Environmental Hazards of Boron and Vanadium Nanoparticles in the Terrestrial Ecosystem—A Case Study with Enchytraeus crypticus
Abstract
:1. Introduction
2. Material and Methods
2.1. Test Organism
2.2. Test Materials and Characterization
2.3. Test Soil and Spiking Procedures
2.4. Enchytraeid Reproduction Test Extension Procedures
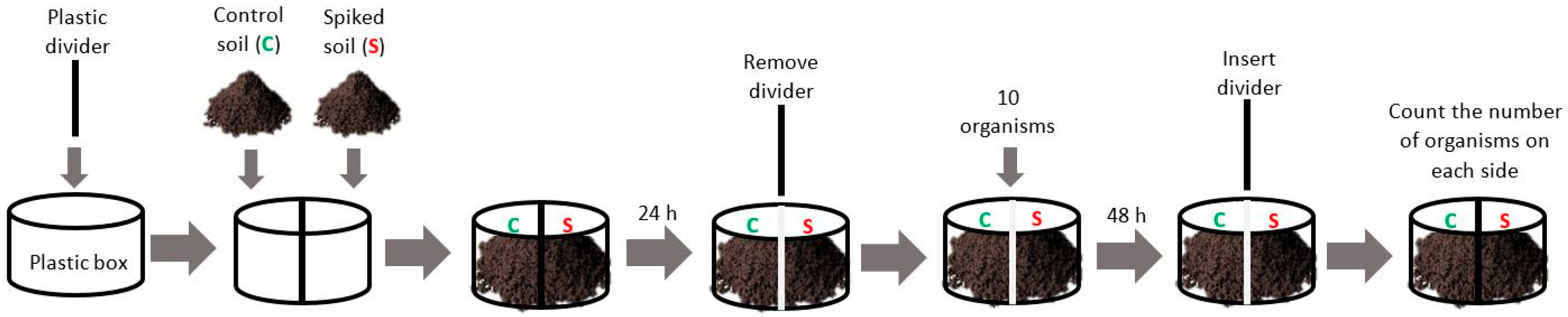
2.5. Avoidance Test Procedures
2.6. Data Analysis
3. Results
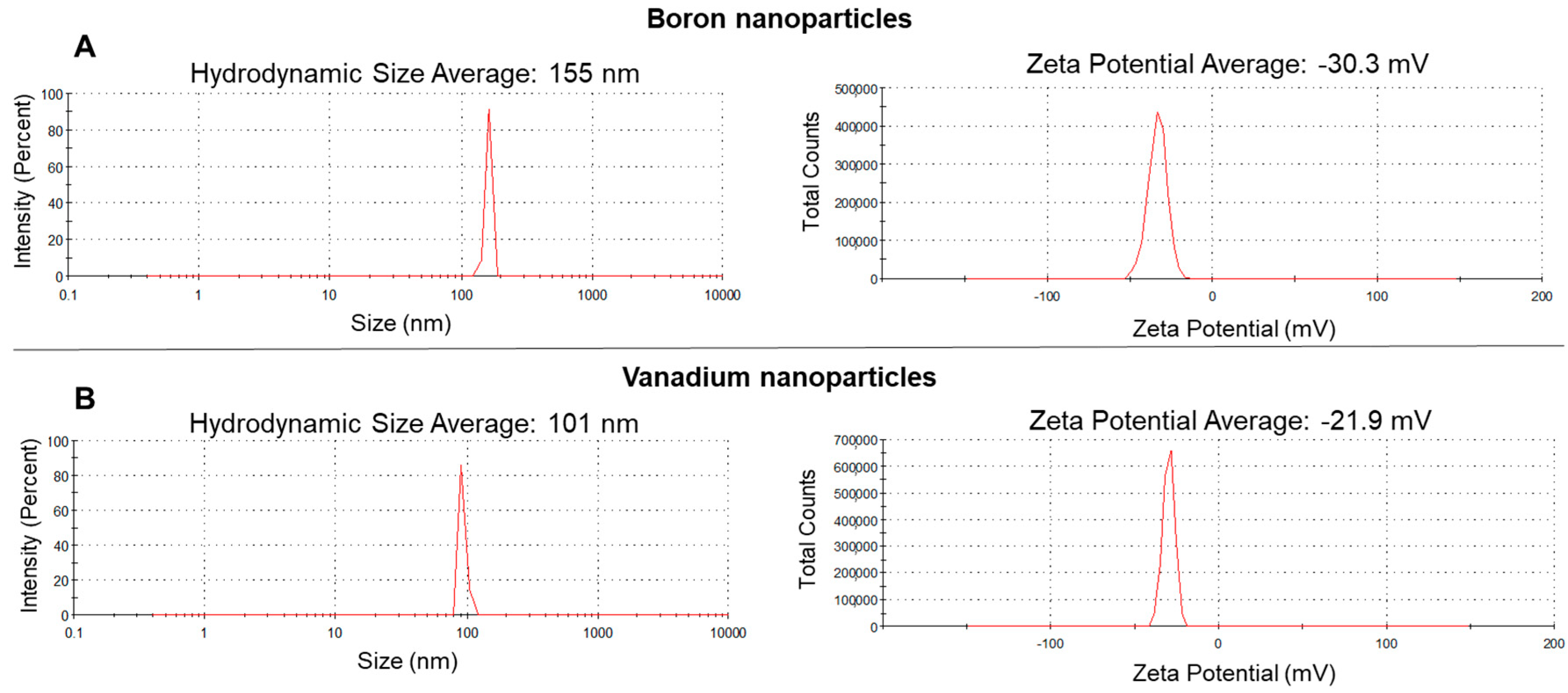
3.1. Characterization of the Test Materials
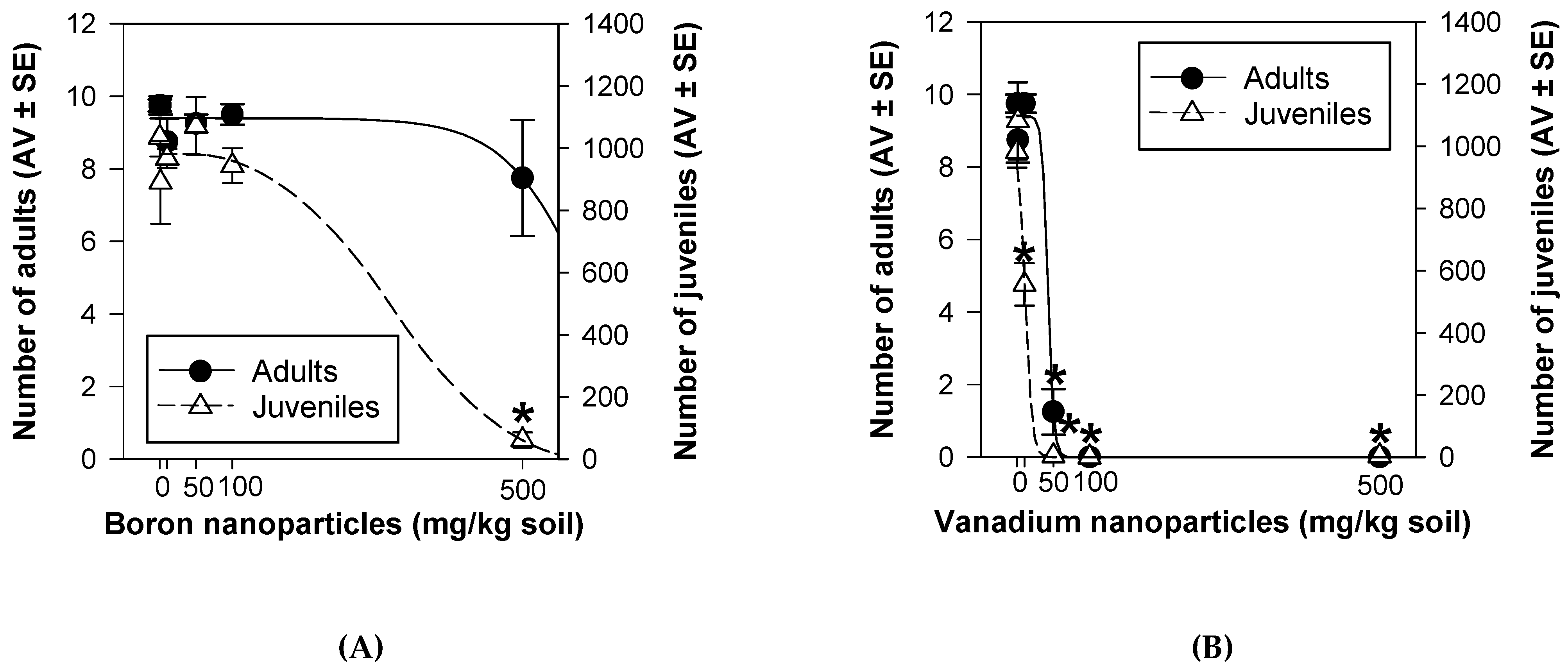
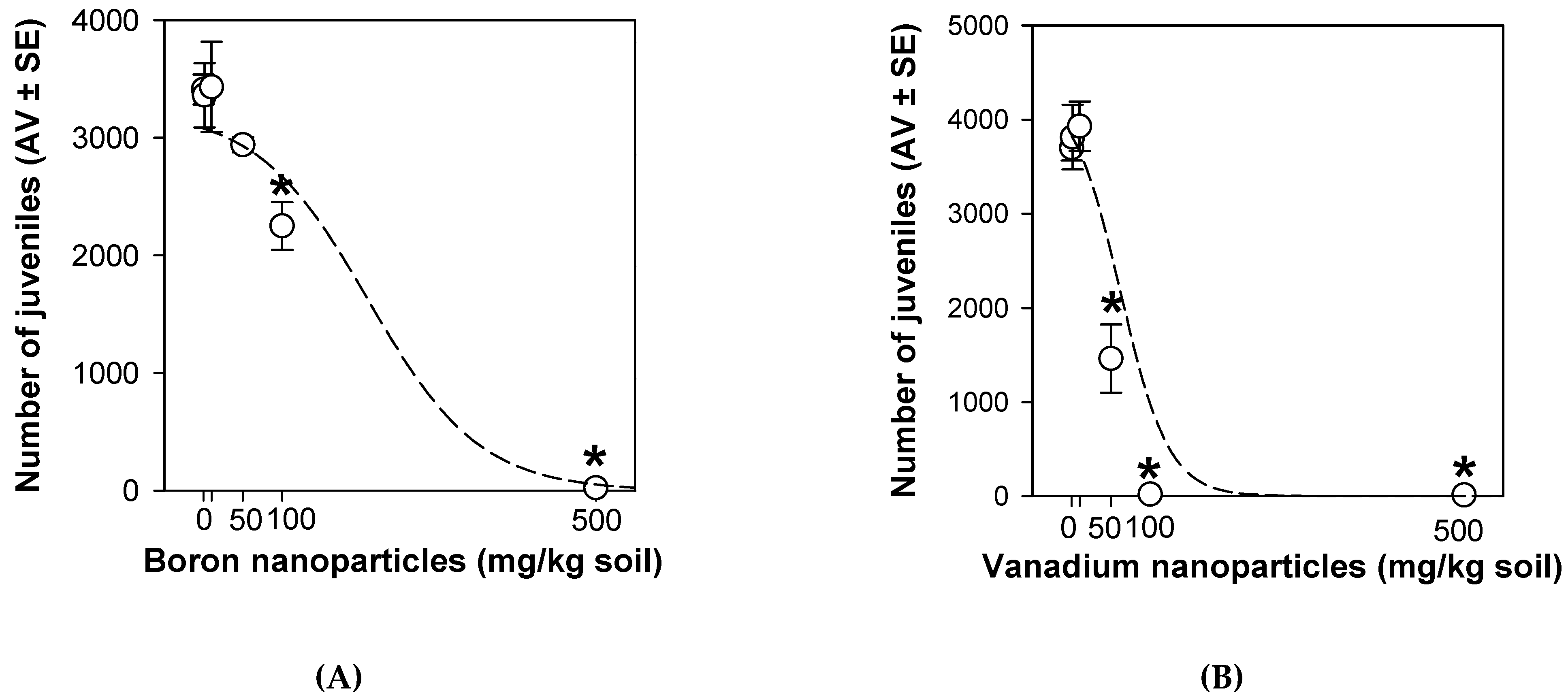
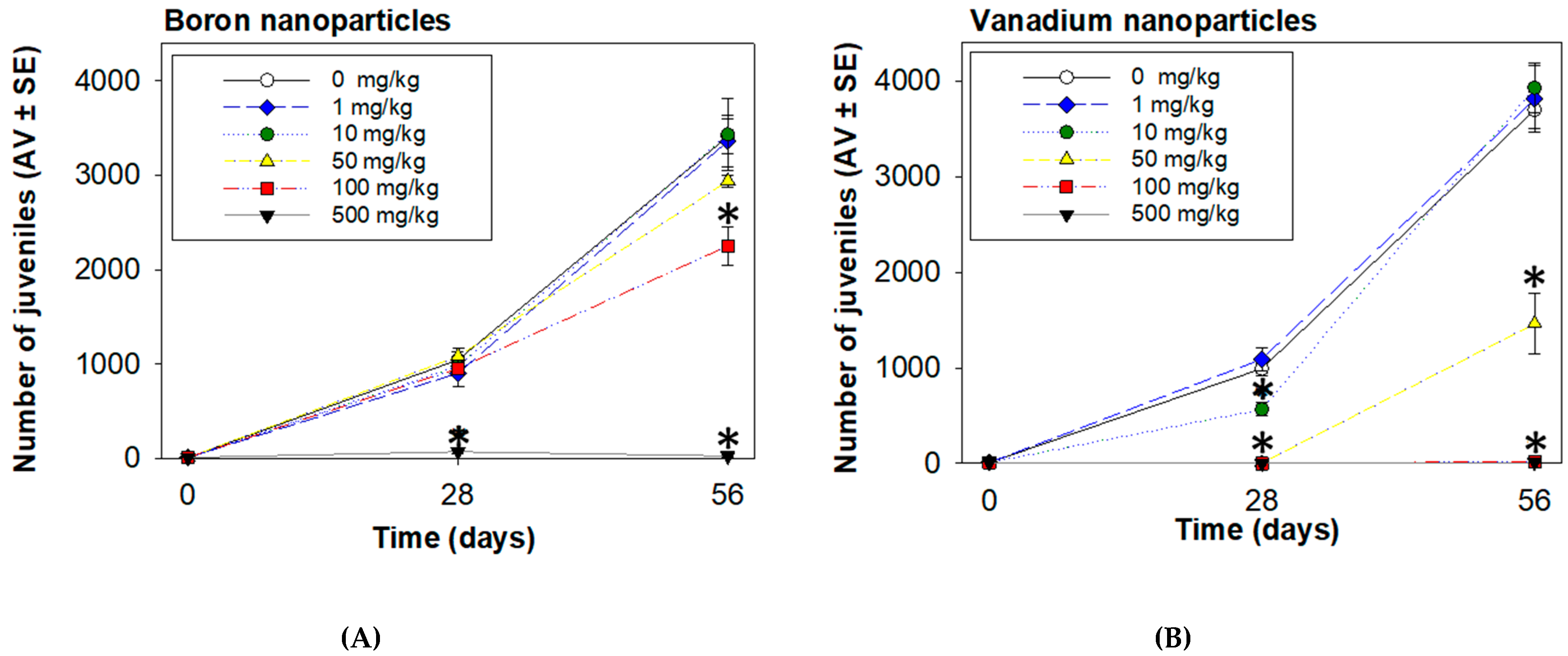
3.2. Enchytraeid Reproduction Test Extension
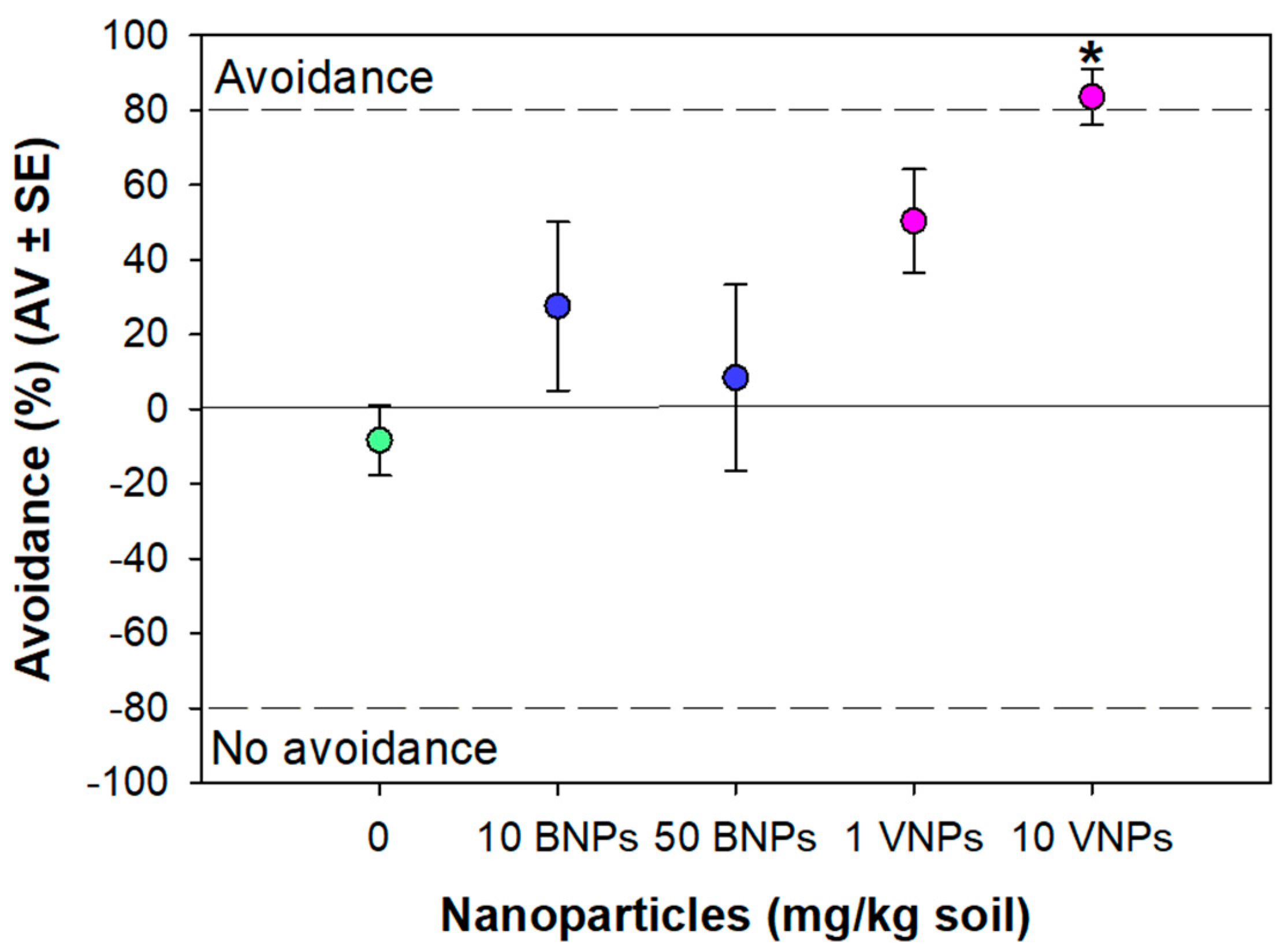
3.3. Avoidance Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbas, Q.; Yousaf, B.; Ali, M.U.; Munir, M.A.M.; El-Naggar, A.; Rinklebe, J.; Naushad, M. Transformation pathways and fate of engineered nanoparticles (ENPs) in distinct interactive environmental compartments: A review. Environ. Int. 2020, 138, 105646. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.J.B.; Fernández-Cruz, M.L.; Hund-Rinke, K.; Scott-Fordsmand, J.J. Environmental hazard testing of nanobiomaterials. Environ. Sci. Eur. 2020, 32, 101. [Google Scholar] [CrossRef]
- Durenkamp, M.; Pawlett, M.; Ritz, K.; Harris, J.A.; Neal, A.L.; McGrath, S.P. Nanoparticles within WWTP sludges have minimal impact on leachate quality and soil microbial community structure and function. Environ. Pollut. 2016, 211, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Klaine, S.J.; Alvarez, P.J.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [CrossRef]
- Lead, J.R.; Batley, G.E.; Alvarez, P.J.J.; Croteau, M.-N.; Handy, R.D.; McLaughlin, M.J.; Judy, J.D.; Schirmer, K. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects—An updated review. Environ. Toxicol. Chem. 2018, 37, 2029–2063. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Q.; Yousaf, B.; Ullah, H.; Ali, M.U.; Ok, Y.S.; Rinklebe, J. Environmental transformation and nano-toxicity of engineered nano-particles (ENPs) in aquatic and terrestrial organisms. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2523–2581. [Google Scholar] [CrossRef]
- Dwivedi, A.D.; Dubey, S.P.; Sillanpää, M.; Kwon, Y.-N.; Lee, C.; Varma, R.S. Fate of engineered nanoparticles: Implications in the environment. Coord. Chem. Rev. 2015, 287, 64–78. [Google Scholar] [CrossRef]
- Petersen, M.S.; Petersen, C.C.; Agger, R.; Sutmuller, M.; Jensen, M.R.; Sørensen, P.G.; Mortensen, M.W.; Hansen, T.; Bjørnholm, T.; Gundersen, H.J.; et al. Boron nanoparticles inhibit tumour growth by boron neutron capture therapy in the Murine B16-OVA model. Anticancer Res. 2008, 28, 571–576. [Google Scholar]
- Tatiya, S.; Pandey, M.; Bhattacharya, S. Nanoparticles containing boron and its compounds—Synthesis and applications: A review. J. Micromanuf. 2020, 3, 159–173. [Google Scholar] [CrossRef]
- Al Zoubi, M.; Farag, H.K.; Endres, F. Sol–gel synthesis of vanadium pentoxide nanoparticles in air- and water-stable ionic liquids. J. Mater. Sci. 2009, 44, 1363–1373. [Google Scholar] [CrossRef]
- Aliyu, A.O.; Garba, S.; Bognet, O. Green synthesis, characterization and antimicrobial activity of vanadium nanoparticles using leaf extract of moringa oleifera. Int. J. Chem. Sci. Res. 2017, 16, 231. [Google Scholar] [CrossRef]
- Daglioglu, Y.; Kabakçi, D.; Akdeniz, G. Toxicity of nano and non-nano boron particles on Apis mellifera (honey bee). Res. J. Chem. Environ. Sci. 2015, 3, 6–13. [Google Scholar]
- Strigul, N.; Vaccari, L.; Galdun, C.; Wazne, M.; Liu, X.; Christodoulatos, C.; Jasinkiewicz, K. Acute toxicity of boron, titanium dioxide, and aluminum nanoparticles to Daphnia magna and Vibrio fischeri. Desalination 2009, 248, 771–782. [Google Scholar] [CrossRef]
- Xi, W.S.; Song, Z.M.; Chen, Z.; Chen, N.; Yan, G.H.; Gao, Y.; Cao, A.; Liu, Y.; Wang, H. Short-term and long-term toxicological effects of vanadium dioxide nanoparticles on A549 cells. Environ. Sci. Nano 2019, 6, 565–579. [Google Scholar] [CrossRef]
- Xi, W.S.; Tang, H.; Liu, Y.Y.; Liu, C.Y.; Gao, Y.; Cao, A.; Liu, Y.; Chen, Z.; Wang, H. Cytotoxicity of vanadium oxide nanoparticles and titanium dioxide-coated vanadium oxide nanoparticles to human lung cells. J. Appl. Toxicol. 2020, 40, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Wörle-Knirsch, J.M.; Kern, K.; Schleh, C.; Adelhelm, C.; Feldmann, C.; Krug, H.F. Nanoparticulate vanadium oxide potentiated vanadium toxicity in human lung cells. Environ. Sci. Technol. 2007, 41, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Zhang, W.; Xing, Y.; Zhang, Q.; Yang, L.; Cao, Q.; Qin, L. Boron Toxicity Causes Multiple Effects on Malus domestica Pollen Tube Growth. Front. Plant Sci. 2016, 7, 208. [Google Scholar] [CrossRef] [Green Version]
- Öz, M.; Yavuz, O.; Bolukbas, F. Histopathology changes in the rainbow trout (Onchorhyncus mykiss) consuming boric acid supplemented fish fodder. J. Trace Elem. Med. Biol. 2020, 62, 126581. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I. Protective effects of dietary antioxidants against vanadium-induced toxicity: A review. Oxid. Med. Cell. Longev. 2020, 2020, 1490316. [Google Scholar] [CrossRef] [PubMed]
- Bouguerra, S.; Gavina, A.; Ksibi, M.; da Graça Rasteiro, M.; Rocha-Santos, T.; Pereira, R. Ecotoxicity of titanium silicon oxide (TiSiO4) nanomaterial for terrestrial plants and soil invertebrate species. Ecotoxicol. Environ. Saf. 2016, 129, 291–301. [Google Scholar] [CrossRef]
- Pereira, C.M.S.; Novais, S.C.; Soares, A.M.V.M.; Amorim, M.J.B. Dimethoate affects cholinesterases in Folsomia candida and their locomotion—False negative results of an avoidance behaviour test. Sci. Total Environ. 2013, 443, 821–827. [Google Scholar] [CrossRef]
- Zidar, P.; Kos, M.; Ilič, E.; Marolt, G.; Drobne, D.; Jemec Kokalj, A. Avoidance behaviour of isopods (Porcellio scaber) exposed to food or soil contaminated with Ag- and CeO2-nanoparticles. Appl. Soil Ecol. 2019, 141, 69–78. [Google Scholar] [CrossRef]
- Santos, J.; Barreto, Â.; Nogueira, J.; Daniel-da-Silva, L.A.; Trindade, T.; Amorim, J.B.M.; Maria, L.V. Effects of Amorphous Silica Nanopowders on the Avoidance Behavior of Five Soil Species—A Screening Study. Nanomaterials 2020, 10, 402. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Plant Protection Products and their Residues (PPR); Ockleford, C.; Adriaanse, P.; Berny, P.; Brock, T.; Duquesne, S.; Grilli, S.; Hernandez-Jerez, A.F.; Bennekou, S.H.; Klein, M.; et al. Scientific Opinion addressing the state of the science on risk assessment of plant protection products for in-soil organisms. EFSA J. 2017, 15, e04690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães, B.; Maria, V.L.; Römbke, J.; Amorim, M.J.B. Multigenerational exposure of Folsomia candida to ivermectin—Using avoidance, survival, reproduction, size and cellular markers as endpoints. Geoderma 2019, 337, 273–279. [Google Scholar] [CrossRef]
- Tourinho, P.S.; van Gestel, C.A.M.; Jurkschat, K.; Soares, A.M.V.M.; Loureiro, S. Effects of soil and dietary exposures to Ag nanoparticles and AgNO3 in the terrestrial isopod Porcellionides pruinosus. Environ. Pollut. 2015, 205, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, M.C.P.; Rodrigues, N.P.; Scott-Fordsmand, J.J.; de Jesus, M.B.; Amorim, M.J.B. The toxicity of silver nanomaterials (NM 300K) is reduced when combined with N-Acetylcysteine: Hazard assessment on Enchytraeus crypticus. Environ. Pollut. 2020, 256, 113484. [Google Scholar] [CrossRef] [PubMed]
- Bicho, R.C.; Santos, F.C.F.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Multigenerational effects of copper nanomaterials (CuONMs) are different of those of CuCl2: Exposure in the soil invertebrate Enchytraeus crypticus. Sci. Rep. 2017, 7, 8457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bicho, R.C.; Ribeiro, T.; Rodrigues, N.P.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Effects of Ag nanomaterials (NM300K) and Ag salt (AgNO3) can be discriminated in a full life cycle long term test with Enchytraeus crypticus. J. Hazard. Mater. 2016, 318, 608–614. [Google Scholar] [CrossRef]
- Bicho, R.C.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Multigenerational Exposure to WCCo Nanomaterials—Epigenetics in the Soil Invertebrate Enchytraeus crypticus. Nanomaterials 2020, 10, 836. [Google Scholar] [CrossRef]
- Bicho, R.C.; Faustino, A.M.R.; Rêma, A.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Confirmatory assays for transient changes of omics in soil invertebrates—Copper materials in a multigenerational exposure. J. Hazard. Mater. 2021, 402, 123500. [Google Scholar] [CrossRef]
- Ribeiro, M.J.; Maria, V.L.; Soares, A.M.V.M.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Fate and effect of nano tungsten carbide cobalt (WCCo) in the soil environment: Observing a nanoparticle specific toxicity in Enchytraeus crypticus. Environ. Sci. Technol. 2018, 52, 11394–11401. [Google Scholar] [CrossRef]
- Ribeiro, M.J.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Multigenerational exposure to cobalt (CoCl2) and WCCo nanoparticles in Enchytraeus crypticus. Nanotoxicology 2019, 13, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.C.F.; Gomes, S.I.L.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Hazard assessment of nickel nanoparticles in soil—The use of a full life cycle test with Enchytraeus crypticus. Environ. Toxicol. Chem. 2017, 36, 2934–2941. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.F.M.; Gomes, S.I.L.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Shorter lifetime of a soil invertebrate species when exposed to copper oxide nanoparticles in a full lifespan exposure test. Sci. Rep. 2017, 7, 1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bicho, R.C.; Santos, F.C.F.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Effects of copper oxide nanomaterials (CuONMs) are life stage dependent—Full life cycle in Enchytraeus crypticus. Environ. Pollut. 2017, 224, 117–124. [Google Scholar] [CrossRef]
- Bicho, R.C.; Roelofs, D.; Mariën, J.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Epigenetic effects of (nano)materials in environmental species—Cu case study in Enchytraeus crypticus. Environ. Int. 2020, 136, 105447. [Google Scholar] [CrossRef]
- Noordhoek, J.W.; Pipicelli, F.; Barone, I.; Franken, O.; Montagne-Wajer, K.; Mariën, J.; Verweij, R.A.; van Gestel, C.A.M.; van Straalen, N.M.; Roelofs, D. Phenotypic and transcriptional responses associated with multi-generation exposure of Folsomia candida to engineered nanomaterials. Environ. Sci. Nano 2018, 5, 2426–2439. [Google Scholar] [CrossRef]
- OECD. Test No. 220: Enchytraeid Reproduction Test; OECD Guidelines for the Testing of Chemicals, Section 2; Organization for Economic Cooperation and Development (OECD): Paris, France, 2016; ISBN 9789264264472. [Google Scholar]
- OECD. Series on the Safety of Manufactured Nanomaterials, No. 36: Guidance on Sample Preparation and Dosimetry for the Safety Testing of Manufactured Nanomaterials; OECD: Paris, France, 2012. [Google Scholar]
- Bicho, R.C.; Santos, F.C.F.; Gonçalves, M.F.M.; Soares, A.M.V.M.; Amorim, M.J.B. Enchytraeid Reproduction TestPLUS: Hatching, growth and full life cycle test—An optional multi-endpoint test with Enchytraeus crypticus. Ecotoxicology 2015, 24, 1053–1063. [Google Scholar] [CrossRef]
- ISO. Soil Quality—Avoidance Test for Determining the Quality of Soils and Effects of Chemicals on Behaviour—Part 1: Test with Earthworms (Eisenia Fetida and Eisenia Andrei); ISO: Geneva, Switzerland, 2008; Volume 25, p. 17512-1. [Google Scholar]
- Bicho, R.C.; Gomes, S.I.L.; Soares, A.M.V.M.; Amorim, M.J.B. Non-avoidance behaviour in enchytraeids to boric acid is related to the GABAergic mechanism. Environ. Sci. Pollut. Res. 2015, 22, 6898–6903. [Google Scholar] [CrossRef]
- Tourinho, P.S.; van Gestel, C.A.M.; Lofts, S.; Svendsen, C.; Soares, A.M.V.M.; Loureiro, S. Metal-based nanoparticles in soil: Fate, behavior, and effects on soil invertebrates. Environ. Toxicol. Chem. 2012, 31, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Hassellöv, M.; Readman, J.W.; Ranville, J.F.; Tiede, K. Nanoparticle analysis and characterization methodologies in environmental risk assessment of engineered nanoparticles. Ecotoxicology 2008, 17, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sis, H.; Birinci, M. Effect of nonionic and ionic surfactants on zeta potential and dispersion properties of carbon black powders. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 341, 60–67. [Google Scholar] [CrossRef]
- Pizzorno, L. Nothing Boring about Boron. Integr. Med. 2015, 14, 35–48. [Google Scholar] [CrossRef]
- Dessordi, R.; Spirlandeli, A.L.; Zamarioli, A.; Volpon, J.B.; Navarro, A.M. Boron supplementation improves bone health of non-obese diabetic mice. J. Trace Elem. Med. Biol. 2017, 39, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Białek, M.; Czauderna, M.; Krajewska, K.A.; Przybylski, W. Selected physiological effects of boron compounds for animals and humans. A review. J. Anim. Feed Sci. 2019, 28, 307–320. [Google Scholar] [CrossRef]
- Naghii, M.R.; Samman, S. The effect of boron on plasma testosterone and plasma lipids in rats. Nutr. Res. 1997, 17, 523–531. [Google Scholar] [CrossRef]
- Naghii, M.R.; Samman, S. The effect of boron supplementation on the distribution of boron in selected tissues and on testosterone synthesis in rats. J. Nutr. Biochem. 1996, 7, 507–512. [Google Scholar] [CrossRef]
- Fail, P.A.; Georg, J.D.; Curtis, J.S.; Grizzle, T.B.; Heindel, J.J. Reproductive toxicity of boric acid in Swiss (CD-1) mice: Assessment using the continuous breeding protocol. Fundam. Appl. Toxicol. 1991, 17, 225–239. [Google Scholar] [CrossRef]
- Domingo, J.L. Vanadium: A review of the reproductive and developmental toxicity. Reprod. Toxicol. 1996, 10, 175–182. [Google Scholar] [CrossRef]
- Boron—Registration Dossier—ECHA. Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/14776 (accessed on 24 June 2021).
- Park, E.J.; Lee, G.H.; Yoon, C.; Kim, D.W. Comparison of distribution and toxicity following repeated oral dosing of different vanadium oxide nanoparticles in mice. Environ. Res. 2016, 150, 154–165. [Google Scholar] [CrossRef]
- Li, L.; Wu, H.; Ji, C.; van Gestel, C.A.M.; Allen, H.E.; Peijnenburg, W.J.G.M. A metabolomic study on the responses of Daphnia magna exposed to silver nitrate and coated silver nanoparticles. Ecotoxicol. Environ. Saf. 2015, 119, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, T.; Ingle, T.; Yan, J.; He, W.; Yin, J.-J.; Chen, T. Differential genotoxicity mechanisms of silver nanoparticles and silver ions. Arch. Toxicol. 2017, 91, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Lemus, M.; Bizarro-Nevares, P.; López-Valdez, N.; González-Villalva, A.; Guerrero-Palomo, G.; Eugenia Cervantes-Valencia, M.; Tavera-Cabrera, O.; Rivera-Fernández, N.; Casarrubias-Tabarez, B.; Ustarroz-Cano, M.; et al. Oxidative Stress and Vanadium. In Genotoxicity and Mutagenicity—Mechanisms and Test Methods; IntechOpen: London, UK, 2021. [Google Scholar]
- Tierney, K.B. Chemical avoidance responses of fishes. Aquat. Toxicol. 2016, 174, 228–241. [Google Scholar] [CrossRef] [PubMed]
| Exposure Characteristics | Assessed Endpoints | Main Findings | Rf |
|---|---|---|---|
| Copper Oxide Nanoparticles (CuONPs) | |||
| Multigenerational (MG) exposure 1 year | - Survival - Reproduction | - CuONPs increased toxicity for EC10 exposed organisms; - CuONPs showed mechanisms of toxicity in the longer-term exposures, not predictable based on short-term studies. | [28] |
| Full life cycle (FLC) test 46 days (d) | - Hatching - Growth - Maturity - Survival - Reproduction | - CuONPs caused toxicity during the juvenile stage, reducing growth, maturation, and reproductive output; - EC50 maturity status (25 d): 3833 mg/kg; - EC50 reproduction (46 d): 1075 mg/kg. | [36] |
| MG exposure 224 d | - Global DNA methylation - Gene-specific methylation - Gene expression | - CuONPs increased global DNA methylation; - Changes in the epigenetic, stress, and detoxification gene targets, also occurring in post-exposure generations. | [37] |
| FLC test + MG exposure 46 and 224 d | - Histology - Immuno-histochemistry | - No tissue alterations; - CuONPs affected the Notch signaling pathway. | [31] |
| Lifespan test 202 d | - Survival - Reproduction | - CuONPs caused shorter life of the adults; - A more amplified effect was found in terms of reproduction. | [35] |
| Nickel Nanoparticles (NiNPs) | |||
| FLC test 46 d | - Hatching - Growth - Maturity - Survival - Reproduction | - Hatching was the most sensitive endpoint, although the organisms recovered; - EC50 hatching (11 d): 870 mg/kg; EC50 growth (25 d): > 3200 mg/kg; EC50 maturity status (25 d): 3946 mg/kg; EC50 survival (46 d): 3627 mg/kg; EC50 reproduction (46 d): 3455 mg/kg. | [34] |
| Silver Nanoparticles (Ag NM300K) | |||
| FLC test 46 d | - Hatching - Growth - Maturity - Survival - Reproduction | - Ag NM300K caused a non-monotonic concentration-response effect; - EC50 hatching (11 d): 61 mg/kg; EC50 maturity status (25 d): 131 mg/kg; EC50 survival (46 d): 99 mg/kg; EC50 reproduction (46 d): 103 mg/kg. | [29] |
| Tungsten Carbide Cobalt Nanoparticles (WCCoNPs) | |||
| Enchytraeid Reproduction Test extension 56 d | - Survival - Reproduction | - WCCoNPs caused no effect on survival; - EC50 reproduction (28 d): 1500 mg/kg; EC50 reproduction (56 d): 128 mg/kg. | [32] |
| MG exposure 224 d | - Survival - Reproduction | - MG exposure did not increase toxicity; - An increase in reproduction at low concentrations of WCCoNPs was found. | [33] |
| MG exposure 224 d | - Global DNA methylation | - MG exposure increased global DNA methylation, which continued in unexposed generations and was associated with an increase in reproduction. | [30] |
| Test Materials | EC20 (mg/kg) | EC50 (mg/kg) | EC80 (mg/kg) |
|---|---|---|---|
| Survival at 28 d | |||
| BNPs | n.e. | n.e. | n.e. |
| VNPs | n.d. | n.d. | n.d. |
| Reproduction | |||
| BNPs | |||
| 28 d | 217.0 ± 79.4 | 319.0 ± 59.5 | 393.8 ± 72.8 |
| 56 d | 111 ± 32.4 | 210.0 ± 70.8 | 308.0 ± 115.1 |
| VNPs | |||
| 28 d | 5.0 ± 1.4 | 11.0 ± 1.5 | 18.0 ± 3.0 |
| 56 d | 19.0 ± 9.8 | 62.0 ± 9.0 | 105.0 ± 15.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreto, A.; Santos, J.; Amorim, M.J.B.; Maria, V.L. Environmental Hazards of Boron and Vanadium Nanoparticles in the Terrestrial Ecosystem—A Case Study with Enchytraeus crypticus. Nanomaterials 2021, 11, 1937. https://doi.org/10.3390/nano11081937
Barreto A, Santos J, Amorim MJB, Maria VL. Environmental Hazards of Boron and Vanadium Nanoparticles in the Terrestrial Ecosystem—A Case Study with Enchytraeus crypticus. Nanomaterials. 2021; 11(8):1937. https://doi.org/10.3390/nano11081937
Chicago/Turabian StyleBarreto, Angela, Joana Santos, Mónica J. B. Amorim, and Vera L. Maria. 2021. "Environmental Hazards of Boron and Vanadium Nanoparticles in the Terrestrial Ecosystem—A Case Study with Enchytraeus crypticus" Nanomaterials 11, no. 8: 1937. https://doi.org/10.3390/nano11081937
APA StyleBarreto, A., Santos, J., Amorim, M. J. B., & Maria, V. L. (2021). Environmental Hazards of Boron and Vanadium Nanoparticles in the Terrestrial Ecosystem—A Case Study with Enchytraeus crypticus. Nanomaterials, 11(8), 1937. https://doi.org/10.3390/nano11081937









