Construction of Chitosan/Alginate Nano-Drug Delivery System for Improving Dextran Sodium Sulfate-Induced Colitis in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of RBITC Labeled CS
2.3. Preparation of NPs
2.4. Characterization of NPs
2.4.1. Particle Size and Zeta Potential
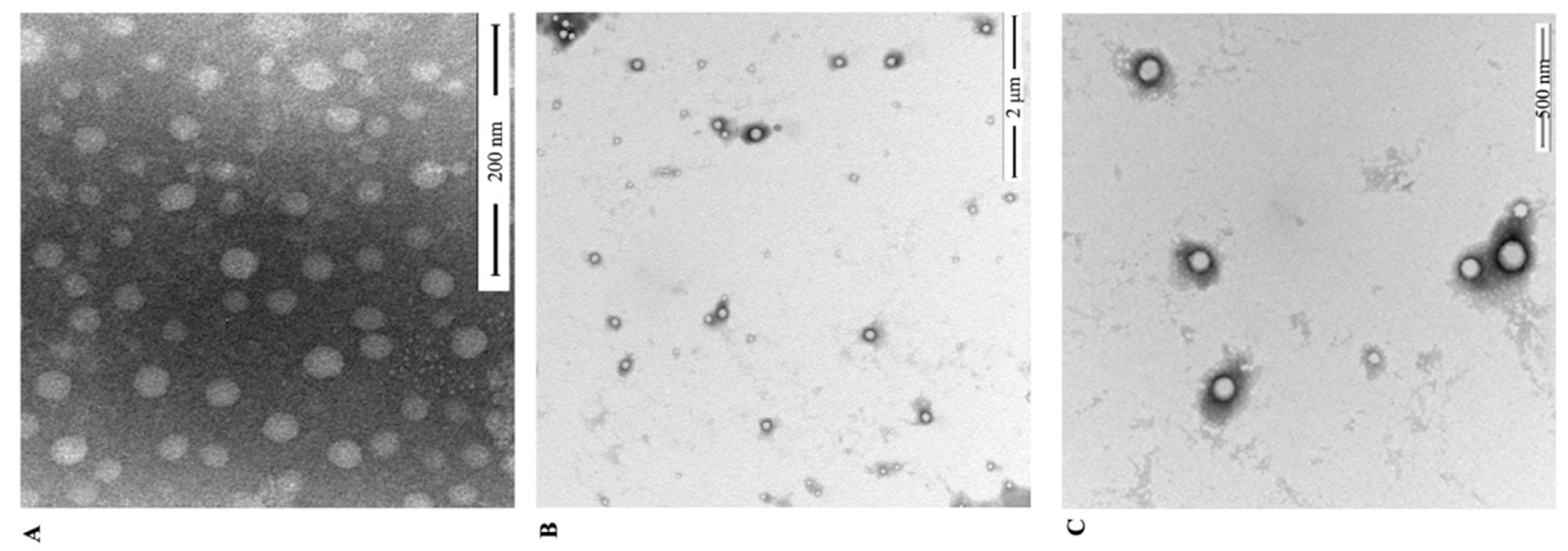
2.4.2. TEM
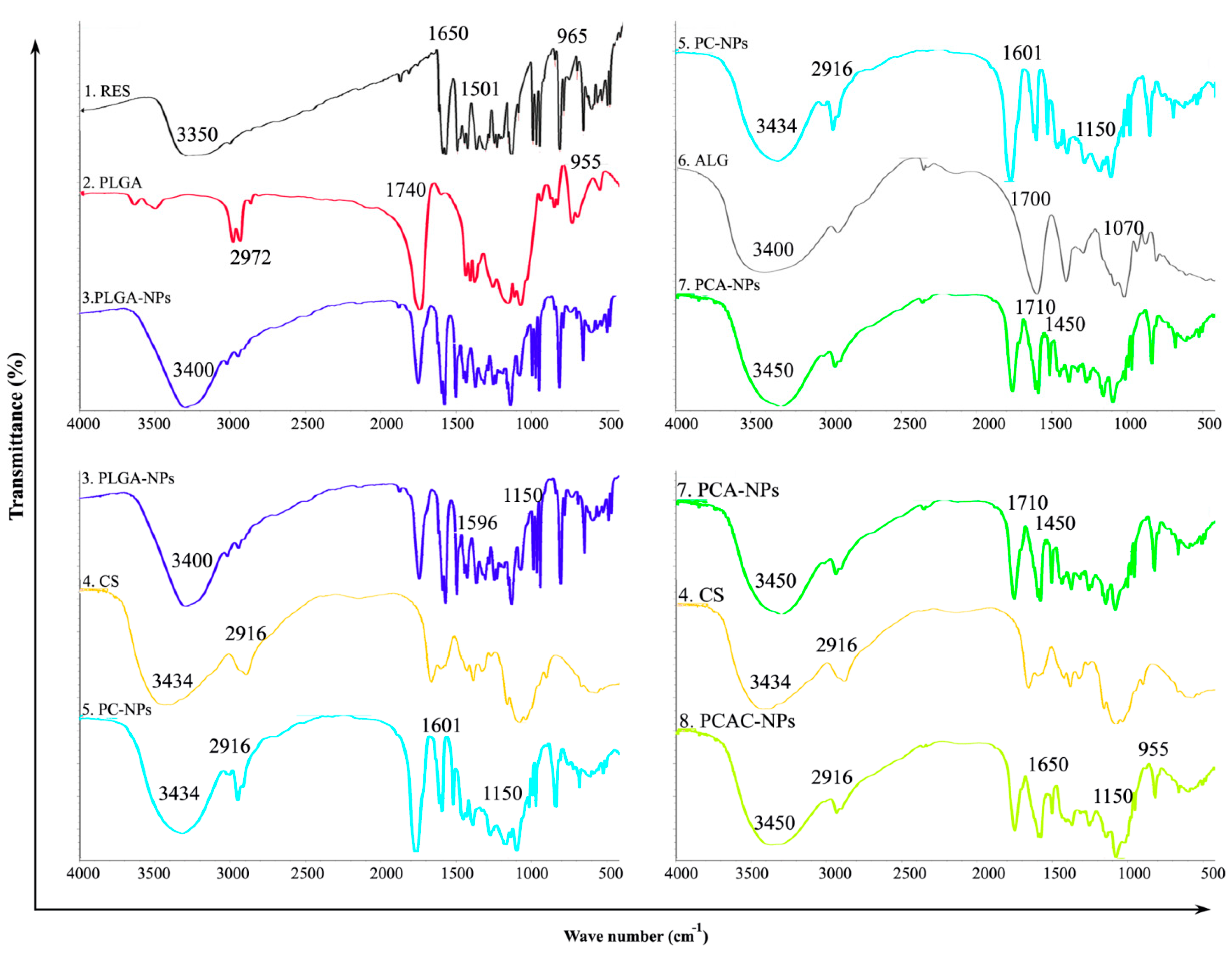
2.4.3. FT-IR
2.4.4. Encapsulation Efficiency (EE) and Drug Loading Capacity (LC)
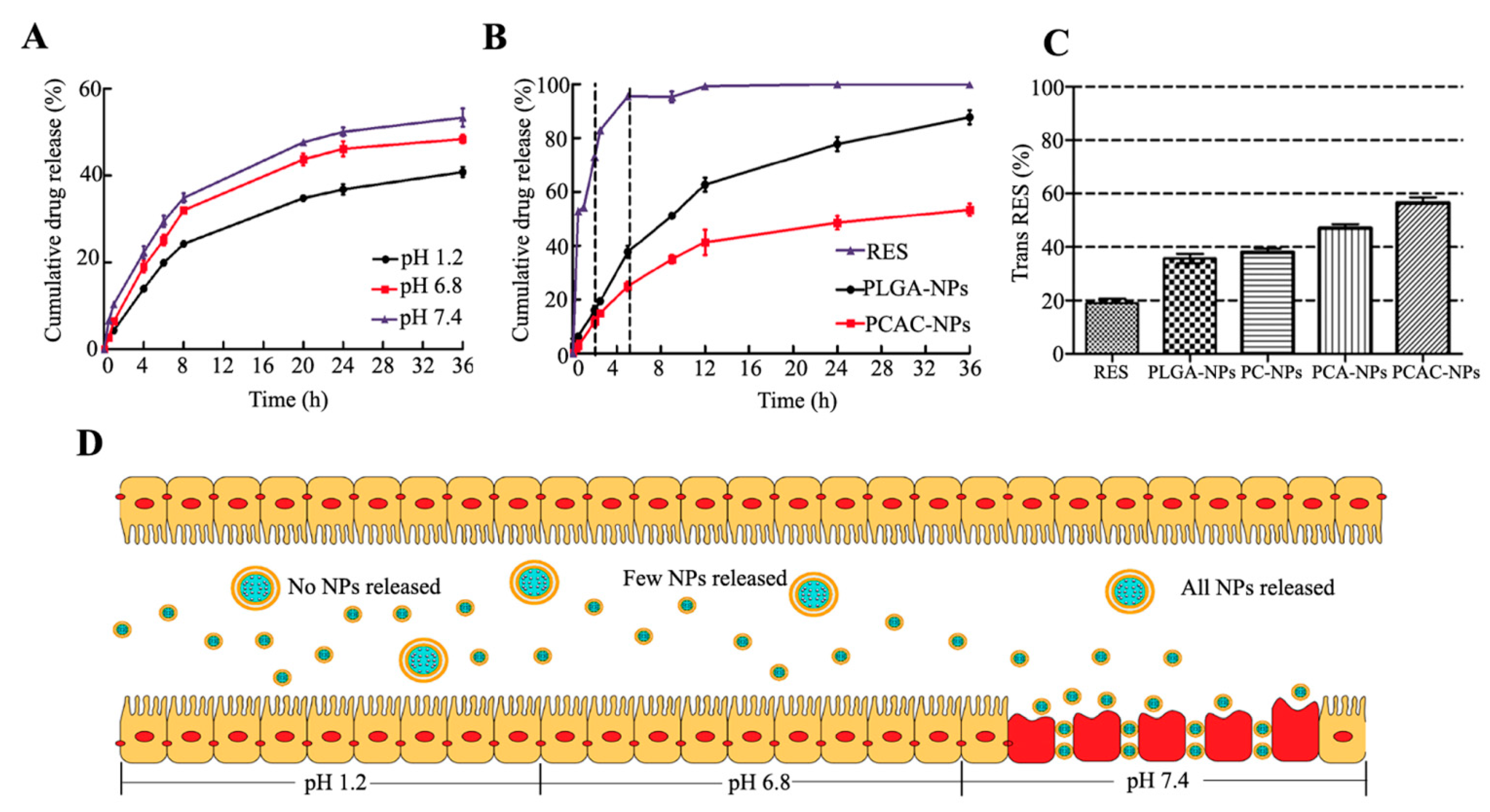
2.4.5. In Vitro Drug Release
2.4.6. RES Protection
2.5. Cell Cultures
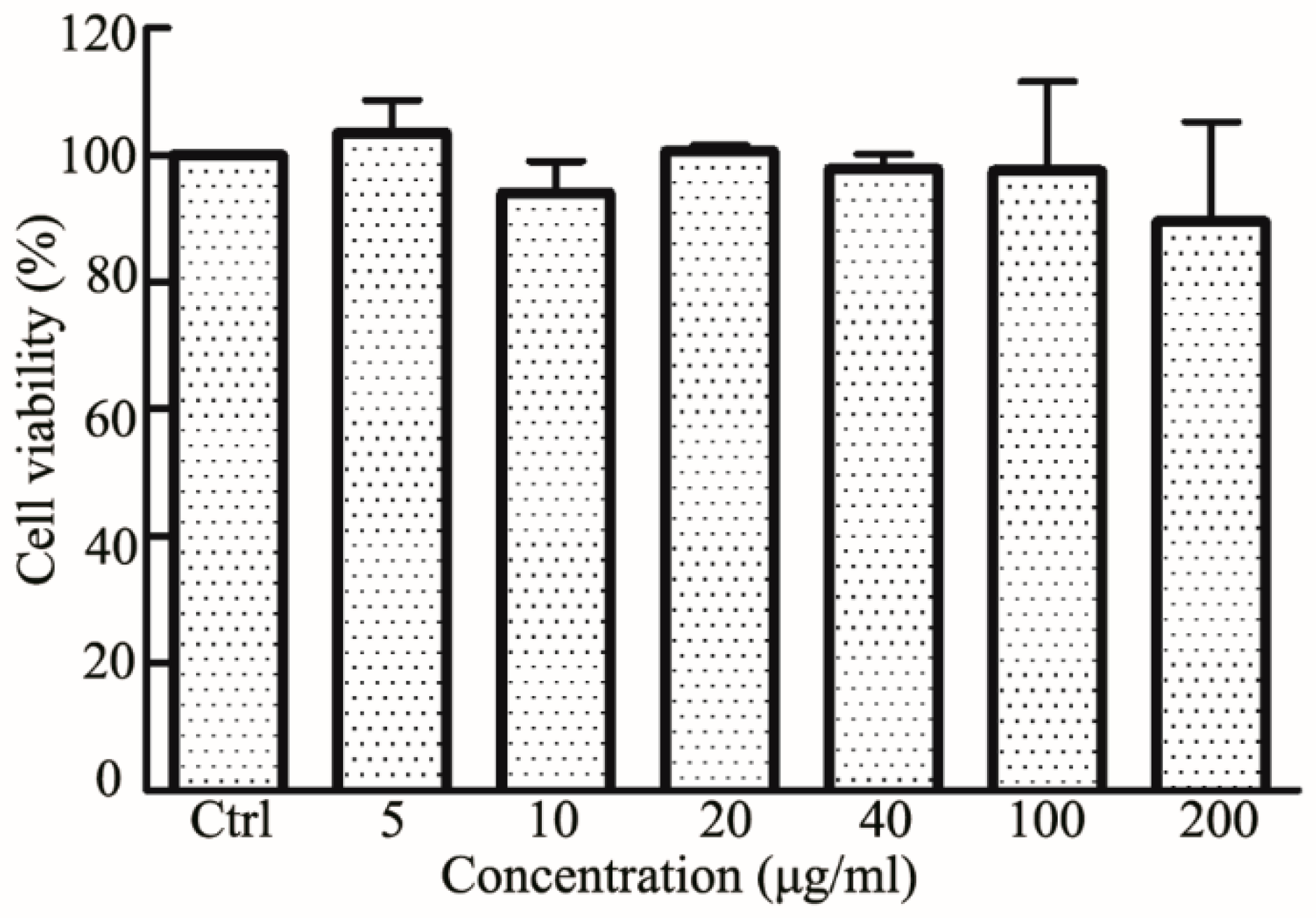
2.6. Biocompatibility Assays
2.7. Cellular Uptake
2.8. Animals
2.8.1. In Vivo Targeted Tracking
2.8.2. Adhesion Experiment
2.8.3. DSS-Induced UC Model
2.8.4. HE Staining and MPO Assay
2.8.5. Antioxidant Capacity Determination
3. Results
3.1. Preparation and Characterization of RES-PCAC-NPs
3.2. FT-IR Analysis
3.3. In Vitro Drug Release
3.4. RES Protection
3.5. Cytotoxicity of RES-PCAC-NPs
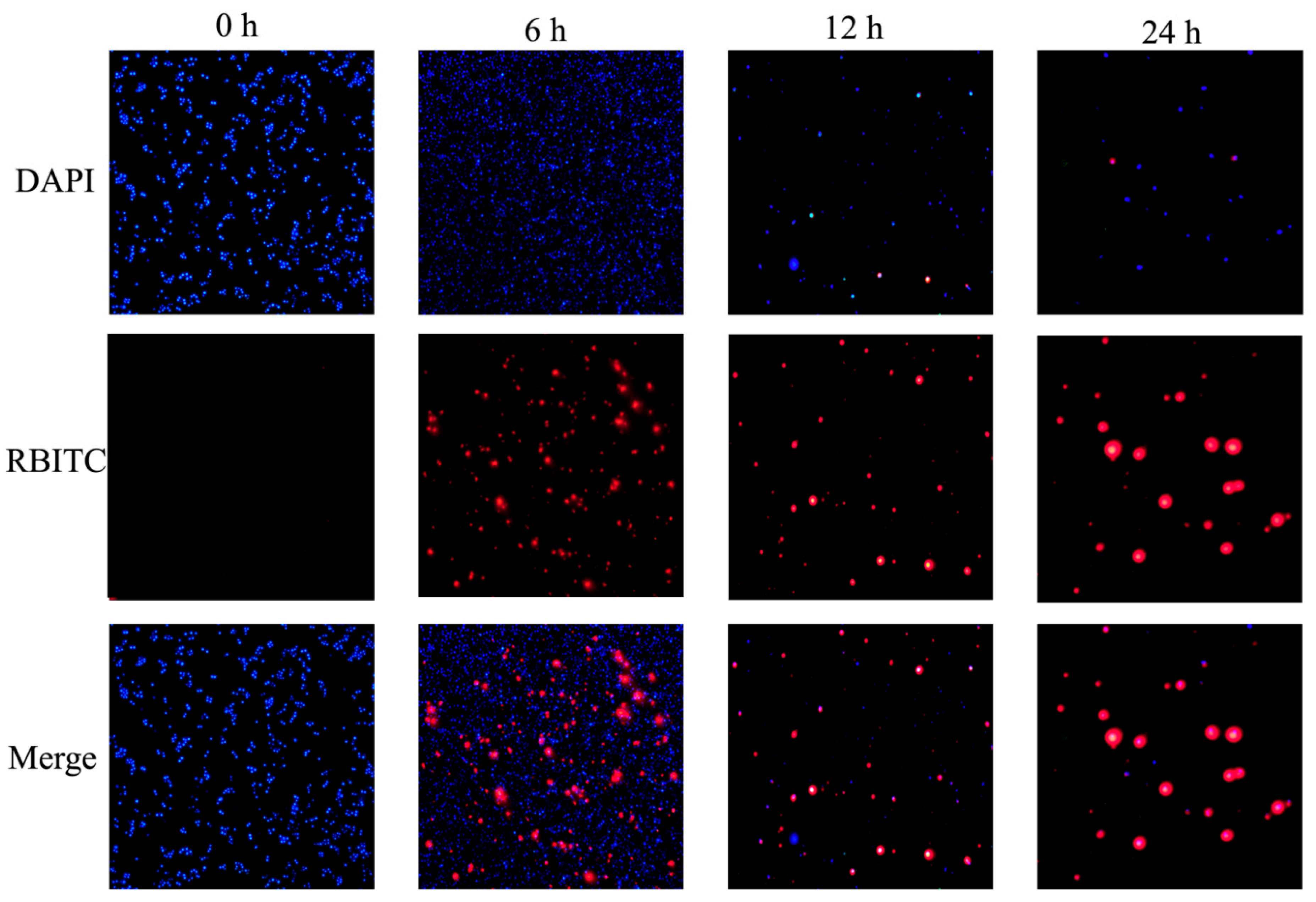
3.6. Cellular Uptake of NPs
3.7. Animals
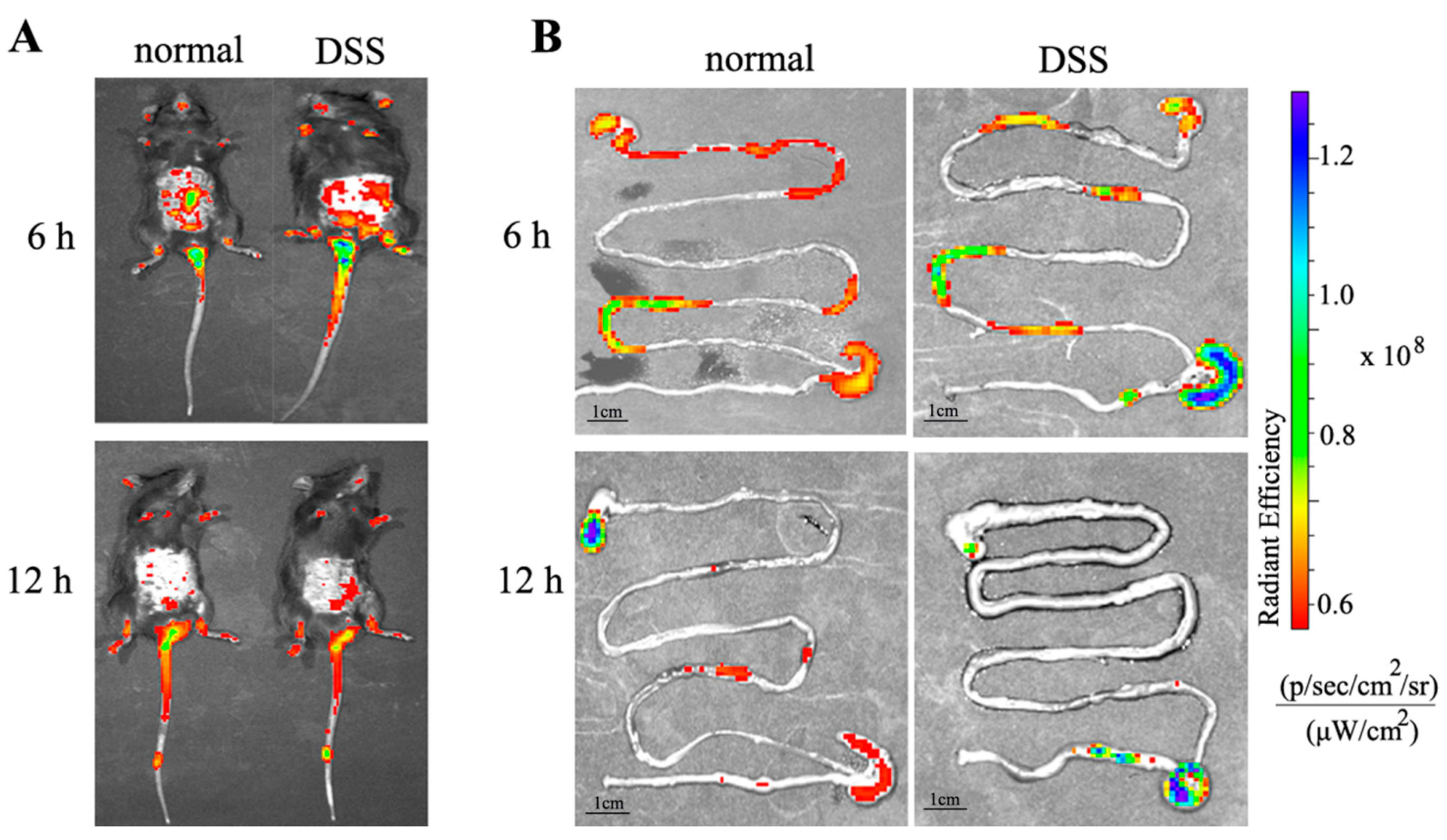
3.7.1. Distribution of NPs in the Body
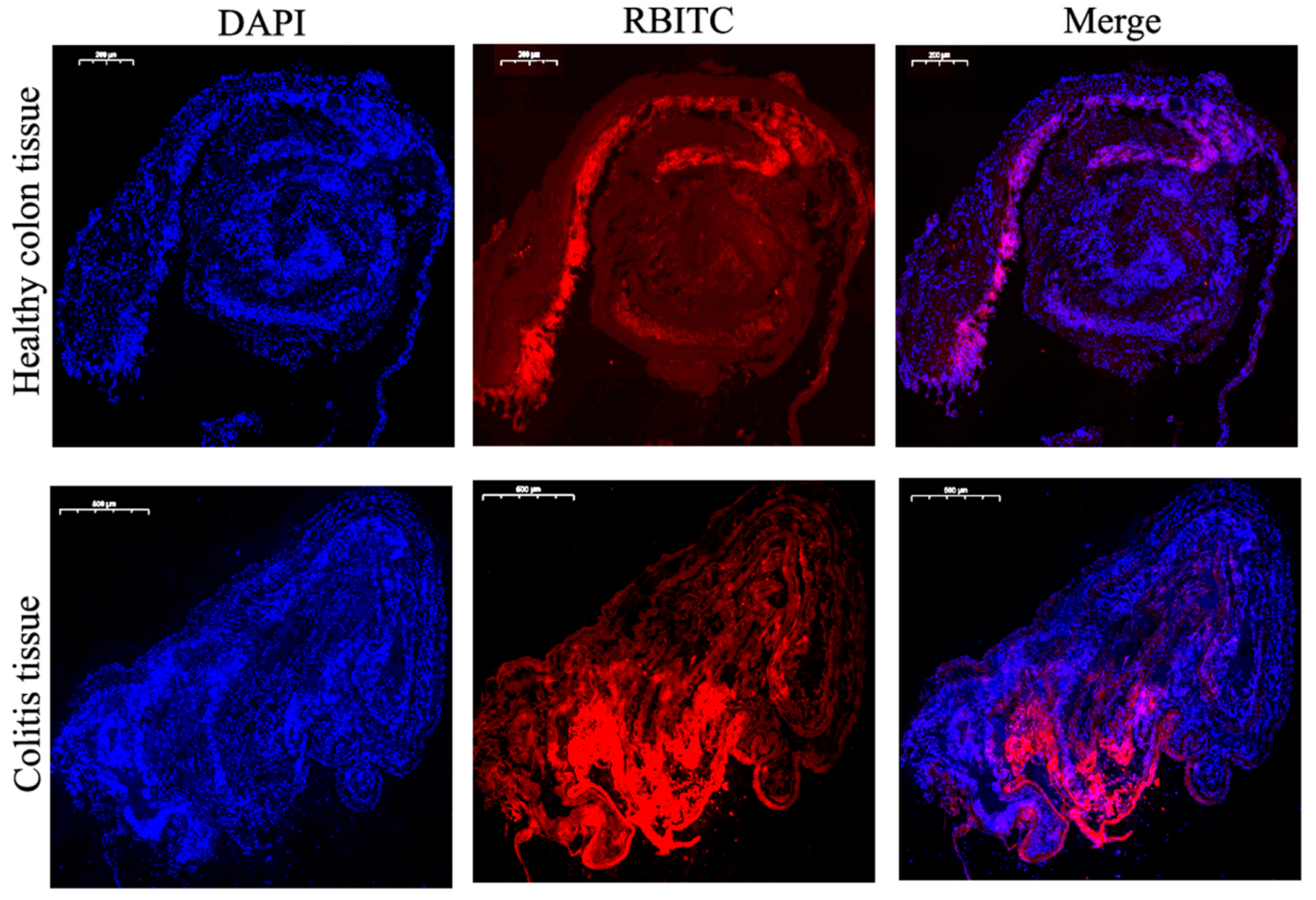
3.7.2. Adhesion and Penetration in Colonic Tissue
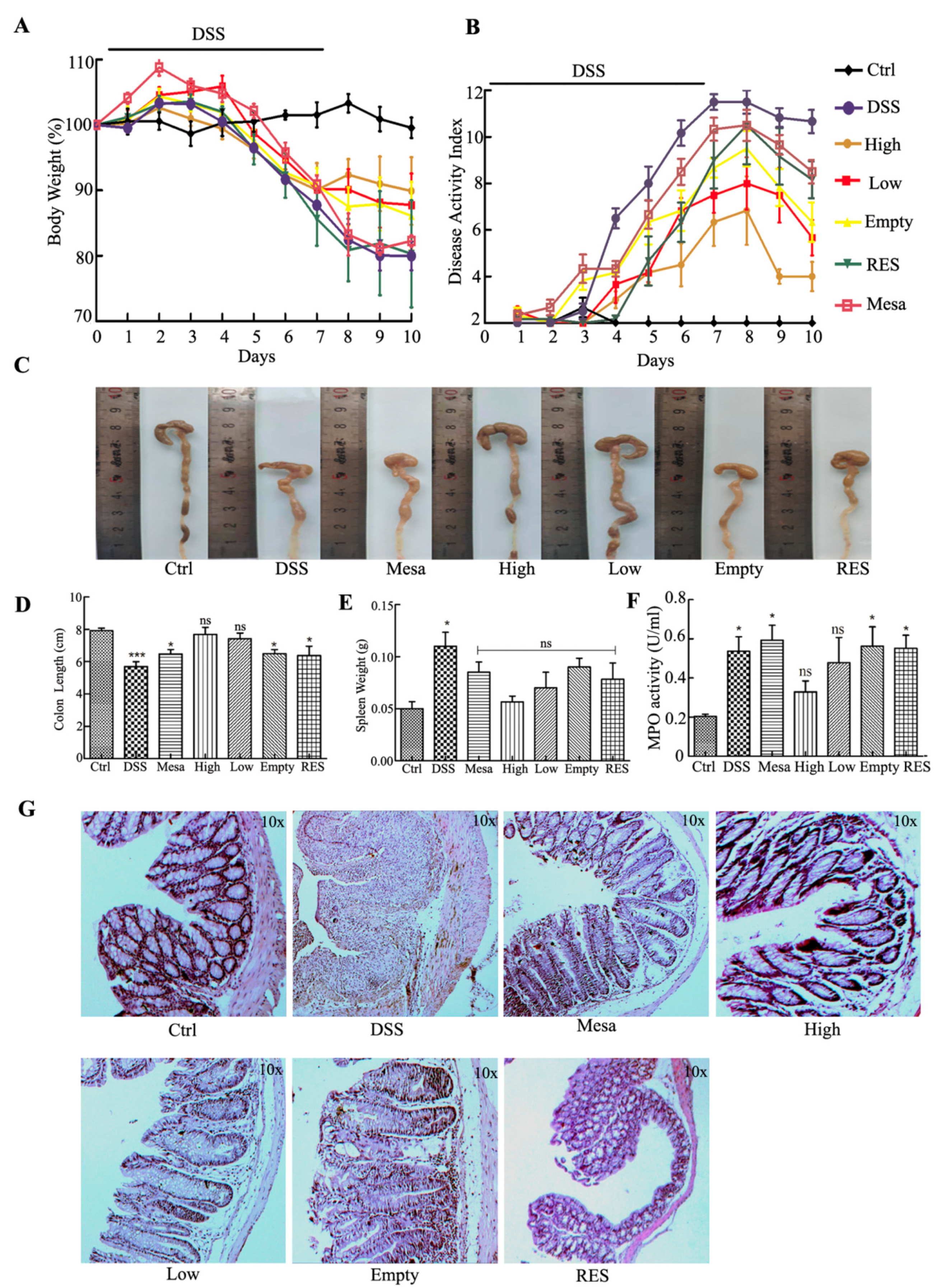
3.7.3. Body Weight and Macroscopic Examination
3.7.4. Histological and MPO Analysis
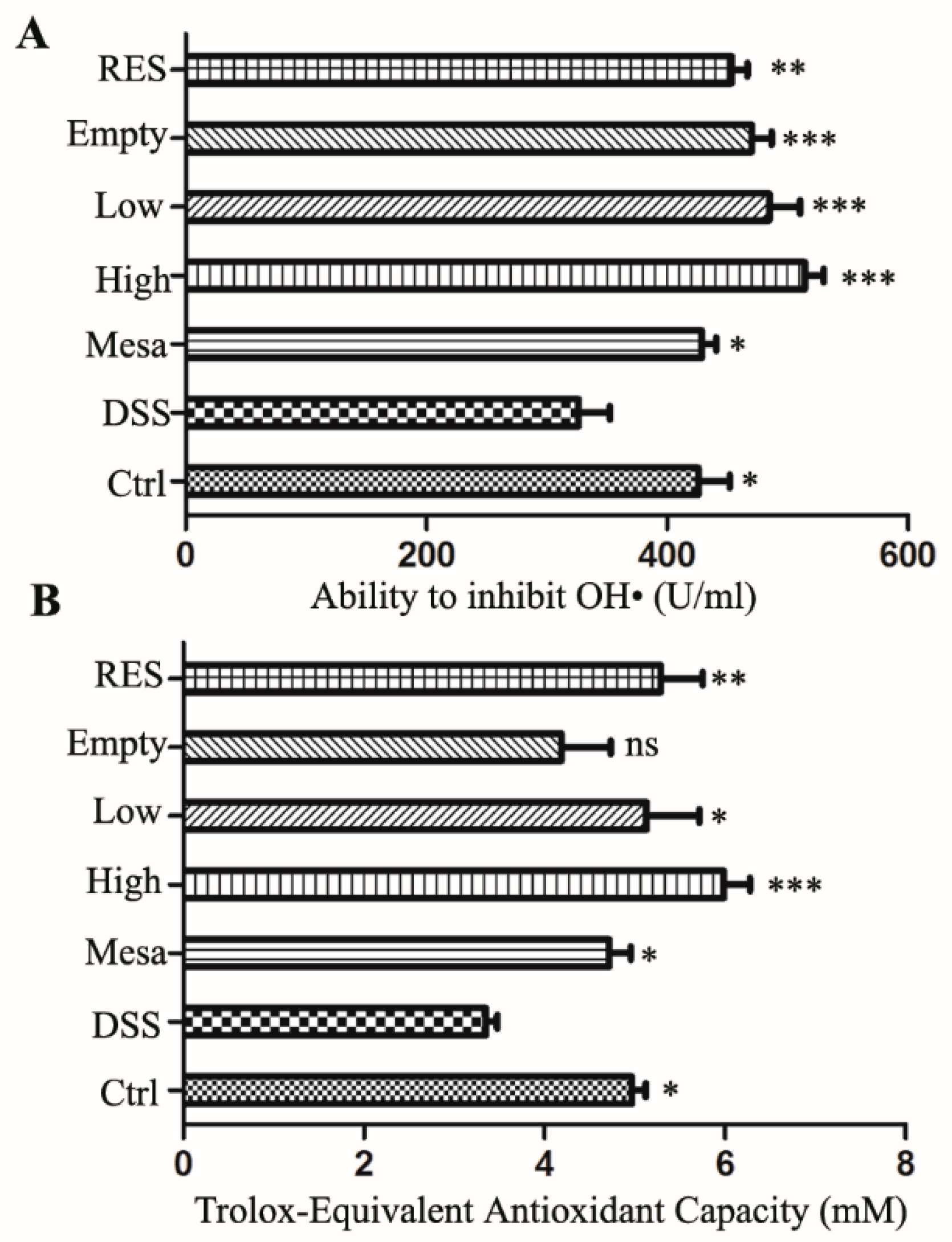
3.7.5. Antioxidant Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Locatelli, M.; Governatori, L.; Carlucci, G.; Genovese, S.; Mollica, A.; Epifano, F. Recent application of analytical methods to phase I and phase II drugs development: A review. Biomed. Chromatogr. 2012, 26, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Furton, K.G.; Tinari, N.; Grossi, L.; Innosa, D.; Macerola, D.; Tartaglia, A.; Di Donato, V.; D’Ovidio, C.; Locatelli, M. Fabric phase sorptive extraction-high performance liquid chromatography-photo diode array detection method for simultaneous monitoring of three inflammatory bowel disease treatment drugs in whole blood, plasma and urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1084, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.; Wu, H.; Neish, A.S.; Champion, J.A. Alginate/chitosan microparticles for gastric passage and intestinal release of therapeutic protein nanoparticles. J. Control. Release 2019, 295, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Laroui, H.; Sitaraman, S.V.; Merlin, D. Gastrointestinal delivery of anti-inflammatory nanoparticles. Methods Enzymol. 2012, 509, 101–125. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Wang, X.; Li, X.; Xiao, B. Effect of particle size on the cellular uptake and anti-inflammatory activity of oral nanotherapeutics. Colloids Surf. B Biointerfaces 2020, 187, 110880. [Google Scholar] [CrossRef]
- Bernstein, C.N. Treatment of IBD: Where we are and where we are going. Am. J. Gastroenterol. 2015, 110, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M. Current and emerging therapeutic targets for IBD. Nature reviews. Gastroenterol. Hepatol. 2017, 14, 688. [Google Scholar] [CrossRef]
- Feagan, B.G.; Chande, N.; MacDonald, J.K. Are there any differences in the efficacy and safety of different formulations of Oral 5-ASA used for induction and maintenance of remission in ulcerative colitis? evidence from cochrane reviews. Inflamm. Bowel Dis. 2013, 19, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, P.; Colombel, J.F.; Aboubakr, A.; Narula, N. Systematic review: Safety of mesalazine in ulcerative colitis. Aliment. Pharmacol. Ther. 2018, 47, 1597–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, A.; Nguyen, T.M.; Parker, C.E.; Feagan, B.G.; MacDonald, J.K. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2020, 8, Cd000544. [Google Scholar] [CrossRef] [PubMed]
- Sanna, V.; Roggio, A.M.; Siliani, S.; Piccinini, M.; Marceddu, S.; Mariani, A.; Sechi, M. Development of novel cationic chitosan-and anionic alginate-coated poly(D,L-lactide-co-glycolide) nanoparticles for controlled release and light protection of resveratrol. Int. J. Nanomed. 2012, 7, 5501–5516. [Google Scholar] [CrossRef] [Green Version]
- Thirumdas, R.; Kothakota, A.; Pandiselvam, R.; Bahrami, A.; Barba, F.J. Role of food nutrients and supplementation in fighting against viral infections and boosting immunity: A review. Trends Food Sci. Technol. 2021, 110, 66–77. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Rout, L.; Jena, M.; Efferth, T.; Bhutia, S.K. Chemotherapeutic efficacy of curcumin and resveratrol against cancer: Chemoprevention, chemoprotection, drug synergism and clinical pharmacokinetics. Semin. Cancer Biol. 2020, 73, 310–320. [Google Scholar] [CrossRef]
- Prysyazhna, O.; Wolhuter, K.; Switzer, C.; Santos, C.; Yang, X.; Lynham, S.; Shah, A.M.; Eaton, P.; Burgoyne, J.R. Blood Pressure-Lowering by the Antioxidant Resveratrol Is Counterintuitively Mediated by Oxidation of cGMP-Dependent Protein Kinase. Circulation 2019, 140, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef]
- Moran, B.W.; Anderson, F.P.; Devery, A.; Cloonan, S.; Butler, W.E.; Varughese, S.; Draper, S.M.; Kenny, P.T. Synthesis, structural characterisation and biological evaluation of fluorinated analogues of resveratrol. Bioorganic Med. Chem. 2009, 17, 4510–4522. [Google Scholar] [CrossRef] [PubMed]
- Ramli, N.Z.; Yahaya, M.F.; Tooyama, I.; Damanhuri, H.A. A Mechanistic Evaluation of Antioxidant Nutraceuticals on Their Potential against Age-Associated Neurodegenerative Diseases. Antioxidants 2020, 9, 1019. [Google Scholar] [CrossRef]
- Jimenez-Gomez, Y.; Mattison, J.A.; Pearson, K.J.; Martin-Montalvo, A.; Palacios, H.H.; Sossong, A.M.; Ward, T.M.; Younts, C.M.; Lewis, K.; Allard, J.S.; et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 2013, 18, 533–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez de Vega, M.J.; Moreno-Fernández, S.; Pontes-Quero, G.M.; González-Amor, M.; Vázquez-Lasa, B.; Sabater-Muñoz, B.; Briones, A.M.; Aguilar, M.R.; Miguel, M.; González-Muñiz, R. Characterization of Novel Synthetic Polyphenols: Validation of Antioxidant and Vasculoprotective Activities. Antioxidants 2020, 9, 787. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Han, Y.; Cai, X.; Gu, M.; Sun, J.; Qi, C.; Goulette, T.; Song, M.; Li, Z.; Xiao, H. Dietary resveratrol attenuated colitis and modulated gut microbiota in dextran sulfate sodium-treated mice. Food Funct. 2020, 11, 1063–1073. [Google Scholar] [CrossRef]
- Jhanji, M.; Rao, C.N.; Sajish, M. Towards resolving the enigma of the dichotomy of resveratrol: Cis- and trans-resveratrol have opposite effects on TyrRS-regulated PARP1 activation. GeroScience 2020, 1–30. [Google Scholar] [CrossRef]
- Signorelli, P.; Ghidoni, R. Resveratrol as an anticancer nutrient: Molecular basis, open questions and promises. J. Nutr. Biochem. 2005, 16, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.S.; Dalmasso, G.; Wang, L.; Sitaraman, S.V.; Merlin, D.; Murthy, N. Orally delivered thioketal nanoparticles loaded with TNF-α-siRNA target inflammation and inhibit gene expression in the intestines. Nat. Mater. 2010, 9, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Xu, Z.; Viennois, E.; Zhang, Y.; Zhang, Z.; Zhang, M.; Han, M.K.; Kang, Y.; Merlin, D. Orally Targeted Delivery of Tripeptide KPV via Hyaluronic Acid-Functionalized Nanoparticles Efficiently Alleviates Ulcerative Colitis. Mol. Ther. 2017, 25, 1628–1640. [Google Scholar] [CrossRef] [Green Version]
- Mundargi, R.C.; Babu, V.R.; Rangaswamy, V.; Patel, P.; Aminabhavi, T.M. Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. J. Control. Release 2008, 125, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Quijano, E.; Liu, Y.; Bahal, R.; Scanlon, S.E.; Song, E.; Hsieh, W.C.; Braddock, D.E.; Ly, D.H.; Saltzman, W.M.; et al. Anti-tumor Activity of miniPEG-γ-Modified PNAs to Inhibit MicroRNA-210 for Cancer Therapy. Molecular therapy. Nucleic Acids 2017, 9, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Han, F.Y.; Thurecht, K.J.; Whittaker, A.K.; Smith, M.T. Bioerodable PLGA-Based Microparticles for Producing Sustained-Release Drug Formulations and Strategies for Improving Drug Loading. Front. Pharmacol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinhold, S.E.; Schwendeman, S.P. Effect of polymer porosity on aqueous self-healing encapsulation of proteins in PLGA microspheres. Macromol. Biosci. 2013, 13, 1700–1710. [Google Scholar] [CrossRef] [Green Version]
- Zaharoff, D.A.; Rogers, C.J.; Hance, K.W.; Schlom, J.; Greiner, J.W. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine 2007, 25, 2085–2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Ratnatilaka Na Bhuket, P.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/alginate nanoparticles as a promising approach for oral delivery of curcumin diglutaric acid for cancer treatment. Mater. Sci. Eng. C Mater. Biol. L Appl. 2018, 93, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Natrajan, D.; Srinivasan, S.; Sundar, K.; Ravindran, A. Formulation of essential oil-loaded chitosan-alginate nanocapsules. J. Food Drug Anal. 2015, 23, 560–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, M.A.; Bourbon, A.I.; Vicente, A.A.; Cerqueira, M.A. Alginate/chitosan nanoparticles for encapsulation and controlled release of vitamin B2. Int. J. Biol. Macromol. 2014, 71, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Xu, C.; Liu, D.; Han, M.K.; Wang, L.; Merlin, D. Oral Delivery of Nanoparticles Loaded with Ginger Active Compound, 6-Shogaol, Attenuates Ulcerative Colitis and Promotes Wound Healing in a Murine Model of Ulcerative Colitis. J. Crohns Colitis 2018, 12, 217–229. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Zhu, C.Y. Pharmacokinetics and correlation between in vitro release and in vivo absorption of bio-adhesive pellets of panax notoginseng saponins. Chin. J. Nat. Med. 2017, 15, 142–151. [Google Scholar] [CrossRef]
- Sinha, P.; Udhumansha, U.; Rathnam, G.; Ganesh, M.; Jang, H.T. Capecitabine encapsulated chitosan succinate-sodium alginate macromolecular complex beads for colon cancer targeted delivery: In vitro evaluation. Int. J. Biol. Macromol. 2018, 117, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Merlin, D. Oral colon-specific therapeutic approaches toward treatment of inflammatory bowel disease. Expert Opin. Drug Deliv. 2012, 9, 1393–1407. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, N.; Galbis, E.; Díaz-Blanco, M.J.; Lucas, R.; Benito, E.; de-Paz, M.V. Nanostructured Chitosan-Based Biomaterials for Sustained and Colon-Specific Resveratrol Release. Int. J. Mol. Sci. 2019, 20, 398. [Google Scholar] [CrossRef] [Green Version]
- Sanna, V.; Siddiqui, I.A.; Sechi, M.; Mukhtar, H. Resveratrol-loaded nanoparticles based on poly(epsilon-caprolactone) and poly(D,L-lactic-co-glycolic acid)-poly(ethylene glycol) blend for prostate cancer treatment. Mol. Pharm. 2013, 10, 3871–3881. [Google Scholar] [CrossRef] [Green Version]
- Laroui, H.; Viennois, E.; Xiao, B.; Canup, B.S.; Geem, D.; Denning, T.L.; Merlin, D. Fab’-bearing siRNA TNFα-loaded nanoparticles targeted to colonic macrophages offer an effective therapy for experimental colitis. J. Control. Release 2014, 186, 41–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, B.; Laroui, H.; Viennois, E.; Ayyadurai, S.; Charania, M.A.; Zhang, Y.; Zhang, Z.; Baker, M.T.; Zhang, B.; Gewirtz, A.T.; et al. Nanoparticles with surface antibody against CD98 and carrying CD98 small interfering RNA reduce colitis in mice. Gastroenterology 2014, 146, 1289–1300.e1281-1219. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Chattopadhyay, M.; Sen, K.K.; Saha, M.K. Development and characterization of alginate coated low molecular weight chitosan nanoparticles as new carriers for oral vaccine delivery in mice. Carbohydr. Polym. 2015, 121, 403–410. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [Green Version]
- Orallo, F. Comparative studies of the antioxidant effects of cis- and trans-resveratrol. Curr. Med. Chem. 2006, 13, 87–98. [Google Scholar] [CrossRef]
- Xu, Y.; Hunt, N.H.; Bao, S. The role of granulocyte macrophage-colony-stimulating factor in acute intestinal inflammation. Cell Res. 2008, 18, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, J.; Shen, P.; Cai, J.; Han, Y.; Zhu, K.; Fu, Y.; Zhang, N.; Zhang, Z.; Cao, Y. Protective Effect of Naringin on DSS-Induced Ulcerative Colitis in Mice. J. Agric. Food Chem. 2018, 66, 13133–13140. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Guo, C.; Li, X. Chitosan Ameliorates DSS-Induced Ulcerative Colitis Mice by Enhancing Intestinal Barrier Function and Improving Microflora. Int. J. Mol. Sci. 2019, 20, 5751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohl, J.L.; Sobba, K. Indications and Options for Surgery in Ulcerative Colitis. Surg. Clin. North Am. 2015, 95, 1211–1232, vi. [Google Scholar] [CrossRef]
- Yang, R.; Liao, Y.; Wang, L.; He, P.; Hu, Y.; Yuan, D.; Wu, Z.; Sun, X. Exosomes Derived From M2b Macrophages Attenuate DSS-Induced Colitis. Front. Immunol. 2019, 10, 2346. [Google Scholar] [CrossRef] [Green Version]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nature reviews. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Vallyathan, V.; Shi, X. The role of oxygen free radicals in occupational and environmental lung diseases. Environm. Health Perspect. 1997, 105 (Suppl. S1), 165–177. [Google Scholar] [CrossRef]
- Li, Y.; Bai, H.; Wang, H.; Shen, Y.; Tang, G.; Ping, Y. Reactive oxygen species (ROS)-responsive nanomedicine for RNAi-based cancer therapy. Nanoscale 2017, 10, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; He, X.; Li, C.; Lin, S.; Chen, H.; Liu, L.; Feng, X. Oral delivery of antioxidant enzymes for effective treatment of inflammatory disease. Biomaterials 2021, 271, 120753. [Google Scholar] [CrossRef]
- Hoffmann, M.H.; Griffiths, H.R. The dual role of Reactive Oxygen Species in autoimmune and inflammatory diseases: Evidence from preclinical models. Free Radic. Biol. Med. 2018, 125, 62–71. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.M.; Choi, H.Y.; Cho, S.G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Lather, V.; Pandita, D. Green synthesis of therapeutic nanoparticles: An expanding horizon. Nanomedicine 2015, 10, 2451–2471. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Lather, V.; Pandita, D. A facile green approach to prepare core-shell hybrid PLGA nanoparticles for resveratrol delivery. Int. J. Biol. Macromol. 2016, 84, 380–384. [Google Scholar] [CrossRef]
- Pujara, N.; Wong, K.Y.; Qu, Z.; Wang, R.; Moniruzzaman, M.; Rewatkar, P.; Kumeria, T.; Ross, B.P.; McGuckin, M.; Popat, A. Oral Delivery of β-Lactoglobulin-Nanosphere-Encapsulated Resveratrol Alleviates Inflammation in Winnie Mice with Spontaneous Ulcerative Colitis. Mol. Pharm. 2021, 18, 627–640. [Google Scholar] [CrossRef]
- Lozano-Pérez, A.A.; Rodriguez-Nogales, A.; Ortiz-Cullera, V.; Algieri, F.; Garrido-Mesa, J.; Zorrilla, P.; Rodriguez-Cabezas, M.E.; Garrido-Mesa, N.; Utrilla, M.P.; De Matteis, L.; et al. Silk fibroin nanoparticles constitute a vector for controlled release of resveratrol in an experimental model of inflammatory bowel disease in rats. Int. J. Nanomed. 2014, 9, 4507–4520. [Google Scholar] [CrossRef] [Green Version]
- Rakotoarisoa, M.; Angelov, B.; Espinoza, S.; Khakurel, K.; Bizien, T.; Angelova, A. Cubic Liquid Crystalline Nanostructures Involving Catalase and Curcumin: BioSAXS Study and Catalase Peroxidatic Function after Cubosomal Nanoparticle Treatment of Differentiated SH-SY5Y Cells. Molecules 2019, 24, 3058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grande, R.; Celia, C.; Mincione, G.; Stringaro, A.; Di Marzio, L.; Colone, M.; Di Marcantonio, M.C.; Savino, L.; Puca, V.; San-toliquido, R.; et al. Detection and Physicochemical Characterization of Membrane Vesicles (MVs) of Lactobacillus reuteri DSM 17938. Front. Microbiol. 2017, 8, 1040. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, M.; Li, S.; Wu, Y.; Li, D.; Han, Y. Construction of Chitosan/Alginate Nano-Drug Delivery System for Improving Dextran Sodium Sulfate-Induced Colitis in Mice. Nanomaterials 2021, 11, 1884. https://doi.org/10.3390/nano11081884
Jin M, Li S, Wu Y, Li D, Han Y. Construction of Chitosan/Alginate Nano-Drug Delivery System for Improving Dextran Sodium Sulfate-Induced Colitis in Mice. Nanomaterials. 2021; 11(8):1884. https://doi.org/10.3390/nano11081884
Chicago/Turabian StyleJin, Mengfei, Shangyong Li, Yanhong Wu, Dandan Li, and Yantao Han. 2021. "Construction of Chitosan/Alginate Nano-Drug Delivery System for Improving Dextran Sodium Sulfate-Induced Colitis in Mice" Nanomaterials 11, no. 8: 1884. https://doi.org/10.3390/nano11081884
APA StyleJin, M., Li, S., Wu, Y., Li, D., & Han, Y. (2021). Construction of Chitosan/Alginate Nano-Drug Delivery System for Improving Dextran Sodium Sulfate-Induced Colitis in Mice. Nanomaterials, 11(8), 1884. https://doi.org/10.3390/nano11081884




