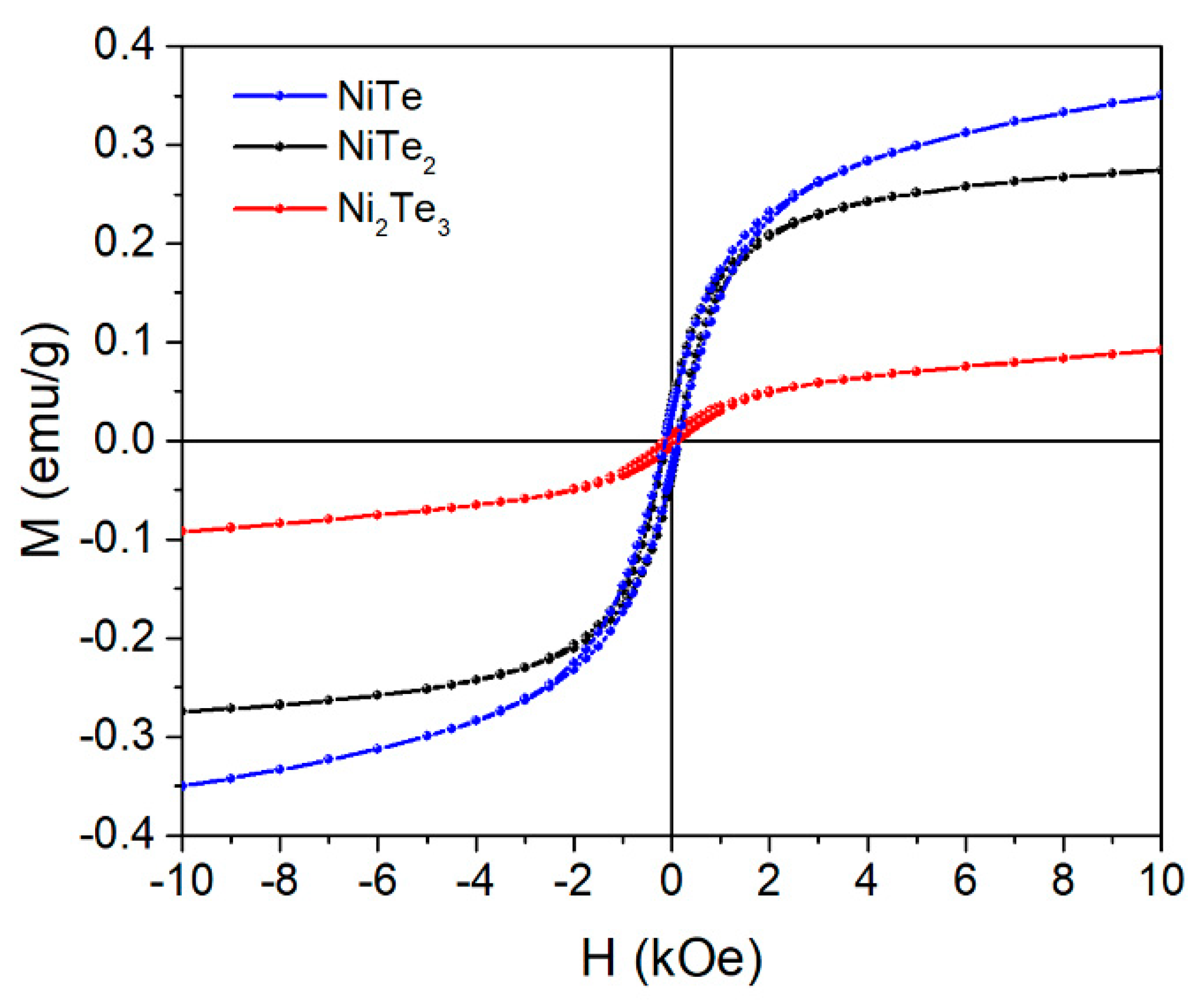
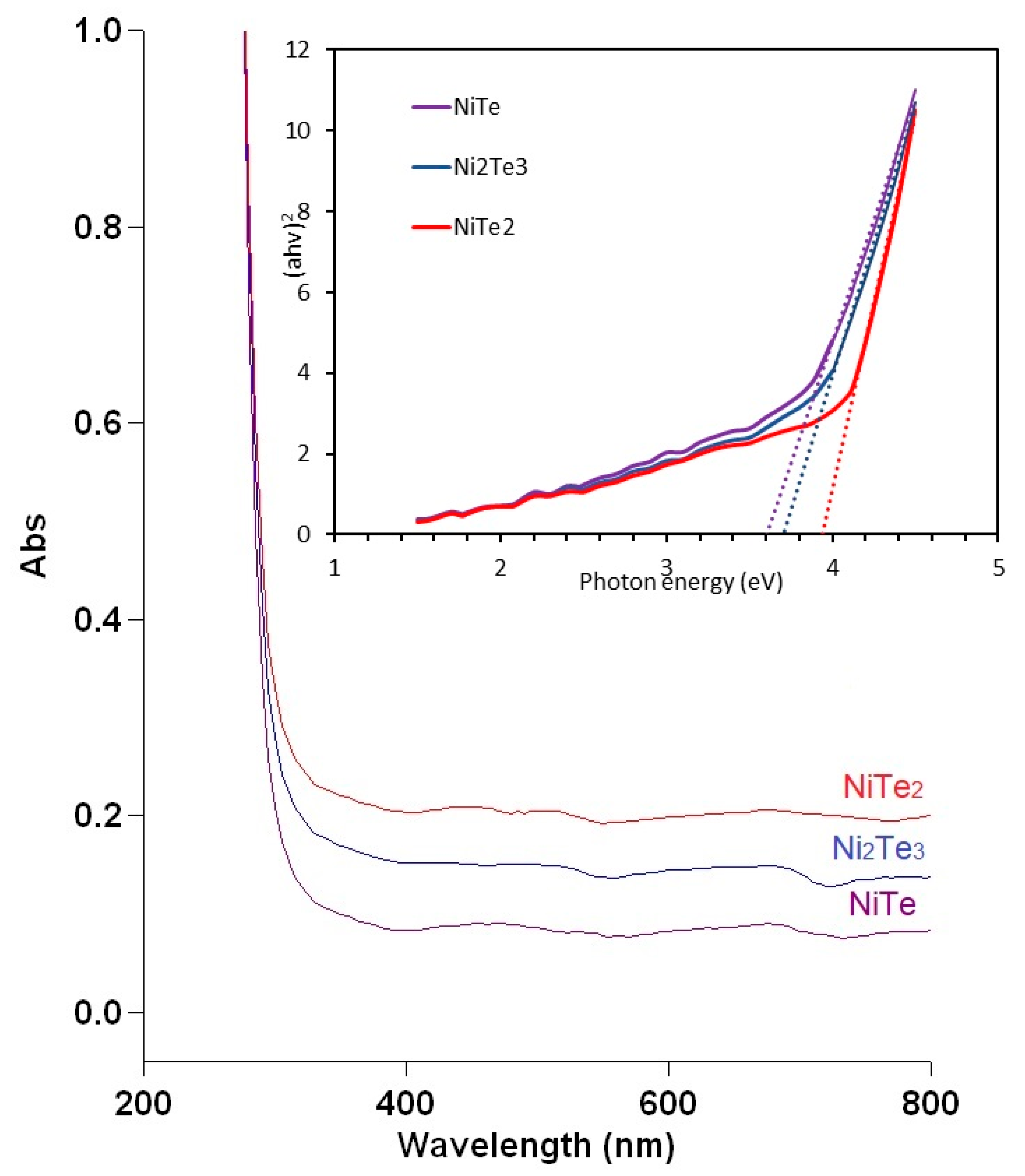
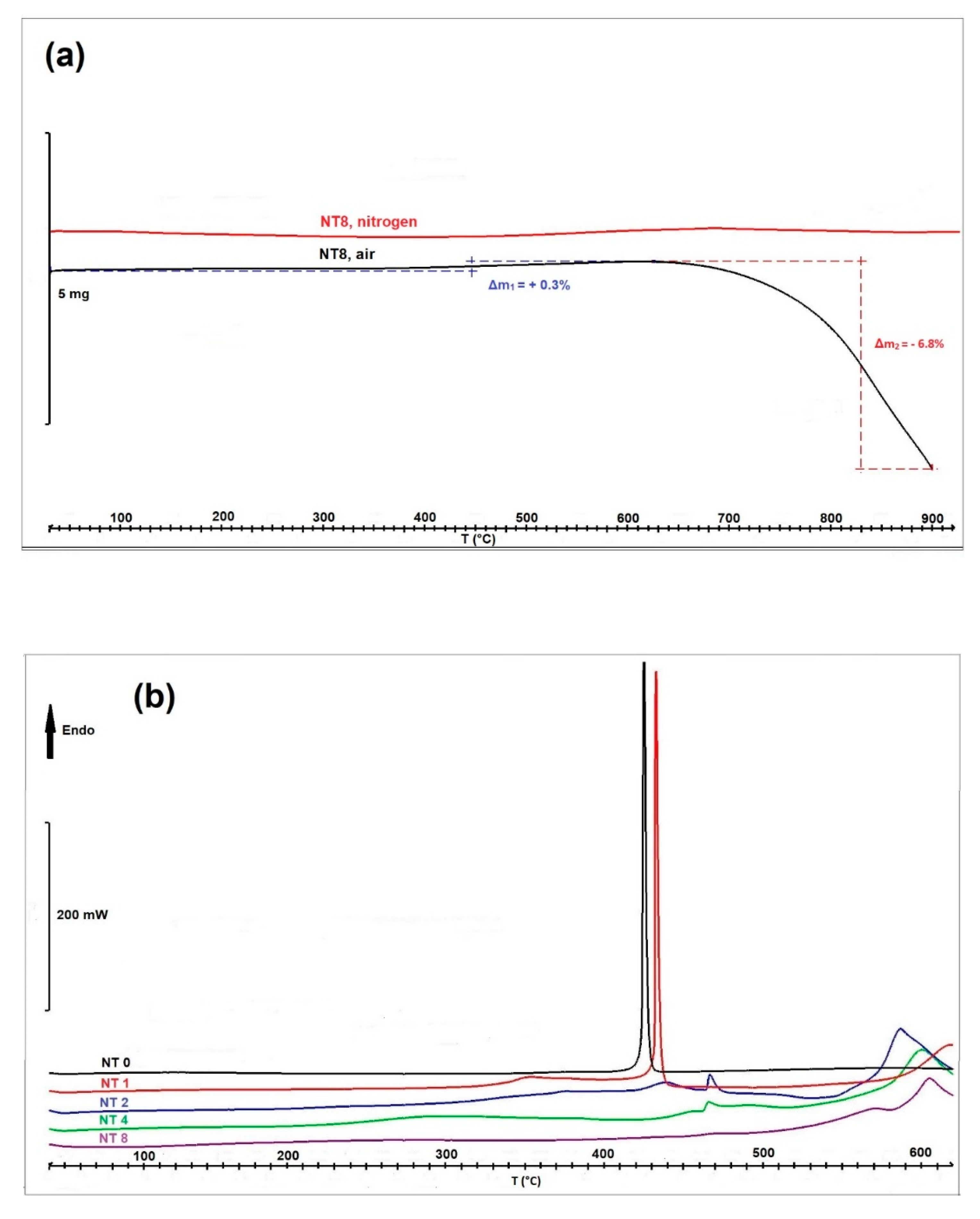
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, B.; Xie, Y.; Huang, J.; Su, H.; Qian, Y. Solvothermal synthesis to NiE2 (E = Se, Te) nanorods at low temperature. Nanostruct. Mater. 1999, 11, 1067–1071. [Google Scholar] [CrossRef]
- Kristl, M.; Dojer, B.; Gyergyek, S.; Kristl, J. Synthesis of nickel and cobalt sulfide nanoparticles using a low cost sonochemical method. Heliyon 2017, 3, e00273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristl, M.; Gyergyek, S.; Kristl, J. Nanostructured nickel sulfides with different stoichiometries prepared by mechanochemical synthesis. Chalcogenide Lett. 2018, 15, 55–61. [Google Scholar]
- Zhang, H.T.; Xiong, Y.M.; Luo, X.G.; Wang, C.H.; Li, S.Y.; Chen, X.C. Hydrothermal synthesis and characterization of NiTe alloy nanocrystallites. J. Cryst. Growth 2002, 242, 259–262. [Google Scholar] [CrossRef]
- Arvhult, C.M.; Guéneau, C.; Gossé, S.; Selleby, M. Thermodynamic assessment of the Ni-Te system. J. Mater. Sci. 2019, 54, 11304–11319. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.X.; Zhou, J.P.; Wang, J.Z.; Miao, N.X.; Guo, Z.Q.; Hassan, Q.U. Novel magnetic properties of uniform NiTex nanorods selectively synthesized by hydrothermal method. Mater. Des. 2017, 117, 390–395. [Google Scholar] [CrossRef]
- Pradhan, S.; Das, R.; Biswas, S.; Das, D.K.; Bhar, R.; Bandyopadhyay, R.; Pramanik, P. Chemical synthesis of nanoparticles of nickel telluride and cobalt telluride and its electrochemical applications for determination of uric acid and adenine. Electrochim. Acta 2017, 238, 185–193. [Google Scholar] [CrossRef]
- Amin, B.G.; de Silva, U.; Masud, J.; Nath, M. Ultrasensitive and Highly Selective Ni3Te2 as a Nonenzymatic Glucose Sensor at Extremely Low Working Potential. ACS Omega 2019, 4, 11152–11162. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.J.; Jiao, S.Q.; Tu, J.G.; Luo, Y.W.; Song, W.L.; Jiao, H.D.; Wang, M.Y.; Chen, H.S.; Fang, D.N. Rechargeable Nickel Telluride/Aluminum Batteries with High Capacity and Enhanced Cycling Performance. ACS Nano 2020, 14, 3469–3476. [Google Scholar] [CrossRef]
- Zhou, P.; Fan, L.; Wu, J.; Gong, C.; Zhang, J.; Tu, Y. Facile hydrothermal synthesis of NiTe and its application as positive electrode material for asymmetric supercapacitor. J. Alloys Compd. 2016, 685, 384–390. [Google Scholar] [CrossRef]
- Manikandan, M.; Subramani, K.; Sathish, M.; Dhanuskodi, S. NiTe Nanorods as Electrode Material for High Performance Supercapacitor Applications. ChemistrySelect 2018, 3, 9034–9040. [Google Scholar] [CrossRef]
- Ye, B.; Huang, M.; Fan, L.; Lin, J.; Wu, J. Co ions doped NiTe electrode material for asymmetric supercapacitor application. J. Alloys Compd. 2019, 776, 993–1001. [Google Scholar] [CrossRef]
- Anand, T.J.S.; Zaidan, M. Electro synthesized NiTe2 Thin Films with the Influence of Additives. Adv. Mater. Res. 2014, 925, 159–163. [Google Scholar] [CrossRef]
- Chia, X.; Sofer, Z.; Luxa, J.; Pumera, M. Unconventionally Layered CoTe2 and NiTe2 as Elactrocatalysts for Hydrogen Evolution. Chem. Eur. J. 2017, 23, 11719–11726. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.S.; Barshilia, H.C.; Nagaraja, H.S. Porous nickel telluride nanostructures as bifunctional electrocatalysts towards hydrogen and oxygen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 24645–24655. [Google Scholar] [CrossRef]
- Oh, J.; Park, H.J.; Bala, A.; Kim, H.S.; Liu, N.; Choo, S.; Lee, M.H.; Kim, S.J.; Kim, S. Nickel telluride vertically aligned thin film by radio-frequency magnetron sputtering for hydrogen evolution reaction. APL Mater. 2020, 8, 121104. [Google Scholar] [CrossRef]
- de Silva, U.; See, J.; Liyanage, W.P.R.; Masud, J.; Wu, J.P.; Yang, W.L.; Chen, W.T.; Prendergast, D.; Nath, M. Understanding the Structural Evolution of a Nickel Chalcogenide Electrocatalyst Surface for Water Oxidation. Energy Fuels 2021, 35, 4387–4403. [Google Scholar] [CrossRef]
- Anantharaj, S.; Karthick, K.; Kundu, S. NiTe2 nanowire Outperforms Pt/C in High-Rate Hydrogen Evolution at Extreme pH Conditions. Inorg. Chem. 2018, 57, 3082–3096. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.C.; Zhang, L.X. Nickel Ditelluride nanosheet Arrays: A Highly Efficient Electrocatalyst for the Oxygen Evolution Reaction. ChemElectroChem 2018, 5, 1153–1158. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, P.; Liu, M.; Guo, C.; Liu, H.; Wei, S.; Zhang, J.; Lu, X. Rational Design of Metallic NiTex (x = 1 or 2) as Bifunctional Electrocatalysts for Efficient Urea Conversion. ACS Appl. Energy Mater. 2019, 2, 3363–3372. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L. In situ growth of NiTe nanosheet film on nickel foam as electrocatalysts for oxygen evolution reaction. Elecrochem. Commun. 2018, 88, 29–33. [Google Scholar] [CrossRef]
- Gulay, L.D.; Olekseyuk, I.D. Crystal structures of the compounds Ni3Te2, Ni3-δTe2 (δ = 0.12) and Ni1.29Te. J. Alloys Compd. 2004, 376, 131–138. [Google Scholar] [CrossRef]
- Umeyama, N.; Tokumoto, M.; Yagi, S.; Tomura, M.; Tokiwa, K.; Fujii, T.; Toda, R.; Miyakawa, N.; Ikeda, S.I. Synthesis and Magnetic Properties of NiSe, NiTe, CoSe, and CoTe. Jpn. J. Appl. Phys. 2012, 51, 053001. [Google Scholar] [CrossRef]
- Parkin, I.P. Solid state metathesis reaction for metal borides, silicides, pnictides and chalcogenides: Ionic or elemental pathways. Chem. Soc. Rev. 1996, 25, 199–207. [Google Scholar] [CrossRef]
- Brennan, J.G.; Siegrist, T.; Stuczynski, S.M.; Steigerwald, M.L. The transition from molecules to solids-molecular syntheses of Ni9Te6(PEt3)8, Ni20Te18(PEt3)12, and NiTe. J. Am. Chem. Soc. 1989, 111, 9240–9241. [Google Scholar] [CrossRef]
- Mu, Y.N.; Li, Q.; Lv, P.; Chen, Y.L.; Ding, D.; Su, S.; Zhou, L.Y.; Fu, W.Y.; Yang, H.B. Fabrication of NiTe films by transformed electrodeposited Te thin films on Ni foils and their electrical properties. RSC Adv. 2014, 4, 54713–54718. [Google Scholar] [CrossRef]
- Zhao, B.; Dang, W.Q.; Liu, Y.; Li, B.; Li, J.; Luo, J.; Zhang, Z.W.; Wu, R.X.; Ma, H.F.; Sun, G.Z.; et al. Synthetic Control of Two-Dimensional NiTe2 Single Crystals with Highly Uniform Thickness Distributions. J. Am. Chem. Soc. 2018, 140, 14217–14223. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Dong, Y.; Li, Y. Synthesis of Uniform CoTe and NiTe Semiconductor Nanocluster Wires through a Novel Coreduction Method. Inorg. Chem. 2003, 42, 2174–2175. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.J.; Liu, J.H.; Huang, X.J. Novel magnetic nickel telluride nanowires decorated with thorns: Synthesis and their intrinsic peroxidase-like activity for detection of glucose. Chem. Commun. 2014, 50, 13589–13591. [Google Scholar] [CrossRef]
- Liu, X.Y.; Hu, R.Z.; Chai, L.L.; Li, H.B.; Gu, J.; Qian, Y.T. Synthesis and Characterization of Hexagonal NiTe2 Nanoplates. J. Nanosci. Nanotechnol. 2009, 9, 2715–2718. [Google Scholar] [CrossRef]
- Jiang, L.; Zhu, Y.J.; Cui, J.B. Cetyltrimethylammonium bromide assisted self-assembly of NiTe2 nanoflakes: Nanoflake arrays and their photoluminescence properties. J. Solid State Chem. 2010, 183, 2358–2364. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Zhang, D.L. Processing of advanced materials using high-energy mechanical milling. Prog. Mater. Sci. 2004, 49, 537–560. [Google Scholar] [CrossRef]
- Matyja, E.; Prusik, K.; Zubko, M.; Dercz, G.; Glowka, K. Structure of the Ni-Co-Mn-In alloy obtained by mechanical alloying and sintering. J. Alloys Compd. 2019, 801, 529–535. [Google Scholar] [CrossRef]
- Gomez-Lopez, P.; Espro, C.; Rodriguez-Padron, D.; Balu, A.M.; Ivars-Barcelo, F.; Moreda, O.I.; Alvarado-Beltran, C.G.; Luque, R. Mechanochemical Preparation of Magnetically Separable Fe and Cu-Based Bimetallic Nanocatalysts for Vanilin Production. Nanomaterials 2021, 11, 1050. [Google Scholar] [CrossRef]
- de Brito Neto, F.M.; Takeno, M.L.; da Costa Pinto, C.; Triches, D.M.; Manzato, L.; de Souza, S.M. Structural and thermal studies of SmNbO4 polymorphs produced by mechanical alloying. Mater. Lett. 2019, 252, 313–316. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.J.; Sayagues, M.J.; Gotor, F.J. A Novel, Simple and Highly Efficient Route to Obtain PrBaMn2O5+δ Double Perovskite: Mechanochemical Synthesis. Nanomaterials 2021, 11, 380. [Google Scholar] [CrossRef]
- Hredzak, S.; Brinacin, J.; Sepelak, V. Mechanochemically Synthesized Coal-Based magnetic Carbon Composites for Removing As(V) and Cd(II) from Aqueous Solutions. Nanomaterials 2019, 9, 100. [Google Scholar]
- Olszewski, R.; Nadolska, M.; Lapinski, M.; Przesniak-Welenc, M.; Cieslik, B.M.; Zelechowska, K. Solvent-Free Synthesis of Phosphonic Graphene Derivative and Its Application in Mercury Ions Adsorption. Nanomaterials 2019, 9, 485. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Wang, H.W.; Liu, C.; Li, D.J.; Kim, H.K.; Harris, C.; Lao, C.Y.; Abdelkader, A.; Xi, K. Facile mechanochemical synthesis of non-stoichiometric silica-carbon composite for enhanced lithium storage properties. J. Alloy. Compd. 2019, 801, 658–665. [Google Scholar] [CrossRef]
- Kristl, M.; Ban, I.; Gyergyek, S. Preparation of Nanosized Copper and Cadmium Chalcogenides by Mechanochemical Synthesis. Mater. Manuf. Process. 2013, 28, 1009–1013. [Google Scholar] [CrossRef]
- Kristl, M.; Gyergyek, S.; Srt, N.; Ban, I. Mechanochemical Route for the Preparation of Nanosized Aluminium and Gallium Sulfide and Selenide. Mater. Manuf. Process. 2016, 31, 1608–1612. [Google Scholar] [CrossRef]
- Weller, D.P.; Morelli, D.T. Rapid synthesis of zinc and nickel co-doped tetrahedrite thermoelectrics by reactive spark plasma sintering and mechanical alloying. J. Alloys Compd. 2017, 710, 794–799. [Google Scholar] [CrossRef]
- Falkenbach, O.; Loeh, M.O.; Wiegand, C.W.; Schmitz, A.; Hartung, D.; Koch, G.; Klar, P.J.; Mueller, E.; Schlecht, S. Structural and Thermoelectric Properties of Nanostructured Nominally Stoichiometric Pb1-xBixTe Prepared by Mechanical Alloying. J. Electron. Mater. 2017, 46, 5781–5791. [Google Scholar] [CrossRef]
- Hu, P.F.; Xie, C.; Mao, Z.H.; Liang, X. A Mechanochemical Route for ZnS Nanocrystals, and Batch Sorting along Size Distribution. Nanomaterials 2019, 9, 1325. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.F.; Sun, Q.X.; Zheng, M.X.; Duan, Z.H.; Wang, Y.H. Solar Sell Applications of Solution-Processed AgInGaSe2 Thin Films and Improved Properties by Sodium Doping. Nanomaterials 2020, 10, 547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, C.E.M. Solid state synthesis and characterization of NiTe nanocrystals. J. Nano Res. 2014, 29, 35–39. [Google Scholar] [CrossRef]
- Unni, G.E.; Sasi, S.; Nair, A.S. Higher open-circuit voltage set by cobalt redox shuttle in SnO2 nanofibers-sensitized CdTe quantum dots solar cells. J. Energy Chem. 2016, 25, 481–488. [Google Scholar] [CrossRef]
- Larichev, Y.V. Application of DLS for metal nanoparticle size determination in supported catalysts. Chem. Pap. 2021, 75, 2059–2066. [Google Scholar] [CrossRef]
- Ban, I.; Markuš, S.; Gyergyek, S.; Drofenik, M.; Korenak, J.; Helix-Nielsen, C.; Petrinić, I. Synthesis of Poly-Sodium_Acrylate (PSA)-Coated Magnetic nanoparticles for Use in Forward Osmosis Draw Solutions. Nanomaterials 2019, 9, 1238. [Google Scholar] [CrossRef] [Green Version]
- Stergar, J.; Maver, U.; Bele, M.; Gradišnik, L.; Kristl, M.; Ban, I. NiCu-silica nanoparticles as a potential drug delivery system. J. Sol. Gel Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Shinde, P.S.; Go, G.H.; Lee, W.J. Facile growth of hierarchical hematite (α-Fe2O3) nanopetals on FTO by pulse reverse electrodeposition for photoelectrochemical water splitting. J. Mater. Chem. 2012, 22, 10469–10471. [Google Scholar] [CrossRef]
- Sadaquat, M.; Manzoor, S.; Nisar, L.; Hassan, A.; Tyagi, D.; Shah, J.H.; Ashiq, M.N.; Joya, K.S.; Alshahrani, T.; Najam-ul-Haq, M. Iron doped nickel ditelluride hierarchical nanoflakes array directly grown on nickel foam as robust electrodes for oxygen evolution reaction. Electrochim. Acta 2021, 371, 137830. [Google Scholar] [CrossRef]
- Woods-Robinson, R.; Han, Y.; Zhang, H.; Ablekim, H.; Khan, I.; Persson, K.A.; Zakutayev, A. Wide Band Gap Chalcogenide Semiconductors. Chem. Rev. 2020, 120, 4007–4055. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kristl, M.; Gyergyek, S.; Škapin, S.D.; Kristl, J. Solvent-Free Mechanochemical Synthesis and Characterization of Nickel Tellurides with Various Stoichiometries: NiTe, NiTe2 and Ni2Te3. Nanomaterials 2021, 11, 1959. https://doi.org/10.3390/nano11081959
Kristl M, Gyergyek S, Škapin SD, Kristl J. Solvent-Free Mechanochemical Synthesis and Characterization of Nickel Tellurides with Various Stoichiometries: NiTe, NiTe2 and Ni2Te3. Nanomaterials. 2021; 11(8):1959. https://doi.org/10.3390/nano11081959
Chicago/Turabian StyleKristl, Matjaž, Sašo Gyergyek, Srečo D. Škapin, and Janja Kristl. 2021. "Solvent-Free Mechanochemical Synthesis and Characterization of Nickel Tellurides with Various Stoichiometries: NiTe, NiTe2 and Ni2Te3" Nanomaterials 11, no. 8: 1959. https://doi.org/10.3390/nano11081959
APA StyleKristl, M., Gyergyek, S., Škapin, S. D., & Kristl, J. (2021). Solvent-Free Mechanochemical Synthesis and Characterization of Nickel Tellurides with Various Stoichiometries: NiTe, NiTe2 and Ni2Te3. Nanomaterials, 11(8), 1959. https://doi.org/10.3390/nano11081959





