Chemical and Physical Characterisation of Human Serum Albumin Nanocolloids: Kinetics, Strength and Specificity of Bonds with 99mTc and 68Ga
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Quality Controls and Labelling Yield
2.2.2. [99mTc]Tc-HSA Labelling Kinetics
2.2.3. [99mTc]Tc-NC Labelling Kinetics
2.2.4. [99mTc]Tc-NC Binding Affinity Studies
2.2.5. [68Ga]Ga-NC Binding Affinity Studies
2.2.6. Competition Evaluation between 68Ga and 99mTc for NC Labelling
2.2.7. Effects of pH on [99mTc]Tc-NC Labelling
2.2.8. [99mTc]Tc -HSA Stability Test via DMSA Competition
2.2.9. [99mTc]Tc-NC Stability Test via DMSA Competition
3. Results
3.1. Quality Controls and Labelling Yield
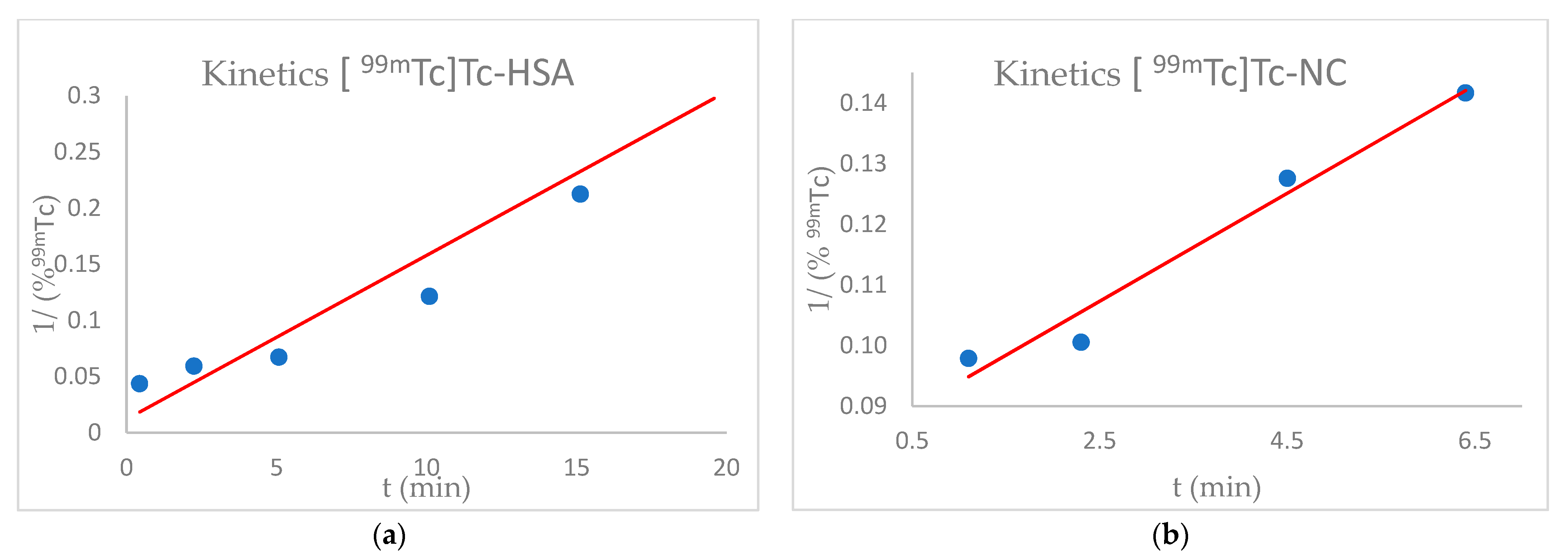
3.2. Kinetics Study of HSA and NC Labelling with 99mTc
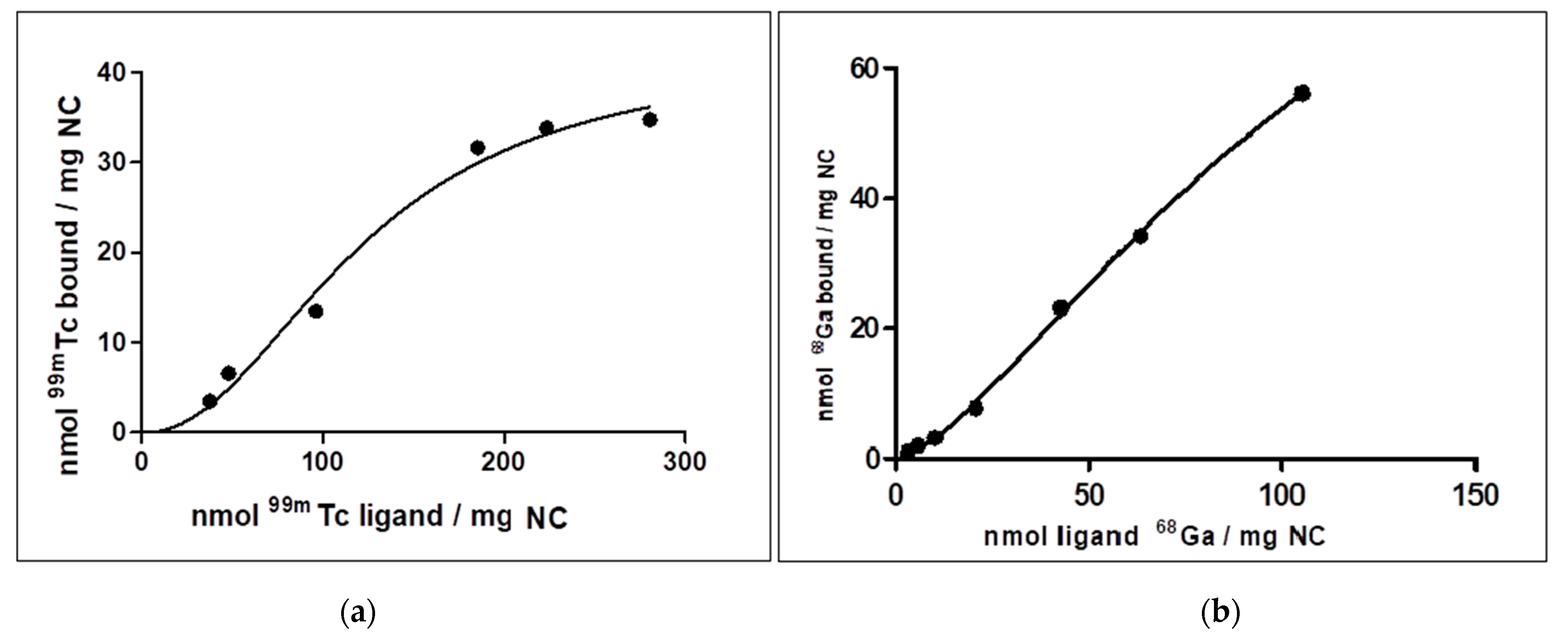
3.3. [99mTc]Tc/[68Ga]Ga-NC Binding Affinities
3.4. 68Ga and 99mTc Competition for NC
3.5. Effects of pH on [99mTc]Tc-NC Labelling
3.6. HSA and NC Competition with DMSA for 99mTc Stability Bond
4. Discussion
4.1. Radiolabelling Kinetics of HSA and NC with 99mTc
4.2. [99mTc]Tc-NC and [68Ga]Ga-NC Binding Affinity Studies
4.3. 68Ga and 99mTc Competition for NC
4.4. Effects of pH on 99mTc Radiolabelling
4.5. HSA and NC Competition with DMSA for 99mTc Stability Bond
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, R.; Heuser, T.; Fitz, H.; Hans-johachim, S.; Esra, W.; Zippel, H.H. Lymphoscintigraphic Sentinel Node Imaging and Gamma Probe Detection in Breast Cancer with Tc-99m Nanocolloidal Albumin. Clin. Nucl. Med. 2001, 26, 293–298. [Google Scholar]
- Warncke, S.H.; Mattei, A.; Fuechsel, F.G.; Z’Brun, S.; Krause, T.; Studer, U.E. Studer, Detection Rate and Operating Time Required for γ Probe-Guided Sentinel Lymph Node Resection after Injection of Technetium-99m Nanocolloid into the Prostate with and without Preoperative Imaging. Europ. Urol. 2007, 52, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Mudun, A.; Unal, S.; Aktay, R.; Akmehmet, S.; Cantez, S. Tc-99m Nanocolloid and Tc-99m MDP Three-Phase Bone Imaging In Osteomyelitis and Septic Arthritis A Comparative Study. Clin. Nucl. Med. 1995, 20, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Abele, J.T.; Allred, K.; Clare, T.; Bedard, E.L. Lymphoscintigraphy in early-stage non-small cell lung cancer with Technetium-99m nanocolloids and hybrid SPECT/TC: A pilot project. Ann. Nucl. Med. 2014, 28, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Fazzi, P.; Albertellu, R.; Grana, M.; Paggiaro, P.L. Lung ventilation scintigraphy in the assessment of obstructive lung disease. Breathe 2009, 5, 252–262. [Google Scholar] [CrossRef]
- Becker, W. The contribution of nuclear medicine to the patient with infection. Eur. J. Nucl. Med. Mol. Imaging 1995, 22, 1195–1211. [Google Scholar] [CrossRef]
- Aboagye, E.O.; Kraeber-Bodéré, F. Highlights lecture EANM 2016: “Embracing molecular imagin and multi-modal imagin: A smart move for nuclear medicine towards personalized medicine”. Eur. J. Nucl. Med. Imaging 2017, 44, 1559–1574. [Google Scholar] [CrossRef] [Green Version]
- Polyak, A.; Ross, T.L. Nanoparticles for SPECT and PET Imaging: Towards Personalized Medicine and Theranostics. Curr. Med. Chem. 2018, 25, 4328–4353. [Google Scholar] [CrossRef]
- Maus, S.; Buchholz, H.G.; Ament, S.; Brochhausen, C.; Bausbacher, N.; Schreckenberger, M. Labelling of commercially available human serum albumin kits with 68Ga as surrogates for 99mTc-MAA microspheres. Appl. Radiat. Isot. 2011, 69, 171–175. [Google Scholar] [CrossRef]
- Persico, M.G.; Marenco, M.; De Matteis, G.; Manfrinato, G.; Cavenaghi, G.; Sgarella, A.; Aprile, C.; Lodola, L. 99mTc-68Ga-ICG-Labelled Macroaggregates and Nanocolloids of Human Serum Albumin: Synthesis Procedures of a Trimodal Imaging Agent Using Commercial Kits. Contrast Media Mol. Imaging 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elzoghby, A.O.; Samy, W.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Persico, M.G.; Lodola, L.; Buroni, F.; Morandotti, M.; Pallavicini, P.; Aprile, C. 99mTc-human serum albumin kits nanocolloids: Particle sizing and radioactivity distribution. J. Label. Compd. Radiopharm 2015, 58, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Aryal, B.P.; Brugarolas, P.; He, C. Binding of ReO4−with an engineered MoO4 2−-binding protein: Towards a new approach in radiopharmaceutical applications. J. Biol. Inorg. Chem. 2011, 17, 97–106. [Google Scholar] [CrossRef]
- Simonin, J.P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Holland, J.P. Chemical Kinetics of Radiolabelling Reactions. Chem.-A Eur. J. 2018, 24, 16472–16483. [Google Scholar] [CrossRef]
- Steigman, J.; Richards, P. Chemistry of technetium 99m. Semin. Nucl. Med. 1974, 4, 269–279. [Google Scholar] [CrossRef]
- Yamasaki, K.; Chuang, V.T.G.; Maruyama, T.; Otagiri, M. Albumin–drug interaction and its clinical implication. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 5435–5443. [Google Scholar] [CrossRef] [PubMed]
- Claessens, R.A.M.J.; Boerman, O.C.; Koenders, E.B.; Oyen, W.J.; Van Der Meer, J.W.M.; Corstens, F.H.M. Technetium-99m labelled hydrazinonicotinamido human non-specific polyclonal immunoglobulin G for detection of infectious foci: A comparison with two other technetium-labelled immunoglobulin preparations. Eur. J. Nucl. Med. Mol. Imaging 1996, 23, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melendez-Alafort, L.; Ramírez, F.D.M.; Ferro-Flores, G.; De Murphy, C.A.; Pedraza-López, M.; Hnatowich, D.J. Lys and Arg in UBI: A specific site for a stable Tc-99m complex? Nucl. Med. Biol. 2003, 30, 605–615. [Google Scholar] [CrossRef]
- Roosen-Runge, F.; Heck, B.S.; Zhang, F.; Kohlbacher, O.; Schreiber, F. Interplay of pH and binding of multivalent metal ions: Charge inversion and reentrant condensation in protein solutions. J. Phys. Chem. B. 2013, 117, 5777–5787. [Google Scholar] [CrossRef] [Green Version]
- Blanck, S.; Maksimoska, J.; Baumeister, J.; Harms, K.; Marmorstein, R.; Meggers, E. The Art of Filling Protein Pockets Efficiently with Octahedral Metal Complexes. Angew. Chem. Int. Ed. 2012, 51, 5244–5246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Kd | Bmax | h | R2 | |
|---|---|---|---|---|
| [99mTc]Tc-NC | 123.1 | 42.32 | 2.158 | 0.9873 |
| [68Ga]Ga-NC | 119.4 | 123.7 | 1.395 | 0.9969 |
| 95% Confidence Intervals | ||||
| [68Ga]Ga-NC | 40.30 to 198.6 | 58.23 to 189.2 | 1.158 to 1.801 | |
| [99mTc]Tc-NC | 40.02 to 206.2 | 20.99 to 63.65 | 0.5075 to 3.809 |
| NC Particles | Atoms Number | HSA Molecules Contained in a Single NC Particle | Atoms Number |
|---|---|---|---|
| 99mTc atoms bound at equilibrium | 2.97(1) 1 × 1010 | 99mTc atoms bound at equilibrium | 18.4(2) |
| 68Ga atoms bound at equilibrium | 9.44(1) 1 × 104 | 68Ga atoms bound at equilibrium | 17.8(1) |
| 99mTc specifically bound | 1.12(1) 1 × 1010 | 99mTc specifically bound | 6.9(1) |
| 68Ga specifically bound | 9.78(1) 1 × 104 | 68Ga specifically bound | 18.5(1) |
| 99mTc Atoms | 68Ga Atoms | 99mTc Atoms | 68Ga Atoms | |
|---|---|---|---|---|
| After 1 h | After 3 h | |||
| Bound molar fraction | 7.98(1) 1 × 109 | 1.82(1) 1 × 109 | 1.23(2) 1 × 109 | 1.06(1) 1 × 109 |
| Unbound molar fraction | 3.19(1) 1 × 109 | 5.98(1) 1 × 109 | 4.02(2) 1 × 109 | 3.91(1) 1 × 109 |
| Method 1 | Method 2 | |||
|---|---|---|---|---|
| pH | bound molar fraction | unbound molar fraction | bound molar fraction | unbound molar fraction |
| 10 | 0.637(1) | 0.363(1) | 0.005(2) | 0.995(2) |
| 6 | 0.912(1) | 0.088(1) | 0.896(1) | 0.104(1) |
| 3 | 0.742(2) | 0.258(2) | 0.632(1) | 0.368(1) |
| [99mTc]Tc-HSA Molar Fraction | [99mTc]Tc-DMSA Molar Fraction | |
|---|---|---|
| Method 1 | 0.013(1) | 0.087(1) |
| Method 2 | 0.071(2) | 0.029(1) |
| [99mTc]Tc-NC Molar Fraction | [99mTc]Tc-DMSA Molar Fraction | |
|---|---|---|
| Method 1 | 0.096(1) | 0.004(1) |
| Method 2 | 0.091(1) | 0.009(2) |
| Method 2 | |
|---|---|
| 99mTc atoms per HSA | 1.95(2) 1 × 10−4 |
| 99mTc atoms per NC | 46.4(2) |
| 99mTc atoms per molecules of Human Serum Albumin in NC | 2.1(2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marenco, M.; Canziani, L.; De Matteis, G.; Cavenaghi, G.; Aprile, C.; Lodola, L. Chemical and Physical Characterisation of Human Serum Albumin Nanocolloids: Kinetics, Strength and Specificity of Bonds with 99mTc and 68Ga. Nanomaterials 2021, 11, 1776. https://doi.org/10.3390/nano11071776
Marenco M, Canziani L, De Matteis G, Cavenaghi G, Aprile C, Lodola L. Chemical and Physical Characterisation of Human Serum Albumin Nanocolloids: Kinetics, Strength and Specificity of Bonds with 99mTc and 68Ga. Nanomaterials. 2021; 11(7):1776. https://doi.org/10.3390/nano11071776
Chicago/Turabian StyleMarenco, Manuela, Letizia Canziani, Gianluca De Matteis, Giorgio Cavenaghi, Carlo Aprile, and Lorenzo Lodola. 2021. "Chemical and Physical Characterisation of Human Serum Albumin Nanocolloids: Kinetics, Strength and Specificity of Bonds with 99mTc and 68Ga" Nanomaterials 11, no. 7: 1776. https://doi.org/10.3390/nano11071776
APA StyleMarenco, M., Canziani, L., De Matteis, G., Cavenaghi, G., Aprile, C., & Lodola, L. (2021). Chemical and Physical Characterisation of Human Serum Albumin Nanocolloids: Kinetics, Strength and Specificity of Bonds with 99mTc and 68Ga. Nanomaterials, 11(7), 1776. https://doi.org/10.3390/nano11071776







