Complete Degradation and Detoxification of Ciprofloxacin by a Micro-/Nanostructured Biogenic Mn Oxide Composite from a Highly Active Mn2+-Oxidizing Pseudomonas Strain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Growth and Manganese Oxidation Activity Determination
2.3. Preparation of BMO/Bacteria Composites
2.4. Electron Microscope and Power X-ray Diffraction Assays
2.5. Degradation of CIP
2.6. Determination of MIC of CIP
2.7. Inhibition Zone Test
2.8. Growth Inhibition Experiments
2.9. Continuous and Repeated Degradation Performance
2.10. Degradation on CIP-Containing Natural Water
3. Results
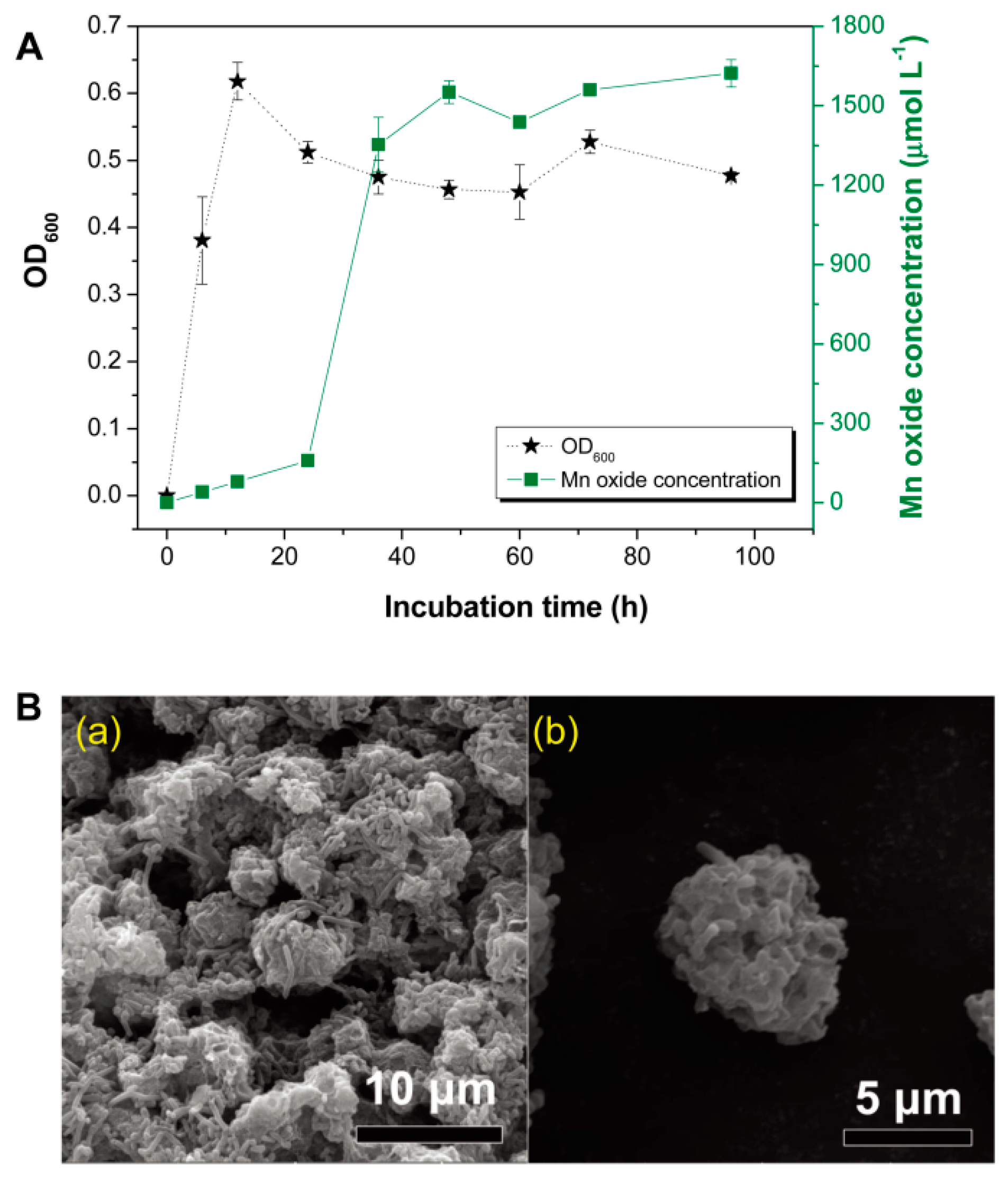
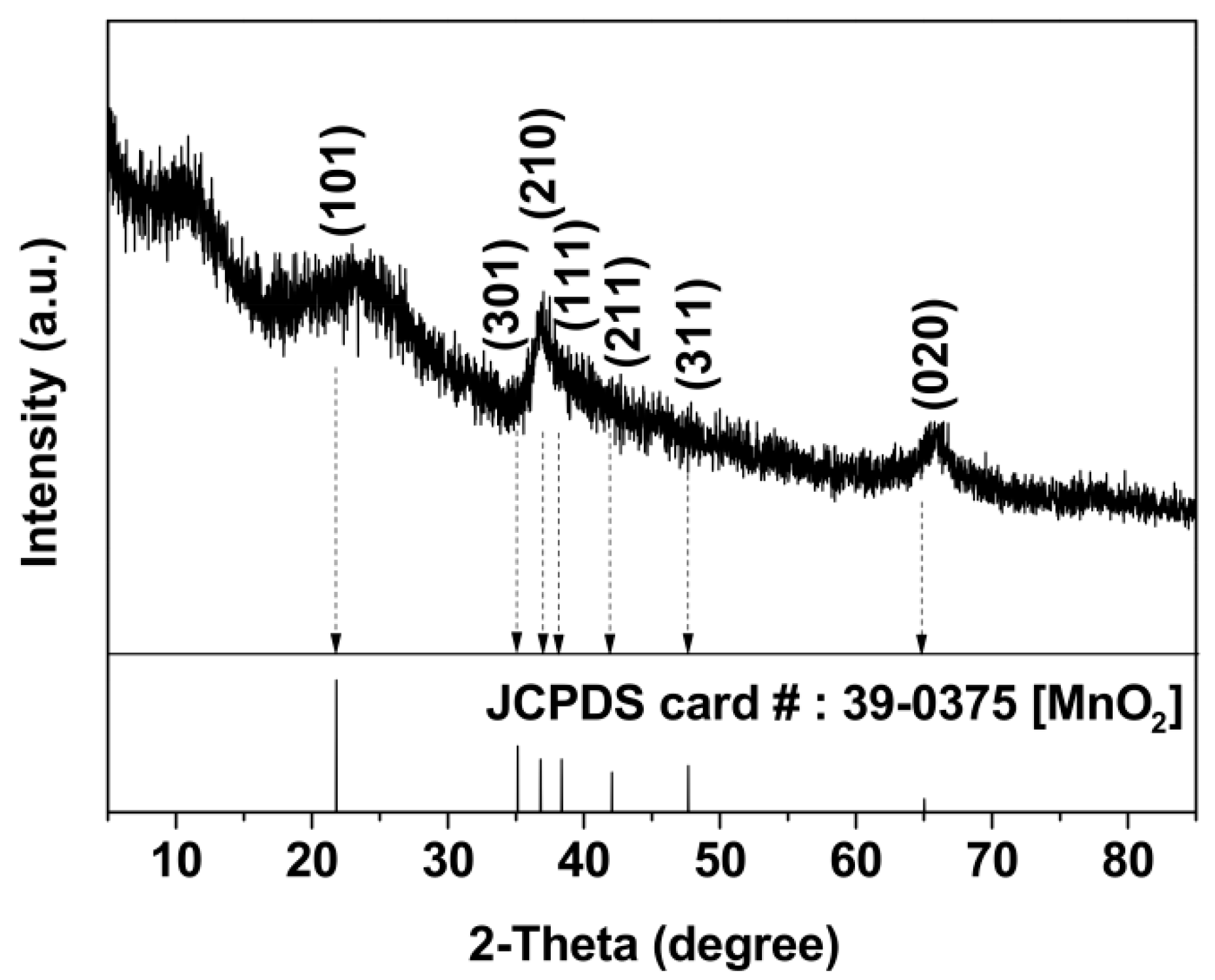
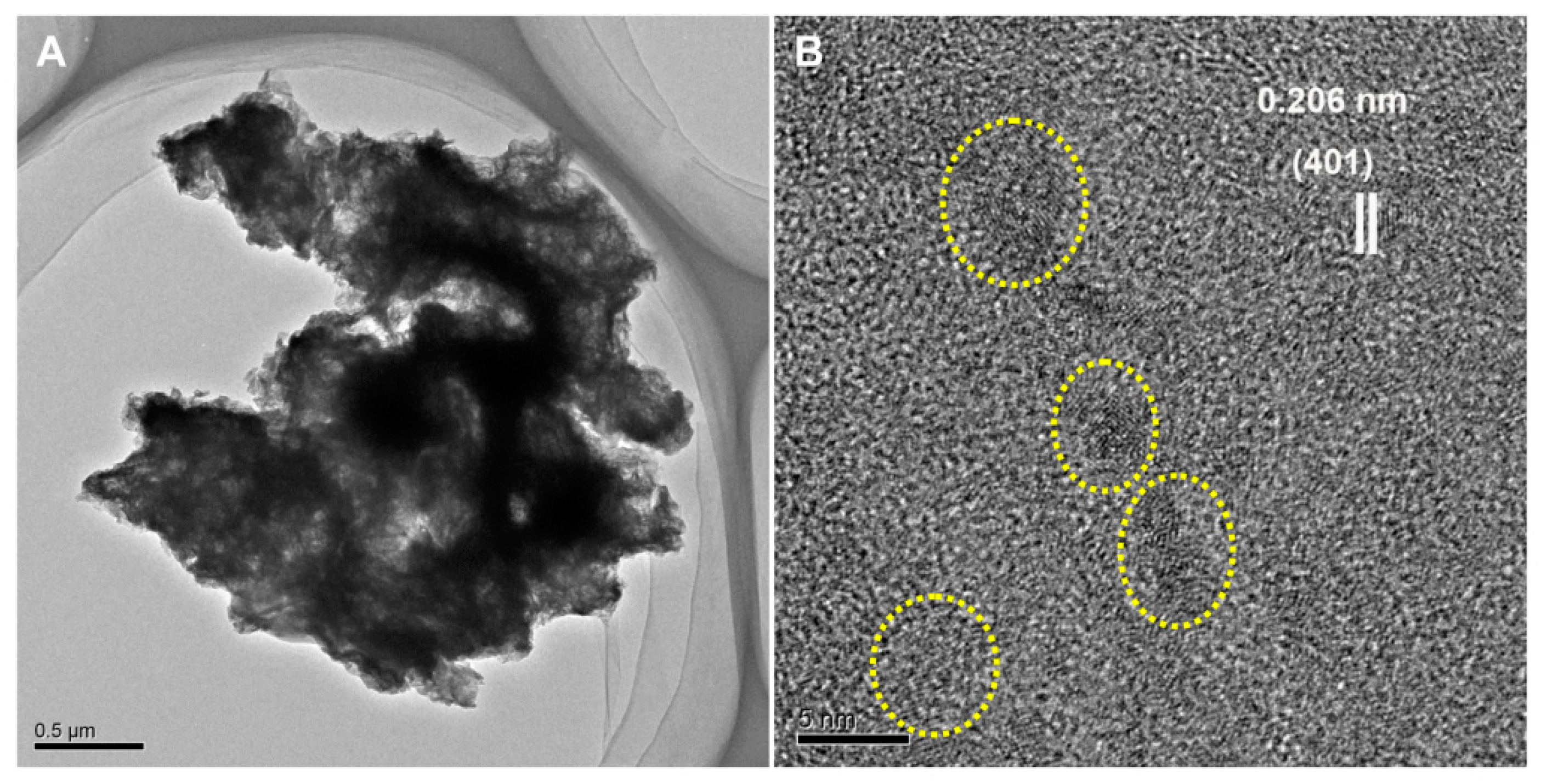
3.1. Formation and Characterization of BMO Aggregate Composites
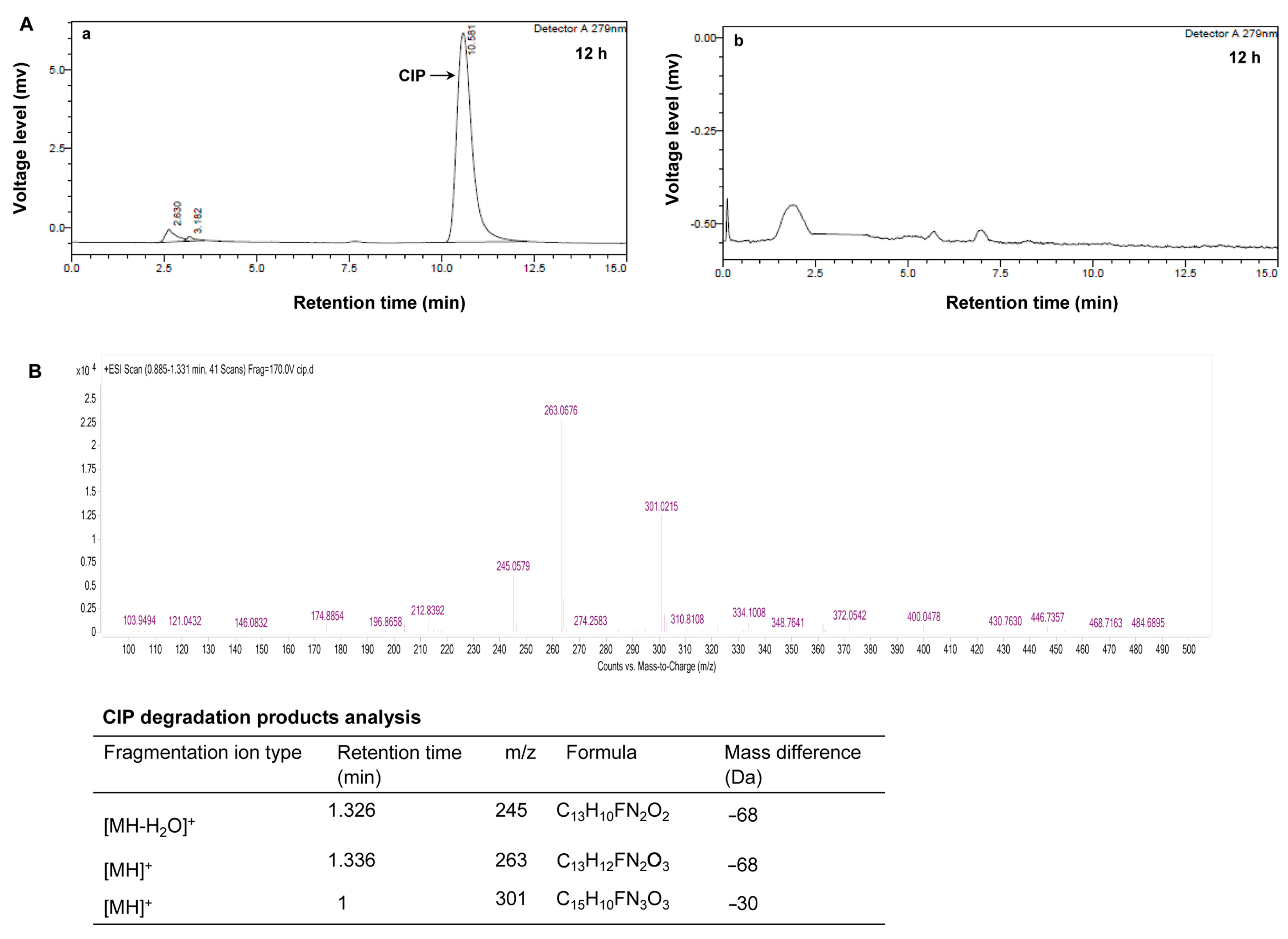
3.2. Degradation of CIP by the BMO Composites and Constructive Degradation-Metabolic Pathway
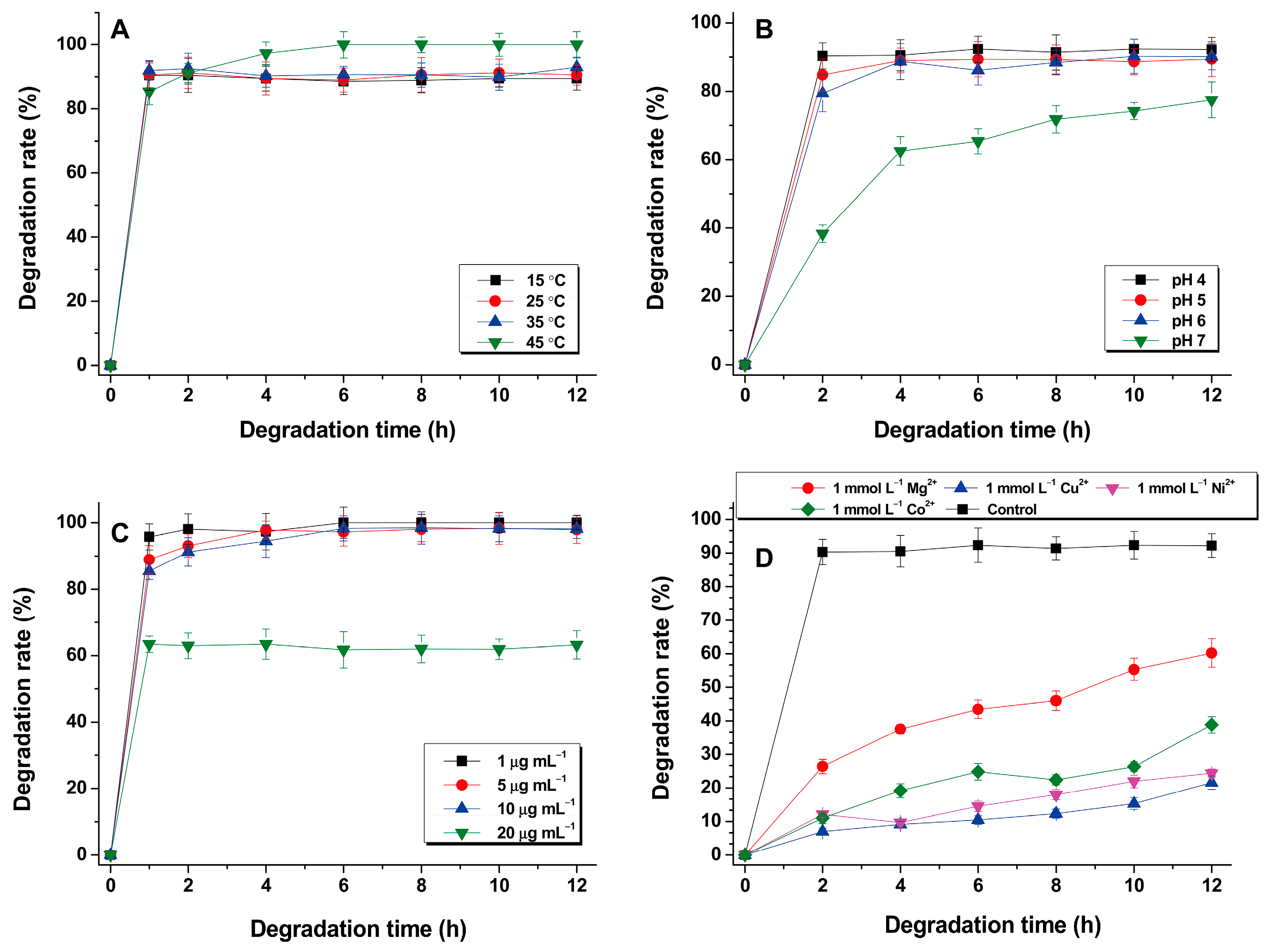
3.3. Effect of Reaction Conditions on CIP Degradation
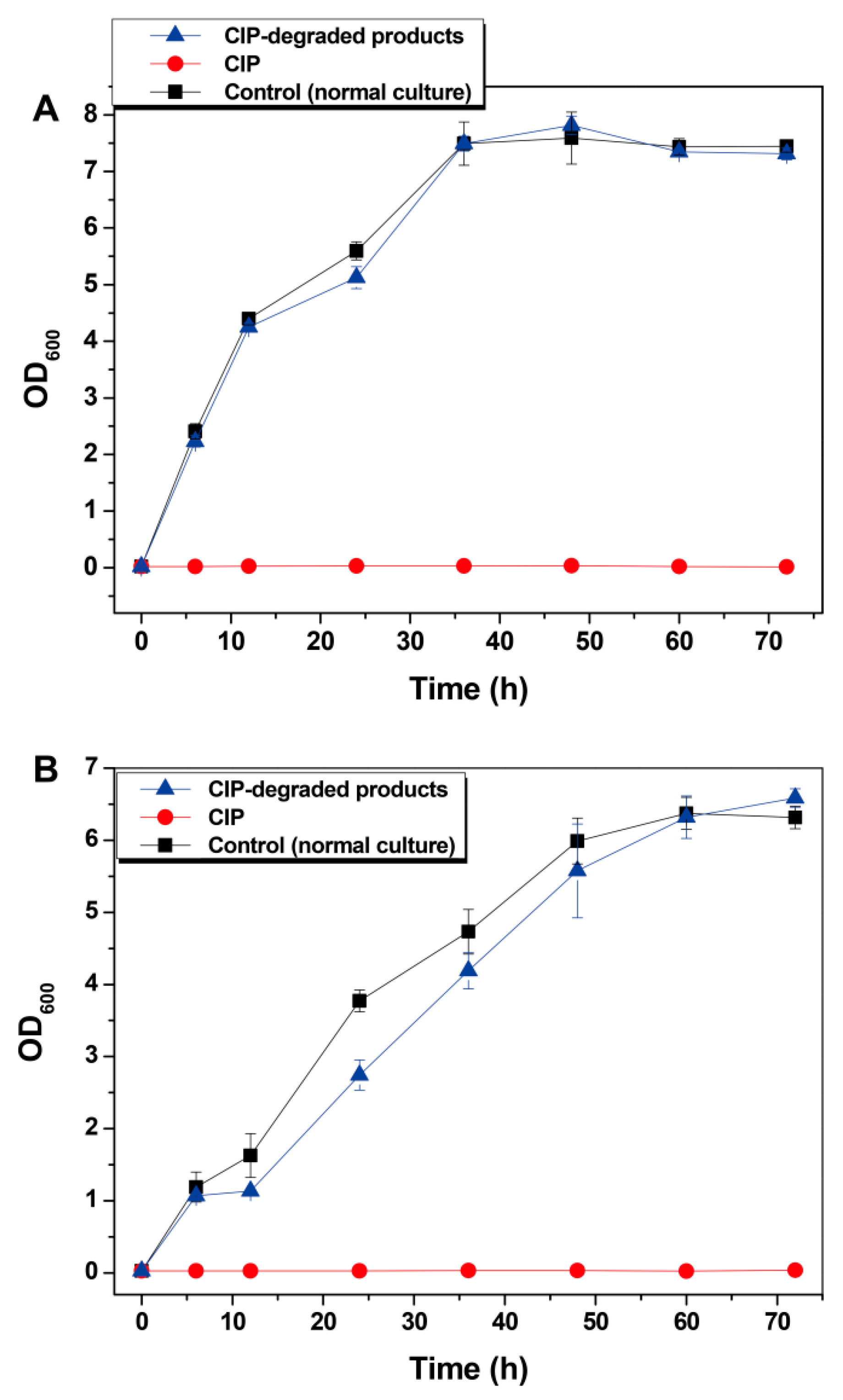
3.4. Antibacterial Activity Evaluation of CIP Degradation Products
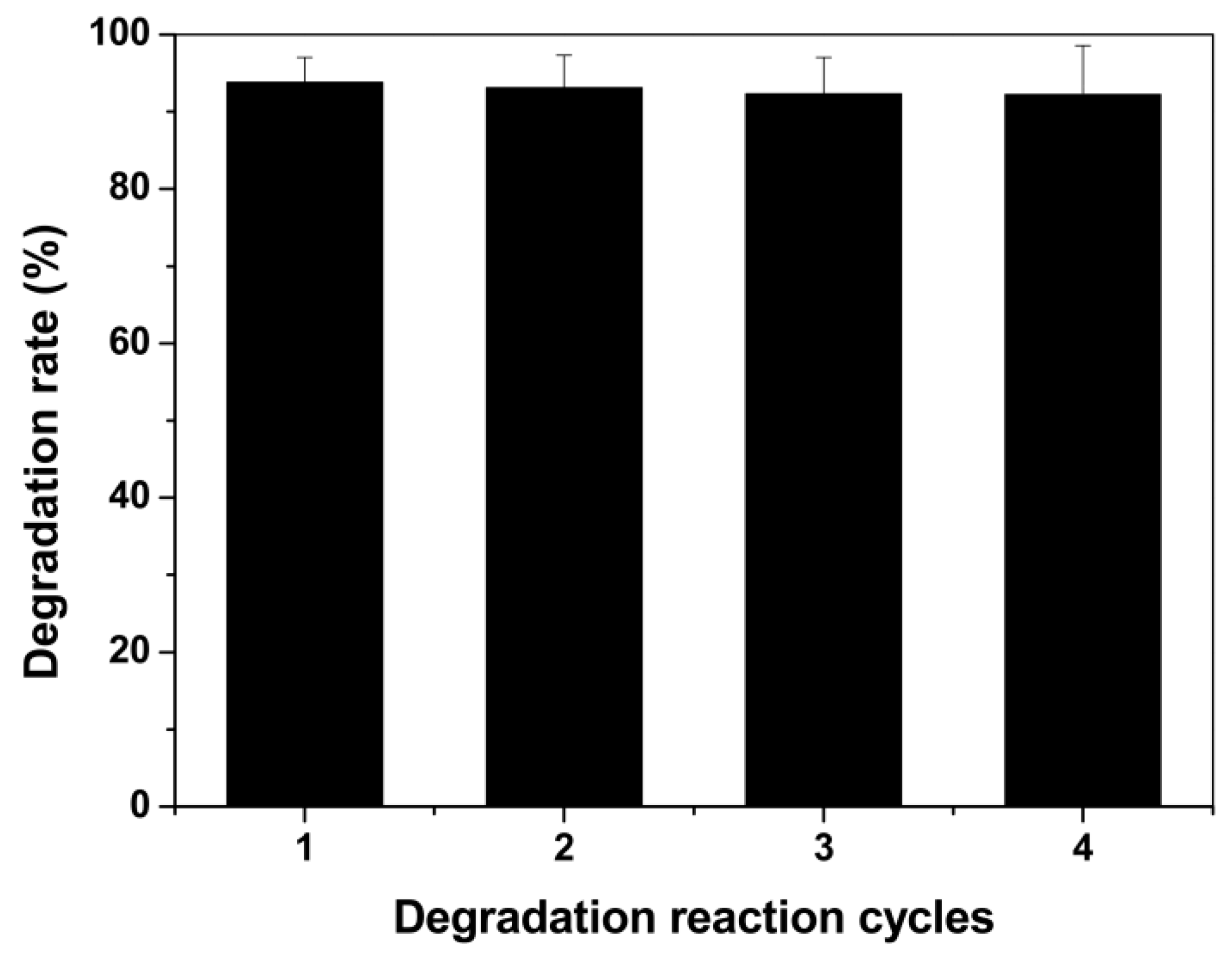
3.5. Continuous/Repeated Degradation
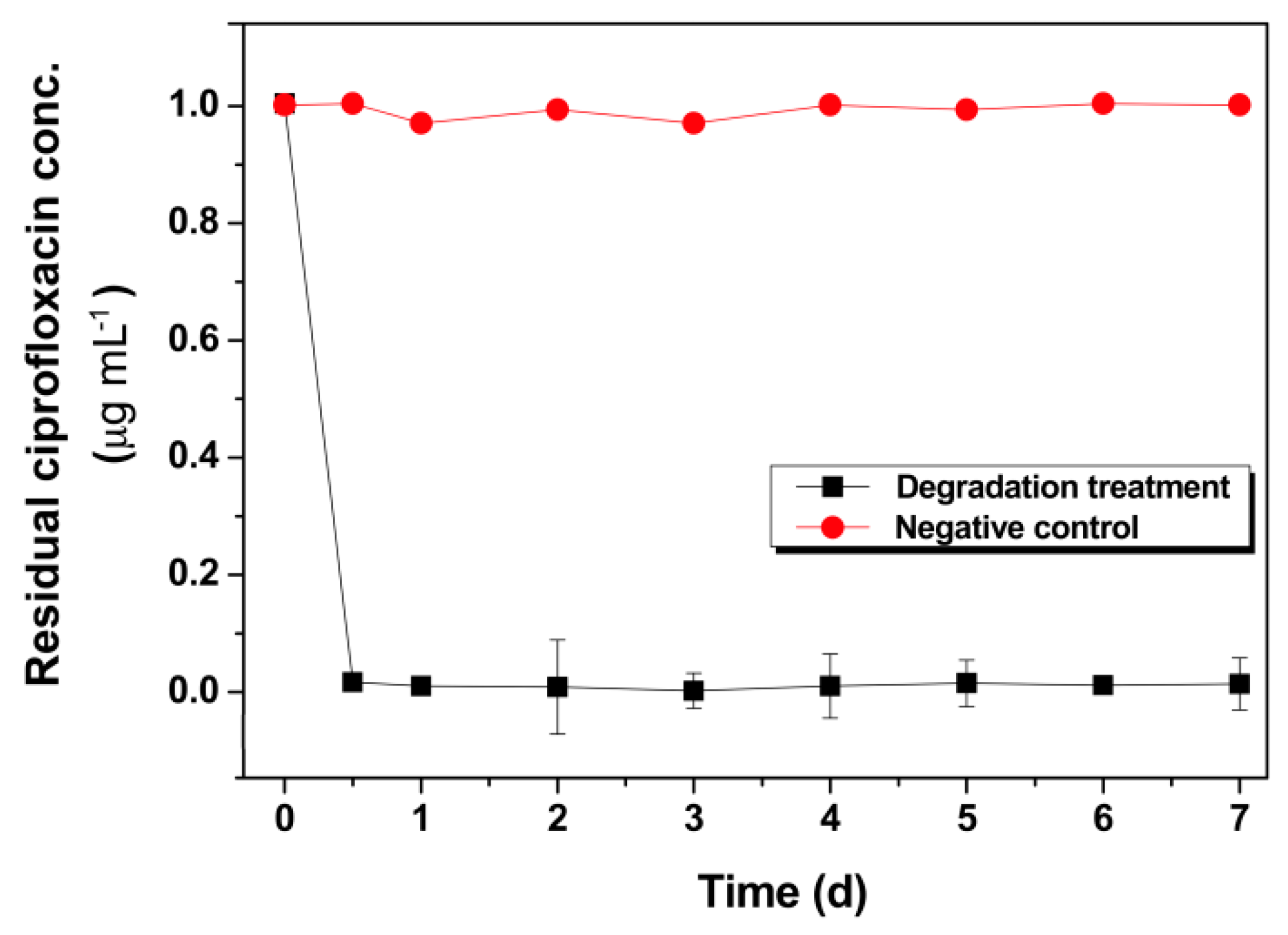
3.6. CIP Degradation on CIP-Containing Natural Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Steele, J.C.; Meng, X.Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef]
- Zhang, G.F.; Liu, X.; Zhang, S.; Pan, B.; Liu, M.L. Ciprofloxacin derivatives and their antibacterial activities. Eur. J. Med. Chem. 2018, 146, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Blanquez, A.; Guillen, F.; Rodriguez, J.; Arias, M.E.; Hernandez, M. The degradation of two fluoroquinolone based antimicrobials by SilA, an alkaline laccase from Streptomyces ipomoeae. World J. Microbiol. Biotechnol. 2016, 32, 52. [Google Scholar] [CrossRef]
- Castro, W.; Navarro, M.; Biot, C. Medicinal potential of ciprofloxacin and its derivatives. Future Med. Chem. 2013, 5, 81–96. [Google Scholar] [CrossRef]
- Girardi, C.; Greve, J.; Lamshoft, M.; Fetzer, I.; Miltner, A.; Schaffer, A.; Kastner, M. Biodegradation of ciprofloxacin in water and soil and its effects on the microbial communities. J. Hazard. Mater. 2011, 198, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Wang, Y.X.; Wang, X.; Yuan, B.; Fu, M.L. Intercalation and adsorption of ciprofloxacin by layered chalcogenides and kinetics study. J. Colloid Interface Sci. 2015, 453, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Nithya, R.; Meenakshi Sundaram, N. Biodegradation and cytotoxicity of ciprofloxacin-loaded hydroxyapatite-polycaprolactone nanocomposite film for sustainable bone implants. Int. J. Nanomed. 2015, 10 (Suppl. 1), 119–127. [Google Scholar]
- Giri, A.S.; Golder, A.K. Decomposition of drug mixture in Fenton and photo-Fenton processes: Comparison to singly treatment, evolution of inorganic ions and toxicity assay. Chemosphere 2015, 127, 254–261. [Google Scholar] [CrossRef]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic manganese oxides: Properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 2004, 21, 287–328. [Google Scholar] [CrossRef] [Green Version]
- Tebo, B.M.; Johnson, H.A.; McCarthy, J.K.; Templeton, A.S. Geomicrobiology of manganese(II) oxidation. Trends Microbiol. 2005, 13, 421–428. [Google Scholar] [CrossRef]
- Miyata, N.; Tani, Y.; Sakata, M.; Iwahori, K. Microbial manganese oxide formation and interaction with toxic metal ions. J. Biosci. Bioeng. 2007, 104, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.E.; Savalia, P.; Davis, R.; Kocar, B.D.; Webb, S.M.; Nealson, K.H.; Fischer, W.W. Real-time manganese phase dynamics during biological and abiotic manganese oxide reduction. Environ. Sci. Technol. 2016, 50, 4248–4258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, X.; Sun, S.P.; McBride, M.B.; Lemley, A.T. Degradation of ciprofloxacin by cryptomelane-type manganese(III/IV) oxides. Environ. Sci. Pollut. Res. Int. 2013, 20, 10–21. [Google Scholar] [CrossRef]
- Tu, J.; Yang, Z.; Hu, C.; Qu, J. Characterization and reactivity of biogenic manganese oxides for ciprofloxacin oxidation. J. Environ. Sci. 2014, 26, 1154–1161. [Google Scholar] [CrossRef]
- Boogerd, F.C.; de Vrind, J.P. Manganese oxidation by Leptothrix discophora. J. Bacteriol. 1987, 169, 489–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krumbein, W.E.; Altmann, H.J. A new method for the detection and enumeration of manganese oxidizing and reducing microorganisms. Helgol. Wiss. Meeresunters. 1973, 25, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Zhang, Z.; Chen, H.; Liu, J.; Ali, M.; Liu, F.; Li, L. Population structure of manganese-oxidizing bacteria in stratified soils and properties of manganese oxide aggregates under manganese-complex medium enrichment. PLoS ONE 2013, 8, e73778. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Ruan, Z.; Liu, J.; Liu, C.; Zhang, F.; Linhardt, R.J.; Li, L. Complete degradation of bisphenol A and nonylphenol by a composite of biogenic manganese oxides and Escherichia coli cells with surface-displayed multicopper oxidase CotA. Chem. Eng. J. 2019, 362, 897–908. [Google Scholar] [CrossRef]
- Zimmermann, E.S.; Torres, B.G.; Dalla Costa, T. Validation of a sensitive HPLC/fluorescence method for assessment of ciprofloxacin levels in plasma and prostate microdialysate samples from rats. Biomed. Chromatogr. 2016, 30, 330–336. [Google Scholar] [CrossRef]
- Liu, J.; Tan, L.; Wang, J.; Wang, Z.; Ni, H.; Li, L. Complete biodegradation of chlorpyrifos by engineered Pseudomonas putida cells expressing surface-immobilized laccases. Chemosphere 2016, 157, 200–207. [Google Scholar] [CrossRef]
- Do, M.T.; Stuckey, D.C. Fate and removal of Ciprofloxacin in an anaerobic membrane bioreactor (AnMBR). Bioresour. Technol. 2019, 289, 121683. [Google Scholar] [CrossRef]
- Araújo, F.M.; Dantas, M.C.S.M.; e Silva, L.S.; Aona, L.Y.S.; Tavares, I.F.; de Souza-Neta, L.C. Antibacterial activity and chemical composition of the essential oil of Croton heliotropiifolius Kunth from Amargosa, Bahia, Brazil. Ind. Crops Prod. 2017, 105, 203–206. [Google Scholar] [CrossRef]
- Sayed, M.; Ismail, M.; Khan, S.; Tabassum, S.; Khan, H.M. Degradation of ciprofloxacin in water by advanced oxidation process: Kinetics study, influencing parameters and degradation pathways. Environ. Technol. 2016, 37, 590–602. [Google Scholar] [CrossRef]
- Ge, L.; Na, G.; Zhang, S.; Li, K.; Zhang, P.; Ren, H.; Yao, Z. New insights into the aquatic photochemistry of fluoroquinolone antibiotics: Direct photodegradation, hydroxyl-radical oxidation, and antibacterial activity changes. Sci. Total Environ. 2015, 527–528, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Gilevska, T.; Wetzig, F.; Dorer, C.; Richnow, H.H.; Vogt, C. Characterization of phenol and cresol biodegradation by compound-specific stable isotope analysis. Environ. Pollut. 2016, 210, 166–173. [Google Scholar] [CrossRef]
- Liao, X.; Li, B.; Zou, R.; Dai, Y.; Xie, S.; Yuan, B. Biodegradation of antibiotic ciprofloxacin: Pathways, influential factors, and bacterial community structure. Environ. Sci. Pollut. Res. Int. 2016, 23, 7911–7918. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; He, Y.L.; Huang, C.H. Oxidation of fluoroquinolone antibiotics and structurally related amines by chlorine dioxide: Reaction kinetics, product and pathway evaluation. Water Res. 2010, 44, 5989–5998. [Google Scholar] [CrossRef]
- Xu, L.; Xu, C.; Zhao, M.; Qiu, Y.; Sheng, G.D. Oxidative removal of aqueous steroid estrogens by manganese oxides. Water Res. 2008, 42, 5038–5044. [Google Scholar] [CrossRef] [PubMed]
- Visvalingam, J.; Palaniappan, K.; Holley, R.A. In Vitro enhancement of antibiotic susceptibility of drug resistant Escherichia coli by cinnamaldehyde. Food Control 2017, 79, 288–291. [Google Scholar] [CrossRef]
- Tao, R.; Ying, G.G.; Su, H.C.; Zhou, H.W.; Sidhu, J.P. Detection of antibiotic resistance and tetracycline resistance genes in Enterobacteriaceae isolated from the Pearl rivers in South China. Environ. Pollut. 2010, 158, 2101–2109. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Liu, J.; Zeng, J.; Li, J.; Liu, Y.; Sun, X.; Xu, L.; Li, L. Complete Degradation and Detoxification of Ciprofloxacin by a Micro-/Nanostructured Biogenic Mn Oxide Composite from a Highly Active Mn2+-Oxidizing Pseudomonas Strain. Nanomaterials 2021, 11, 1660. https://doi.org/10.3390/nano11071660
Li L, Liu J, Zeng J, Li J, Liu Y, Sun X, Xu L, Li L. Complete Degradation and Detoxification of Ciprofloxacin by a Micro-/Nanostructured Biogenic Mn Oxide Composite from a Highly Active Mn2+-Oxidizing Pseudomonas Strain. Nanomaterials. 2021; 11(7):1660. https://doi.org/10.3390/nano11071660
Chicago/Turabian StyleLi, Li, Jin Liu, Jie Zeng, Jiaoqing Li, Yongxuan Liu, Xiaowen Sun, Liangzheng Xu, and Lin Li. 2021. "Complete Degradation and Detoxification of Ciprofloxacin by a Micro-/Nanostructured Biogenic Mn Oxide Composite from a Highly Active Mn2+-Oxidizing Pseudomonas Strain" Nanomaterials 11, no. 7: 1660. https://doi.org/10.3390/nano11071660
APA StyleLi, L., Liu, J., Zeng, J., Li, J., Liu, Y., Sun, X., Xu, L., & Li, L. (2021). Complete Degradation and Detoxification of Ciprofloxacin by a Micro-/Nanostructured Biogenic Mn Oxide Composite from a Highly Active Mn2+-Oxidizing Pseudomonas Strain. Nanomaterials, 11(7), 1660. https://doi.org/10.3390/nano11071660





