Silver Nanoparticles Stabilized with Phosphorus-Containing Heterocyclic Surfactants: Synthesis, Physico-Chemical Properties, and Biological Activity Determination
Abstract
1. Introduction
2. Materials and Methods
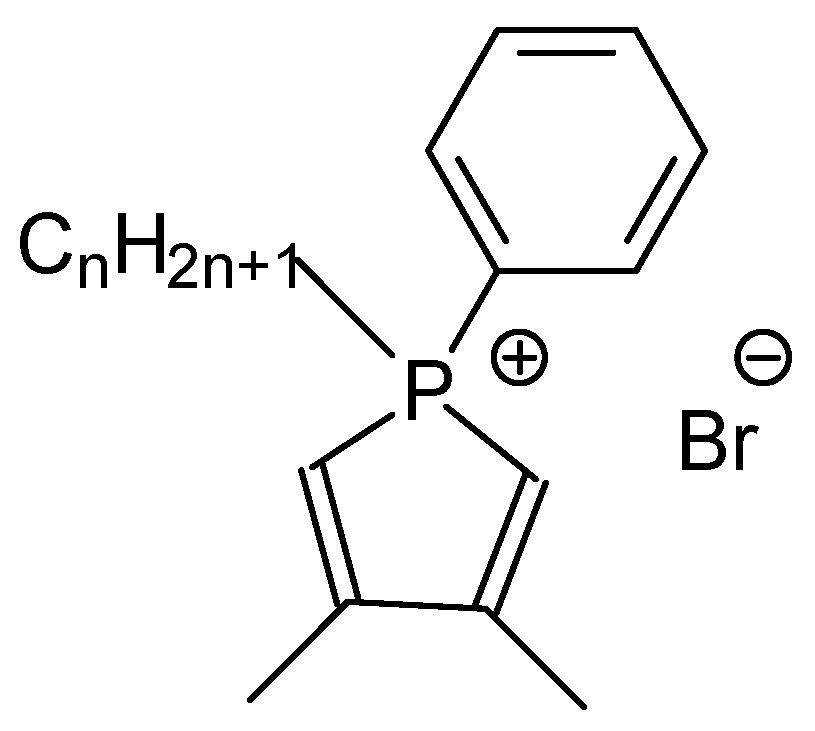
2.1. Used Surfactants and Synthesis of AgNPs
2.2. Spectroscopic Measurements
2.3. Dynamic Light Scattering and Scanning Electron Microscopy
2.4. Zeta Potential Measurements
2.5. Determination of Biological Activities
3. Results and Discussion
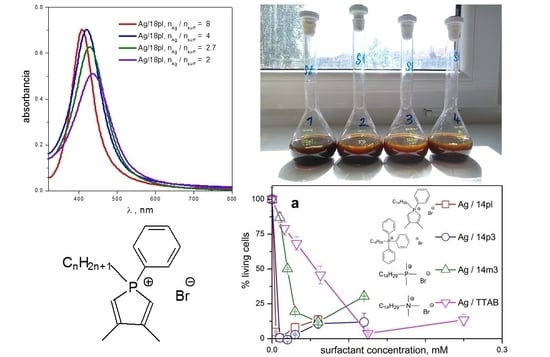
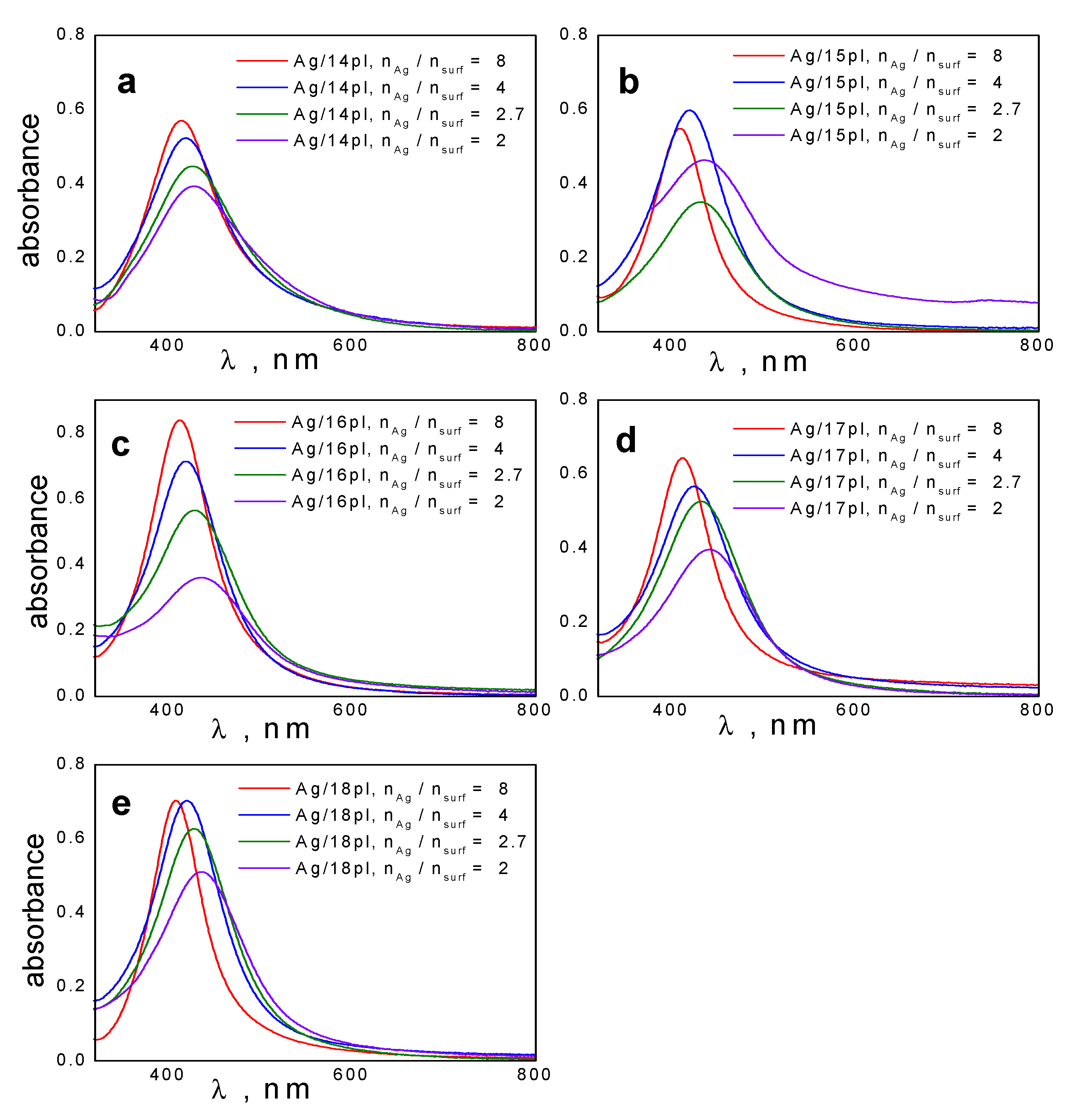
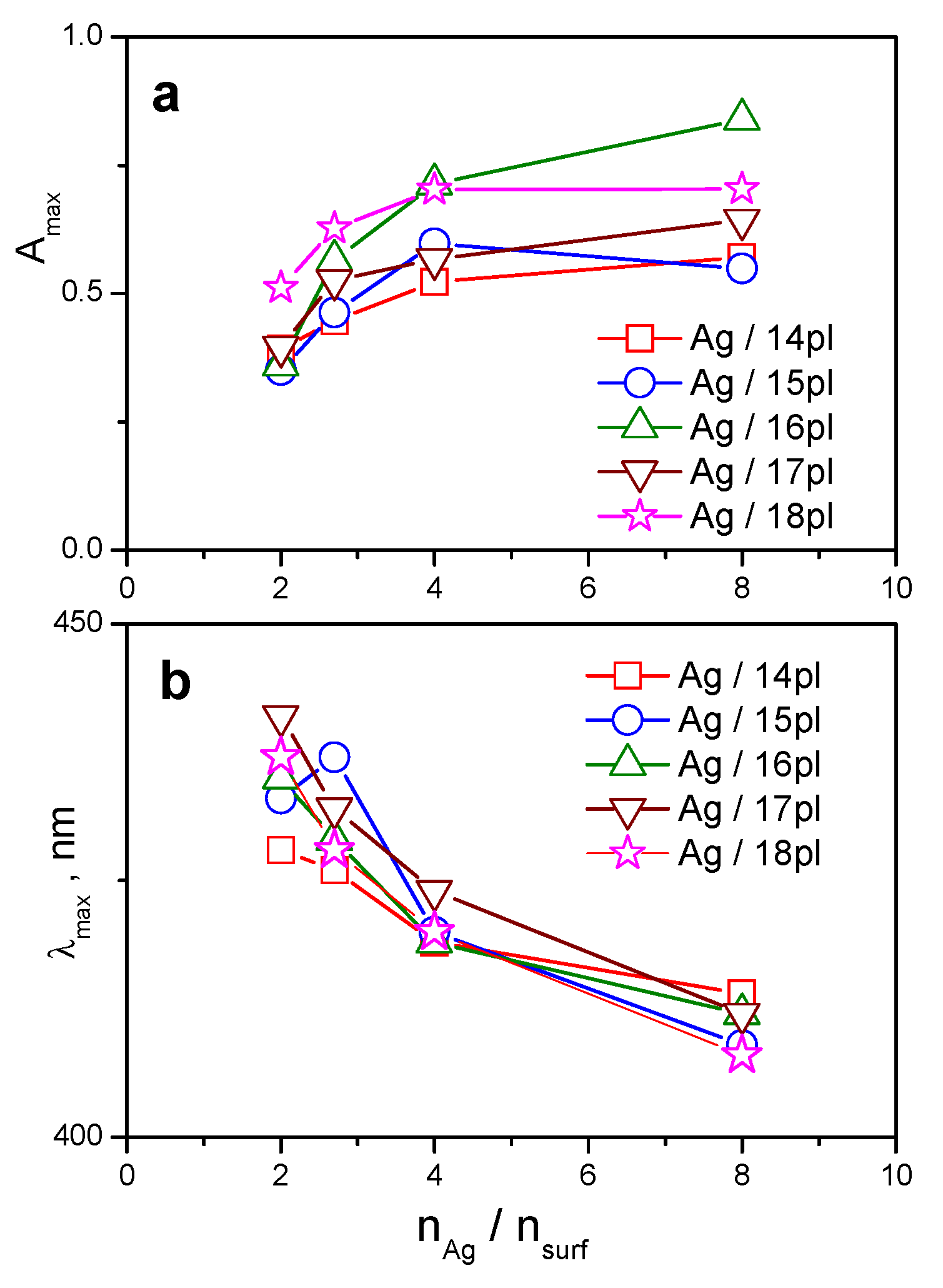
3.1. UV-VIS Spectra
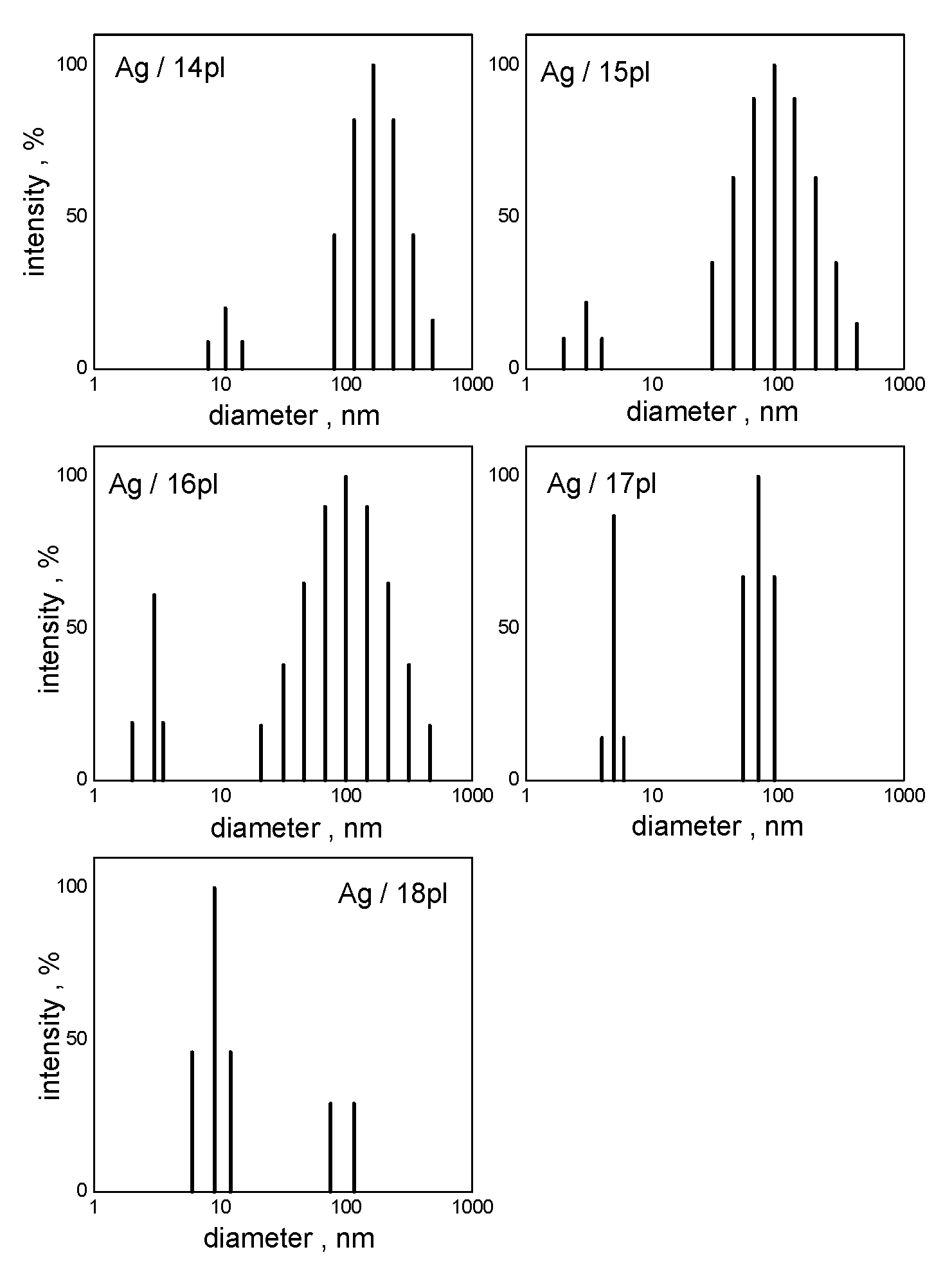
3.2. Nanoparticle Size and Zeta Potential
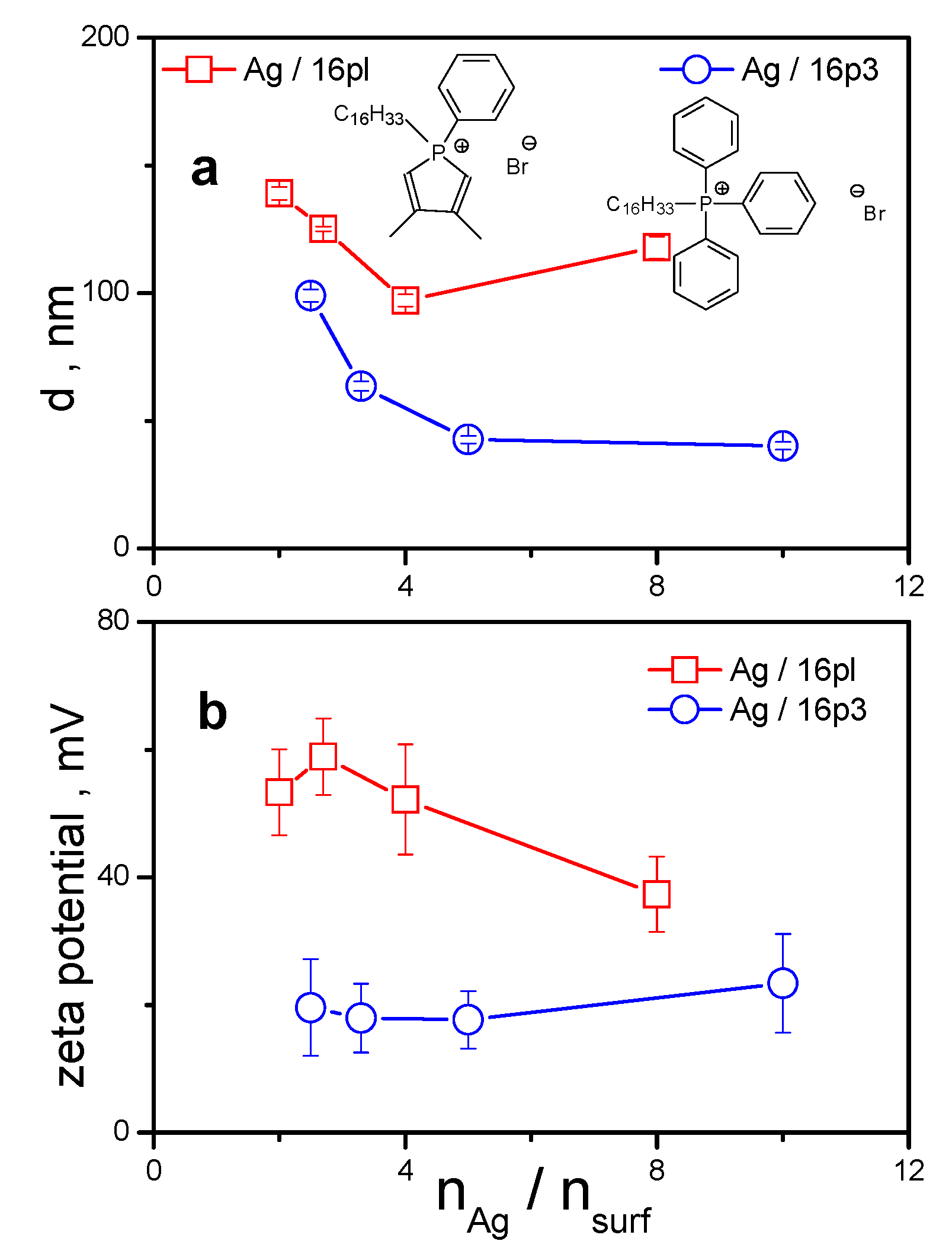
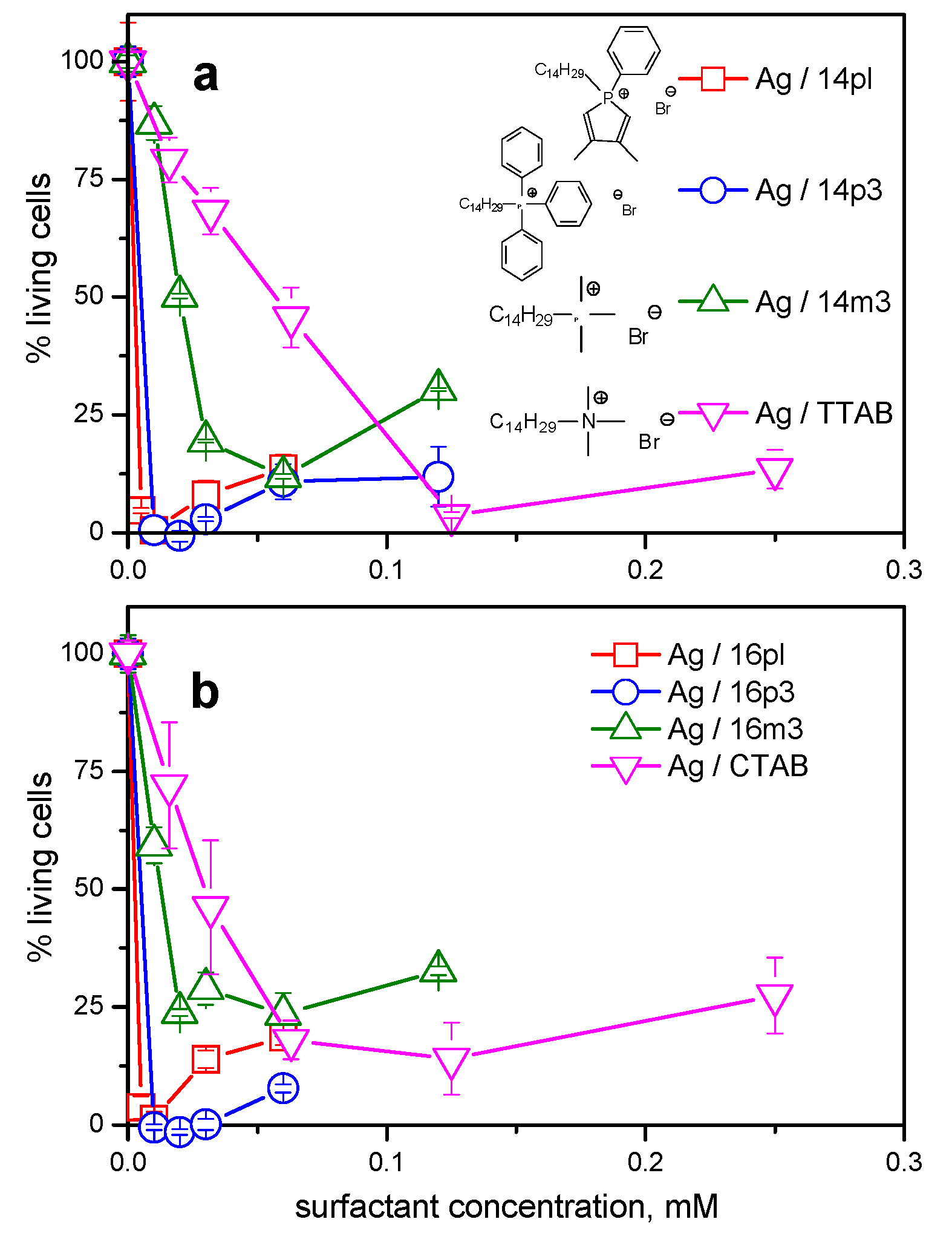
3.3. Comparison with Non-Heterocyclic Phosphonium Surfactants
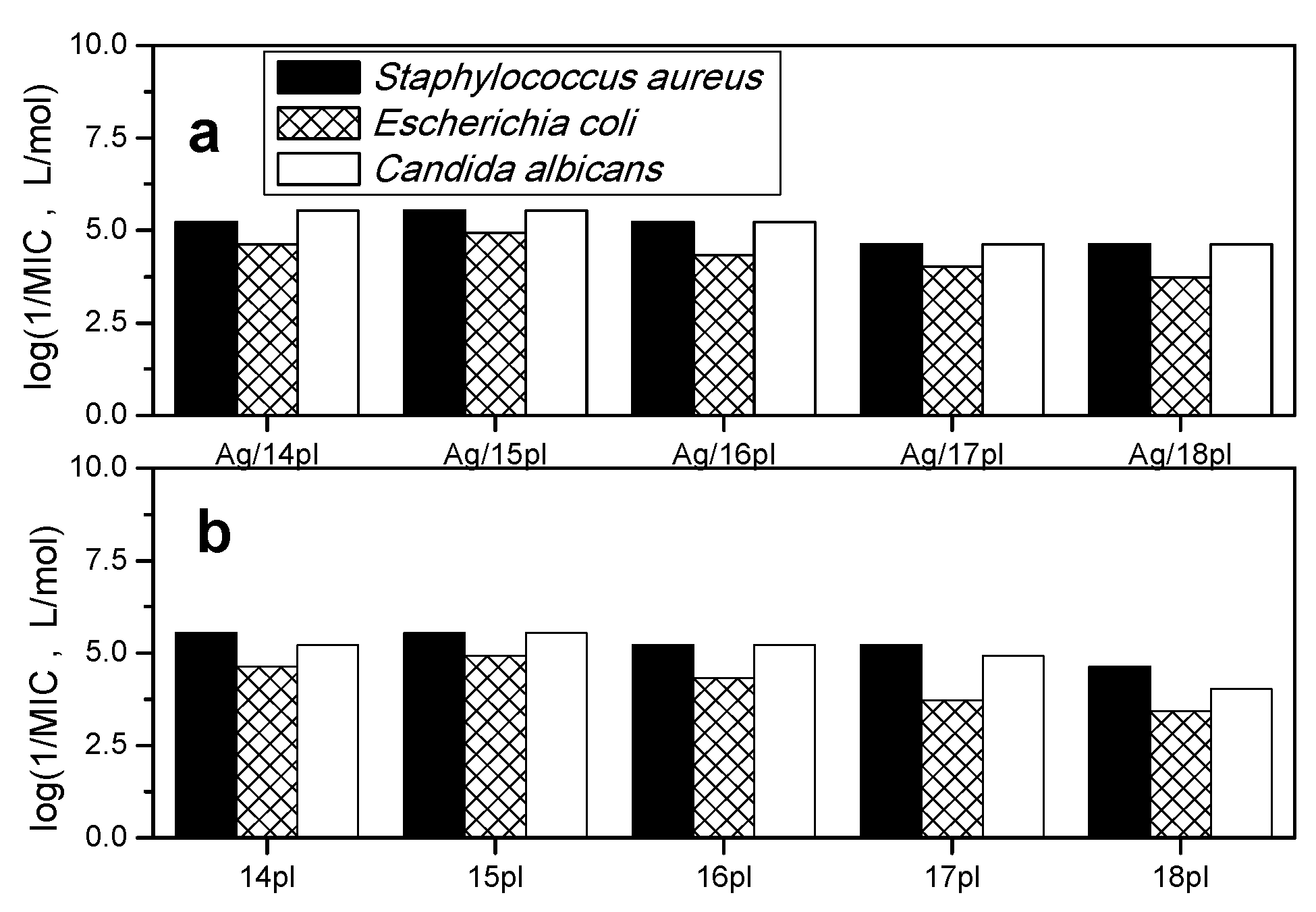
3.4. Antimicrobial Activity
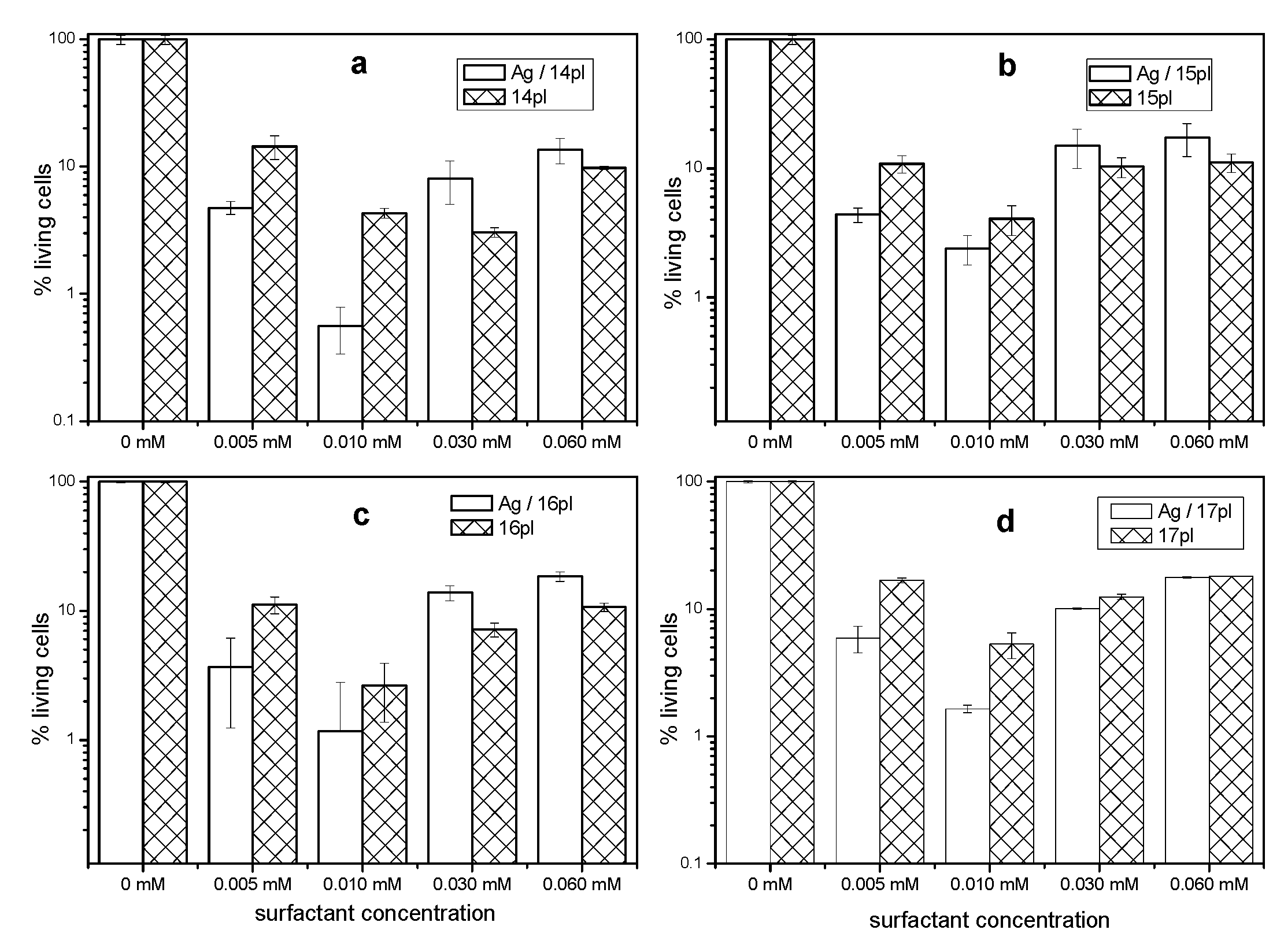
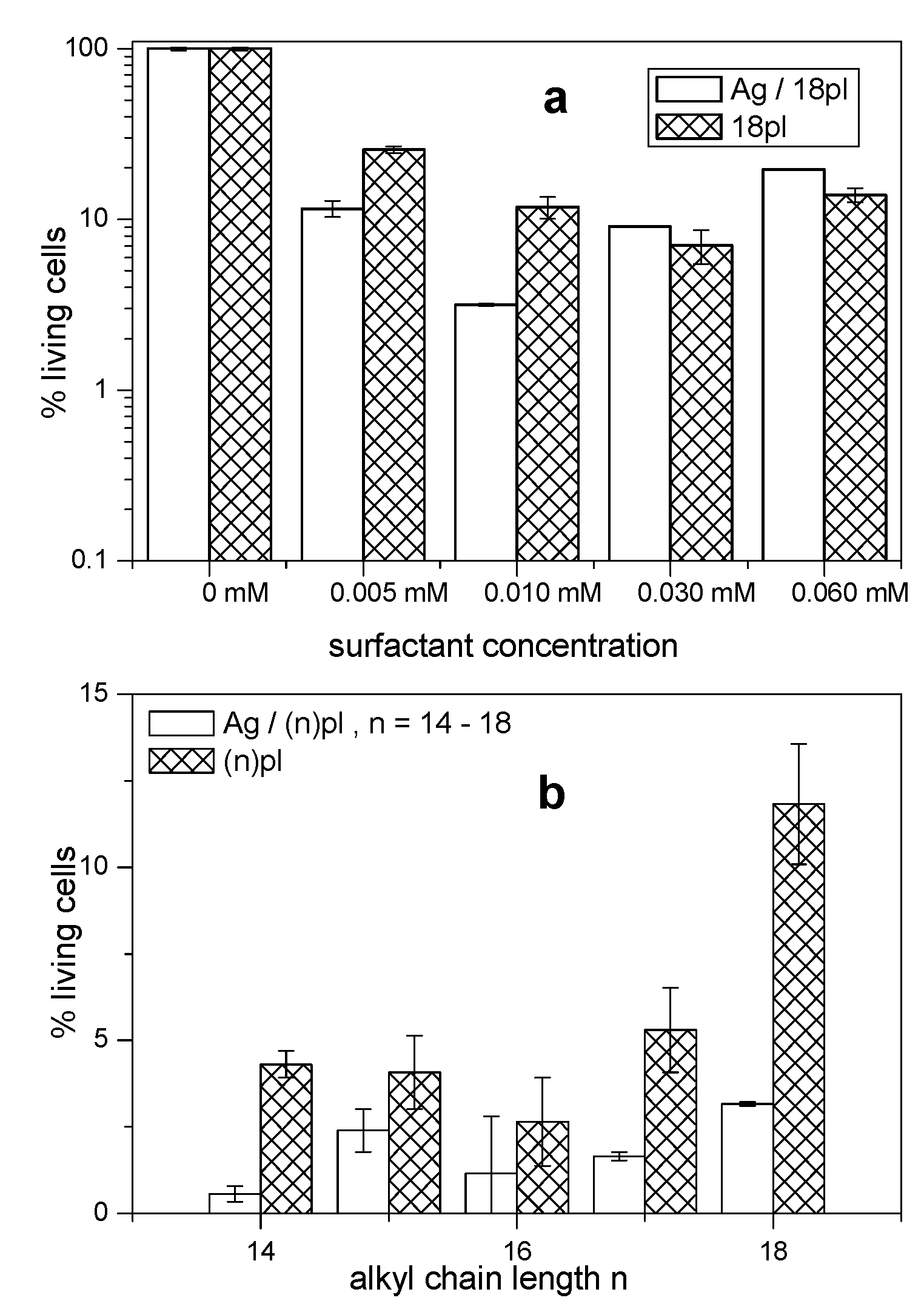
3.5. Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, P.; Hu, Y.; Gao, Z.; Zhai, J.; Fang, D.; Yue, T.; Zhang, C.; Sun, W. Discovery of a Novel Cationic Surfactant: Tributyltetradecyl-Phosphonium Chloride for Iron Ore Flotation: From Prediction to Experimental Verification. Minerals 2017, 7, 240. [Google Scholar] [CrossRef]
- Rodríguez-Palmeiro, I.; Rodríguez-Escontrela, I.; Rodríguez, O.; Soto, A.; Reichmann, S.; Amro, M.M. Tributyl(Tetradecyl)Phosphonium Chloride Ionic Liquid for Surfactant-Enhanced Oil Recovery. Energy Fuels 2017, 31, 6758–6765. [Google Scholar] [CrossRef]
- Schnee, V.P.; Palmer, C.P. Characterization of a Cationic Phosphonium Surfactant for Micellar Electrokinetic Chromatography: Using the Linear Solvation Energy Relationships Model. Electrophoresis 2008, 29, 761–766. [Google Scholar] [CrossRef]
- Tawfik, S.M.; Sayed, A.; Aiad, I. Corrosion Inhibition by Some Cationic Surfactants in Oil Fields. J. Surfactants Deterg. 2012, 15, 577–585. [Google Scholar] [CrossRef]
- Aiad, I.A.; Tawfik, S.M.; Shaban, S.M.; Abd-Elaal, A.A.; El-Shafie, M. Enhancing of Corrosion Inhibition and the Biocidal Effect of Phosphonium Surfactant Compounds for Oil Field Equipment. J. Surfactants Deterg. 2014, 17, 391–401. [Google Scholar] [CrossRef]
- Dos Santos, E.; Fook, M.; Malta, O.; de Lima Silva, S.; Leite, I. Role of Surfactants in the Properties of Poly(Ethylene Terephthalate)/Purified Clay Nanocomposites. Materials 2018, 11, 1397. [Google Scholar] [CrossRef]
- Xie, W.; Xie, R.; Pan, W.-P.; Hunter, D.; Koene, B.; Tan, L.-S.; Vaia, R. Thermal Stability of Quaternary Phosphonium Modified Montmorillonites. Chem. Mater. 2002, 14, 4837–4845. [Google Scholar] [CrossRef]
- Hedley, C.; Yuan, G.; Theng, B. Thermal Analysis of Montmorillonites Modified with Quaternary Phosphonium and Ammonium Surfactants. Appl. Clay Sci. 2007, 35, 180–188. [Google Scholar] [CrossRef]
- Durazo, S.A.; Kompella, U.B. Functionalized Nanosystems for Targeted Mitochondrial Delivery. Mitochondrion 2012, 12, 190–201. [Google Scholar] [CrossRef]
- Boddapati, S.V.; D’Souza, G.G.M.; Erdogan, S.; Torchilin, V.P.; Weissig, V. Organelle-Targeted Nanocarriers: Specific Delivery of Liposomal Ceramide to Mitochondria Enhances Its Cytotoxicity In Vitro and In Vivo. Nano Lett. 2008, 8, 2559–2563. [Google Scholar] [CrossRef] [PubMed]
- Boddapati, S.V.; D’Souza, G.G.M.; Weissig, V. Liposomes for Drug Delivery to Mitochondria. In Liposomes; Weissig, V., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 605, pp. 295–303. ISBN 978-1-60327-359-6. [Google Scholar]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef]
- Chang, L.; Zhang, X.; Shi, X.; Zhao, L.; Liu, X.M. Synthesis and Characterization of a Novel Fibrous Antibacterial Fiber with Organophorsphor Functional Groups. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Nonaka, T.; Hua, L.; Ogata, T.; Kurihara, S. Synthesis of Water-Soluble Thermosensitive Polymers Having Phosphonium Groups from Methacryloyloxyethyl Trialkyl Phosphonium Chlorides-N-Isopropylacrylamide Copolymers and Their Functions. J. Appl. Polym. Sci. 2003, 87, 386–393. [Google Scholar] [CrossRef]
- Kenawy, E. Biologically Active Polymers: Synthesis and Antimicrobial Activity of Modified Glycidyl Methacrylate Polymers Having a Quaternary Ammonium and Phosphonium Groups. J. Control. Release 1998, 50, 145–152. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Abdel-Hay, F.I.; El-Shanshoury, A.E.-R.R.; El-Newehy, M.H. Biologically Active Polymers. V. Synthesis and Antimicrobial Activity of Modified Poly(Glycidyl Methacrylate-Co-2-Hydroxyethyl Methacrylate) Derivatives with Quaternary Ammonium and Phosphonium Salts. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2384–2393. [Google Scholar] [CrossRef]
- El-Newehy, M.H.; Kenawy, E.-R.; Al-Deyab, S.S. Biocidal Polymers: Preparation and Antimicrobial Assessment of Immobilized Onium Salts onto Modified Chitosan. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 758–766. [Google Scholar] [CrossRef]
- Qiu, T.; Zeng, Q.; Ao, N. Preparation and Characterization of Chlorinated Nature Rubber (CNR) Based Polymeric Quaternary Phosphonium Salt Bactericide. Mater. Lett. 2014, 122, 13–16. [Google Scholar] [CrossRef]
- Selva, M.; Perosa, A.; Noè, M. Phosphonium salts and P-ylides. In Organophosphorus Chemistry; Allen, D.W., Loakes, D., Tebby, J.C., Eds.; Royal Society of Chemistry: Cambridge, UK, 2016; Volume 45, pp. 132–169. ISBN 978-1-78262-433-2. [Google Scholar]
- Bakshi, M.S.; Kaur, I. Triphenyl- and Tributyl-Phosphonium Head Groups Contributions in Mixed Cationic Surfactants and Anionic Polyelectrolytes Aggregates. Colloids Surf. A Physicochem. Eng. Asp. 2003, 227, 9–19. [Google Scholar] [CrossRef]
- Bakshi, M.S.; Singh, J.; Singh, K.; Kaur, G. Mixed Micelles of Cationic Gemini with Tetraalkyl Ammonium and Phosphonium Surfactants: The Head Group and Hydrophobic Tail Contributions. Colloids Surf. A Physicochem. Eng. Asp. 2004, 234, 77–84. [Google Scholar] [CrossRef]
- Prasad, M.; Moulik, S.P.; Wardian, A.A.; Moore, S.; van Bommel, A.; Palepu, R. Alkyl (C10, C12, C14 and C16) Triphenyl Phosphonium Bromide Influenced Cloud Points of Nonionic Surfactants (Triton X 100, Brij 56 and Brij 97) and the Polymer (Polyvinyl Methyl Ether). Colloid Polym. Sci. 2005, 283, 887–897. [Google Scholar] [CrossRef]
- Moore, S.A.; Glenn, K.M.; MacDonald, A.M.; Palepu, R.M. Micellar and Associated Thermodynamic Properties of Binary Mixtures of Alkyl Triphenyl Phosphonium Bromides in Ethylene Glycol and Water Mixtures. Colloid Polym. Sci. 2007, 285, 543–552. [Google Scholar] [CrossRef]
- Shrivastava, A.; Ghosh, K.K. Micellization of Cetyl Triphenyl Phosphonium Bromide Surfactant in Binary Aqueous Solvents. J. Surfactants Deterg. 2008, 11, 287–292. [Google Scholar] [CrossRef]
- Verma, S.K.; Ghosh, K.K. Micellar and Surface Properties of Some Monomeric Surfactants and a Gemini Cationic Surfactant. J. Surfactants Deterg. 2011, 14, 347–352. [Google Scholar] [CrossRef]
- Gainanova, G.A.; Vagapova, G.I.; Syakaev, V.V.; Ibragimova, A.R.; Valeeva, F.G.; Tudriy, E.V.; Galkina, I.V.; Kataeva, O.N.; Zakharova, L.Y.; Latypov, S.K.; et al. Self-Assembling Systems Based on Amphiphilic Alkyltriphenylphosphonium Bromides: Elucidation of the Role of Head Group. J. Colloid Interface Sci. 2012, 367, 327–336. [Google Scholar] [CrossRef]
- Vagapova, G.I.; Valeeva, F.G.; Gainanova, G.A.; Syakaev, V.V.; Galkina, I.V.; Zakharova, L.Y.; Latypov, S.K.; Konovalov, A.I. Novel Self-Assembling Systems Based on Amphiphilic Phosphonium Salt and Polyethylene Glycol. Kinetic Arguments for Synergetic Aggregation Behavior. Colloids Surf. A Physicochem. Eng. Asp. 2013, 419, 186–193. [Google Scholar] [CrossRef]
- Lu, F.; Shi, L.; Gu, Y.; Yang, X.; Zheng, L. Aggregation Behavior of Alkyl Triphenyl Phosphonium Bromides in Aprotic and Protic Ionic Liquids. Colloid Polym. Sci. 2013, 291, 2375–2384. [Google Scholar] [CrossRef]
- Lukáč, M.; Devínsky, F.; Pisárčik, M.; Papapetropoulou, A.; Bukovský, M.; Horváth, B. Novel Phospholium-Type Cationic Surfactants: Synthesis, Aggregation Properties and Antimicrobial Activity. J. Surfactants Deterg. 2017, 20, 159–171. [Google Scholar] [CrossRef]
- Gamarra, A.; Urpí, L.; Martínez de Ilarduya, A.; Muñoz-Guerra, S. Crystalline Structure and Thermotropic Behavior of Alkyltrimethylphosphonium Amphiphiles. Phys. Chem. Chem. Phys. 2017, 19, 4370–4382. [Google Scholar] [CrossRef]
- Rauber, D.; Heib, F.; Schmitt, M.; Hempelmann, R. Trioctylphosphonium Room Temperature Ionic Liquids with Perfluorinated Groups—Physical Properties and Surface Behavior in Comparison with the Nonfluorinated Analogues. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 116–125. [Google Scholar] [CrossRef]
- Slepička, P.; Slepičková Kasálková, N.; Siegel, J.; Kolská, Z.; Švorčík, V. Methods of Gold and Silver Nanoparticles Preparation. Materials 2019, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and Antibacterial Activity of Silver Nanoparticles against Gram-Positive and Gram-Negative Bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 37–45. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Trevino, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver Nanoparticles Are Broad-Spectrum Bactericidal and Virucidal Compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef]
- Ortiz, C.; Torres, R.; Paredes, D. Synthesis, Characterization, and Evaluation of Antibacterial Effect of Ag Nanoparticles against Escherichia Coli O157:H7 and Methicillin- Resistant Staphylococcus Aureus (MRSA). IJN 2014, 1717. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna, M.; Hudy, D. Biological Activity of Silver Nanoparticles and Their Applications in Anticancer Therapy. In Silver Nanoparticles—Fabrication, Characterization and Applications; Maaz, K., Ed.; InTech: London, UK, 2018; ISBN 978-1-78923-478-7. [Google Scholar]
- Buttacavoli, M.; Albanese, N.N.; Cara, G.D.; Alduina, R.; Faleri, C.; Gallo, M.; Pizzolanti, G.; Gallo, G.; Feo, S.; Baldi, F.; et al. Anticancer Activity of Biogenerated Silver Nanoparticles: An Integrated Proteomic Investigation. Oncotarget 2018, 9, 9685. [Google Scholar] [CrossRef] [PubMed]
- Yeşilot, Ş.; Aydın Acar, Ç. Silver Nanoparticles; a New Hope in Cancer Therapy? East. J. Med. 2019, 24, 111–116. [Google Scholar] [CrossRef]
- Soni, N.; Dhiman, R.C. Phytochemical, Anti-Oxidant, Larvicidal, and Antimicrobial Activities of Castor (Ricinus Communis) Synthesized Silver Nanoparticles. Chin. Herb. Med. 2017, 9, 289–294. [Google Scholar] [CrossRef]
- Arumai Selvan, D.; Mahendiran, D.; Senthil Kumar, R.; Kalilur Rahiman, A. Garlic, Green Tea and Turmeric Extracts-Mediated Green Synthesis of Silver Nanoparticles: Phytochemical, Antioxidant and in Vitro Cytotoxicity Studies. J. Photochem. Photobiol. B Biol. 2018, 180, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yu, S.; Li, X.; Ma, C.; Li, A. Evaluation of Local Anesthetic Effects of Lidocaine-Ibuprofen Ionic Liquid Stabilized Silver Nanoparticles in Male Swiss Mice. J. Photochem. Photobiol. B Biol. 2018, 178, 367–370. [Google Scholar] [CrossRef]
- Karthik, C.S.; Manukumar, H.M.; Ananda, A.P.; Nagashree, S.; Rakesh, K.P.; Mallesha, L.; Qin, H.-L.; Umesha, S.; Mallu, P.; Krishnamurthy, N.B. Synthesis of Novel Benzodioxane Midst Piperazine Moiety Decorated Chitosan Silver Nanoparticle against Biohazard Pathogens and as Potential Anti-Inflammatory Candidate: A Molecular Docking Studies. Int. J. Biol. Macromol. 2018, 108, 489–502. [Google Scholar] [CrossRef]
- Domeradzka-Gajda, K.; Nocuń, M.; Roszak, J.; Janasik, B.; Quarles, C.D.; Wąsowicz, W.; Grobelny, J.; Tomaszewska, E.; Celichowski, G.; Ranoszek-Soliwoda, K.; et al. A Study on the in Vitro Percutaneous Absorption of Silver Nanoparticles in Combination with Aluminum Chloride, Methyl Paraben or Di-n-Butyl Phthalate. Toxicol. Lett. 2017, 272, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Kraeling, M.E.K.; Topping, V.D.; Keltner, Z.M.; Belgrave, K.R.; Bailey, K.D.; Gao, X.; Yourick, J.J. In Vitro Percutaneous Penetration of Silver Nanoparticles in Pig and Human Skin. Regul. Toxicol. Pharmacol. 2018, 95, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Pannerselvam, B.; Dharmalingam Jothinathan, M.K.; Rajenderan, M.; Perumal, P.; Pudupalayam Thangavelu, K.; Kim, H.J.; Singh, V.; Rangarajulu, S.K. An in Vitro Study on the Burn Wound Healing Activity of Cotton Fabrics Incorporated with Phytosynthesized Silver Nanoparticles in Male Wistar Albino Rats. Eur. J. Pharm. Sci. 2017, 100, 187–196. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, R.-C. Facile and Eco-Friendly Fabrication of AgNPs Coated Silk for Antibacterial and Antioxidant Textiles Using Honeysuckle Extract. J. Photochem. Photobiol. B Biol. 2018, 178, 463–471. [Google Scholar] [CrossRef]
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable Hybrid Nanocomposites of Chitosan/Gelatin and Silver Nanoparticles for Active Food Packaging Applications. Food Packag. Shelf Life 2018, 16, 178–184. [Google Scholar] [CrossRef]
- Padnya, P.; Gorbachuk, V.; Stoikov, I. The Role of Calix[n]Arenes and Pillar[n]Arenes in the Design of Silver Nanoparticles: Self-Assembly and Application. Int. J. Mol. Sci. 2020, 21, 1425. [Google Scholar] [CrossRef]
- Pisárčik, M.; Jampílek, J.; Lukáč, M.; Horáková, R.; Devínsky, F.; Bukovský, M.; Kalina, M.; Tkacz, J.; Opravil, T. Silver Nanoparticles Stabilised by Cationic Gemini Surfactants with Variable Spacer Length. Molecules 2017, 22, 1794. [Google Scholar] [CrossRef]
- Brycki, B.; Szulc, A.; Babkova, M. Synthesis of Silver Nanoparticles with Gemini Surfactants as Efficient Capping and Stabilizing Agents. Appl. Sci. 2020, 11, 154. [Google Scholar] [CrossRef]
- Pisárčik, M.; Lukáč, M.; Jampílek, J.; Bilka, F.; Bilková, A.; Pašková, Ľ.; Devínsky, F.; Horáková, R.; Opravil, T. Silver Nanoparticles Stabilised with Cationic Single-Chain Surfactants. Structure-Physical Properties-Biological Activity Relationship Study. J. Mol. Liq. 2018, 272, 60–72. [Google Scholar] [CrossRef]
- Pisárčik, M.; Lukáč, M.; Jampílek, J.; Bilka, F.; Bilková, A.; Pašková, Ľ.; Devínsky, F.; Horáková, R.; Opravil, T. Phosphonium Surfactant Stabilised Silver Nanoparticles. Correlation of Surfactant Structure with Physical Properties and Biological Activity of Silver Nanoparticles. J. Mol. Liq. 2020, 314, 113683. [Google Scholar] [CrossRef]
- He, S.; Chen, H.; Guo, Z.; Wang, B.; Tang, C.; Feng, Y. High-Concentration Silver Colloid Stabilized by a Cationic Gemini Surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2013, 429, 98–105. [Google Scholar] [CrossRef]
- Cheng, W.; Dong, S.; Wang, E. Studies of Electrochemical Quantized Capacitance Charging of Surface Ensembles of Silver Nanoparticles. Electrochem. Commun. 2002, 4, 412–416. [Google Scholar] [CrossRef]
- Jiang, X.C.; Chen, W.M.; Chen, C.Y.; Xiong, S.X.; Yu, A.B. Role of Temperature in the Growth of Silver Nanoparticles Through a Synergetic Reduction Approach. Nanoscale Res. Lett. 2010. [Google Scholar] [CrossRef]
- Lukáč, M.; Lacko, I.; Bukovský, M.; Kyselová, Z.; Karlovská, J.; Horváth, B.; Devínsky, F. Synthesis and Antimicrobial Activity of a Series of Optically Active Quaternary Ammonium Salts Derived from Phenylalanine. Open Chem. 2010, 8, 194–201. [Google Scholar] [CrossRef]
- Raspotnig, G.; Fauler, G.; Jantscher, A.; Windischhofer, W.; Schachl, K.; Leis, H.J. Colorimetric Determination of Cell Numbers by Janus Green Staining. Anal. Biochem. 1999, 275, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gu, B. Preparation and Characterization of Silver Nanoparticles at High Concentrations. In Concentrated Dispersions: Theory, Experiments, and Applications; ACS Symposium Series 878; Oxford University Press: Oxford, UK, 2004; Chapter 1; pp. 1–14. [Google Scholar]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Epiotis, N.D.; Cherry, W. On the Aromaticity of Phospholes and Arsoles. J. Am. Chem. Soc. 1976, 98, 4365–4370. [Google Scholar] [CrossRef]
- Chesnut, D.B.; Quin, L.D. The Important Role of the Phosphorus Lone Pair in Phosphole Aromaticity. Heteroat. Chem. 2007, 18, 754–758. [Google Scholar] [CrossRef]
- Yu, H.; Narusawa, H.; Itoh, K.; Oshi, A.; Yoshino, N.; Ohbu, K.; Shirakawa, T.; Fukada, K.; Fujii, M.; Kato, T.; et al. Hydrophilicity of Polar and Apolar Domains of Amphiphiles. J. Colloid Interface Sci. 2000, 229, 375–390. [Google Scholar] [CrossRef]
- Yu, D.; Huang, X.; Deng, M.; Lin, Y.; Jiang, L.; Huang, J.; Wang, Y. Effects of Inorganic and Organic Salts on Aggregation Behavior of Cationic Gemini Surfactants. J. Phys. Chem. B 2010, 114, 14955–14964. [Google Scholar] [CrossRef]
- Balgavý, P.; Devínsky, F. Cut-off Effects in Biological Activities of Surfactants. Adv. Colloid Interface Sci. 1996, 66, 23–63. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, S.; Yu, J.; Chen, X.; Lei, Q.; Fang, W. Antibacterial Activity, in Vitro Cytotoxicity, and Cell Cycle Arrest of Gemini Quaternary Ammonium Surfactants. Langmuir 2015, 31, 12161–12169. [Google Scholar] [CrossRef] [PubMed]
- Wypij, M.; Czarnecka, J.; Świecimska, M.; Dahm, H.; Rai, M.; Golinska, P. Synthesis, Characterization and Evaluation of Antimicrobial and Cytotoxic Activities of Biogenic Silver Nanoparticles Synthesized from Streptomyces Xinghaiensis OF1 Strain. World J. Microbiol. Biotechnol. 2018, 34. [Google Scholar] [CrossRef] [PubMed]
- Janowska, S.; Paneth, A.; Wujec, M. Cytotoxic Properties of 1,3,4-Thiadiazole Derivatives—A Review. Molecules 2020, 25, 4309. [Google Scholar] [CrossRef]
- Senff-Ribeiro, A.; Echevarria, A.; Silva, E.F.; Franco, C.R.C.; Veiga, S.S.; Oliveira, M.B.M. Cytotoxic Effect of a New 1,3,4-Thiadiazolium Mesoionic Compound (MI-D) on Cell Lines of Human Melanoma. Br. J. Cancer 2004, 91, 297–304. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisárčik, M.; Lukáč, M.; Jampílek, J.; Bilka, F.; Bilková, A.; Pašková, Ľ.; Devínsky, F.; Horáková, R.; Březina, M.; Opravil, T. Silver Nanoparticles Stabilized with Phosphorus-Containing Heterocyclic Surfactants: Synthesis, Physico-Chemical Properties, and Biological Activity Determination. Nanomaterials 2021, 11, 1883. https://doi.org/10.3390/nano11081883
Pisárčik M, Lukáč M, Jampílek J, Bilka F, Bilková A, Pašková Ľ, Devínsky F, Horáková R, Březina M, Opravil T. Silver Nanoparticles Stabilized with Phosphorus-Containing Heterocyclic Surfactants: Synthesis, Physico-Chemical Properties, and Biological Activity Determination. Nanomaterials. 2021; 11(8):1883. https://doi.org/10.3390/nano11081883
Chicago/Turabian StylePisárčik, Martin, Miloš Lukáč, Josef Jampílek, František Bilka, Andrea Bilková, Ľudmila Pašková, Ferdinand Devínsky, Renáta Horáková, Matěj Březina, and Tomáš Opravil. 2021. "Silver Nanoparticles Stabilized with Phosphorus-Containing Heterocyclic Surfactants: Synthesis, Physico-Chemical Properties, and Biological Activity Determination" Nanomaterials 11, no. 8: 1883. https://doi.org/10.3390/nano11081883
APA StylePisárčik, M., Lukáč, M., Jampílek, J., Bilka, F., Bilková, A., Pašková, Ľ., Devínsky, F., Horáková, R., Březina, M., & Opravil, T. (2021). Silver Nanoparticles Stabilized with Phosphorus-Containing Heterocyclic Surfactants: Synthesis, Physico-Chemical Properties, and Biological Activity Determination. Nanomaterials, 11(8), 1883. https://doi.org/10.3390/nano11081883