Eco-Friendly Production of AuNPs and Their Impact on the Oil Oxidative Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Vegetable Oil
2.2. Production of AuNPs
2.3. Characterization of AuNPs by TEM and EDS
2.4. Quantification of AuNPs by ICP-OES
2.5. Oxidative Stability
2.6. Fatty Acid Composition Determination
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, P.; Han, G.; De, M.; Kim, C.; Rotello, V. Gold Nanoparticles in Delivery Applications. Adv. Drug Deliv. Rev. 2008, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, X.; Hu, W.; Bai, Y.; Zhang, L. Preparation and Evaluation of Naringenin-Loaded Sulfobutylether-β-Cyclodextrin/Chitosan Nanoparticles for Ocular Drug Delivery. Carbohydr. Polym. 2016, 149. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, A.M.; Farag, M.M.; El-Rashedi, A.M.I. Bioactive Glass Nanoparticles Designed for Multiple Deliveries of Lithium Ions and Drugs: Curative and Restorative Bone Treatment. Eur. J. Pharm. Sci. 2016, 91. [Google Scholar] [CrossRef]
- Braun, K.; Pochert, A.; Lindén, M.; Davoudi, M.; Schmidtchen, A.; Nordström, R.; Malmsten, M. Membrane Interactions of Mesoporous Silica Nanoparticles as Carriers of Antimicrobial Peptides. J. Colloid Interface Sci. 2016, 475. [Google Scholar] [CrossRef] [PubMed]
- Chitemere, R.P.; Stafslien, S.; Rasulev, B.; Webster, D.C.; Quadir, M. Soysome: A Surfactant-Free, Fully Biobased, Self-Assembled Platform for Nanoscale Drug Delivery Applications. ACS Appl. Bio Mater. 2018, 1. [Google Scholar] [CrossRef]
- Suter, F.; Schmid, D.; Wandrey, F.; Zülli, F. Heptapeptide-Loaded Solid Lipid Nanoparticles for Cosmetic Anti-Aging Applications. Eur. J. Pharm. Biopharm. 2016, 108. [Google Scholar] [CrossRef] [PubMed]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid Nanoparticles (SLN, NLC) in Cosmetic and Pharmaceutical Dermal Products. Int. J. Pharm. 2009, 366. [Google Scholar] [CrossRef]
- Black, C. Health and Safety Issues With Antistatic Agents. In Handbook of Antistatics; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Song, H.-T.; Choi, J.; Huh, Y.-M.; Kim, S.; Jun, Y.; Suh, J.-S.; Cheon, J. Surface Modulation of Magnetic Nanocrystals in the Development of Highly Efficient Magnetic Resonance Probes for Intracellular Labeling. J. Am. Chem. Soc. 2005, 127. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Yonezawa, T. Sputtering onto a Liquid: Interesting Physical Preparation Method for Multi-Metallic Nanoparticles. Sci. Technol. Adv. Mater. 2018, 19. [Google Scholar] [CrossRef]
- Wender, H.; Migowski, P.; Feil, A.F.; Teixeira, S.R.; Dupont, J. Sputtering Deposition of Nanoparticles onto Liquid Substrates: Recent Advances and Future Trends. Coord. Chem. Rev. 2013, 257. [Google Scholar] [CrossRef]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Khezri, K.; Saeedi, M.; Maleki Dizaj, S. Application of Nanoparticles in Percutaneous Delivery of Active Ingredients in Cosmetic Preparations. Biomed. Pharmacother. 2018, 106. [Google Scholar] [CrossRef]
- Frizzo, M.S.; Feuser, P.E.; Berres, P.H.; Ricci-Júnior, E.; Campos, C.E.M.; Costa, C.; de Araújo, P.H.H.; Sayer, C. Simultaneous Encapsulation of Zinc Oxide and Octocrylene in Poly (Methyl Methacrylate-Co-Styrene) Nanoparticles Obtained by Miniemulsion Polymerization for Use in Sunscreen Formulations. Colloids Surf. A Physicochem. Eng. Asp. 2019, 561. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Lopedota, A.; Fini, P.; Laurenzana, A.; Fibbi, G.; Fanelli, F.; Petrella, A.; Laquintana, V.; Denora, N.; et al. One Pot Environmental Friendly Synthesis of Gold Nanoparticles Using Punica Granatum Juice: A Novel Antioxidant Agent for Future Dermatological and Cosmetic Applications. J. Colloid Interface Sci. 2018, 521. [Google Scholar] [CrossRef]
- Ballarin, B.; Mignani, A.; Mogavero, F.; Gabbanini, S.; Morigi, M. Hybrid Material Based on ZnAl Hydrotalcite and Silver Nanoparticles for Deodorant Formulation. Appl. Clay Sci. 2015, 114. [Google Scholar] [CrossRef]
- Dhal, S.; Pal, K.; Giri, S. Transdermal Delivery of Gold Nanoparticles by a Soybean Oil-Based Oleogel under Iontophoresis. ACS Appl. Bio Mater. 2020, 3. [Google Scholar] [CrossRef]
- Gupta, R.; Rai, B. Effect of Size and Surface Charge of Gold Nanoparticles on Their Skin Permeability: A Molecular Dynamics Study. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Omrani, E.; Siddaiah, A.; Moghadam, A.D.; Garg, U.; Rohatgi, P.; Menezes, P.L. Ball Milled Graphene Nano Additives for Enhancing Sliding Contact in Vegetable Oil. Nanomaterials 2021, 11. [Google Scholar] [CrossRef]
- Pinto, F.; de Barros, D.P.C.; Fonseca, L.P. Design of Multifunctional Nanostructured Lipid Carriers Enriched with α-Tocopherol Using Vegetable Oils. Ind. Crop. Prod. 2018, 118. [Google Scholar] [CrossRef]
- Lacatusu, I.; Niculae, G.; Badea, N.; Stan, R.; Popa, O.; Oprea, O.; Meghea, A. Design of Soft Lipid Nanocarriers Based on Bioactive Vegetable Oils with Multiple Health Benefits. Chem. Eng. J. 2014, 246. [Google Scholar] [CrossRef]
- Park, H.; Yang, S.; Kang, J.Y.; Park, M.-H. On-Demand Drug Delivery System Using Micro-Organogels with Gold Nanorods. ACS Med. Chem. Lett. 2016, 7. [Google Scholar] [CrossRef]
- Vergallo, C. Nutraceutical Vegetable Oil Nanoformulations for Prevention and Management of Diseases. Nanomaterials 2020, 10, 1232. [Google Scholar] [CrossRef]
- Dario, M.F.; Oliveira, F.F.; Marins, D.S.S.; Baby, A.R.; Velasco, M.V.R.; Löbenberg, R.; Bou-Chacra, N.A. Synergistic Photoprotective Activity of Nanocarrier Containing Oil of Acrocomia Aculeata (Jacq.) Lodd. Ex. Martius—Arecaceae. Ind. Crops Prod. 2018, 112. [Google Scholar] [CrossRef]
- Tehranifar, A.; Selahvarzi, Y.; Kharrazi, M.; Bakhsh, V.J. High Potential of Agro-Industrial by-Products of Pomegranate (Punica Granatum L.) as the Powerful Antifungal and Antioxidant Substances. Ind. Crop. Prod. 2011, 34. [Google Scholar] [CrossRef]
- Dhavamani, S.; Poorna Chandra Rao, Y.; Lokesh, B.R. Total Antioxidant Activity of Selected Vegetable Oils and Their Influence on Total Antioxidant Values in Vivo: A Photochemiluminescence Based Analysis. Food Chem. 2014, 164. [Google Scholar] [CrossRef] [PubMed]
- Wender, H.; de Oliveira, L.F.; Feil, A.F.; Lissner, E.; Migowski, P.; Meneghetti, M.R.; Teixeira, S.R.; Dupont, J. Synthesis of Gold Nanoparticles in a Biocompatible Fluid from Sputtering Deposition onto Castor Oil. Chem. Commun. 2010, 46. [Google Scholar] [CrossRef] [PubMed]
- Wender, H.; Gonçalves, R.V.; Feil, A.F.; Migowski, P.; Poletto, F.S.; Pohlmann, A.R.; Dupont, J.; Teixeira, S.R. Sputtering onto Liquids: From Thin Films to Nanoparticles. J. Phys. Chem. C 2011, 115. [Google Scholar] [CrossRef]
- Wender, H.; de Oliveira, L.F.; Migowski, P.; Feil, A.F.; Lissner, E.; Prechtl, M.H.G.; Teixeira, S.R.; Dupont, J. Ionic Liquid Surface Composition Controls the Size of Gold Nanoparticles Prepared by Sputtering Deposition. J. Phys. Chem. C 2010, 114. [Google Scholar] [CrossRef]
- Wender, H.; Migowski, P.; Feil, A.F.; de Oliveira, L.F.; Prechtl, M.H.G.; Leal, R.; Machado, G.; Teixeira, S.R.; Dupont, J. On the Formation of Anisotropic Gold Nanoparticles by Sputtering onto a Nitrile Functionalised Ionic Liquid. Phys. Chem. Chem. Phys. 2011, 13. [Google Scholar] [CrossRef]
- Castro, H.P.S.; Wender, H.; Alencar, M.A.R.C.; Teixeira, S.R.; Dupont, J.; Hickmann, J.M. Third-Order Nonlinear Optical Response of Colloidal Gold Nanoparticles Prepared by Sputtering Deposition. J. Appl. Phys. 2013, 114. [Google Scholar] [CrossRef]
- Ishida, Y.; Nakabayashi, R.; Corpuz, R.D.; Yonezawa, T. Water-Dispersible Fluorescent Silver Nanoparticles via Sputtering Deposition over Liquid Polymer Using a Very Short Thiol Ligand. Colloids Surf. A Physicochem. Eng. Asp. 2017, 518. [Google Scholar] [CrossRef]
- Venables, J.A.; Spiller, G.D.T.; Hanbucken, M. Nucleation and Growth of Thin Films. Rep. Prog. Phys. 1984, 47. [Google Scholar] [CrossRef]
- Yu, S.-J.; Zhang, Y.-J.; Chen, M.-G. Comparision of Stree Relief Mechanisms of Metal Films Deposited on Liquid Substrates by Thermal Evaporating and Sputtering. Int. J. Mod. Phys. B 2010, 24. [Google Scholar] [CrossRef]
- Fan, J.H.; Hung, W.I.; Li, W.T.; Yeh, J.M. Biocompatibility Study of Gold Nanoparticles to Human Cells. In Proceedings of the 13th International Conference on Biomedical Engineering, Singapore, 3–6 December 2008; Springer: Berlin/Heidelberg, Gremany, 2009; pp. 870–873. [Google Scholar]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of Gold Nanoparticles and Their Endocytotic Fate Inside the Cellular Compartment: A Microscopic Overview. Langmuir 2005, 21. [Google Scholar] [CrossRef] [PubMed]
- Mieszawska, A.J.; Mulder, W.J.M.; Fayad, Z.A.; Cormode, D.P. Multifunctional Gold Nanoparticles for Diagnosis and Therapy of Disease. Mol. Pharm. 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, Y.; Li, J.; Tang, L.; Hu, J.; Deng, Z. Evaluating and Predicting the Oxidative Stability of Vegetable Oils with Different Fatty Acid Compositions. J. Food Sci. 2013, 78. [Google Scholar] [CrossRef]
- Pardauil, J.J.R.; Souza, L.K.C.; Molfetta, F.A.; Zamian, J.R.; Rocha Filho, G.N.; da Costa, C.E.F. Determination of the Oxidative Stability by DSC of Vegetable Oils from the Amazonian Area. Bioresour. Technol. 2011, 102. [Google Scholar] [CrossRef]
- Trindade, A.S.N.; Dantas, A.F.; Lima, D.C.; Ferreira, S.L.C.; Teixeira, L.S.G. Multivariate Optimization of Ultrasound-Assisted Extraction for Determination of Cu, Fe, Ni and Zn in Vegetable Oils by High-Resolution Continuum Source Atomic Absorption Spectrometry. Food Chem. 2015, 185. [Google Scholar] [CrossRef]
- Nunes, L.S.; Barbosa, J.T.P.; Fernandes, A.P.; Lemos, V.A.; dos Santos, W.N.L.; Korn, M.G.A.; Teixeira, L.S.G. Multi-Element Determination of Cu, Fe, Ni and Zn Content in Vegetable Oils Samples by High-Resolution Continuum Source Atomic Absorption Spectrometry and Microemulsion Sample Preparation. Food Chem. 2011, 127. [Google Scholar] [CrossRef]
- Zhu, F.; Fan, W.; Wang, X.; Qu, L.; Yao, S. Health Risk Assessment of Eight Heavy Metals in Nine Varieties of Edible Vegetable Oils Consumed in China. Food Chem. Toxicol. 2011, 49. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Michels, F.S.; Trindade, M.A.G.; Falcão, E.A.; Guimarães, R.C.A.; Oliveira, S.L.; Caires, A.R.L. The Effect of the Excitation Light Intensity during On-Line Monitoring of Biodiesel by Fluorescence Spectroscopy. Fuel 2017, 193. [Google Scholar] [CrossRef]
- British Standards Institution. BS EN 14112:2003 Fatty Acid Methyl Esters (FAME)-Determination of Oxidation Stability (Accelerated Oxidation Test). Eur. Comm. Stand 2003, 3, 18. [Google Scholar]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 2871. [Google Scholar] [CrossRef]
- Ye, G.; Zhang, Q.; Feng, C.; Ge, H.; Jiao, Z. Structural and Electrical Properties of a Metallic Rough-Thin-Film System Deposited on Liquid Substrates. Phys. Rev. B 1996, 54. [Google Scholar] [CrossRef]
- Dupont, J.; Scholten, J.D. On the Structural and Surface Properties of Transition-Metal Nanoparticles in Ionic Liquids. Chem. Soc. Rev. 2010, 39. [Google Scholar] [CrossRef]
- Yuan, Q.; Williams, R.A. CO-Stabilisation Mechanisms of Nanoparticles and Surfactants in Pickering Emulsions Produced by Membrane Emulsification. J. Membr. Sci. 2016, 497. [Google Scholar] [CrossRef]
- Migowski, P.; Teixeira, S.R.; Machado, G.; Alves, M.C.M.; Geshev, J.; Dupont, J. Structural and Magnetic Characterization of Ni Nanoparticles Synthesized in Ionic Liquids. J. Electron. Spectrosc. Relat. Phenom. 2007, 156–158. [Google Scholar] [CrossRef][Green Version]
- Zhang, W.; Li, Y.; Niu, J.; Chen, Y. Photogeneration of Reactive Oxygen Species on Uncoated Silver, Gold, Nickel, and Silicon Nanoparticles and Their Antibacterial Effects. Langmuir 2013, 29. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15. [Google Scholar] [CrossRef]
- Martinez-Torres, A.C.; Lorenzo-Anota, H.Y.; García-Juárez, M.G.; Zárate-Triviño, D.G.; Rodriguez-Padilla, C. Chitosan Gold Nanoparticles Induce Different ROS-Dependent Cell Death Modalities in Leukemic Cells. Int. J. Nanomed. 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Piryazev, A.P.; Azizova, O.A.; Aseichev, A.V.; Dudnik, L.B.; Sergienko, V.I. Effect of Gold Nanoparticles on Production of Reactive Oxygen Species by Human Peripheral Blood Leukocytes Stimulated with Opsonized Zymosan. Bull. Exp. Biol. Med. 2013, 156. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, X.; Chen, P.; Zhang, L.; Yang, C.S.; Zhang, J. Selenium Nanoparticles Are More Efficient than Sodium Selenite in Producing Reactive Oxygen Species and Hyper-Accumulation of Selenium Nanoparticles in Cancer Cells Generates Potent Therapeutic Effects. Free Radic. Biol. Med. 2018, 126. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Lin, L.; Fu, Y.; Alenius, H.; Lindsey, K.; Chen, C. Silver Nanoparticles Regulate Arabidopsis Root Growth by Concentration-Dependent Modification of Reactive Oxygen Species Accumulation and Cell Division. Ecotoxicol. Environ. Saf. 2020, 190. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.A.; Muk Cho, H.; Masjuki, H.H.; Kalam, M.A.; Ong, H.C.; Gul, M.; Harith, M.H.; Yusoff, M.N.A.M. Critical Review on Sesame Seed Oil and Its Methyl Ester on Cold Flow and Oxidation Stability. Energy Rep. 2020, 6. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12. [Google Scholar] [CrossRef]
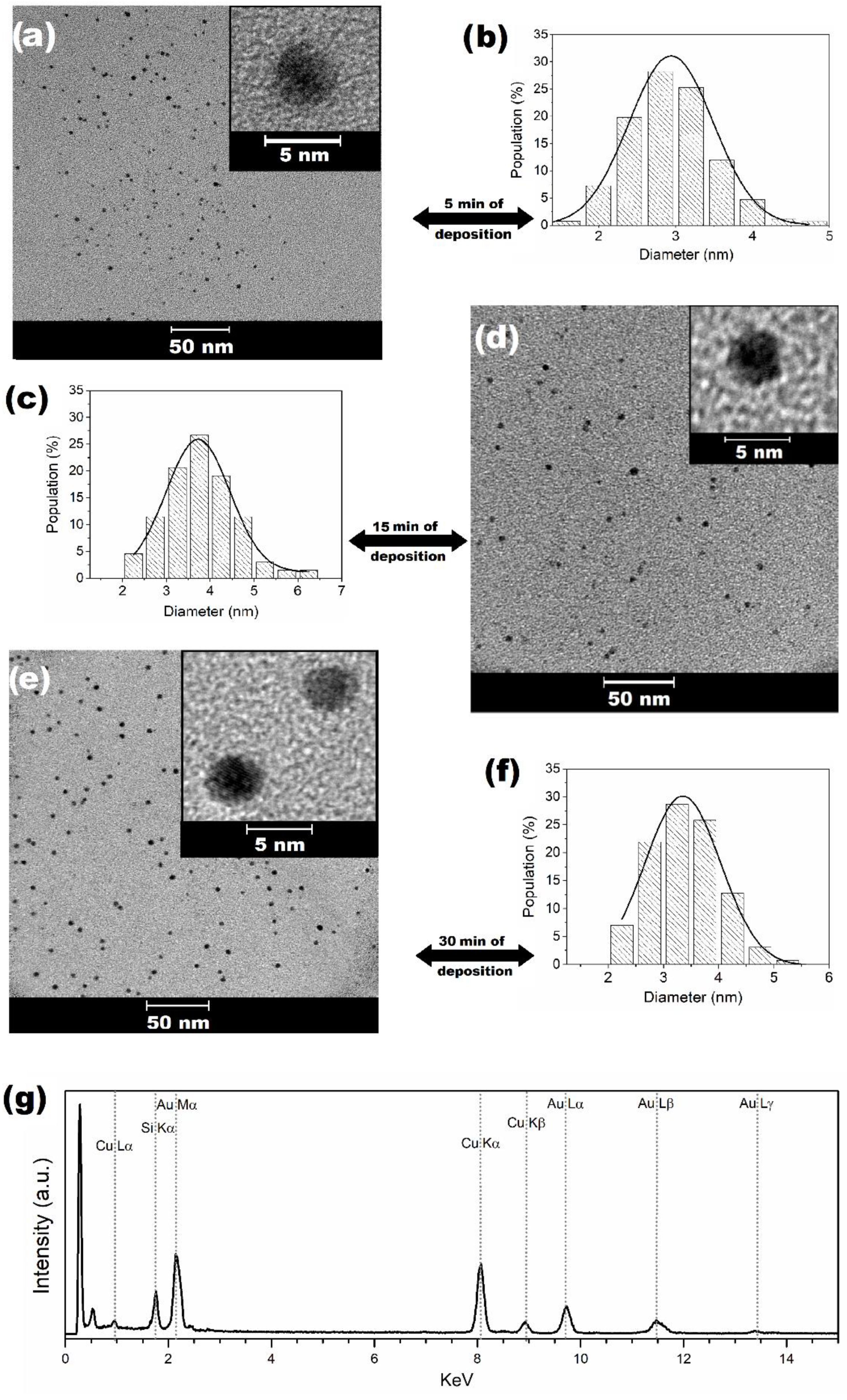
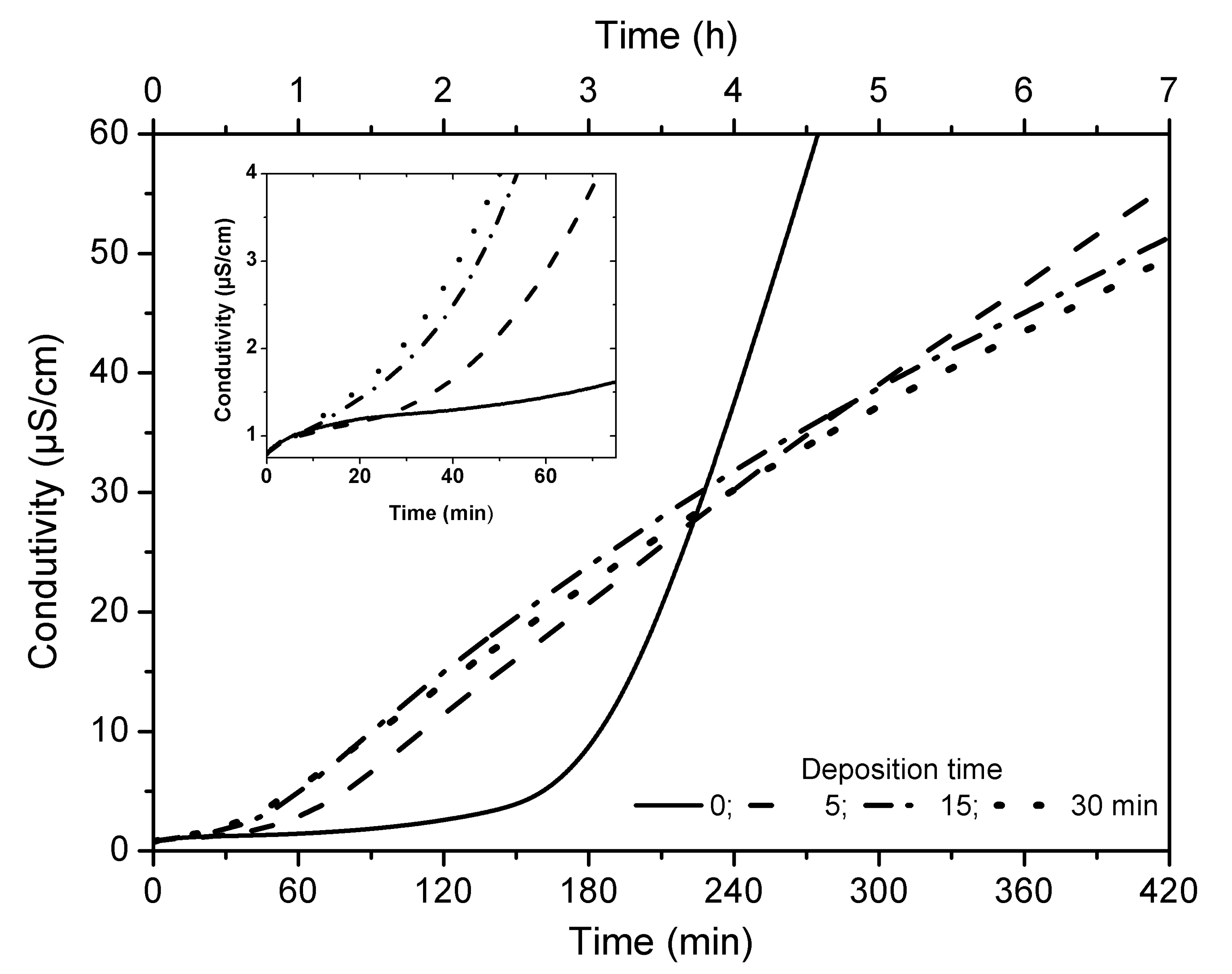
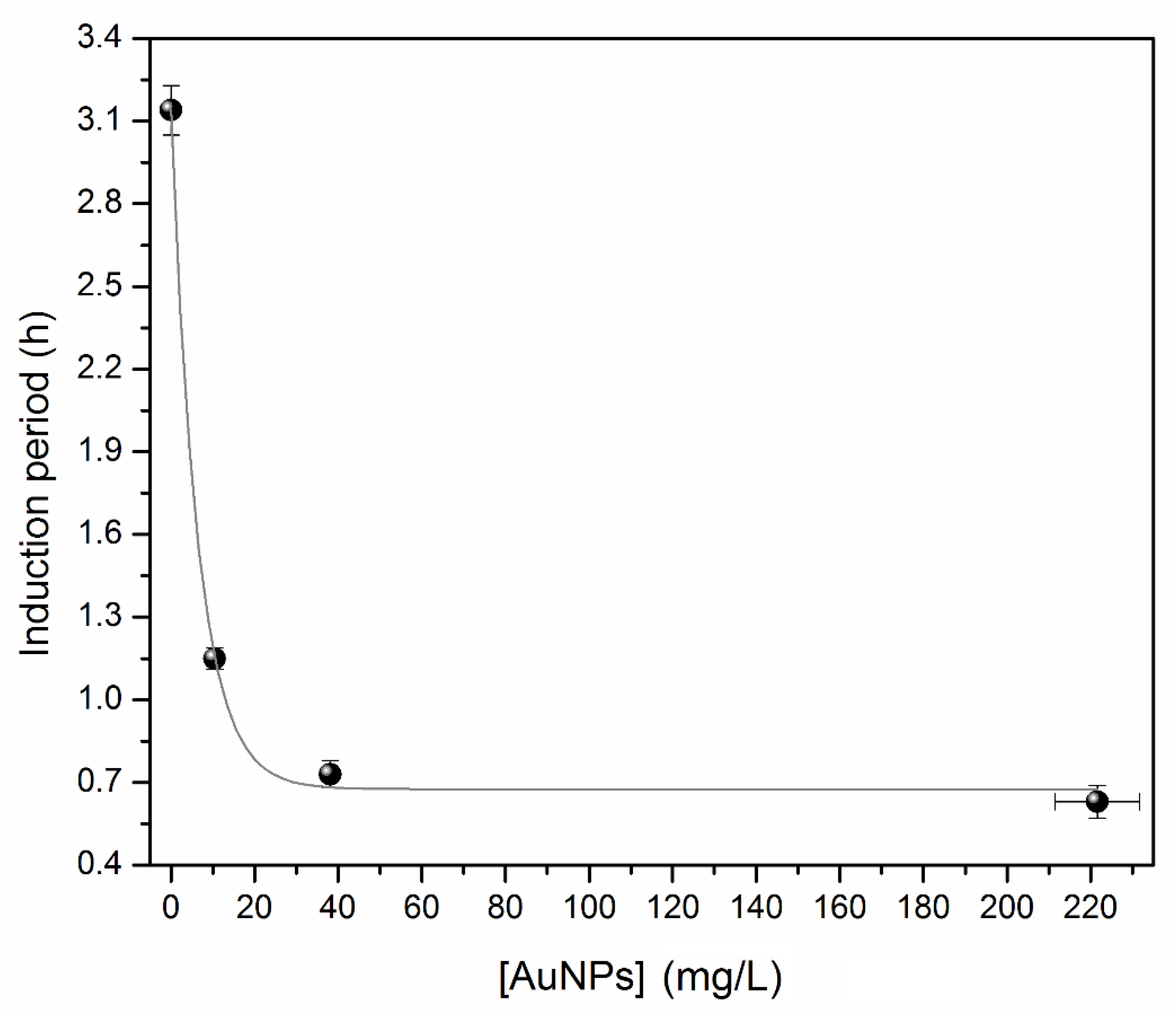
| Step | Temperature (°C) | Time (Min) | Pressure (Bar) |
|---|---|---|---|
| 1 | 120 | 10 | 30 |
| 2 | 180 | 15 | 30 |
| 3 | 250 | 10 | 30 |
| 4 | 50 | 10 | 20 |
| Deposition Time (Min) | Concentration (mg/L) | %RSD |
|---|---|---|
| 5.0 | 10.52 ± 0.09 | 0.87 |
| 15.0 | 38.07 ± 0.14 | 0.37 |
| 30.0 | 224.58 ± 10.06 | 4.54 |
| Fatty Acids | % |
|---|---|
| Palmitic, C16:0 | 5.9 ± 0.3 1 |
| Palmitoleic, C16:1 | 0.2 ± 0.0 |
| Stearic, C18:0 | 3.2 ± 0.1 |
| Oleic, C18:1 | 36.8 ± 0.3 |
| Linoleic, C18:2 | 49.5 ± 0.1 |
| Linolenic, C18:3 | 0.2 ± 0.0 |
| Arachidic, C20:0 | 0.2 ± 0.0 |
| Cis-11-Eicosenoic, C20:1 | 0.2 ± 0.0 |
| Ducosanoic, C22:0 | 0.6 ± 0.1 |
| ∑ Saturated | 9.9 |
| ∑ Unsaturated | 86.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michels, F.S.; Gonçalves, P.J.; Nascimento, V.A.; Oliveira, S.L.; Wender, H.; Caires, A.R.L. Eco-Friendly Production of AuNPs and Their Impact on the Oil Oxidative Stability. Nanomaterials 2021, 11, 1668. https://doi.org/10.3390/nano11071668
Michels FS, Gonçalves PJ, Nascimento VA, Oliveira SL, Wender H, Caires ARL. Eco-Friendly Production of AuNPs and Their Impact on the Oil Oxidative Stability. Nanomaterials. 2021; 11(7):1668. https://doi.org/10.3390/nano11071668
Chicago/Turabian StyleMichels, Flávio S., Pablo J. Gonçalves, Valter A. Nascimento, Samuel L. Oliveira, Heberton Wender, and Anderson R. L. Caires. 2021. "Eco-Friendly Production of AuNPs and Their Impact on the Oil Oxidative Stability" Nanomaterials 11, no. 7: 1668. https://doi.org/10.3390/nano11071668
APA StyleMichels, F. S., Gonçalves, P. J., Nascimento, V. A., Oliveira, S. L., Wender, H., & Caires, A. R. L. (2021). Eco-Friendly Production of AuNPs and Their Impact on the Oil Oxidative Stability. Nanomaterials, 11(7), 1668. https://doi.org/10.3390/nano11071668









