Liposomes Loaded with Unsaponifiable Matter from Amaranthus hypochondriacus as a Source of Squalene and Carrying Soybean Lunasin Inhibited Melanoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
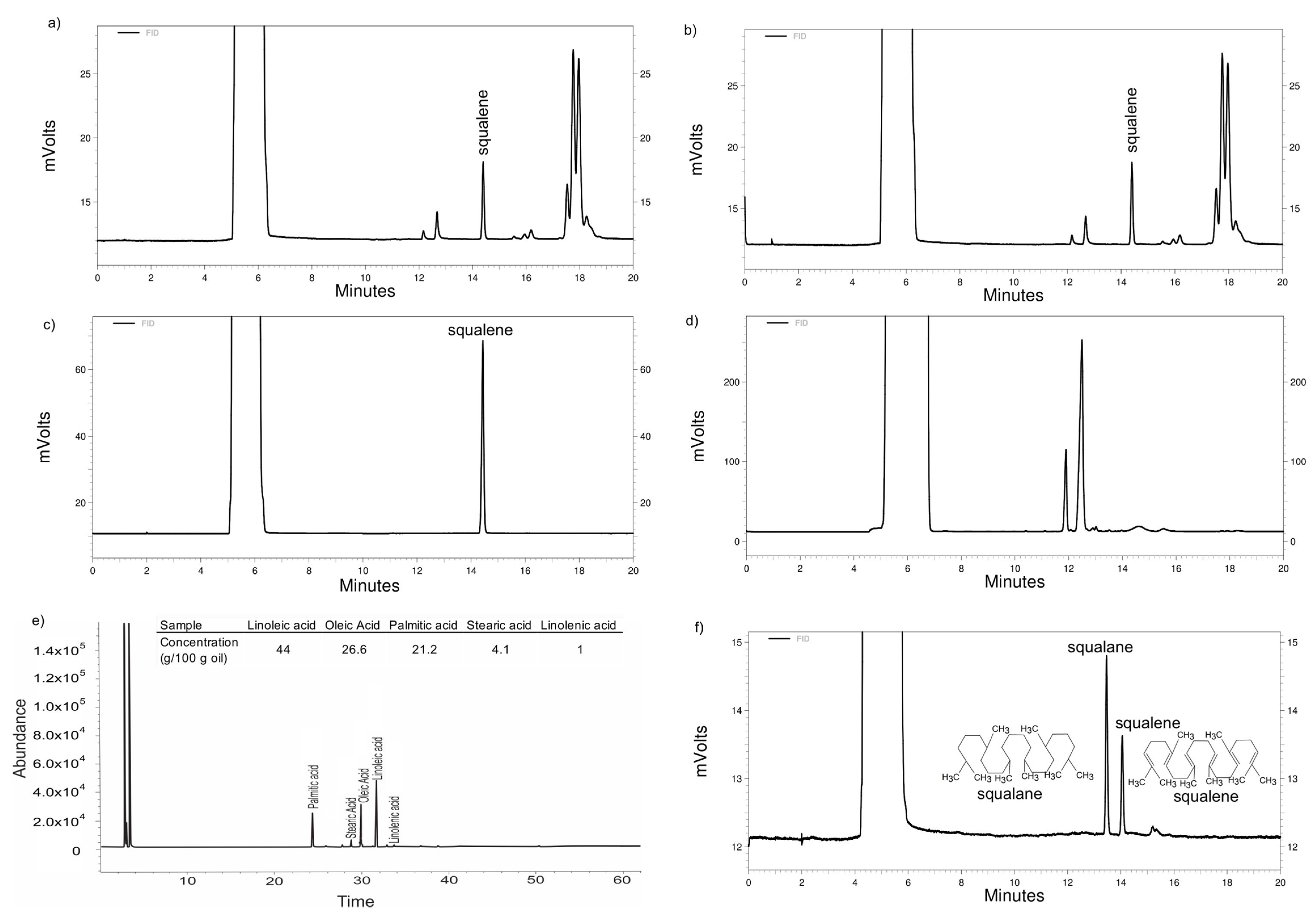
2.2.1. Amaranth oil Supercritical Fluid Extraction (SFE) and Squalene Determination in Oil Using Supercritical Fluid Chromatography (SFC)
2.2.2. Quantification of Fatty Acid Contained in the Amaranth Oil by Gas Chromatography (GC)
2.2.3. Extraction and Characterization of Amaranth Unsaponifiable Matter by SFC
2.2.4. Soybean Lunasin Purification by Anion Exchange Chromatography
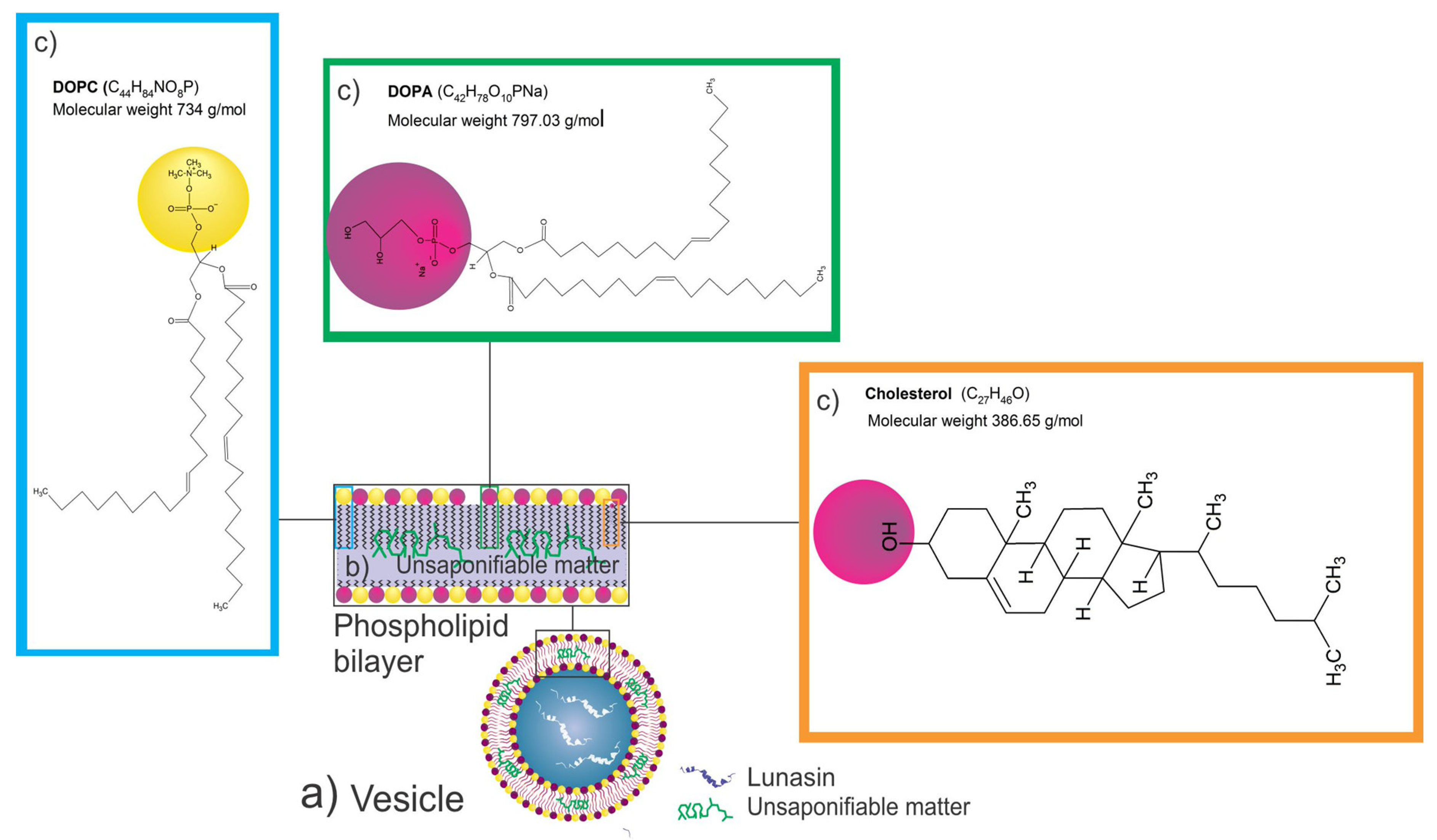
2.2.5. Preparation of Liposomes Loaded with the Unsaponifiable Matter for Optimization and with Lunasin after Optimization
2.2.6. Liposome Characterization
Particle Size, Polydispersity Index (PDI), and Zeta Potential
Encapsulation Efficiency (EE)
Transmission Electron Microscopy (TEM)
2.2.7. Determination of Cell Cytotoxicity after Treatments with Empty Liposomes, Loaded with the Unsaponifiable Matter and Loaded with Lunasin
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. Amaranth Oil Extracted by Supercritical Fluid Extraction and Squalene Determination using Supercritical Fluid Chromatography
3.2. Fatty Acid Transesterification and Extraction of Unsaponifiable Matter from Amaranth Oil
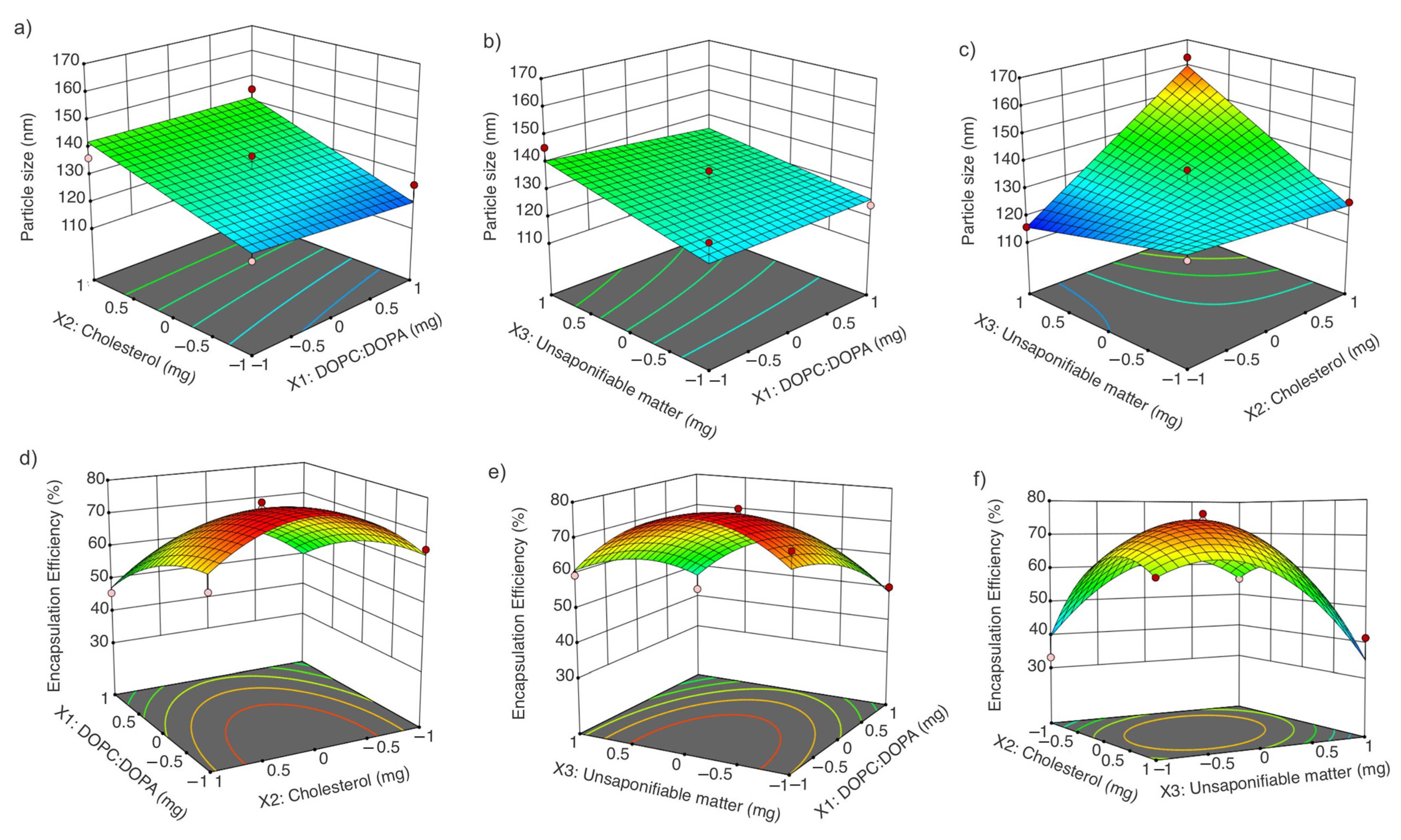
3.3. Liposome Optimization with Amaranth Unsaponifiable Matter Using Response Surface Methodology (Box–Behnken Design)
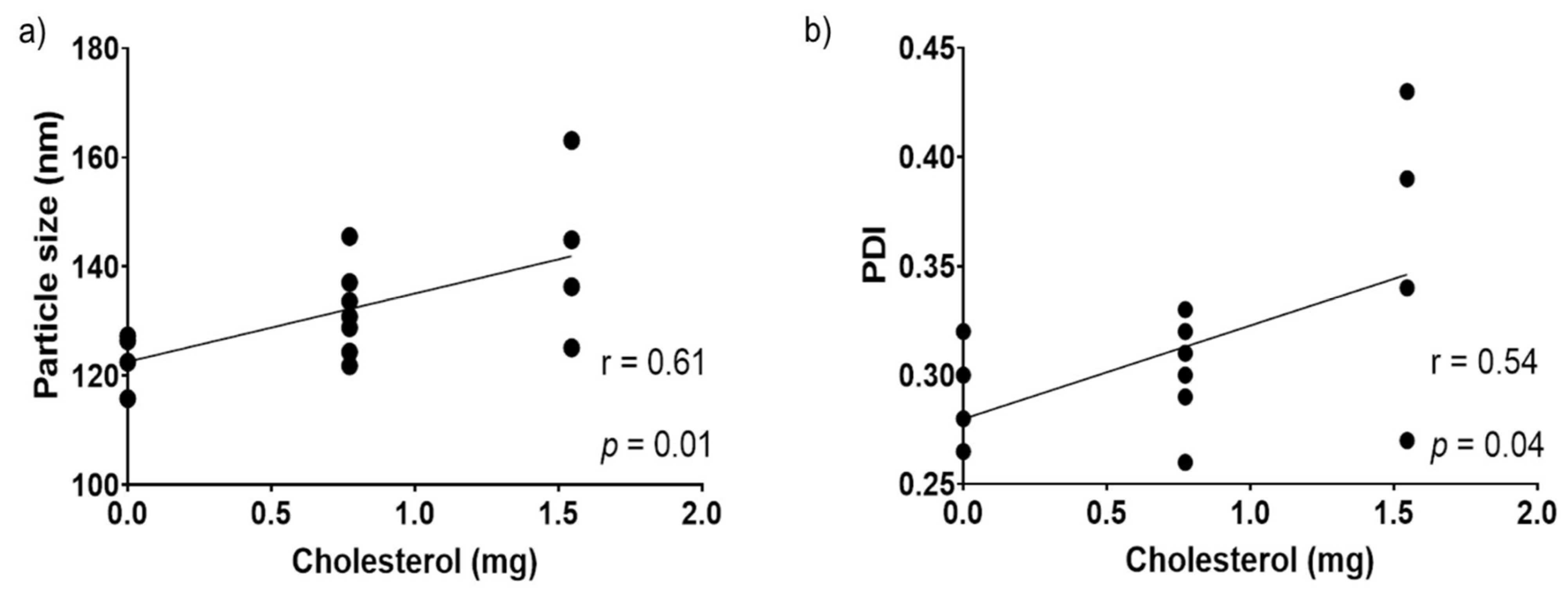
3.3.1. Effect of DOPC:DOPA Ratio, Cholesterol, and Amaranth Unsaponifiable Matter on Particle Size
3.3.2. Effect of DOPC:DOPA Ratio, Cholesterol, and Amaranth Unsaponifiable Matter on Encapsulation Efficiency
3.3.3. Optimization of Liposomes Loaded with the Unsaponifiable Matter
3.4. Transmission Electron Microscopy (TEM) Images of Empty Liposomes, Optimized Liposomes, and Lunasin-Loaded Liposomes
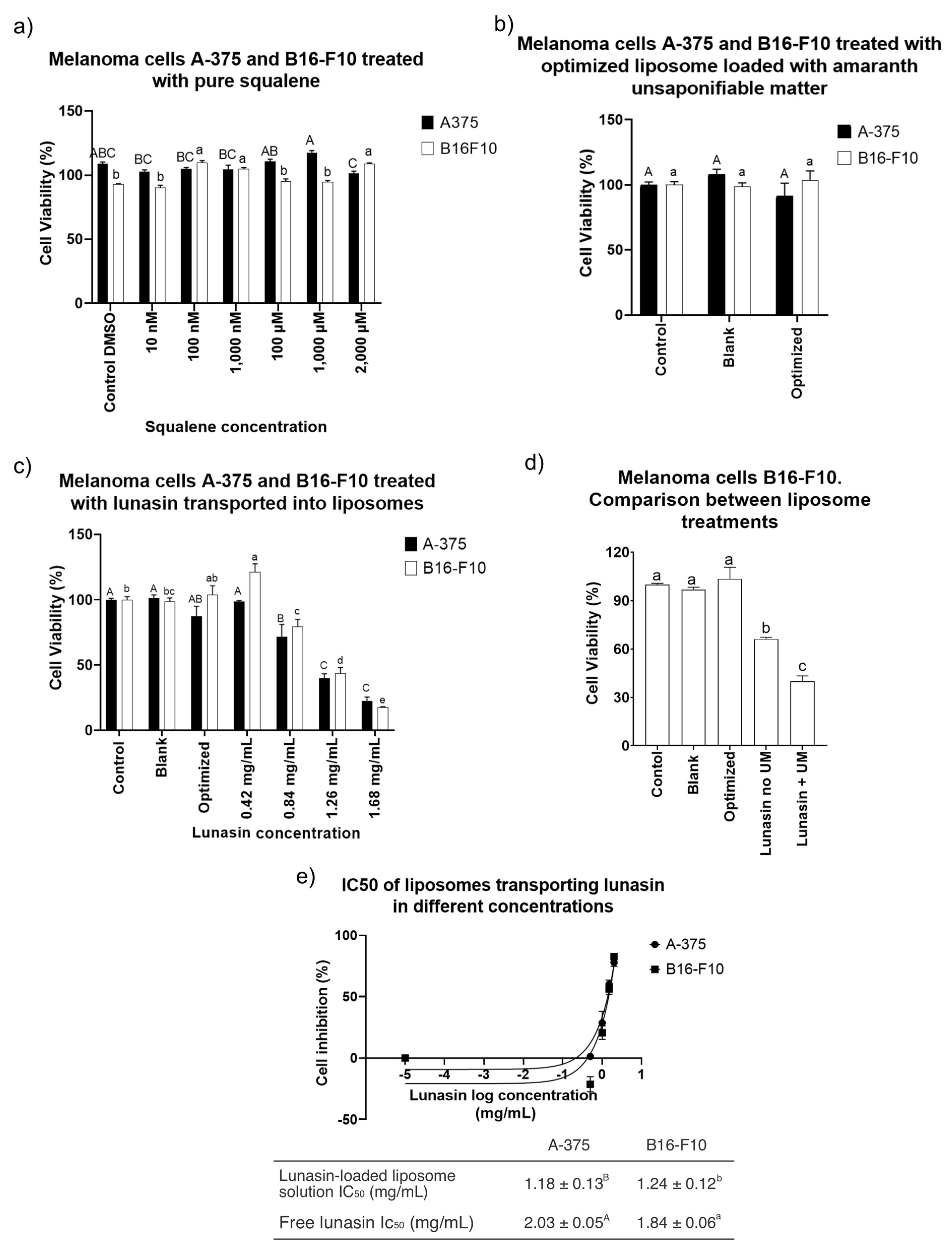
3.5. Melanoma B16-F10 and A-375 Cell Viability Treated with Empty Liposomes, Liposomes Loaded with Unsaponifiable Matter (Optimized), and Lunasin-Loaded Liposomes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coelho, L.M.; Silva, P.M.; Martins, J.T.; Pinheiro, A.C.; Vicente, A.A. Emerging opportunities in exploring the nutritional/functional value of amaranth. Food Funct. 2018, 9, 5499–5512. [Google Scholar] [CrossRef]
- Krulj, J.; Brlek, T.; Pezo, L.; Brkljača, J.; Popović, S.; Zeković, Z.; Bodroža Solarov, M. Extraction methods of Amaranthus sp. grain oil isolation. J. Sci. Food Agric. 2016, 96, 3552–3558. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Sicairos, E.S.; Domínguez-Rodríguez, M.; Montoya-Rodríguez, A.; Milán-Noris, A.K.; Reyes-Moreno, C.; Milán-Carrillo, J. Phytochemical compounds and antioxidant activity modified by germination and hydrolysis in mexican Amaranth. Plant Foods Hum. Nutr. 2020, 75, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Rosales García, T.; Jimenez Martínez, C.; Cardador Martínez, A.; Martín Del Campo, S.T.; Galicia Luna, L.A.; Téllez Medina, D.I.; Dávila Ortiz, G. Squalene extraction by supercritical fluids from traditionally puffed Amaranthus hypochondriacus seeds. J. Food Qual. 2017, 2017, 6879712. [Google Scholar] [CrossRef]
- Rosales García, T.; Jiménez Martínez, C.; Dávila Ortiz, G. Squalene extraction: Biological sources and extraction methods. Int. J. Environ. Agric. Biotechnol. 2017, 2, 1662–1670. [Google Scholar] [CrossRef]
- Lozano-Grande, M.A.; Dávila-Ortiz, G.; García-Dávila, J.; Ríos-Cortés, G.; Espitia-Rangel, E.; Martínez-Ayala, A.L. Optimisation of microwave-assisted extraction of squalene from Amaranthus spp. seeds. J. Microw. Power Electromagn. Energy 2019, 53, 243–258. [Google Scholar] [CrossRef]
- Shimizu, N.; Ito, J.; Kato, S.; Otoki, Y.; Goto, M.; Eitsuka, T.; Miyazawa, T.; Nakagawa, K. Oxidation of squalene by singlet oxygen and free radicals results in different compositions of squalene monohydroperoxide isomers. Sci. Rep. 2018, 8, 9116. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzińska, M.; Raczyk, M.; Mišina, I.; Segliņa, D. Impact of cultivar on profile and concentration of lipophilic bioactive compounds in kernel oils recovered from sweet cherry (Prunus avium L.) by-products. Plant Foods Hum. Nutr. 2019, 74, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Lozano Grande, M.A.; Gorinstein, S.; Espitia Rangel, E.; Dávila Ortiz, G.; Martínez Ayala, A.L. Plant sources, extraction methods, and uses of squalene. Int. J. Agron. 2018, 2018. [Google Scholar] [CrossRef]
- Naziri, E.; Glisic, S.B.; Mantzouridou, F.T.; Tsimidou, M.Z.; Nedovic, V.; Bugarski, B. Advantages of supercritical fluid extraction for recovery of squalene from wine lees. J. Supercrit. Fluids 2016, 107, 560–565. [Google Scholar] [CrossRef]
- Liu, G.; Fu, J. Squalene synthase cloning and functional identification in wintersweet plant (Chimonanthus zhejiangensis). Bot. Stud. 2018, 59. [Google Scholar] [CrossRef]
- Dąbrowski, G.; Czaplicki, S.; Konopka, I. Fractionation of sterols, tocols and squalene in flaxseed oils under the impact of variable conditions of supercritical CO2 extraction. J. Food Compos. Anal. 2019, 83, 103261. [Google Scholar] [CrossRef]
- Kraujalis, P.; Venskutonis, P.R. Optimisation of supercritical carbon dioxide extraction of amaranth seeds by response surface methodology and characterization of extracts isolated from different plant cultivars. J. Supercrit. Fluids 2013, 73, 80–86. [Google Scholar] [CrossRef]
- Tamkutė, L.; Liepuoniūtė, R.; Pukalskienė, M.; Venskutonis, P.R. Recovery of valuable lipophilic and polyphenolic fractions from cranberry pomace by consecutive supercritical CO2 and pressurized liquid extraction. J. Supercrit. Fluids 2020, 159, 104755. [Google Scholar] [CrossRef]
- Özkal, S.G.; Yener, M.E. Supercritical carbon dioxide extraction of flaxseed oil: Effect of extraction parameters and mass transfer modeling. J. Supercrit. Fluids 2016, 112, 76–80. [Google Scholar] [CrossRef]
- Dąbrowski, G.; Konopka, I.; Czaplicki, S. Supercritical CO2 extraction in chia oils production: Impact of process duration and co-solvent addition. Food Sci. Biotechnol. 2018, 27, 677–686. [Google Scholar] [CrossRef]
- Danlami, J.M.; Arsad, A.; Zaini, M.A.A.; Sulaiman, H. A comparative study of various oil extraction techniques from plants. Rev. Chem. Eng. 2014, 30, 605–626. [Google Scholar] [CrossRef]
- Bhilwade, H.N.; Tatewaki, N.; Konishi, T.; Nishida, M.; Eitsuka, T.; Yasui, H.; Inanami, O.; Handa, O.; Naito, Y.; Ikekawa, N.; et al. The adjuvant effect of squalene, an active ingredient of functional foods, on doxorubicin-treated allograft mice. Nutr. Cancer 2019, 71, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Eid, J.; El Achkar, T.; Fourmentin, S.; Greige-Gerges, H.; Jraij, A. First investigation of liposomes behavior and phospholipids organization in choline chloride-based deep eutectic solvents by atomic force microscopy. J. Mol. Liq. 2020, 306, 112851. [Google Scholar] [CrossRef]
- Wu, P.T.; Lin, C.W.C.L.; Lin, C.W.C.L.; Chang, N.C.; Tsai, W.B.; Yu, J. Methylene-blue-encapsulated liposomes as photodynamic therapy nano agents for breast cancer cells. Nanomaterials 2019, 9, 14. [Google Scholar] [CrossRef]
- Castañeda-Reyes, E.D.; Perea-Flores, M.d.J.; Davila-Ortiz, G.; Lee, Y.; Gonzalez de Mejia, E. Development, characterization and use of liposomes as amphipathic transporters of bioactive compounds for melanoma treatment and reduction of skin inflammation: A review. Int. J. Nanomed. 2020, 15, 7627–7650. [Google Scholar] [CrossRef]
- Tonggu, L.; Wang, L. Cryo-EM sample preparation method for extremely low concentration liposomes. Ultramicroscopy 2020, 208, 112849. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; Schiborr, C.; Kocher, A.; Meins, J.; Behnam, D.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Transepithelial transport of curcumin in Caco-2 cells is significantly enhanced by micellar solubilisation. Plant Foods Hum. Nutr. 2017, 72, 48–53. [Google Scholar] [CrossRef]
- Toopkanloo, S.P.; Tan, T.B.; Abas, F.; Alharthi, F.A.; Nehdi, I.A.; Tan, C.P. Impact of quercetin encapsulation with added phytosterols on bilayer membrane and photothermal-alteration of novel mixed soy lecithin-based liposome. Nanomaterials 2020, 10, 2432. [Google Scholar] [CrossRef] [PubMed]
- Houshmand, M.; Garello, F.; Circosta, P.; Stefania, R.; Aime, S.; Saglio, G.; Giachino, C. Nanocarriers as magic bullets in the treatment of leukemia. Nanomaterials 2020, 10, 276. [Google Scholar] [CrossRef]
- Roesch, A.; Berking, C. Melanoma. In Braun-Falco’s Dermatology; Plewig, G., French, L., Ruzicka, T., Kaufmann, R., Hertl, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–17. ISBN 978-3-662-58713-3. [Google Scholar]
- Davoodvandi, A.; Darvish, M.; Borran, S.; Nejati, M.; Mazaheri, S.; Reza Tamtaji, O.; Hamblin, M.R.; Masoudian, N.; Mirzaei, H. The therapeutic potential of resveratrol in a mouse model of melanoma lung metastasis. Int. Immunopharmacol. 2020, 88, 106905. [Google Scholar] [CrossRef]
- Gong, C.; Xia, H. Resveratrol suppresses melanoma growth by promoting autophagy through inhibiting the PI3K/AKT/mTOR signaling pathway. Exp. Ther. Med. 2019, 19, 1878–1886. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yoo, E.S.; Woo, J.S.; Han, S.H.; Lee, J.H.; Jung, S.H.; Kim, H.J.; Jung, J.Y. Antitumor and apoptotic effects of quercetin on human melanoma cells involving JNK/P38 MAPK signaling activation. Eur. J. Pharmacol. 2019, 860, 172568. [Google Scholar] [CrossRef] [PubMed]
- Shidal, C.; Al-Rayyan, N.; Yaddanapudi, K.; Davis, K.R. Lunasin is a novel therapeutic agent for targeting melanoma cancer stem cells. Oncotarget 2016, 7, 84128–84141. [Google Scholar] [CrossRef]
- Gonzalez De Mejia, E.; Castañeda-Reyes, E.D.; Mojica, L.; Dia, V.; Wang, H.; Wang, T.; Johnson, L.A. Potential health benefits associated with lunasin concentration in dietary supplements and lunasin-enriched soy extract. Nutrients 2021, 13, 1618. [Google Scholar] [CrossRef]
- Shidal, C.; Inaba, J.I.; Yaddanapudi, K.; Davis, K.R. The soy-derived peptide lunasin inhibits invasive potential of melanoma initiating cells. Oncotarget 2017, 8, 25525–25541. [Google Scholar] [CrossRef]
- Devapatla, B.; Shidal, C.; Yaddanapudi, K.; Davis, K.R. Validation of syngeneic mouse models of melanoma and non-small cell lung cancer for investigating the anticancer effects of the soy-derived peptide Lunasin. F1000Research 2017, 5, 2432. [Google Scholar] [CrossRef]
- King, J.W.; Johnson, J.H.; Eller, F.J. Effect of supercritical carbon dioxide pressurized with helium on solute solubility during supercritical fluid extraction. Anal. Chem. 1995, 67, 2288–2291. [Google Scholar] [CrossRef]
- Eller, F.J.; Taylor, S.L.; Palmquist, D.E. Supercritical fluid chromatographic analysis for on-line monitoring of hexane removal from soybean oil miscella using liquid carbon dioxide. J. Chromatogr. A 2005, 1094, 183–186. [Google Scholar] [CrossRef]
- Eller, F.J.; King, J.W. Supercritical CO2 extraction of fat: Comparison of gravimetric and GC-FAME methods. J. Agric. Food Chern. 1998, 46, 3657–3661. [Google Scholar] [CrossRef]
- Eller, F.J.; Cermak, S.C.; Taylor, S.L. Supercritical carbon dioxide extraction of cuphea seed oil. Ind. Crops Prod. 2011, 33, 554–557. [Google Scholar] [CrossRef]
- American Oil Chemists Society AOCS Official method Ca 6a-40. In Official Methods and Recommended Practices of the AOCS; Urbana: Champaign, IL, USA, 2011.
- Mahira, S.; Kommineni, N.; Doppalapudi, S.; Khan, W. Edge activated ultradeformable liposomes of psoralen and its derivatives: Development and comparative evaluation for vitiligo therapy. J. Drug Deliv. Sci. Technol. 2019, 52, 83–95. [Google Scholar] [CrossRef]
- Pettinato, M.; Trucillo, P.; Campardelli, R.; Perego, P.; Reverchon, E. Bioactives extraction from spent coffee grounds and liposome encapsulation by a combination of green technologies. Chem. Eng. Process. Process Intensif. 2020, 151, 107911. [Google Scholar] [CrossRef]
- Obrzut, D.L.; Bell, P.W.; Roberts, C.B.; Duke, S.R. Effect of process conditions on the spray characteristics of a PLA + methylene chloride solution in the supercritical antisolvent precipitation process. J. Supercrit. Fluids 2007, 42, 299–309. [Google Scholar] [CrossRef]
- Kraujalis, P.; Venskutonis, P.R. Supercritical carbon dioxide extraction of squalene and tocopherols from amaranth and assessment of extracts antioxidant activity. J. Supercrit. Fluids 2013, 80, 78–85. [Google Scholar] [CrossRef]
- Shukla, A.; Srivastava, N.; Suneja, P.; Yadav, S.K.; Hussain, Z.; Rana, J.C.; Yadav, S. Untapped amaranth (Amaranthus spp.) genetic diversity with potential for nutritional enhancement. Genet. Resour. Crop Evol. 2018, 65, 243–253. [Google Scholar] [CrossRef]
- León-Camacho, M.; García-González, D.L.; Aparicio, R. A detailed and comprehensive study of amaranth (Amaranthus cruentus L.) oil fatty profile. Eur. Food Res. Technol. 2001, 213, 349–355. [Google Scholar] [CrossRef]
- De Vita, D.; Messore, A.; Toniolo, C.; Frezza, C.; Scipione, L.; Bertea, C.M.; Micera, M.; Di Sarno, V.; Madia, V.N.; Pindinello, I.; et al. Towards a new application of amaranth seed oil as an agent against Candida albicans. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef]
- Dhellot, J.R.; Matouba, E.; Maloumbi, M.G.; Nzikou, J.M.; Ngoma, D.G.S.; Linder, M.; Desobry, S.; Parmentier, M. Extraction, chemical composition and nutrional characterization of vegetable oils: Case of Amaranthus hybridus (var 1 and 2) of Congo Brazzaville. Afr. J. Biotechnol. 2006, 5, 1095–1101. [Google Scholar]
- He, H.P.; Corke, H. Oil and squalene in Amaranthus grain and leaf. J. Agric. Food Chem. 2003, 51, 7913–7920. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, A.A.; Balanč, B.D.; Ota, A.; Ahlin Grabnar, P.; Djordjević, V.B.; Šavikin, K.P.; Bugarski, B.M.; Nedović, V.A.; Poklar Ulrih, N. Comparative effects of cholesterol and β-sitosterol on the liposome membrane characteristics. Eur. J. Lipid Sci. Technol. 2018, 120, 1800039. [Google Scholar] [CrossRef]
- Drabik, D.; Przybyło, M.; Chodaczek, G.; Iglič, A.; Langner, M. The modified fluorescence based vesicle fluctuation spectroscopy technique for determination of lipid bilayer bending properties. Biochim. Biophys. Acta Biomembr. 2016, 1858, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Singh, S.; Sharma, D.; Webster, T.; Shafaat, K.; Faruk, A. Elastic liposomes as novel carriers: Recent advances in drug delivery. Int. J. Nanomed. 2017, 12, 5087–5108. [Google Scholar] [CrossRef]
- Cambrea, L.R.; Haque, F.; Schieler, J.L.; Rochet, J.C.; Hovis, J.S. Effect of ions on the organization of phosphatidylcholine/phosphatidic acid bilayers. Biophys. J. 2007, 93, 1630–1638. [Google Scholar] [CrossRef][Green Version]
- Kaddah, S.; Khreich, N.; Kaddah, F.; Charcosset, C.; Greige-Gerges, H. Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule. Food Chem. Toxicol. 2018, 113, 40–48. [Google Scholar] [CrossRef]
- Shaker, S.; Gardouh, A.; Ghorab, M. Factors affecting liposomes particle size prepared by ethanol injection method. Res. Pharm. Sci. 2017, 12, 346. [Google Scholar] [CrossRef]
- Hudiyanti, D.; Aminah, S.; Hikmahwati, Y.; Siahaan, P. Cholesterol implications on coconut liposomes encapsulation of beta-carotene and vitamin C. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012037. [Google Scholar] [CrossRef]
- Mare, R.; Da, H.; Fresta, M.; Cosco, D.; Awasthi, V. Anchoring property of a novel hydrophilic lipopolymer, HDAS-SHP, post-inserted in preformed liposomes. Nanomaterials 2019, 9, 1185. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.J. The effect of tocopherol on the structure and permeability of phosphatidylcholine liposomes. J. Control. Release 2012, 160, 158–163. [Google Scholar] [CrossRef]
- Hudiyanti, D.; Fawrin, H.; Siahaan, P. Simultant encapsulation of vitamin C and beta-carotene in sesame (Sesamum indicum l.) liposomes. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Yogyakarta, Indonesia, 20 September 2017; Institute of Physics Publishing: Bristol, UK, 2018; Volume 349, p. 012014. [Google Scholar]
- Briuglia, M.-L.L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef]
- Mohan, A.; Rajendran, S.R.C.K.; Thibodeau, J.; Bazinet, L.; Udenigwe, C.C. Liposome encapsulation of anionic and cationic whey peptides: Influence of peptide net charge on properties of the nanovesicles. LWT 2018, 87, 40–46. [Google Scholar] [CrossRef]
- Colletier, J.P.; Chaize, B.; Winterhalter, M.; Fournier, D. Protein encapsulation in liposomes: Efficiency depends on interactions between protein and phospholipid bilayer. BMC Biotechnol. 2002, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Zidan, A.S.; Mokhtar Ibrahim, M.; Megrab, N.A.E. Optimization of methotrexate loaded niosomes by Box–Behnken design: An understanding of solvent effect and formulation variability. Drug Dev. Ind. Pharm. 2017, 43, 1450–1459. [Google Scholar] [CrossRef]
- Lopes, N.A.; Barreto Pinilla, C.M.; Brandelli, A. Antimicrobial activity of lysozyme-nisin co-encapsulated in liposomes coated with polysaccharides. Food Hydrocoll. 2019, 93, 1–9. [Google Scholar] [CrossRef]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Importance of physicochemical characterization of nanoparticles in pharmaceutical product development. In Basic Fundamentals of Drug Delivery; Academic Press, Elsevier: San Diego, CA, USA, 2018; pp. 369–400. ISBN 9780128179093. [Google Scholar]
- Tuoriniemi, J.; Johnsson, A.C.J.H.; Holmberg, J.P.; Gustafsson, S.; Gallego-Urrea, J.A.; Olsson, E.; Pettersson, J.B.C.; Hassellöv, M. Intermethod comparison of the particle size distributions of colloidal silica nanoparticles. Sci. Technol. Adv. Mater. 2014, 15, 35009. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Tatewaki, N.; Konishi, T.; Nakajima, Y.; Nishida, M.; Saito, M.; Eitsuka, T.; Sakamaki, T.; Ikekawa, N.; Nishida, H. Squalene inhibits ATM-dependent signaling in γIR-induced DNA damage response through induction of Wip1 phosphatase. PLoS ONE 2016, 11, e0147570. [Google Scholar] [CrossRef] [PubMed]
- Warleta, F.; Campos, M.; Allouche, Y.; Sánchez Quesada, C.; Ruiz Mora, J.; Beltrán, G.; Gaforio, J.J. Squalene protects against oxidative DNA damage in MCF10A human mammary epithelial cells but not in MCF7 and MDA-MB-231 human breast cancer cells. Food Chem. Toxicol. 2010, 48, 1092–1100. [Google Scholar] [CrossRef]
- Kilian, G.; Davids, H.; Milne, P.J.; Kilian, G. Anticancer activity of the liposome-encapsulated cyclic dipeptides, cyclo(His-Gly) and cyclo(His-Ala). Pharmazie 2013, 68, 207–211. [Google Scholar] [CrossRef] [PubMed]

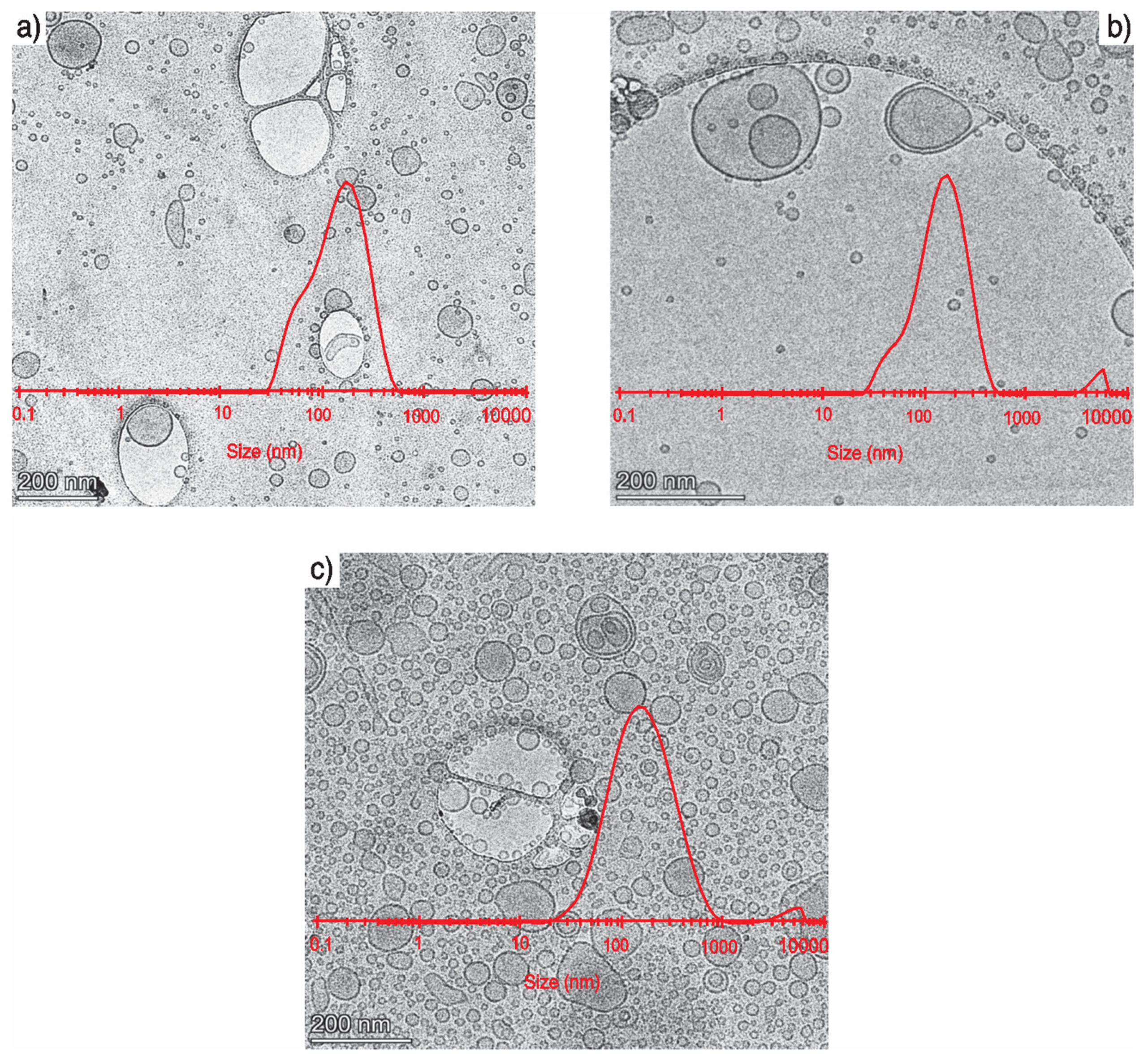
| # | Factors | Size (nm) | Squalene EE (%) | Zeta Potential (mV) | PDI | ||||
|---|---|---|---|---|---|---|---|---|---|
| DOPC:DOPA X1 (mg) | Cholesterol X2 (mg) | Amaranth Unsaponifiable Matter X3 (mg) | Observed | Predicted | Observed | Predicted | |||
| 1 | −1 (9.50) | 0 (0.77) | 1 (1.11) | 145.50 ± 2.17 | 140.61 | 59.45 ± 5.64 | 45.11 | −86.43 ± 1.50 | 0.33 ± 0.03 |
| 2 | −1 (9.50) | 1 (1.55) | 0 (0.89) | 136.30 ± 2.87 | 142.17 | 62.01 ± 10.93 | 58.1 | −76.62 ± 1.42 | 0.34 ± 0.03 |
| 3 | 0 (12.67) | −1 (0.00) | 1 (1.11) | 115.80 ± 8.77 | 115.77 | 55.16 ± 1.81 | 56.03 | −84.18 ± 6.32 | 0.28 ± 0.02 |
| 4 | 1 (15.83) | −1 (0.00) | 0 (0.89) | 126.40 ± 3.67 | 119.94 | 54.87 ± 3.66 | 51.11 | −81.92 ± 3.52 | 0.30 ± 0.00 |
| 5 | 0 (12.67) | 0 (0.77) | 0 (0.89) | 128.80 ± 2.73 | 132.23 | 72.58 ± 7.10 | 50.77 | −82.42 ± 2.52 | 0.31 ± 0.04 |
| 6 | −1 (9.50) | −1 (0.00) | 0 (0.89) | 122.48 ± 1.65 | 125.16 | 65.47 ± 6.16 | 47.98 | −82.22 ± 0.55 | 0.27 ± 0.00 |
| 7 | 0 (12.67) | 0 (0.77) | 0 (0.89) | 121.82 ± 1.05 | 132.23 | 74.12 ± 0.81 | 50.77 | −80.57 ± 1.70 | 0.26 ± 0.00 |
| 8 | 0 (12.67) | 1 (1.55) | 1 (1.11) | 163.12 ± 15.68 | 159.90 | 41.29 ± 16.34 | 38.19 | −95.30 ± 7.40 | 0.43 ± 0.02 |
| 9 | 0 (12.67) | −1 (0.00) | −1 (0.66) | 127.32 ± 5.02 | 129.33 | 33.14 ± 13.18 | 43.06 | −84.95 ± 1.95 | 0.32 ± 0.04 |
| 10 | 0 (12.67) | 1 (1.55) | −1 (0.66) | 125.10 ± 3.43 | 123.92 | 59.83 ± 13.58 | 65.78 | −82.43 ± 6.03 | 0.27 ± 0.01 |
| 11 | 1 (15.83) | 1 (1.55) | 0 (0.89) | 144.92 ± 11.95 | 141.65 | 45.65 ± 0.00 | 45.87 | −92.92 ± 3.15 | 0.39 ± 0.08 |
| 12 | −1 (9.50) | 0 (0.77) | −1 (0.66) | 133.65 ± 3.48 | 126.72 | 72.63 ± 0.37 | 60.97 | −80.90 ± 1.03 | 0.30 ± 0.03 |
| 13 | 1 (15.83) | 0 (0.77) | 1 (1.11) | 130.82 ± 0.05 | 135.06 | 42.34 ± 9.27 | 49.11 | −80.25 ± 5.25 | 0.29 ± 0.02 |
| 14 | 0 (12.67) | 0 (0.77) | 0 (0.89) | 137.10 ± 15.73 | 132.23 | 76.08 ± 14.75 | 50.77 | −82.67 ± 3.77 | 0.32 ± 0.07 |
| 15 | 1 (15.83) | 0 (0.77) | −1 (0.66) | 124.33 ± 3.07 | 126.53 | 50.16 ± 8.86 | 47.87 | −82.77 ± 0.30 | 0.29 ± 0.02 |
| Encapsulation Efficiency (Quadratic) | Particle Size (Two Factors Interaction) | |||||||
|---|---|---|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | p-Value | Sum of Squares | df | Mean Square | p-Value | |
| Model | 2280.95 | 9.00 | 253.44 | 0.02 | 1643.29 | 6.00 | 273.88 | 0.01 |
| X1 (DOPC:DOPA) | 553.45 | 1.00 | 553.45 | 0.01 | 16.44 | 1.00 | 16.44 | 0.56 |
| X2 (Cholesterol) | 0.00 | 1.00 | 0.00 | 0.99 | 749.49 | 1.00 | 749.49 | <0.01 |
| X3 (Unsaponifiable matter) | 38.37 | 1.00 | 38.37 | 0.35 | 251.25 | 1.00 | 251.25 | 0.04 |
| X1X2 | 8.29 | 1.00 | 8.29 | 0.65 | 5.52 | 1.00 | 5.52 | 0.73 |
| X1X3 | 7.18 | 1.00 | 7.18 | 0.68 | 7.20 | 1.00 | 7.20 | 0.69 |
| X2X3 | 411.28 | 1.00 | 411.28 | 0.02 | 613.39 | 1.00 | 613.39 | 0.01 |
| X12 | 66.22 | 1.00 | 66.22 | 0.24 | - | - | - | - |
| X22 | 626.40 | 1.00 | 626.40 | 0.01 | - | - | - | - |
| X32 | 711.34 | 1.00 | 711.34 | 0.01 | - | - | - | - |
| Residual | 182.25 | 5.00 | 36.45 | - | 348.51 | 8.00 | 43.56 | - |
| Lack of Fit | 176.10 | 3.00 | 58.70 | >0.05 | 231.43 | 6.00 | 38.57 | 0.71 |
| Pure Error | 6.15 | 2.00 | 3.08 | - | 117.08 | 2.00 | 58.54 | - |
| Correlation Matrix | DOPC:DOPA Ratio | Cholesterol | Unsaponifiable Matter | Particle Size | Encapsulation Efficiency | PDI | Zeta Potential |
|---|---|---|---|---|---|---|---|
| DOPC:DOPA ratio | 1.00 | ||||||
| Cholesterol | 0.00 | 1.00 | |||||
| Unsaponifiable matter | 0.00 | 0.00 | 1.00 | ||||
| Particle size | −0.09 | 0.61 * | 0.36 | 1.00 | |||
| Encapsulation efficiency | −0.47 | 0.86 | −0.12 | −0.25 | 1.00 | ||
| PDI | 0.07 | 0.54 * | 0.31 | 0.91 | −0.43 | 1.00 | |
| Zeta Potential | −0.23 | −0.28 | −0.30 | −0.68 | 0.50 | −0.76 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañeda-Reyes, E.D.; Gonzalez de Mejia, E.; Eller, F.J.; Berhow, M.A.; Perea-Flores, M.d.J.; Dávila-Ortíz, G. Liposomes Loaded with Unsaponifiable Matter from Amaranthus hypochondriacus as a Source of Squalene and Carrying Soybean Lunasin Inhibited Melanoma Cells. Nanomaterials 2021, 11, 1960. https://doi.org/10.3390/nano11081960
Castañeda-Reyes ED, Gonzalez de Mejia E, Eller FJ, Berhow MA, Perea-Flores MdJ, Dávila-Ortíz G. Liposomes Loaded with Unsaponifiable Matter from Amaranthus hypochondriacus as a Source of Squalene and Carrying Soybean Lunasin Inhibited Melanoma Cells. Nanomaterials. 2021; 11(8):1960. https://doi.org/10.3390/nano11081960
Chicago/Turabian StyleCastañeda-Reyes, Erick Damian, Elvira Gonzalez de Mejia, Fred Joseph Eller, Mark A. Berhow, María de Jesús Perea-Flores, and Gloria Dávila-Ortíz. 2021. "Liposomes Loaded with Unsaponifiable Matter from Amaranthus hypochondriacus as a Source of Squalene and Carrying Soybean Lunasin Inhibited Melanoma Cells" Nanomaterials 11, no. 8: 1960. https://doi.org/10.3390/nano11081960
APA StyleCastañeda-Reyes, E. D., Gonzalez de Mejia, E., Eller, F. J., Berhow, M. A., Perea-Flores, M. d. J., & Dávila-Ortíz, G. (2021). Liposomes Loaded with Unsaponifiable Matter from Amaranthus hypochondriacus as a Source of Squalene and Carrying Soybean Lunasin Inhibited Melanoma Cells. Nanomaterials, 11(8), 1960. https://doi.org/10.3390/nano11081960









