Conical Nanotubes Synthesized by Atomic Layer Deposition of Al2O3, TiO2, and SiO2 in Etched Ion-Track Nanochannels
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
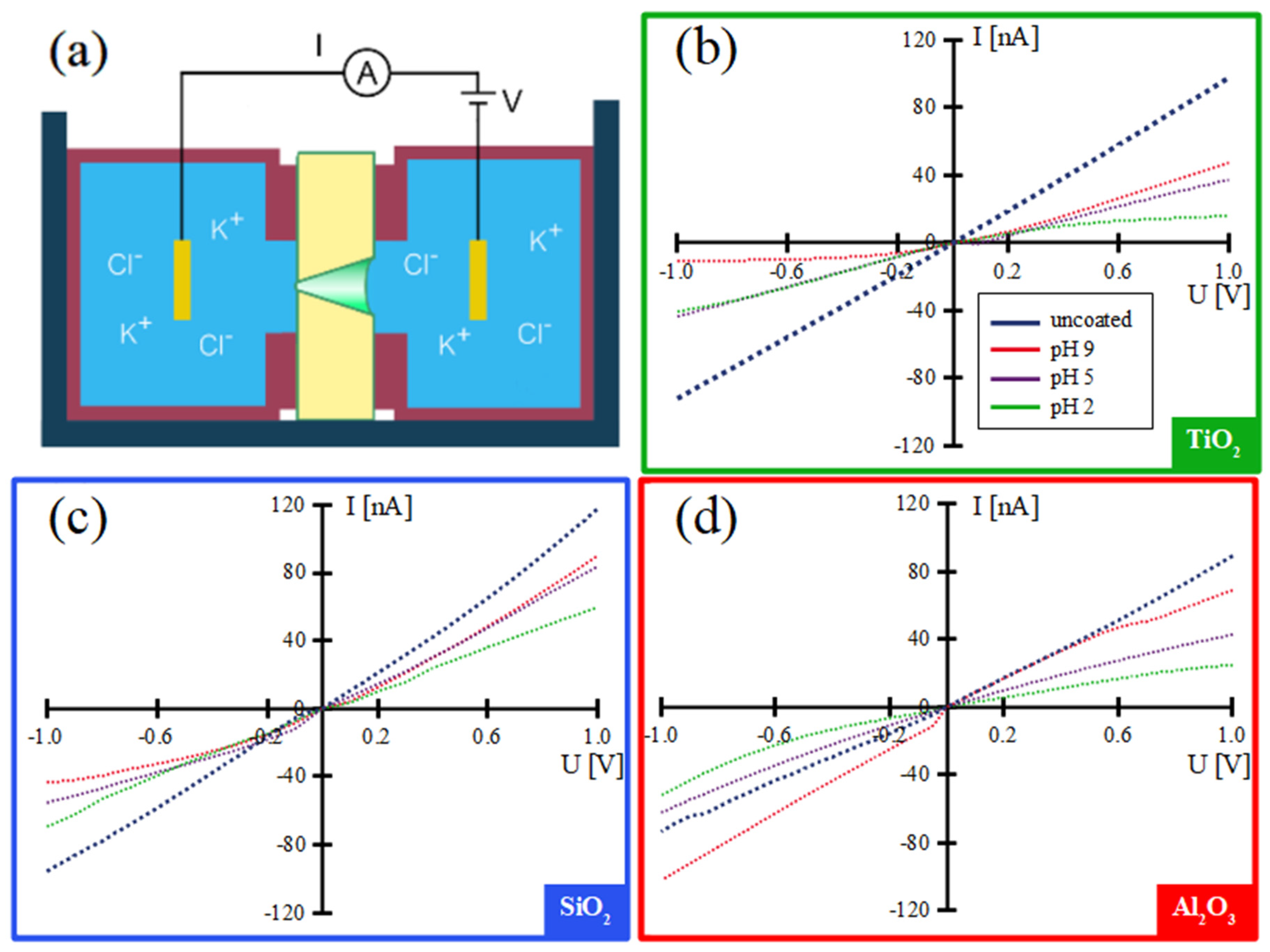
3.1. Transport Properties of ALD-Coated Single Conical Nanochannels
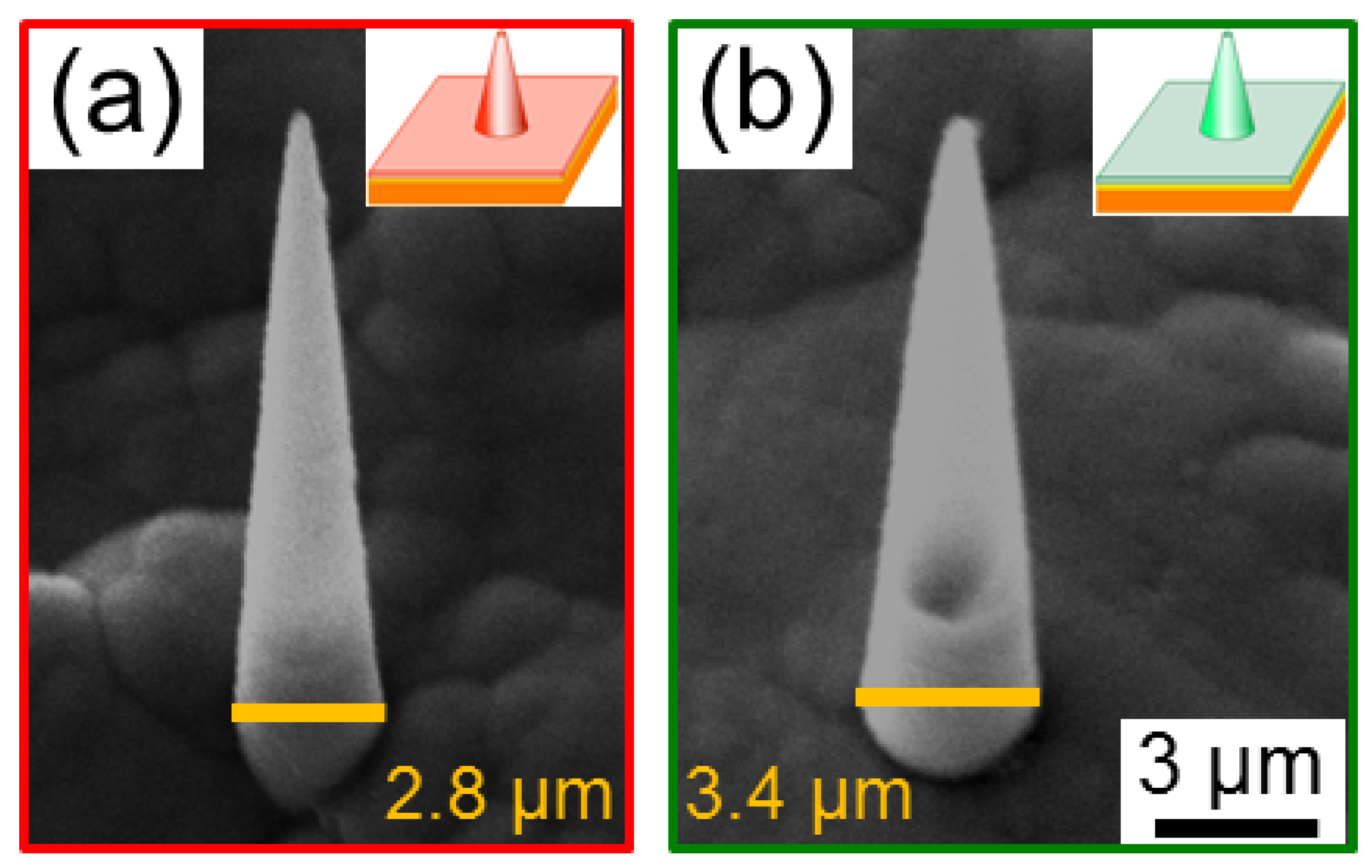
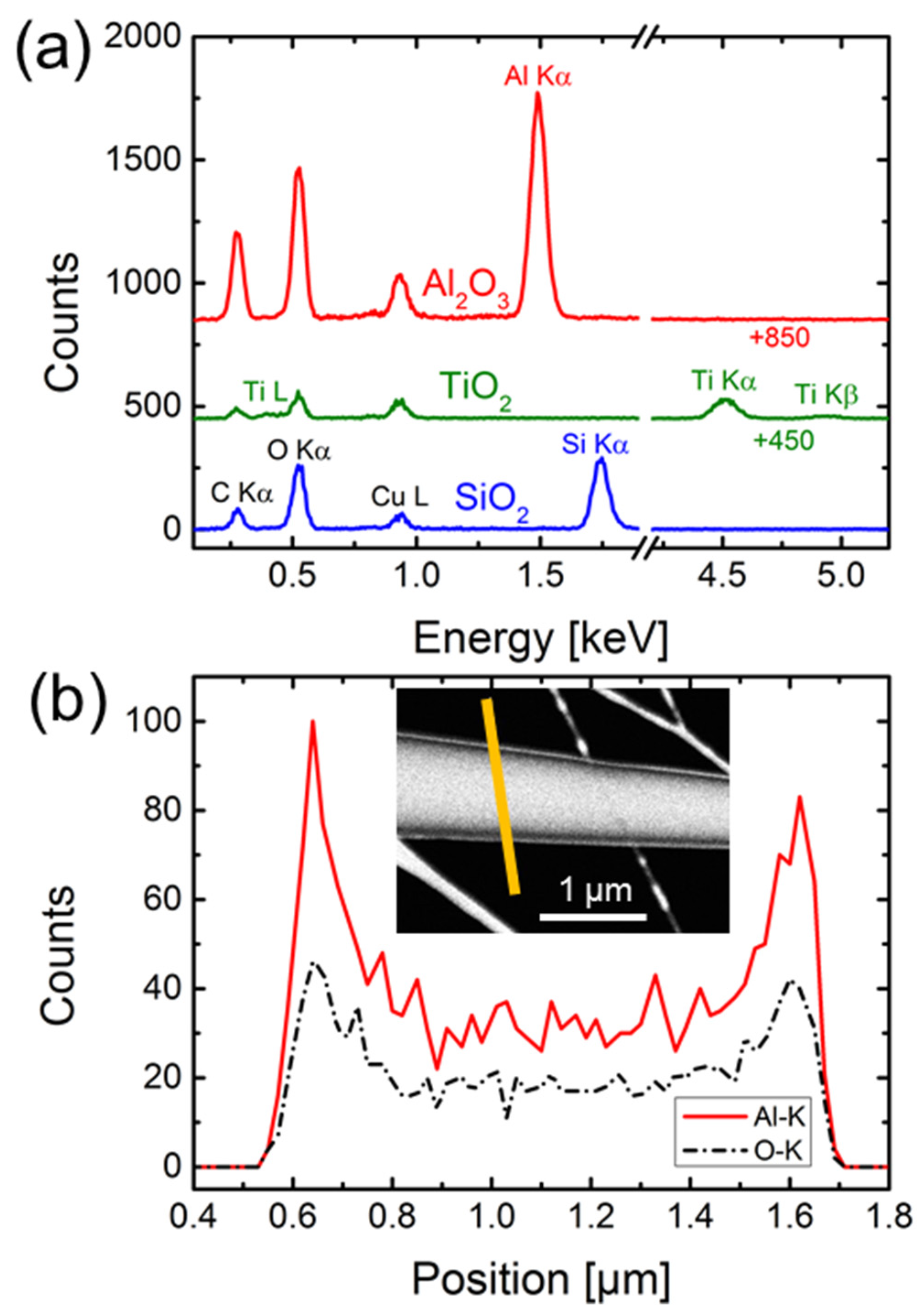
3.2. Geometrical and Compositional Characterization
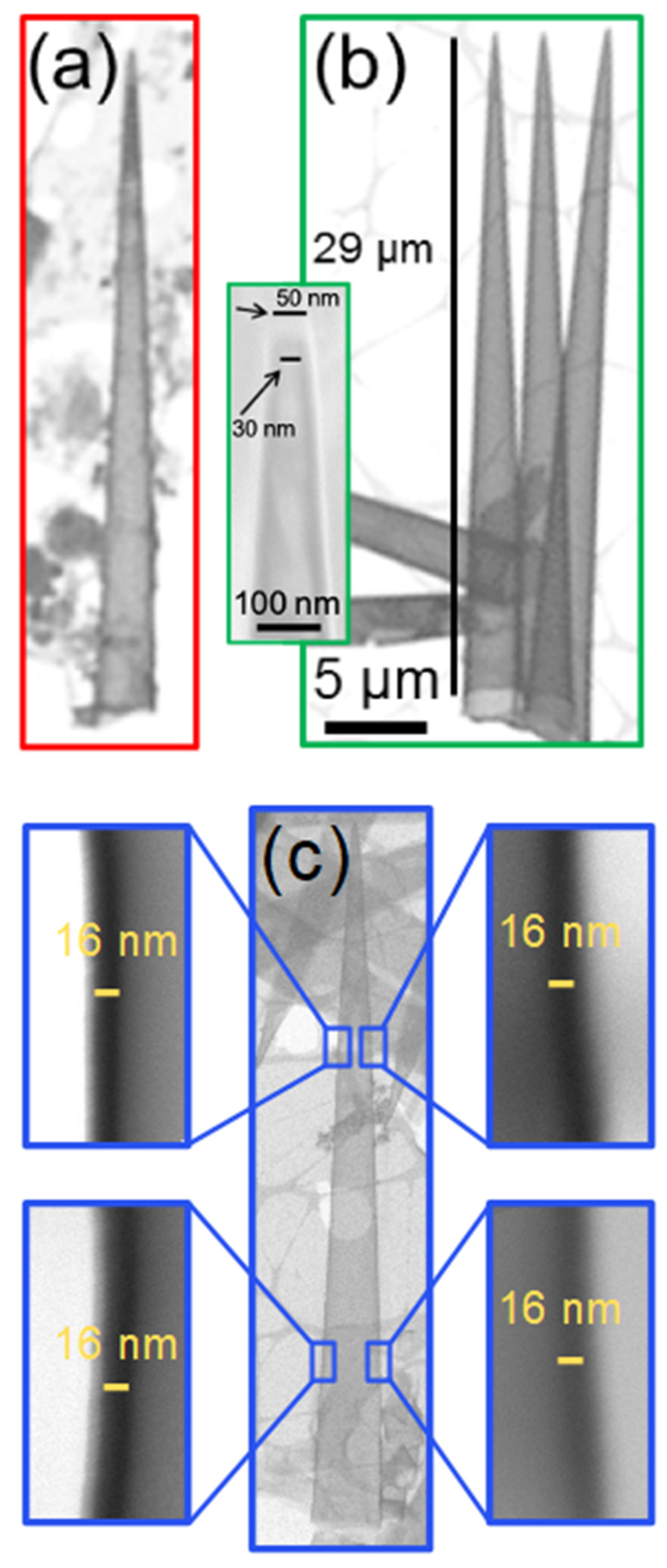
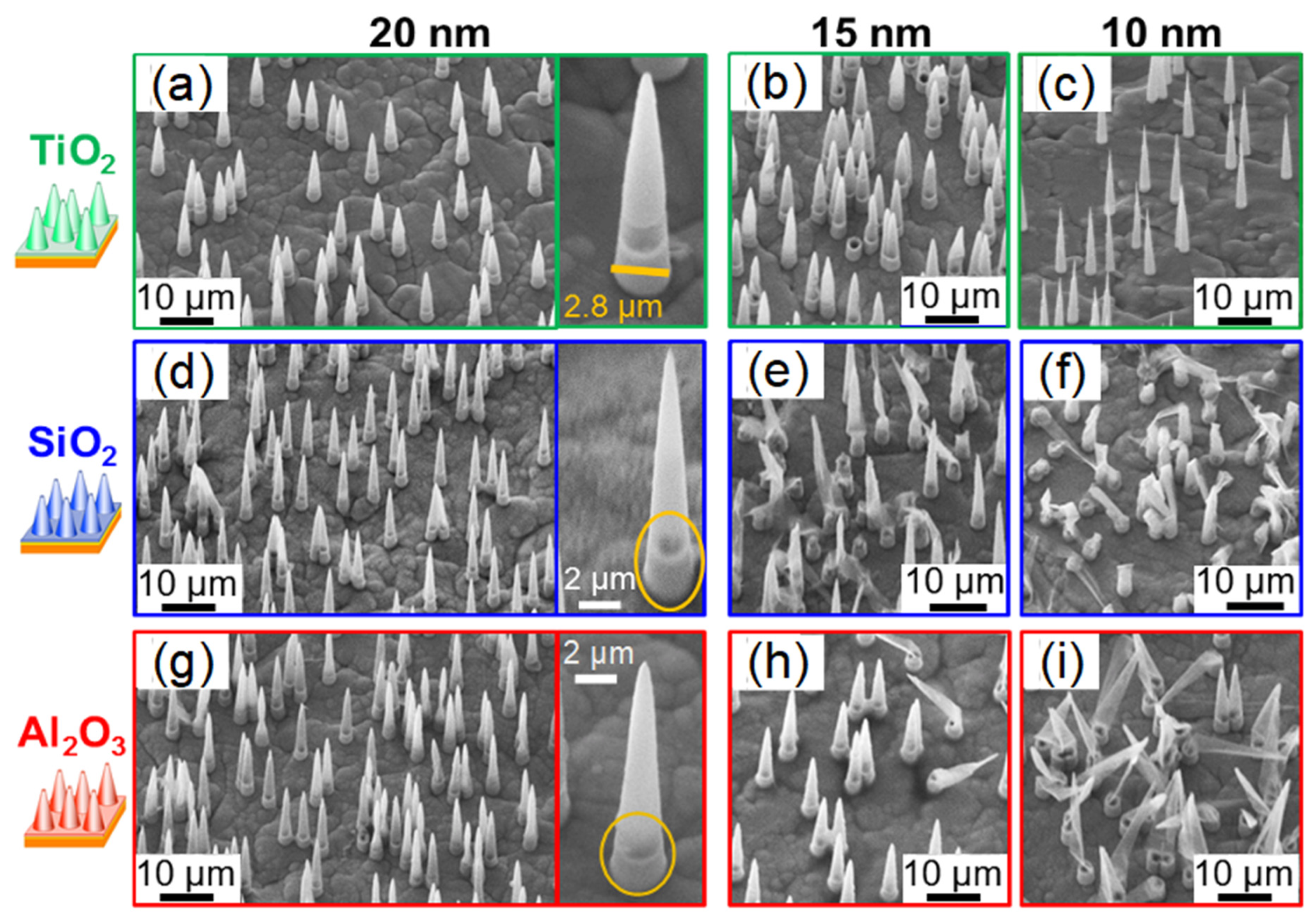
3.3. Free-Standing Conical Nanotube Arrays
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Rauwel, E.; Nilsen, O.; Rauwel, P.; Walmsley, J.C.; Frogner, H.B.; Rytter, E.; Fjellvåg, H. Oxide Coating of Alumina Nanoporous Structure Using ALD to Produce Highly Porous Spinel. Chem. Vap. Depos. 2012, 18, 315–325. [Google Scholar] [CrossRef]
- Rauwel, E.; Galeckas, A.; Rauwel, P.; Nilsen, O.; Walmsley, J.C.; Rytter, E.; Fjellvag, H. ALD Applied to Conformal Coating of Nanoporous γ-Alumina: Spinel Formation and Luminescence Induced by Europium Doping. J. Electrochem. Soc. 2012, 159, P45–P49. [Google Scholar] [CrossRef]
- Ali, R.; Saleem, M.R.; Roussey, M.; Turunen, J.; Honkanen, S. Fabrication of buried nanostructures by atomic layer deposition. Sci. Rep. 2018, 8, 15098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Kong, Y.; Zhang, Z.; Huang, G.; Mei, Y. Atomic layer–deposited nanostructures and their applications in energy storage and sensing. J. Mater. Res. 2020, 35, 701–719. [Google Scholar] [CrossRef]
- Elam, J.W.; Routkevitch, D.; Mardilovich, P.P.; George, S. Conformal Coating on Ultrahigh-Aspect-Ratio Nanopores of Anodic Alumina by Atomic Layer Deposition. Chem. Mater. 2003, 15, 3507–3517. [Google Scholar] [CrossRef]
- Romero, V.; Vega, V.; García, J.; Zierold, R.; Nielsch, K.; de la Prida, V.; Hernando, B.; Benavente, J. Changes in Morphology and Ionic Transport Induced by ALD SiO2 Coating of Nanoporous Alumina Membranes. ACS Appl. Mater. Interfaces 2013, 5, 3556–3564. [Google Scholar] [CrossRef]
- VanDersarl, J.J.; Xu, A.; Melosh, N.A. Nanostraws for Direct Fluidic Intracellular Access. Nano Lett. 2012, 12, 3881–3886. [Google Scholar] [CrossRef]
- Shin, H.; Jeong, D.-K.; Lee, J.; Sung, M.M.; Kim, J. Formation of TiO2 and ZrO2 Nanotubes Using Atomic Layer Deposition with Ultraprecise Control of the Wall Thickness. Adv. Mater. 2004, 16, 1197–1200. [Google Scholar] [CrossRef]
- Bae, C.; Kim, S.; Ahn, B.; Kim, J.; Sung, M.M.; Shin, H. Template-directed gas-phase fabrication of oxide nanotubes. J. Mater. Chem. 2008, 18, 1362–1367. [Google Scholar] [CrossRef]
- Groner, M.D.; Fabreguette, F.H.; Elam, A.J.W.; George, S. Low-Temperature Al2O3 Atomic Layer Deposition. Chem. Mater. 2004, 16, 639–645. [Google Scholar] [CrossRef]
- Minton, T.K.; Wu, B.; Zhang, J.; Lindholm, N.F.; Abdulagatov, A.I.; O’Patchen, J.; George, S.; Groner, M.D. Protecting Polymers in Space with Atomic Layer Deposition Coatings. ACS Appl. Mater. Interfaces 2010, 2, 2515–2520. [Google Scholar] [CrossRef]
- Cooper, R.; Upadhyaya, H.; Minton, T.K.; Berman, M.R.; Du, X.; George, S. Protection of polymer from atomic-oxygen erosion using Al2O3 atomic layer deposition coatings. Thin Solid Films 2008, 516, 4036–4039. [Google Scholar] [CrossRef]
- Sobel, N.; Hess, C.; Lukas, M.; Spende, A.; Stühn, B.; Toimil-Molares, M.E.; Trautmann, C. Conformal SiO2 coating of sub-100 nm diameter channels of polycarbonate etched ion-track channels by atomic layer deposition. Beilstein J. Nanotechnol. 2015, 6, 472–479. [Google Scholar] [CrossRef] [Green Version]
- Spende, A.; Sobel, N.; Lukas, M.; Zierold, R.; Riedl, J.C.; Gura, L.; Schubert, I.; Moreno, J.M.M.; Nielsch, K.; Stühn, B.; et al. TiO2, SiO2, and Al2O3 coated nanopores and nanotubes produced by ALD in etched ion-track membranes for transport measurements. Nanotechnology 2015, 26, 335301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruff, P.; Carrillo-Solano, M.; Ulrich, N.; Hadley, A.; Kluth, P.; Toimil-Molares, M.E.; Trautmann, C.; Hess, C. Nanoscale Structuring in Confined Geometries using Atomic Layer Deposition: Conformal Coating and Nanocavity Formation. Z. Phys. Chem. 2018, 232, 1147–1171. [Google Scholar] [CrossRef] [Green Version]
- Chaaya, A.A.; Le Poitevin, M.; Cabello-Aguilar, S.; Balme, S.; Bechelany, M.; Kraszewski, S.; Picaud, F.; Cambedouzou, J.; Balanzat, E.; Janot, J.-M.; et al. Enhanced Ionic Transport Mechanism by Gramicidin A Confined Inside Nanopores Tuned by Atomic Layer Deposition. J. Phys. Chem. C 2013, 117, 15306–15315. [Google Scholar] [CrossRef]
- Fink, D. Ion Tracks in Polymers. In Superconductivity; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2004; Volume 63, pp. 171–206. [Google Scholar]
- Fleischer, R.L. Nuclear Tracks in Solids: Principles and Applications; University of California Press: Berkeley, CA, USA, 1975. [Google Scholar]
- Cornelius, T.; Apel, P.; Schiedt, B.; Trautmann, C.; Toimil-Molares, M.; Karim, S.; Neumann, R. Investigation of nanopore evolution in ion track-etched polycarbonate membranes. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2007, 265, 553–557. [Google Scholar] [CrossRef]
- Toulemonde, M.; Trautmann, C.; Balanzat, E.; Hjort, K.; Weidinger, A. Track Formation and Fabrication of Nanostructures with MeV-Ion Beams. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2004, 216, 1–8. [Google Scholar] [CrossRef]
- Steckenreiter, T.; Balanzat, E.; Fuess, H.; Trautmann, C. Pyrolytic effects induced by energetic ions in polymers. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1999, 151, 161–168. [Google Scholar] [CrossRef]
- Apel, P.; Dmitriev, S.N. Micro- and nanoporous materials produced using accelerated heavy ion beams. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 013002. [Google Scholar] [CrossRef]
- Spohr, R. Status of ion track technology—Prospects of single tracks. Radiat. Meas. 2005, 40, 191–202. [Google Scholar] [CrossRef]
- Trautmann, C. Ion Beams in Nanoscience and Technology; Hellborg, R., Whitlow, H.J., Zhang, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Toimil-Molares, M.E. Characterization and properties of micro- and nanowires of controlled size, composition, and geometry fabricated by electrodeposition and ion-track technology. Beilstein J. Nanotechnol. 2012, 3, 860–883. [Google Scholar] [CrossRef] [PubMed]
- Apel, P.; Korchev, Y.; Siwy, Z.; Spohr, R.; Yoshida, M. Diode-like single-ion track membrane prepared by electro-stopping. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2001, 184, 337–346. [Google Scholar] [CrossRef]
- Siwy, Z.; Apel, P.; Dobrev, D.; Neumann, R.; Spohr, R.; Trautmann, C.; Voss, K. Ion transport through asymmetric nanopores prepared by ion track etching. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2003, 208, 143–148. [Google Scholar] [CrossRef]
- Apel, P.; Blonskaya, I.V.; Dmitriev, S.N.; Orelovitch, O.L.; Presz, A.; Sartowska, B.A. Fabrication of nanopores in polymer foils with surfactant-controlled longitudinal profiles. Nanotechnology 2007, 18, 305302. [Google Scholar] [CrossRef]
- Laucirica, G.; Albesa, A.G.; Toimil-Molares, M.E.; Trautmann, C.; Marmisollé, W.A.; Azzaroni, O. Shape matters: Enhanced osmotic energy harvesting in bullet-shaped nanochannels. Nano Energy 2020, 71, 104612. [Google Scholar] [CrossRef]
- Pérez-Mitta, G.; Trautmann, C.; Toimil-Molares, M.E.; Azzaroni, O. 3—Single Ion Track-Etched Nanochannels for Analytical Applications. In Chemically Modified Nanopores and Nanochannels; Tagliazucchi, M., Szleifer, I., Eds.; William Andrew Publishing: Boston, MA, USA, 2017; pp. 61–83. [Google Scholar]
- Duan, J.L.; Lei, D.; Chen, F.; Lau, S.P.; Milne, W.I.; Toimil-Molares, M.E.; Trautmann, C.; Liu, J. Vertically-Aligned Single-Crystal Nanocone Arrays: Controlled Fabrication and Enhanced Field Emission. ACS Appl. Mater. Interfaces 2015, 8, 472–479. [Google Scholar] [CrossRef]
- Serbun, P.; Jordan, F.; Navitski, A.; Müller, G.; Alber, I.; Toimil-Molares, M.E.; Trautmann, C. Copper nanocones grown in polymer ion-track membranes as field emitters. Eur. Phys. J. Appl. Phys. 2012, 58, 10402. [Google Scholar] [CrossRef]
- Siwy, Z.; Apel, P.; Baur, D.; Dobrev, D.D.; Korchev, Y.; Neumann, R.; Spohr, R.; Trautmann, C.; Voss, K.-O. Preparation of synthetic nanopores with transport properties analogous to biological channels. Surf. Sci. 2003, 532–535, 1061–1066. [Google Scholar] [CrossRef]
- Schiedt, B.; Healy, K.; Morrison, A.; Neumann, R.; Siwy, Z. Transport of ions and biomolecules through single asymmetric nanopores in polymer films. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2005, 236, 109–116. [Google Scholar] [CrossRef]
- Davenport, M.; Rodriguez, A.; Shea, K.J.; Siwy, Z.S. Squeezing Ionic Liquids through Nanopores. Nano Lett. 2009, 9, 2125–2128. [Google Scholar] [CrossRef]
- Pérez-Mitta, G.; Toimil-Molares, M.E.; Trautmann, C.; Marmisollé, W.A.; Azzaroni, O. Molecular Design of Solid-State Nanopores: Fundamental Concepts and Applications. Adv. Mater. 2019, 31, e1901483. [Google Scholar] [CrossRef]
- Mara, A.; Siwy, Z.; Trautmann, C.; Wan, A.J.; Kamme, F. An Asymmetric Polymer Nanopore for Single Molecule Detection. Nano Lett. 2004, 4, 497–501. [Google Scholar] [CrossRef]
- Harrell, C.C.; Choi, Y.; Horne, L.P.; Baker, L.A.; Siwy, A.Z.S.; Martin, C.R. Resistive-Pulse DNA Detection with a Conical Nanopore Sensor. Langmuir 2006, 22, 10837–10843. [Google Scholar] [CrossRef]
- Healy, K. Nanopore-based single-molecule DNA analysis. Nanomedicine 2007, 2, 459–481. [Google Scholar] [CrossRef]
- Xiao, K.; Wen, L.; Jiang, L. Biomimetic Solid-State Nanochannels: From Fundamental Research to Practical Applications. Small 2016, 12, 2810–2831. [Google Scholar] [CrossRef] [PubMed]
- Laucirica, G.; Terrones, Y.T.; Cayón, V.M.; Cortez, M.L.; Toimil-Molares, M.E.; Trautmann, C.; Marmisollé, W.A.; Azzaroni, O. High-sensitivity detection of dopamine by biomimetic nanofluidic diodes derivatized with poly(3-aminobenzylamine). Nanoscale 2020, 12, 18390–18399. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mitta, G.; Peinetti, A.S.; Cortez, M.L.; Toimil-Molares, M.E.; Trautmann, C.; Azzaroni, O. Highly Sensitive Biosensing with Solid-State Nanopores Displaying Enzymatically Reconfigurable Rectification Properties. Nano Lett. 2018, 18, 3303–3310. [Google Scholar] [CrossRef] [PubMed]
- Vlassiouk, I.; Siwy, Z.S. Nanofluidic Diode. Nano Lett. 2007, 7, 552–556. [Google Scholar] [CrossRef]
- Siwy, Z.; Gu, Y.; Spohr, H.A.; Baur, D.; Wolf-Reber, A.; Spohr, R.; Apel, P.; Korchev, Y. Rectification and voltage gating of ion currents in a nanofabricated pore. EPL Europhys. Lett. 2002, 60, 349–355. [Google Scholar] [CrossRef]
- Alcaraz, A.; Ramírez, P.; García-Giménez, E.; López, M.L.; Andrio, A.; Aguilella, V.M. A pH-Tunable Nanofluidic Diode: Electrochemical Rectification in a Reconstituted Single Ion Channel. J. Phys. Chem. B 2006, 110, 21205–21209. [Google Scholar] [CrossRef] [PubMed]
- Siwy, Z.; Fuliński, A. A nanodevice for rectification and pumping ions. Am. J. Phys. 2004, 72, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Hulteen, J.C.; Jirage, A.K.B.; Martin, C.R. Introducing Chemical Transport Selectivity into Gold Nanotubule Membranes. J. Am. Chem. Soc. 1998, 120, 6603–6604. [Google Scholar] [CrossRef]
- Ali, M.; Yameen, B.; Cervera, J.; Ramírez, P.; Neumann, R.; Ensinger, W.; Knoll, W.; Azzaroni, O. Layer-by-Layer Assembly of Polyelectrolytes into Ionic Current Rectifying Solid-State Nanopores: Insights from Theory and Experiment. J. Am. Chem. Soc. 2010, 132, 8338–8348. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, M.; Azzaroni, O.; Szleifer, I. Responsive Polymers End-Tethered in Solid-State Nanochannels: When Nanoconfinement Really Matters. J. Am. Chem. Soc. 2010, 132, 12404–12411. [Google Scholar] [CrossRef]
- Yameen, B.; Ali, M.; Neumann, R.; Ensinger, W.; Knoll, W.; Azzaroni, O. Proton-regulated rectified ionic transport through solid-state conical nanopores modified with phosphate-bearing polymer brushes. Chem. Commun. 2010, 46, 1908–1910. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fu, Q.; Wang, X.; Kong, D.; Sheng, Q.; Wang, Y.; Chen, Q.; Xue, J. Atomic Layer Deposition Modified Track-Etched Conical Nanochannels for Protein Sensing. Anal. Chem. 2015, 87, 8227–8233. [Google Scholar] [CrossRef]
- Ali, M.; Ramirez, P.; Mafe, S.; Neumann, R.; Ensinger, W. A pH-Tunable Nanofluidic Diode with a Broad Range of Rectifying Properties. ACS Nano 2009, 3, 603–608. [Google Scholar] [CrossRef]
- Ali, M.; Yameen, B.; Neumann, R.; Ensinger, W.; Knoll, W.; Azzaroni, O. Biosensing and Supramolecular Bioconjugation in Single Conical Polymer Nanochannels. Facile Incorporation of Biorecognition Elements into Nanoconfined Geometries. J. Am. Chem. Soc. 2008, 130, 16351–16357. [Google Scholar] [CrossRef]
- Ali, M.; Schiedt, B.; Healy, K.; Neumann, R.; Ensinger, W. Modifying the surface charge of single track-etched conical nanopores in polyimide. Nanotechnology 2008, 19, 085713. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Zhang, H.; Xie, G.; Xiao, K.; Wen, L.; Li, S.; Tian, Y.; Jiang, L. Ultratrace detection of glucose with enzyme-functionalized single nanochannels. J. Mater. Chem. A 2014, 2, 19131–19135. [Google Scholar] [CrossRef]
- Apel, P.Y.; Blonskaya, I.V.; Orelovitch, O.L.; Ramírez, P.; Sartowska, B.A. Effect of nanopore geometry on ion current rectification. Nanotechnology 2011, 22, 175302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Y.; Vlassiouk, I.; Hinkle, T.; Toimil-Molares, M.E.; Levine, A.J.; Siwy, Z.S. Role of Particle Focusing in Resistive-Pulse Technique: Direction-Dependent Velocity in Micropores. ACS Nano 2016, 10, 3509–3517. [Google Scholar] [CrossRef]
- Du, Y.; Du, X.; George, S. SiO2 film growth at low temperatures by catalyzed atomic layer deposition in a viscous flow reactor. Thin Solid Films 2005, 491, 43–53. [Google Scholar] [CrossRef]
- Jakschik, S.; Schroeder, U.; Hecht, T.; Gutsche, M.; Seidl, H.; Bartha, J.W. Crystallization behavior of thin ALD-Al2O3 films. Thin Solid Films 2003, 425, 216–220. [Google Scholar] [CrossRef]
- Aarik, J.; Aidla, A.; Uustare, T.; Ritala, M.; Leskela, M. Titanium isopropoxide as a precursor for atomic layer deposition: Characterization of titanium dioxide growth process. Appl. Surf. Sci. 2000, 161, 385–395. [Google Scholar] [CrossRef]
- Sneh, O.; Wise, M.; Ott, A.; Okada, L.; George, S. Atomic layer growth of SiO2 on Si(100) using SiCl4 and H2O in a binary reaction sequence. Surf. Sci. 1995, 334, 135–152. [Google Scholar] [CrossRef]
- Kim, K. Chemical and electrical characterization of virgin and protein-fouled polycarbonate track-etched membranes by FTIR and streaming-potential measurements. J. Membr. Sci. 1997, 134, 199–208. [Google Scholar] [CrossRef]
- Carter, C.B.; Norton, M.G. Ceramic Materials: Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2007; Volume 716. [Google Scholar]
- Schoch, R.B.; Han, J.; Renaud, P. Transport phenomena in nanofluidics. Rev. Mod. Phys. 2008, 80, 839–883. [Google Scholar] [CrossRef] [Green Version]
- Rückerl, A.; Zeisel, R.; Mandl, M.; Costina, I.; Schroeder, T.; Zoellner, M.H. Characterization and prevention of humidity related degradation of atomic layer deposited Al2O3. J. Appl. Phys. 2017, 121, 025306. [Google Scholar] [CrossRef]
- Lide, D. CRC Handbook of Chemistry and Physics; CRC Press: Bocca Raton, FL, USA, 2005. [Google Scholar]
- Pérez-Mitta, G.; Marmisollé, W.A.; Azzaroni, O.; Trautmann, C.; Toimil-Molares, M.E. An All-Plastic Field-Effect Nanofluidic Diode Gated by a Conducting Polymer Layer. Adv. Mater. 2017, 29, 1700972. [Google Scholar] [CrossRef]
- Wang, X.; Xue, J.; Wang, L.; Guo, W.; Zhang, W.; Wang, Y.; Liu, Q.; Ji, H.; Ouyang, Q. How the geometric configuration and the surface charge distribution influence the ionic current rectification in nanopores. J. Phys. D Appl. Phys. 2007, 40, 7077–7084. [Google Scholar] [CrossRef]
- Momotenko, D.; Josserand, J.; Girault, H.; Cortés-Salazar, F.; Liu, S.; Shao, Y. Ion current rectification and rectification inversion in conical nanopores: A perm-selective view. Phys. Chem. Chem. Phys. 2011, 13, 5430–5440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heins, E.A.; Siwy, Z.S.; Baker, L.; Martin, C.R. Detecting Single Porphyrin Molecules in a Conically Shaped Synthetic Nanopore. Nano Lett. 2005, 5, 1824–1829. [Google Scholar] [CrossRef]
- Müller, S.; Schötz, C.; Picht, O.; Sigle, W.; Kopold, P.; Rauber, M.; Alber, I.; Neumann, R.; Toimil-Molares, M.E.; Schubert, I. Electrochemical Synthesis of Bi1–xSbx Nanowires with Simultaneous Control on Size, Composition, and Surface Roughness. Cryst. Growth Des. 2011, 12, 615–621. [Google Scholar] [CrossRef]
- Hatty, V.; Kahn, H.; Heuer, A. Fracture Toughness, Fracture Strength, and Stress Corrosion Cracking of Silicon Dioxide Thin Films. J. Microelectromech. Syst. 2008, 17, 943–947. [Google Scholar] [CrossRef]
- Li, J.; Forberg, S.; Hermansson, L. Caption of the Mechanical Properties of Hot Isostatically Pressed Titania and Titania-Calcium Phosphate Composites Powder Preparation. Biomaterials 1991, 12, 438–440. [Google Scholar] [CrossRef]
- Claussen, N. Fracture Toughness of Al2O3 with an Unstabilized ZrO2 Dispersed Phase. J. Am. Ceram. Soc. 1976, 59, 49–51. [Google Scholar] [CrossRef]
- Basu, B.; Balani, K. Advanced Structural Ceramics; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]

| Coating Material | Tip Diameter Uncoated | Tip Diameter Coated |
|---|---|---|
| TiO2 | 107 nm | 71 nm |
| SiO2 | 106 nm | 72 nm |
| Al2O3 | 103 nm | 74 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulrich, N.; Spende, A.; Burr, L.; Sobel, N.; Schubert, I.; Hess, C.; Trautmann, C.; Toimil-Molares, M.E. Conical Nanotubes Synthesized by Atomic Layer Deposition of Al2O3, TiO2, and SiO2 in Etched Ion-Track Nanochannels. Nanomaterials 2021, 11, 1874. https://doi.org/10.3390/nano11081874
Ulrich N, Spende A, Burr L, Sobel N, Schubert I, Hess C, Trautmann C, Toimil-Molares ME. Conical Nanotubes Synthesized by Atomic Layer Deposition of Al2O3, TiO2, and SiO2 in Etched Ion-Track Nanochannels. Nanomaterials. 2021; 11(8):1874. https://doi.org/10.3390/nano11081874
Chicago/Turabian StyleUlrich, Nils, Anne Spende, Loïc Burr, Nicolas Sobel, Ina Schubert, Christian Hess, Christina Trautmann, and Maria Eugenia Toimil-Molares. 2021. "Conical Nanotubes Synthesized by Atomic Layer Deposition of Al2O3, TiO2, and SiO2 in Etched Ion-Track Nanochannels" Nanomaterials 11, no. 8: 1874. https://doi.org/10.3390/nano11081874
APA StyleUlrich, N., Spende, A., Burr, L., Sobel, N., Schubert, I., Hess, C., Trautmann, C., & Toimil-Molares, M. E. (2021). Conical Nanotubes Synthesized by Atomic Layer Deposition of Al2O3, TiO2, and SiO2 in Etched Ion-Track Nanochannels. Nanomaterials, 11(8), 1874. https://doi.org/10.3390/nano11081874






