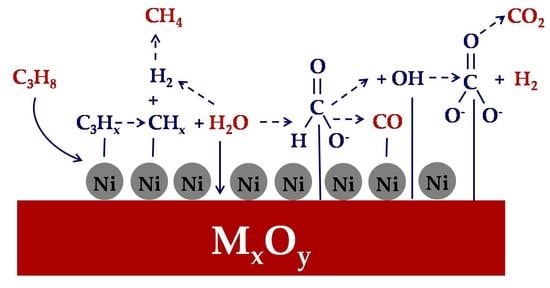
Support Effects on the Activity of Ni Catalysts for the Propane Steam Reforming Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation and Characterization
2.2. Catalytic Performance Tests and Kinetic Measurements
2.3. In Situ FTIR Spectroscopy
3. Results
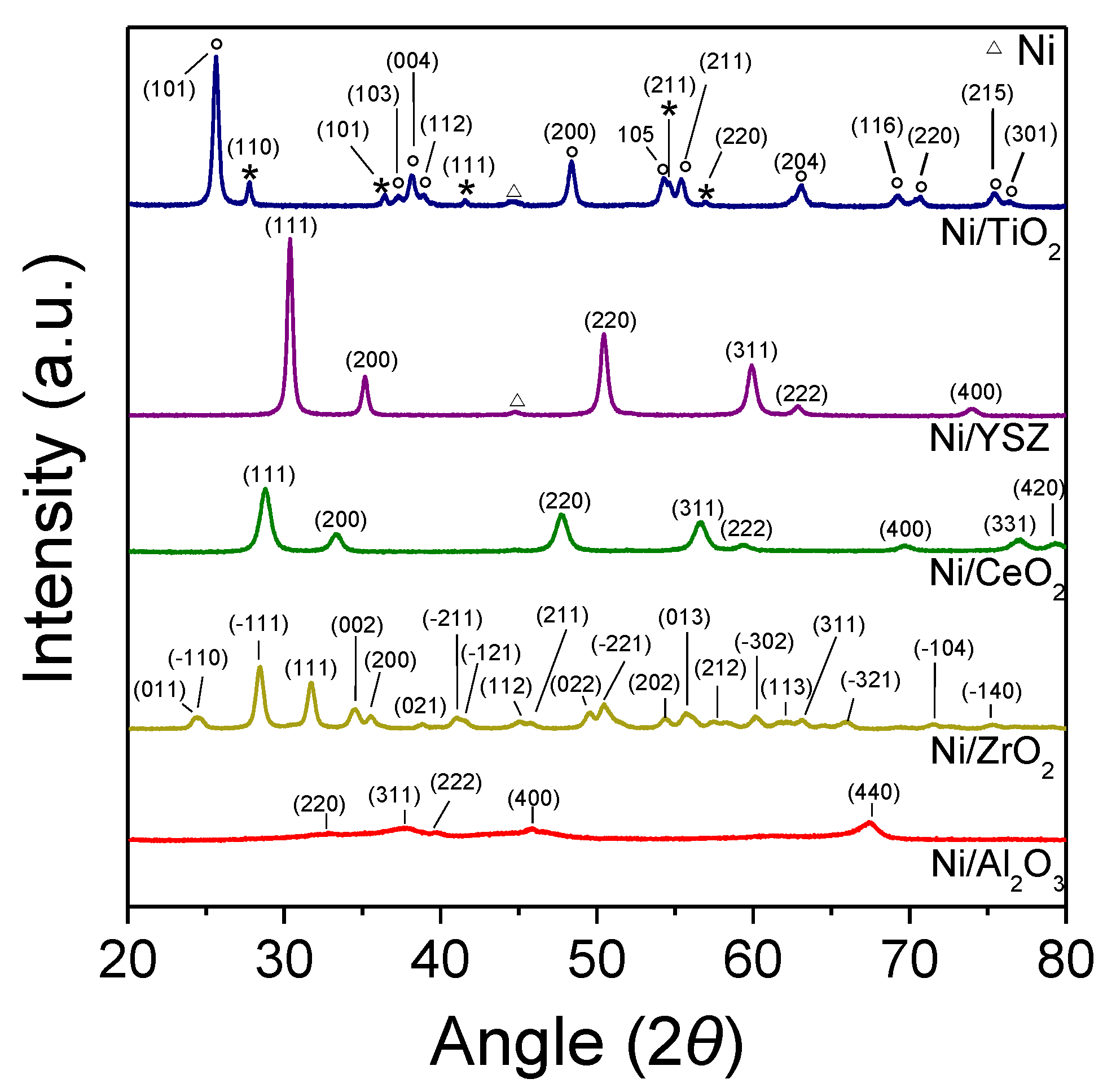
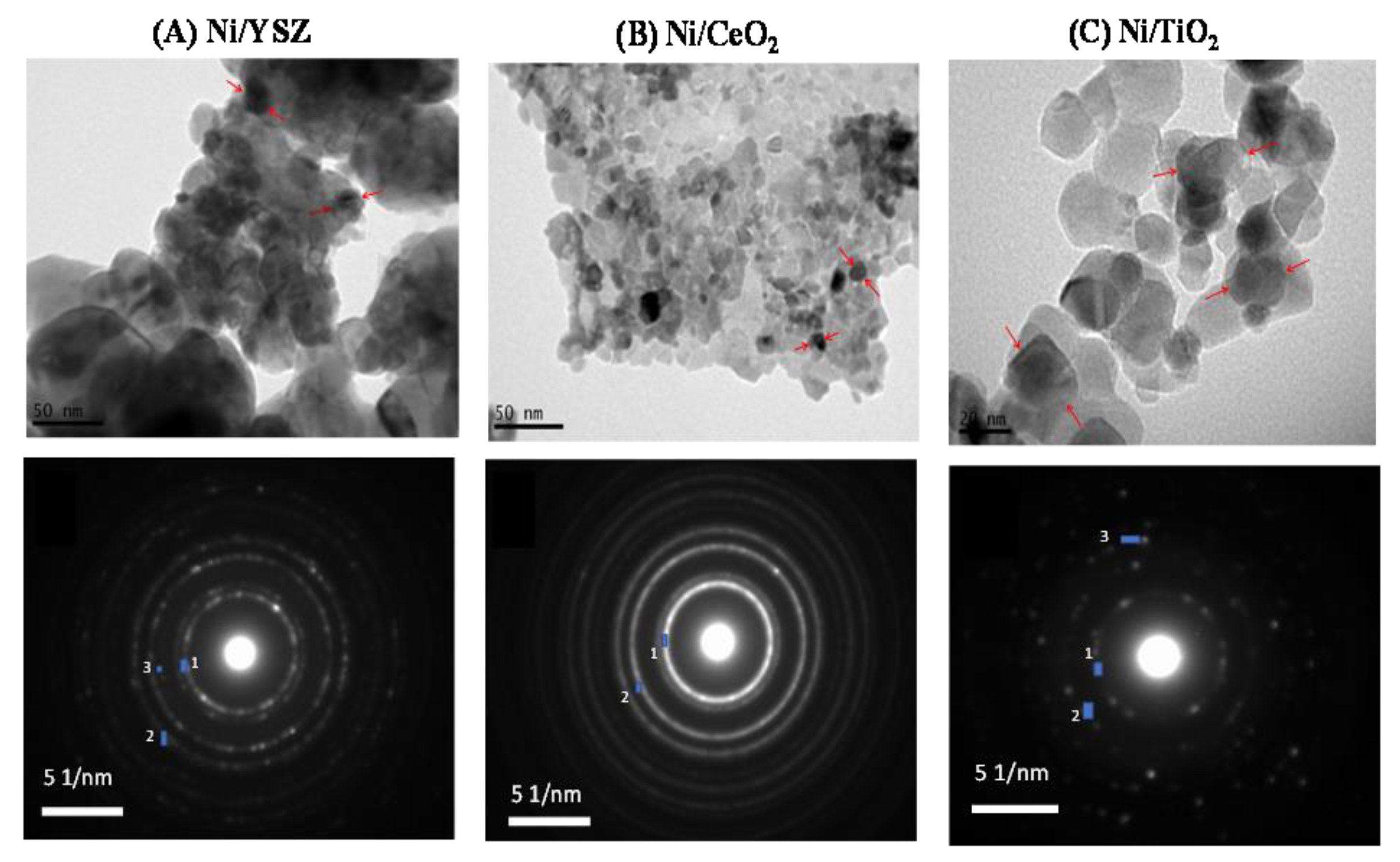
3.1. Catalyst Characterization
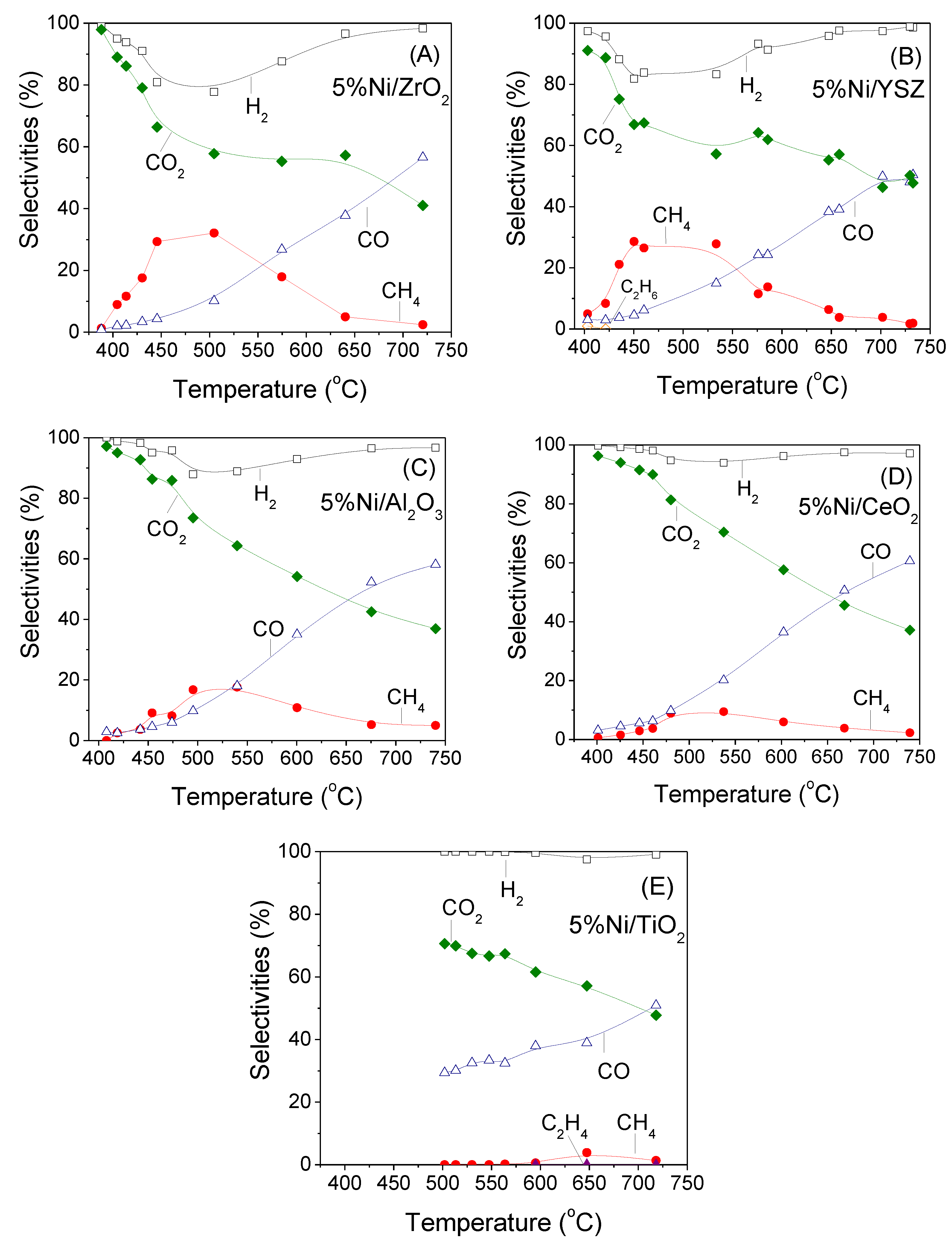
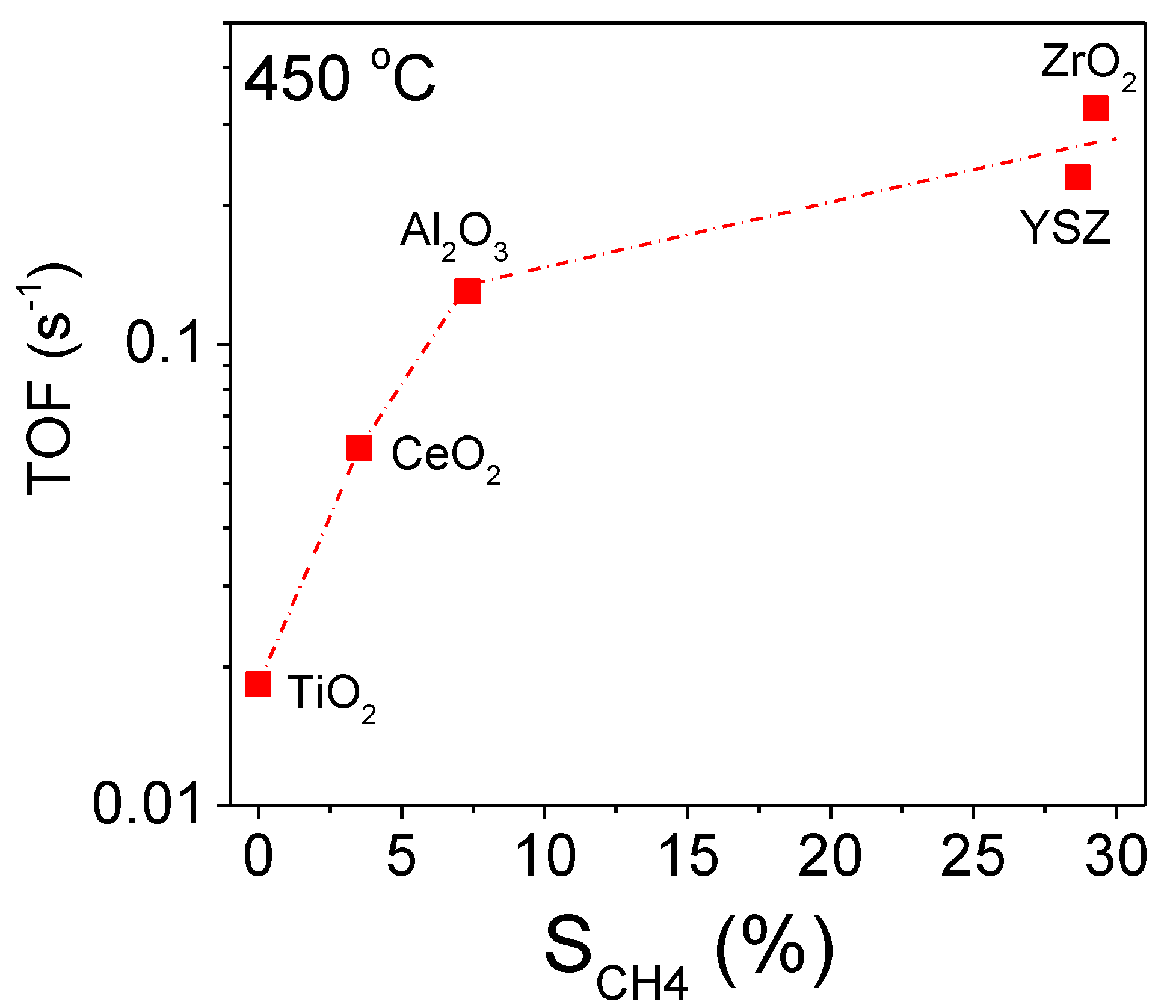
3.2. Influence of the Nature of the Support on Catalytic Activity
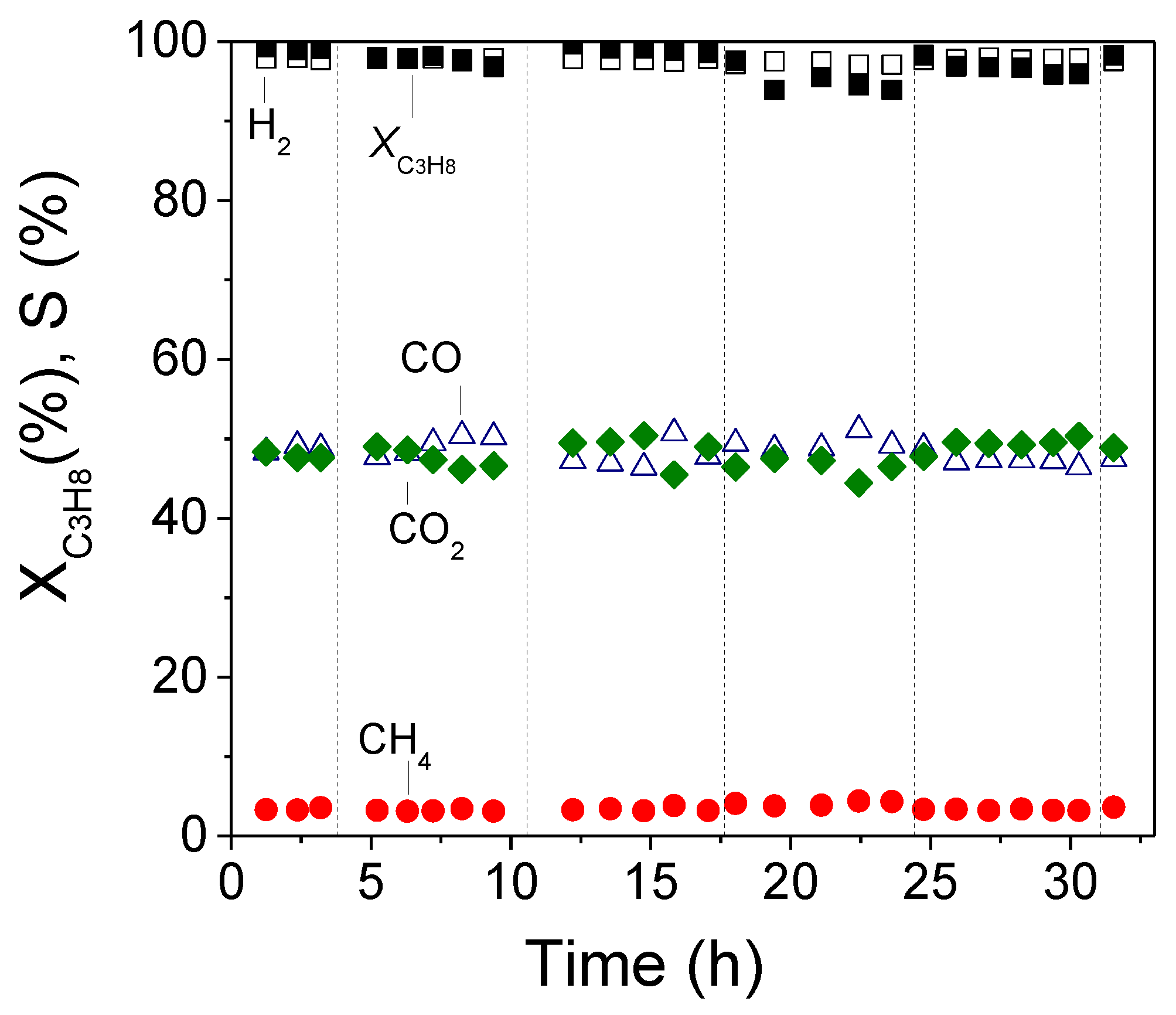
3.3. Long Term Stability Test
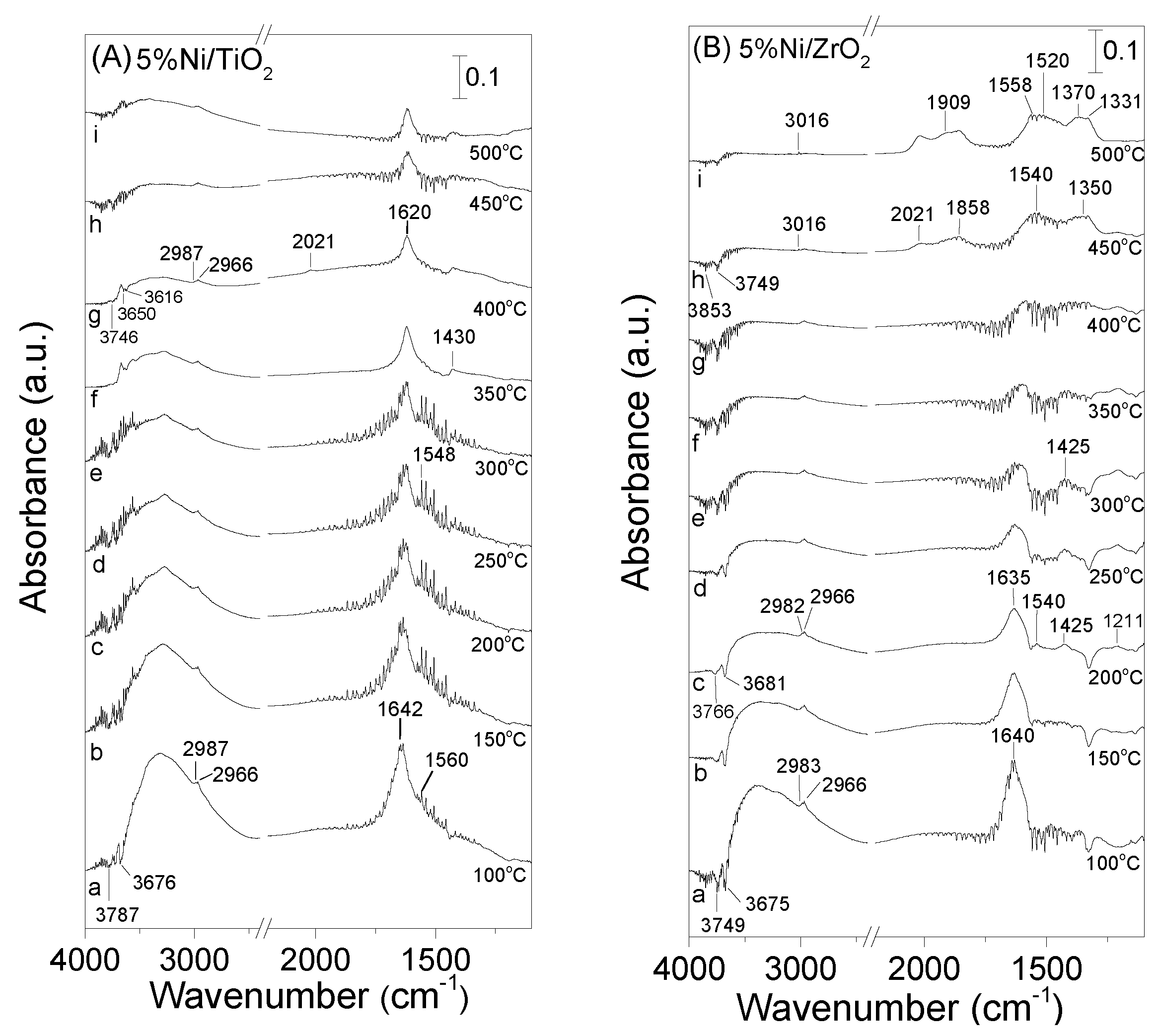
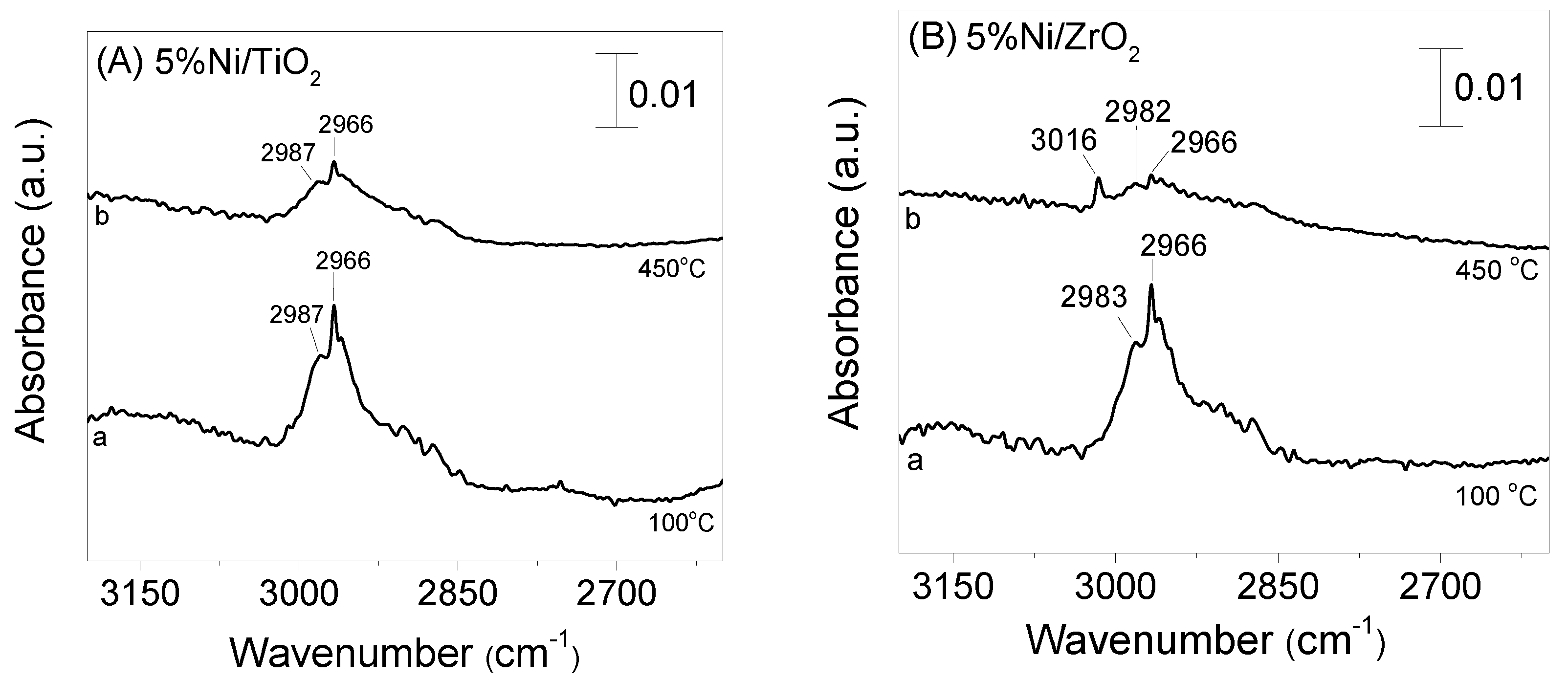
3.4. DRIFT Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen Production Technologies: Current State and Future Developments. Conf. Pap. Energy 2013, 2013, 690627. [Google Scholar] [CrossRef] [Green Version]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Im, Y.; Lee, J.H.; Kwak, B.S.; Do, J.Y.; Kang, M. Effective hydrogen production from propane steam reforming using M/NiO/YSZ catalysts (M = Ru, Rh, Pd, and Ag). Catal. Today 2018, 303, 168–176. [Google Scholar] [CrossRef]
- Santamaria, L.; Lopez, G.; Arregi, A.; Amutio, M.; Artetxe, M.; Bilbao, J.; Olazar, M. Influence of the support on Ni catalysts performance in the in-line steam reforming of biomass fast pyrolysis derived volatiles. Appl. Catal. B Environ. 2018, 229, 105–113. [Google Scholar] [CrossRef]
- Santamaria, L.; Lopez, G.; Arregi, A.; Amutio, M.; Artetxe, M.; Bilbao, J.; Olazar, M. Stability of different Ni supported catalysts in the in-line steam reforming of biomass fast pyrolysis volatiles. Appl. Catal. B Environ. 2019, 242, 109–120. [Google Scholar] [CrossRef]
- Miyazawa, T.; Kimura, T.; Nishikawa, J.; Kado, S.; Kunimori, K.; Tomishige, K. Catalytic performance of supported Ni catalysts in partial oxidation and steam reforming of tar derived from the pyrolysis of wood biomass. Catal. Today 2006, 115, 254–262. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Hassan, M.; Djama, M.; Khaleel, A. Hydrogen Production by Steam Reforming of Commercially Available LPG in UAE. Chem. Eng. Commun. 2017, 204, 141–148. [Google Scholar] [CrossRef]
- Do, J.Y.; Lee, J.H.; Park, N.-K.; Lee, T.J.; Lee, S.T.; Kang, M. Synthesis and characterization of Ni2−xPdxMnO4/γ-Al2O3 catalysts for hydrogen production via propane steam reforming. Chem. Eng. J. 2018, 334, 1668–1678. [Google Scholar] [CrossRef]
- Do, J.Y.; Park, N.-K.; Lee, T.J.; Lee, S.T.; Kang, M. Effective hydrogen productions from propane steam reforming over spinel-structured metal-manganese oxide redox couple catalysts. Int. J. Energy Res. 2018, 42, 429–446. [Google Scholar] [CrossRef]
- Barzegari, F.; Kazemeini, M.; Farhadi, F.; Rezaei, M.; Keshavarz, A. Preparation of mesoporous nanostructure NiO–MgO–SiO2 catalysts for syngas production via propane steam reforming. Int. J. Hydrogen Energy 2020, 45, 6604–6620. [Google Scholar] [CrossRef]
- Kokka, A.; Katsoni, A.; Yentekakis, I.V.; Panagiotopoulou, P. Hydrogen production via steam reforming of propane over supported metal catalysts. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Kokka, A.; Ramantani, T.; Panagiotopoulou, P. Effect of Operating Conditions on the Performance of Rh/TiO2 Catalyst for the Reaction of LPG Steam Reforming. Catalysts 2021, 11, 374. [Google Scholar] [CrossRef]
- Yu, L.; Sato, K.; Nagaoka, K. Rh/Ce0.25Zr0.75O2 Catalyst for Steam Reforming of Propane at Low Temperature. ChemCatChem 2019, 11, 1472–1479. [Google Scholar] [CrossRef]
- Karakaya, C.; Karadeniz, H.; Maier, L.; Deutschmann, O. Surface Reaction Kinetics of the Oxidation and Reforming of Propane over Rh/Al2O3 Catalysts. ChemCatChem 2017, 9, 685–695. [Google Scholar] [CrossRef]
- Aghamiri, A.R.; Alavi, S.M.; Bazyari, A.; Azizzadeh Fard, A. Effects of simultaneous calcination and reduction on performance of promoted Ni/SiO2 catalyst in steam reforming of propane. Int. J. Hydrogen Energy 2019, 44, 9307–9315. [Google Scholar] [CrossRef]
- Harshini, D.; Yoon, C.W.; Han, J.; Yoon, S.P.; Nam, S.W.; Lim, T.-H. Catalytic Steam Reforming of Propane over Ni/LaAlO3 Catalysts: Influence of Preparation Methods and OSC on Activity and Stability. Catal. Lett. 2012, 142, 205–212. [Google Scholar] [CrossRef]
- Azizzadeh Fard, A.; Arvaneh, R.; Alavi, S.M.; Bazyari, A.; Valaei, A. Propane steam reforming over promoted Ni–Ce/MgAl2O4 catalysts: Effects of Ce promoter on the catalyst performance using developed CCD model. Int. J. Hydrogen Energy 2019, 44, 21607–21622. [Google Scholar] [CrossRef]
- Kim, K.M.; Kwak, B.S.; Park, N.-K.; Lee, T.J.; Lee, S.T.; Kang, M. Effective hydrogen production from propane steam reforming over bimetallic co-doped NiFe/Al2O3 catalyst. J. Ind. Eng. Chem. 2017, 46, 324–336. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Sangtongkitcharoen, W.; Assabumrungrat, S. Catalytic steam reforming of ethane and propane over CeO2-doped Ni/Al2O3 at SOFC temperature: Improvement of resistance toward carbon formation by the redox property of doping CeO2. Fuel 2006, 85, 323–332. [Google Scholar] [CrossRef]
- Natesakhawat, S.; Oktar, O.; Ozkan, U.S. Effect of lanthanide promotion on catalytic performance of sol–gel Ni/Al2O3 catalysts in steam reforming of propane. J. Mol. Catal. A Chem. 2005, 241, 133–146. [Google Scholar] [CrossRef]
- Matsumura, Y.; Nakamori, T. Steam reforming of methane over nickel catalysts at low reaction temperature. Appl. Catal. A Gen. 2004, 258, 107–114. [Google Scholar] [CrossRef]
- Guo, J.; Lou, H.; Zhao, H.; Chai, D.; Zheng, X. Dry reforming of methane over nickel catalysts supported on magnesium aluminate spinels. Appl. Catal. A Gen. 2004, 273, 75–82. [Google Scholar] [CrossRef]
- Lee, D.S.; Min, D.J. A Kinetics of Hydrogen Reduction of Nickel Oxide at Moderate Temperature. Met. Mater. Int. 2019, 25, 982–990. [Google Scholar] [CrossRef] [Green Version]
- Richardson, J.T.; Scates, R.; Twigg, M.V. X-ray diffraction study of nickel oxide reduction by hydrogen. Appl. Catal. A Gen. 2003, 246, 137–150. [Google Scholar] [CrossRef]
- Richardson, J.T.; Scates, R.M.; Twigg, M.V. X-ray diffraction study of the hydrogen reduction of NiO/α-Al2O3 steam reforming catalysts. Appl. Catal. A Gen. 2004, 267, 35–46. [Google Scholar] [CrossRef]
- Almasan, V.; Gaeumann, T.; Lazar, M.; Marginean, P.; Aldea, N. Hydrogen spillover effect over the oxide surfaces in supported nickel catalysts. In Studies in Surface Science and Catalysis; Froment, G.F., Waugh, K.C., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; Volume 109, pp. 547–552. [Google Scholar]
- Kramer, R.; Andre, M. Adsorption of atomic hydrogen on alumina by hydrogen spillover. J. Catal. 1979, 58, 287–295. [Google Scholar] [CrossRef]
- Hatzisymeon, M.; Petala, A.; Panagiotopoulou, P. Carbon Dioxide Hydrogenation over Supported Ni and Ru Catalysts. Catal. Lett. 2021, 151, 888–900. [Google Scholar] [CrossRef]
- Kolb, G.; Zapf, R.; Hessel, V.; Löwe, H. Propane steam reforming in micro-channels—results from catalyst screening and optimisation. Appl. Catal. A Gen. 2004, 277, 155–166. [Google Scholar] [CrossRef]
- Modafferi, V.; Panzera, G.; Baglio, V.; Frusteri, F.; Antonucci, P.L. Propane reforming on Ni–Ru/GDC catalyst: H2 production for IT-SOFCs under SR and ATR conditions. Appl. Catal. A Gen. 2008, 334, 1–9. [Google Scholar] [CrossRef]
- Malaibari, Z.O.; Croiset, E.; Amin, A.; Epling, W. Effect of interactions between Ni and Mo on catalytic properties of a bimetallic Ni-Mo/Al2O3 propane reforming catalyst. Appl. Catal. A Gen. 2015, 490, 80–92. [Google Scholar] [CrossRef]
- Helali, Z.; Jedidi, A.; Syzgantseva, O.A.; Calatayud, M.; Minot, C. Scaling reducibility of metal oxides. Theor. Chem. Acc. 2017, 136, 100–115. [Google Scholar] [CrossRef]
- Alphonse, P.; Ansart, F. Catalytic coatings on steel for low-temperature propane prereforming to solid oxide fuel cell (SOFC) application. J. Colloid Interface Sci. 2009, 336, 658–666. [Google Scholar] [CrossRef] [Green Version]
- Panagiotopoulou, P.; Kondarides, D.I.; Verykios, X.E. Mechanistic aspects of the selective methanation of CO over Ru/TiO2 catalyst. Catal. Today 2012, 181, 138–147. [Google Scholar] [CrossRef]
- Panagiotopoulou, P. Methanation of CO2 over alkali-promoted Ru/TiO2 catalysts: II. Effect of alkali additives on the reaction pathway. Appl. Catal. B Environ. 2018, 236, 162–170. [Google Scholar] [CrossRef]
- Wang, B.; Wu, X.; Ran, R.; Si, Z.; Weng, D. IR characterization of propane oxidation on Pt/CeO2–ZrO2: The reaction mechanism and the role of Pt. J. Mol. Catal. A Chem. 2012, 356, 100–105. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Christodoulakis, A.; Kondarides, D.I.; Boghosian, S. Particle size effects on the reducibility of titanium dioxide and its relation to the water–gas shift activity of Pt/TiO2 catalysts. J. Catal. 2006, 240, 114–125. [Google Scholar] [CrossRef]
- Kokka, A.; Ramantani, T.; Petala, A.; Panagiotopoulou, P. Effect of the nature of the support, operating and pretreatment conditions on the catalytic performance of supported Ni catalysts for the selective methanation of CO. Catal. Today 2020, 355, 832–843. [Google Scholar] [CrossRef]
- Karelovic, A.; Ruiz, P. Mechanistic study of low temperature CO2 methanation over Rh/TiO2 catalysts. J. Catal. 2013, 301, 141–153. [Google Scholar] [CrossRef]
- Liao, L.F.; Lien, C.F.; Shieh, D.L.; Chen, M.T.; Lin, J.L. FTIR Study of Adsorption and Photoassisted Oxygen Isotopic Exchange of Carbon Monoxide, Carbon Dioxide, Carbonate, and Formate on TiO2. J. Phys. Chem. B 2002, 106, 11240–11245. [Google Scholar] [CrossRef]
- Marwood, M.; Doepper, R.; Renken, A. In-situ surface and gas phase analysis for kinetic studies under transient conditions The catalytic hydrogenation of CO2. Appl. Catal. A Gen. 1997, 151, 223–246. [Google Scholar] [CrossRef] [Green Version]
- Mino, L.; Spoto, G.; Ferrari, A.M. CO2 Capture by TiO2 Anatase Surfaces: A Combined DFT and FTIR Study. J. Phys. Chem. C 2014, 118, 25016–25026. [Google Scholar] [CrossRef]
- Aldana, P.A.U.; Ocampo, F.; Kobl, K.; Louis, B.; Thibault-Starzyk, F.; Daturi, M.; Bazin, P.; Thomas, S.; Roger, A.C. Catalytic CO2 valorization into CH4 on Ni-based ceria-zirconia. Reaction mechanism by operando IR spectroscopy. Catal. Today 2013, 215, 201–207. [Google Scholar] [CrossRef]
- Westermann, A.; Azambre, B.; Bacariza, M.C.; Graça, I.; Ribeiro, M.F.; Lopes, J.M.; Henriques, C. Insight into CO2 methanation mechanism over NiUSY zeolites: An operando IR study. Appl. Catal. B Environ. 2015, 174–175, 120–125. [Google Scholar] [CrossRef]
- Mohamed, Z.; Dasireddy, V.D.B.C.; Singh, S.; Friedrich, H.B. The preferential oxidation of CO in hydrogen rich streams over platinum doped nickel oxide catalysts. Appl. Catal. B Environ. 2016, 180, 687–697. [Google Scholar] [CrossRef]
- Konishcheva, M.V.; Potemkin, D.I.; Badmaev, S.D.; Snytnikov, P.V.; Paukshtis, E.A.; Sobyanin, V.A.; Parmon, V.N. On the Mechanism of CO and CO2 Methanation Over Ni/CeO2 Catalysts. Top. Catal. 2016, 59, 1424–1430. [Google Scholar] [CrossRef]
- Köck, E.-M.; Kogler, M.; Bielz, T.; Klötzer, B.; Penner, S. In Situ FT-IR Spectroscopic Study of CO2 and CO Adsorption on Y2O3, ZrO2, and Yttria-Stabilized ZrO2. J. Phys. Chem. C 2013, 117, 17666–17673. [Google Scholar] [CrossRef]
- Bachiller-Baeza, B.; Rodriguez-Ramos, I.; Guerrero-Ruiz, A. Interaction of Carbon Dioxide with the Surface of Zirconia Polymorphs. Langmuir 1998, 14, 3556–3564. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Hülsey, M.J.; Yan, N. Zirconia phase effect in Pd/ZrO2 catalyzed CO2 hydrogenation into formate. Mol. Catal. 2019, 475, 110461. [Google Scholar] [CrossRef]
- Föttinger, K.; Emhofer, W.; Lennon, D.; Rupprechter, G. Adsorption and Reaction of CO on (Pd–)Al2O3 and (Pd–)ZrO2: Vibrational Spectroscopy of Carbonate Formation. Topics Catal. 2017, 60, 1722–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pokrovski, K.; Jung, K.T.; Bell, A.T. Investigation of CO and CO2 Adsorption on Tetragonal and Monoclinic Zirconia. Langmuir 2001, 17, 4297–4303. [Google Scholar] [CrossRef]
- Anderson, J.A.; Daza, L.; Fierro, J.L.G.; Rodrigo, M.T. Influence of preparation method on the characteristics of nickel/sepiolite catalysts. J. Chem. Soc. Faraday Trans. 1993, 89, 3651–3657. [Google Scholar] [CrossRef]
- Wu, R.-f.; Zhang, Y.; Wang, Y.-z.; Gao, C.-g.; Zhao, Y.-x. Effect of ZrO2 promoter on the catalytic activity for CO methanation and adsorption performance of the Ni/SiO2 catalyst. J. Fuel Chem. Technol. 2009, 37, 578–582. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Cui, K.; Jia, A.; Hu, G.; Jiao, Z.; Liu, Y.; Zhang, X. Role of surface Ni and Ce species of Ni/CeO2 catalyst in CO2 methanation. Appl. Surf. Sci. 2016, 383, 248–252. [Google Scholar] [CrossRef]
- Faria, E.C.; Rabelo-Neto, R.C.; Colman, R.C.; Ferreira, R.A.R.; Hori, C.E.; Noronha, F.B. Steam Reforming of LPG over Ni/Al2O3 and Ni/CexZr1—xO2/Al2O3 Catalysts. Catal. Lett. 2016, 146, 2229–2241. [Google Scholar] [CrossRef]

| Catalyst | SSA (a) (m2/g) | dMxOy (b) (nm) | DNi (c) (%) | dNi (c) (nm) | Activation Energy (kJ/mol) |
|---|---|---|---|---|---|
| 5%Ni/ZrO2 | 39 | 15.0 | 5.7 | 17.8 | 154 |
| 5%Ni/YSZ | 11 | 20.9 | 4.7 | 21.4 | 140 |
| 5%Ni/Al2O3 | 66 | 6.0 | 4.0 | 25.5 | 121 |
| 5%Ni/CeO2 | 39 | 10.5 | 11.9 | 8.5 | 102 |
| 5%Ni/TiO2 | 41 | 21.8 | 2.8 | 36.1 | 127 |
| Catalyst | Spot | d-Spacing (Å) | Formula | Crystallographic Plane (h k l) | JCPDS No |
|---|---|---|---|---|---|
| 5%Ni/YSZ | 1 | 3.0 | Y0.15Zr0.85O1.93 | (111) | 30-1468 |
| 2 | 2.6 | Y0.15Zr0.85O1.93 | (200) | 30-1468 | |
| 3 | 2.1 | Ni | (111) | 1-1258 | |
| 5%Ni/CeO2 | 1 | 3.1 | CeO2 | (111) | 1-800 |
| 2 | 2.7 | CeO2 | (200) | 1-800 | |
| 3 | 2.1 | Ni | (111) | 1-1258 | |
| 5%Ni/TiO2 | 1 | 3.5 | TiO2 | (101) | 1-562 |
| 2 | 2.4 | TiO2 | (103) | 1-562 | |
| 3 | 2.1 | Ni | (111) | 1-1258 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokka, A.; Petala, A.; Panagiotopoulou, P. Support Effects on the Activity of Ni Catalysts for the Propane Steam Reforming Reaction. Nanomaterials 2021, 11, 1948. https://doi.org/10.3390/nano11081948
Kokka A, Petala A, Panagiotopoulou P. Support Effects on the Activity of Ni Catalysts for the Propane Steam Reforming Reaction. Nanomaterials. 2021; 11(8):1948. https://doi.org/10.3390/nano11081948
Chicago/Turabian StyleKokka, Aliki, Athanasia Petala, and Paraskevi Panagiotopoulou. 2021. "Support Effects on the Activity of Ni Catalysts for the Propane Steam Reforming Reaction" Nanomaterials 11, no. 8: 1948. https://doi.org/10.3390/nano11081948
APA StyleKokka, A., Petala, A., & Panagiotopoulou, P. (2021). Support Effects on the Activity of Ni Catalysts for the Propane Steam Reforming Reaction. Nanomaterials, 11(8), 1948. https://doi.org/10.3390/nano11081948