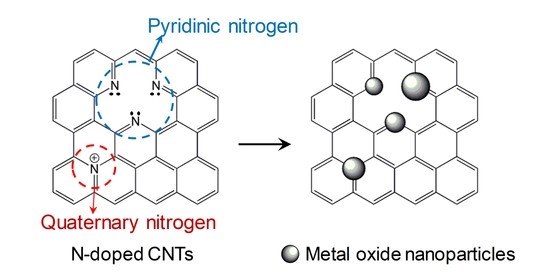
N-Dopant-Mediated Growth of Metal Oxide Nanoparticles on Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanopatterned Catalysts for NCNT Growth
2.3. Growth of Vertically Aligned NCNTs
2.4. Synthesis of MnOx and RuOx Nanoparticles Supported on NCNTs
2.5. Characterization
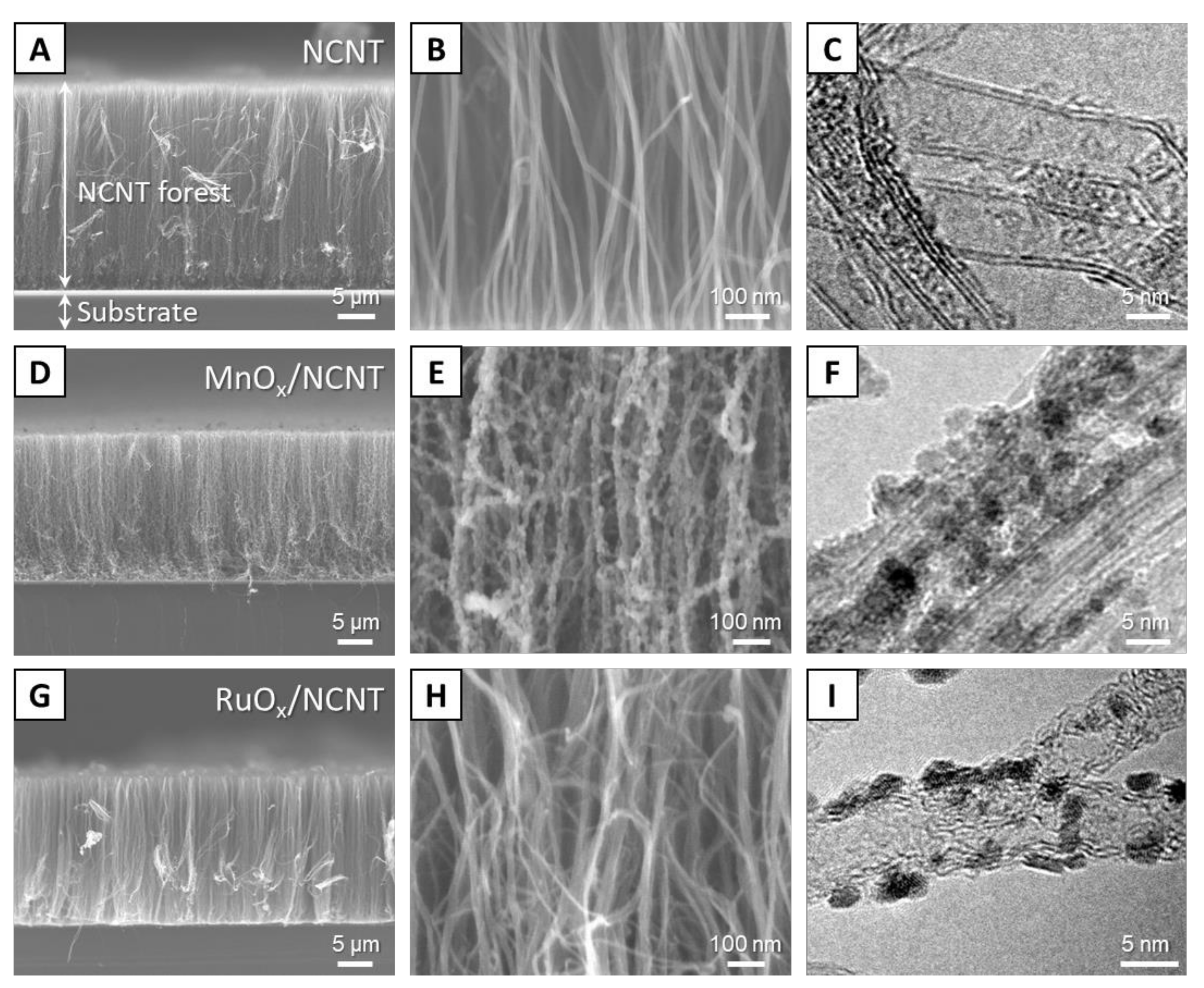
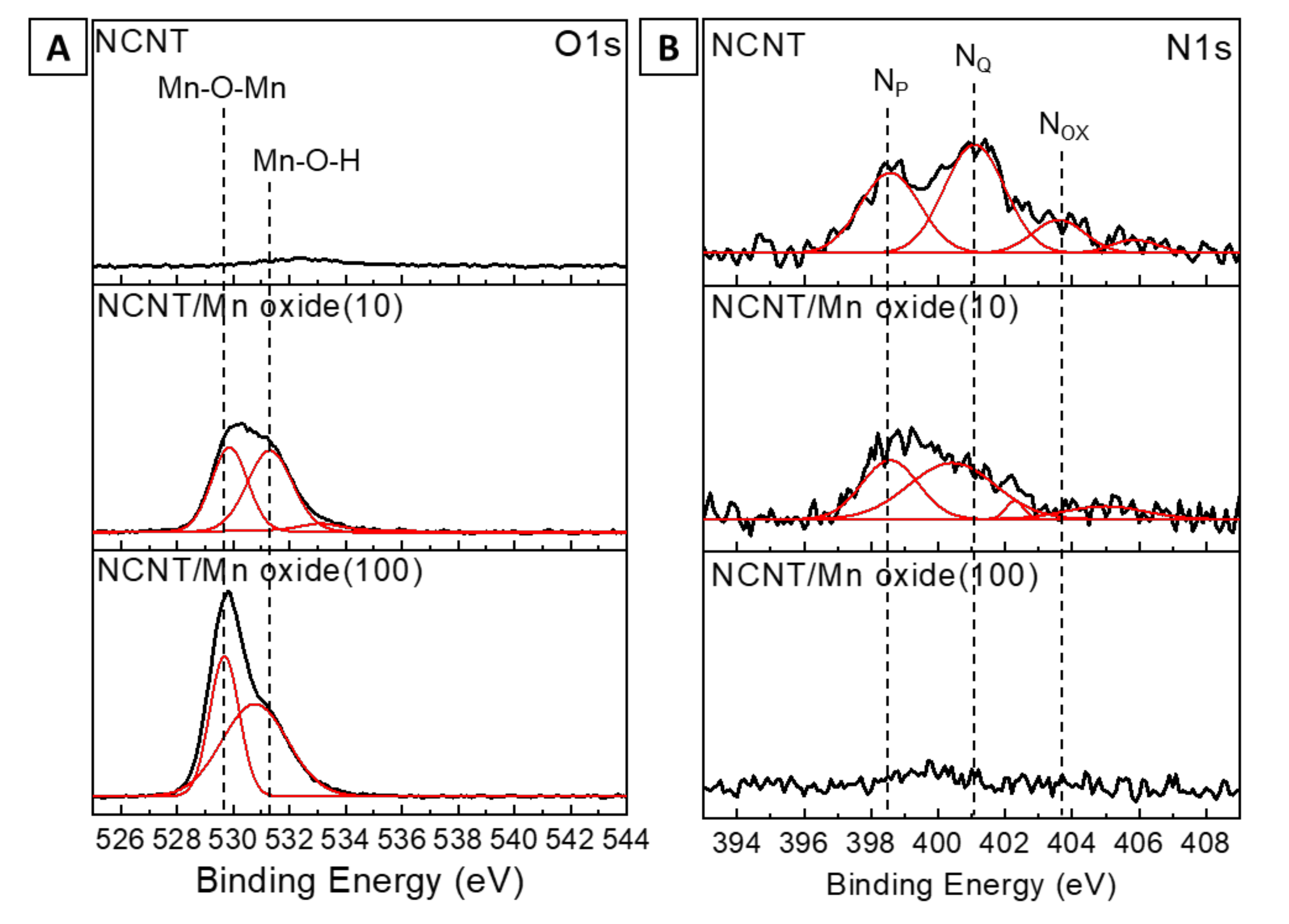
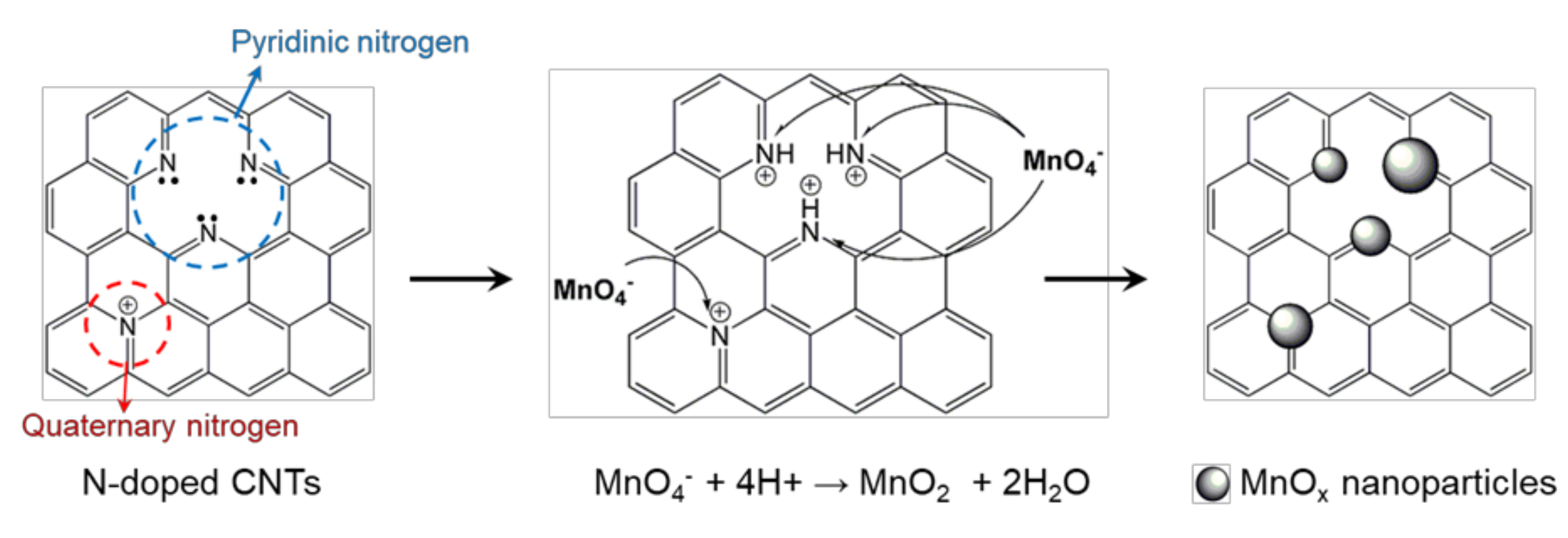
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maiti, U.N.; Lee, W.J.; Lee, J.M.; Oh, Y.; Kim, J.Y.; Kim, J.E.; Shim, J.; Han, T.H.; Kim, S.O. 25th anniversary article: Chemically modified/doped carbon nanotubes & graphene for optimized nanostructures & nanodevices. Adv. Mater. 2014, 26, 40–67. [Google Scholar] [PubMed]
- Lee, W.J.; Maiti, U.N.; Lee, J.M.; Lim, J.; Han, T.H.; Kim, S.O. Nitrogen-doped carbon nanotubes and graphene composite structures for energy and catalytic applications. Chem. Commun. 2014, 50, 6818–6830. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Yuan, J.; Giannozzi, P. Gas adsorption on graphene doped with B, N, Al, and S: A theoretical study. Appl. Phys. Lett. 2009, 95, 232105. [Google Scholar] [CrossRef]
- Lim, J.; Jung, J.-W.; Kim, N.-Y.; Lee, G.Y.; Lee, H.J.; Lee, Y.; Choi, D.S.; Yoon, K.R.; Kim, Y.-H.; Kim, I.-D. N2-dopant of graphene with electrochemically switchable bifunctional ORR/OER catalysis for Zn-air battery. Energy Storage Mater. 2020, 32, 517–524. [Google Scholar] [CrossRef]
- Li, D.J.; Maiti, U.N.; Lim, J.; Choi, D.S.; Lee, W.J.; Oh, Y.; Lee, G.Y.; Kim, S.O. Molybdenum sulfide/N-doped CNT forest hybrid catalysts for high-performance hydrogen evolution reaction. Nano Lett. 2014, 14, 1228–1233. [Google Scholar] [CrossRef]
- Haq, A.U.; Lim, J.; Yun, J.M.; Lee, W.J.; Han, T.H.; Kim, S.O. Direct Growth of Polyaniline Chains from N-Doped Sites of Carbon Nanotubes. Small 2013, 9, 3829–3833. [Google Scholar] [CrossRef]
- Parambhath, V.B.; Nagar, R.; Ramaprabhu, S. Effect of nitrogen doping on hydrogen storage capacity of palladium decorated graphene. Langmuir 2012, 28, 7826–7833. [Google Scholar] [CrossRef]
- Nandi, D.; Mohan, V.B.; Bhowmick, A.K.; Bhattacharyya, D. Metal/metal oxide decorated graphene synthesis and application as supercapacitor: A review. J. Mater. Sci. 2020, 55, 6375–6400. [Google Scholar] [CrossRef]
- Salice, P.; Rossi, E.; Pace, A.; Maity, P.; Carofiglio, T.; Menna, E.; Maggini, M. Chemistry of Carbon Nanotubes in Flow. J. Flow Chem. 2014, 4, 79–85. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.-H.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [Green Version]
- Lou, X.W.; Deng, D.; Lee, J.Y.; Feng, J.; Archer, L.A. Self-supported formation of needlelike Co3O4 nanotubes and their application as lithium-ion battery electrodes. Adv. Mater. 2008, 20, 258–262. [Google Scholar] [CrossRef]
- Hu, C.-C.; Chang, K.-H.; Lin, M.-C.; Wu, Y.-T. Design and tailoring of the nanotubular arrayed architecture of hydrous RuO2 for next generation supercapacitors. Nano Lett. 2006, 6, 2690–2695. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Peng, S.; Yang, X.; Strong, J.; Sarkar, B.; Jiang, Q.; Peng, F.; Liu, D.; Wang, H. MnO2-decorated N-doped carbon nanotube with boosted activity for low-temperature oxidation of formaldehyde. J. Hazard. Mater. 2020, 396, 122750. [Google Scholar] [CrossRef]
- Amini, K.; Gostick, J.; Pritzker, M.D. Metal and metal oxide electrocatalysts for redox flow batteries. Adv. Func. Mater. 2020, 30, 1910564. [Google Scholar] [CrossRef]
- Zhu, J.; Tu, W.; Pan, H.; Zhang, H.; Liu, B.; Cheng, Y.; Deng, Z.; Zhang, H. Self-Templating Synthesis of Hollow Co3O4 Nanoparticles Embedded in N, S-Dual-Doped Reduced Graphene Oxide for Lithium Ion Batteries. ACS Nano 2020, 14, 5780–5787. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.G.; Sefidi, P.Y.; Aydin, Z.; Kinayyigit, S. Toward enhancing the photoelectrochemical water splitting efficiency of organic acid doped polyaniline-WO3 photoanode by photo-assisted electrochemically reduced graphene oxide. Electrochim. Acta 2020, 333, 135475. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z.; Ma, Y.; Qu, Y. Graphene and their hybrid electrocatalysts for water splitting. ChemCatChem 2017, 9, 1554–1568. [Google Scholar] [CrossRef]
- Blaudeck, T.; Preuß, A.; Scharf, S.; Notz, S.; Kossmann, A.; Hartmann, S.; Kasper, L.; Mendes, R.G.; Gemming, T.; Hermann, S. Photosensitive Field-Effect Transistors Made from Semiconducting Carbon Nanotubes and Non-Covalently Attached Gold Nanoparticles. Phys. Status Solidi A 2019, 216, 1900030. [Google Scholar] [CrossRef]
- Carroll, S.; Seeger, K.; Palmer, R. Trapping of size-selected Ag clusters at surface steps. Appl. Phys. Lett. 1998, 72, 305–307. [Google Scholar] [CrossRef]
- Muhulet, A.; Miculescu, F.; Voicu, S.I.; Schütt, F.; Thakur, V.K.; Mishra, Y.K. Fundamentals and scopes of doped carbon nanotubes towards energy and biosensing applications. Mater. Today Energy 2018, 9, 154–186. [Google Scholar] [CrossRef]
- Pan, J.; Tian, X.L.; Zaman, S.; Dong, Z.; Liu, H.; Park, H.S.; Xia, B.Y. Recent Progress on Transition Metal Oxides as Bifunctional Catalysts for Lithium-Air and Zinc-Air Batteries. Batter. Supercaps 2019, 2, 336–347. [Google Scholar] [CrossRef]
- Tabassum, H.; Mahmood, A.; Zhu, B.; Liang, Z.; Zhong, R.; Guo, S.; Zou, R. Recent advances in confining metal-based nanoparticles into carbon nanotubes for electrochemical energy conversion and storage devices. Energy Environ. Sci. 2019, 12, 2924–2956. [Google Scholar] [CrossRef]
- Lim, J.; Maiti, U.N.; Kim, N.-Y.; Narayan, R.; Lee, W.J.; Choi, D.S.; Oh, Y.; Lee, J.M.; Lee, G.Y.; Kang, S.H. Dopant-specific unzipping of carbon nanotubes for intact crystalline graphene nanostructures. Nat. Commun. 2016, 7, 10364. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Huang, T.; Tang, Y.; Liu, J.; Xue, L.; Zhuang, J.; Yu, A. Factors influencing MnO2/multi-walled carbon nanotubes composite’s electrochemical performance as supercapacitor electrode. Electrochim. Acta 2009, 54, 7173–7179. [Google Scholar] [CrossRef]
- Liu, X.; Huber, T.A.; Kopac, M.C.; Pickup, P.G. Ru oxide/carbon nanotube composites for supercapacitors prepared by spontaneous reduction of Ru (VI) and Ru (VII). Electrochim. Acta 2009, 54, 7141–7147. [Google Scholar] [CrossRef]
- Tong, H.; Li, T.; Liu, J.; Gong, D.; Xiao, J.; Shen, L.; Ding, B.; Zhang, X. Fabrication of the Oxygen Vacancy Amorphous MnO2/Carbon Nanotube as Cathode for Advanced Aqueous Zinc-Ion Batteries. Energy Technol. 2021, 9, 2000769. [Google Scholar] [CrossRef]
- Rani, J.; Thangavel, R.; Kim, M.; Lee, Y.S.; Jang, J.-H. Ultra-High Energy Density Hybrid Supercapacitors Using MnO2/Reduced Graphene Oxide Hybrid Nanoscrolls. Nanomaterials 2020, 10, 2049. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, L.; Qian, W.; Xie, F.; Dong, C. Directly grown multiwall carbon nanotube and hydrothermal MnO2 composite for high-performance supercapacitor electrodes. Nanomaterials 2019, 9, 703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, G.; Liu, X.; Li, Q.; Lin, H.; Li, Y.; Nie, M.; Qin, L. The evolution of α-MnO2 from hollow cubes to hollow spheres and their electrochemical performance for supercapacitors. J. Mater. Sci. 2017, 52, 10915–10926. [Google Scholar] [CrossRef]
- Lim, J.; Lee, G.Y.; Lee, H.J.; Cha, S.K.; Choi, D.S.; Koo, S.H.; Lee, W.J.; Kim, S.O. Open porous graphene nanoribbon hydrogel via additive-free interfacial self-assembly: Fast mass transport electrodes for high-performance biosensing and energy storage. Energy Storage Mater. 2019, 16, 251–258. [Google Scholar] [CrossRef]
- Yang, H.B.; Miao, J.; Hung, S.-F.; Chen, J.; Tao, H.B.; Wang, X.; Zhang, L.; Chen, R.; Gao, J.; Chen, H.M. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: Development of highly efficient metal-free bifunctional electrocatalyst. Sci. Adv. 2016, 2, e1501122. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Zhao, X.; Qiao, Z.; Jung, J.; Zhu, Y.; Lu, Y.; Zhang, L.L.; MacDonald, A.H.; Ruoff, R.S. Capacitance of carbon-based electrical double-layer capacitors. Nat. Commun. 2014, 5, 3317. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Du, Y.; Xiao, P.; Cheng, J.; Yuan, S.; Chen, Y.; Yuan, J.; Chen, J. Transition Metal Oxide Electrode Materials for Supercapacitors: A Review of Recent Developments. Nanomaterials 2021, 11, 1248. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Lee, J.-W.; Yeo, I.-H.; Park, J.; Mho, S.-I. The role of cations of the electrolyte for the pseudocapacitive behavior of metal oxide electrodes, MnO2 and RuO2. Electrochim. Acta 2004, 50, 849–855. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.A.; Lee, W.J.; Lim, J.; Kim, S.O. N-Dopant-Mediated Growth of Metal Oxide Nanoparticles on Carbon Nanotubes. Nanomaterials 2021, 11, 1882. https://doi.org/10.3390/nano11081882
Lee JA, Lee WJ, Lim J, Kim SO. N-Dopant-Mediated Growth of Metal Oxide Nanoparticles on Carbon Nanotubes. Nanomaterials. 2021; 11(8):1882. https://doi.org/10.3390/nano11081882
Chicago/Turabian StyleLee, Jin Ah, Won Jun Lee, Joonwon Lim, and Sang Ouk Kim. 2021. "N-Dopant-Mediated Growth of Metal Oxide Nanoparticles on Carbon Nanotubes" Nanomaterials 11, no. 8: 1882. https://doi.org/10.3390/nano11081882
APA StyleLee, J. A., Lee, W. J., Lim, J., & Kim, S. O. (2021). N-Dopant-Mediated Growth of Metal Oxide Nanoparticles on Carbon Nanotubes. Nanomaterials, 11(8), 1882. https://doi.org/10.3390/nano11081882