A Perspective on the Application of Covalent Organic Frameworks for Detection and Water Treatment
Abstract
:1. Introduction
2. COF Processing
2.1. COF-Based Membranes
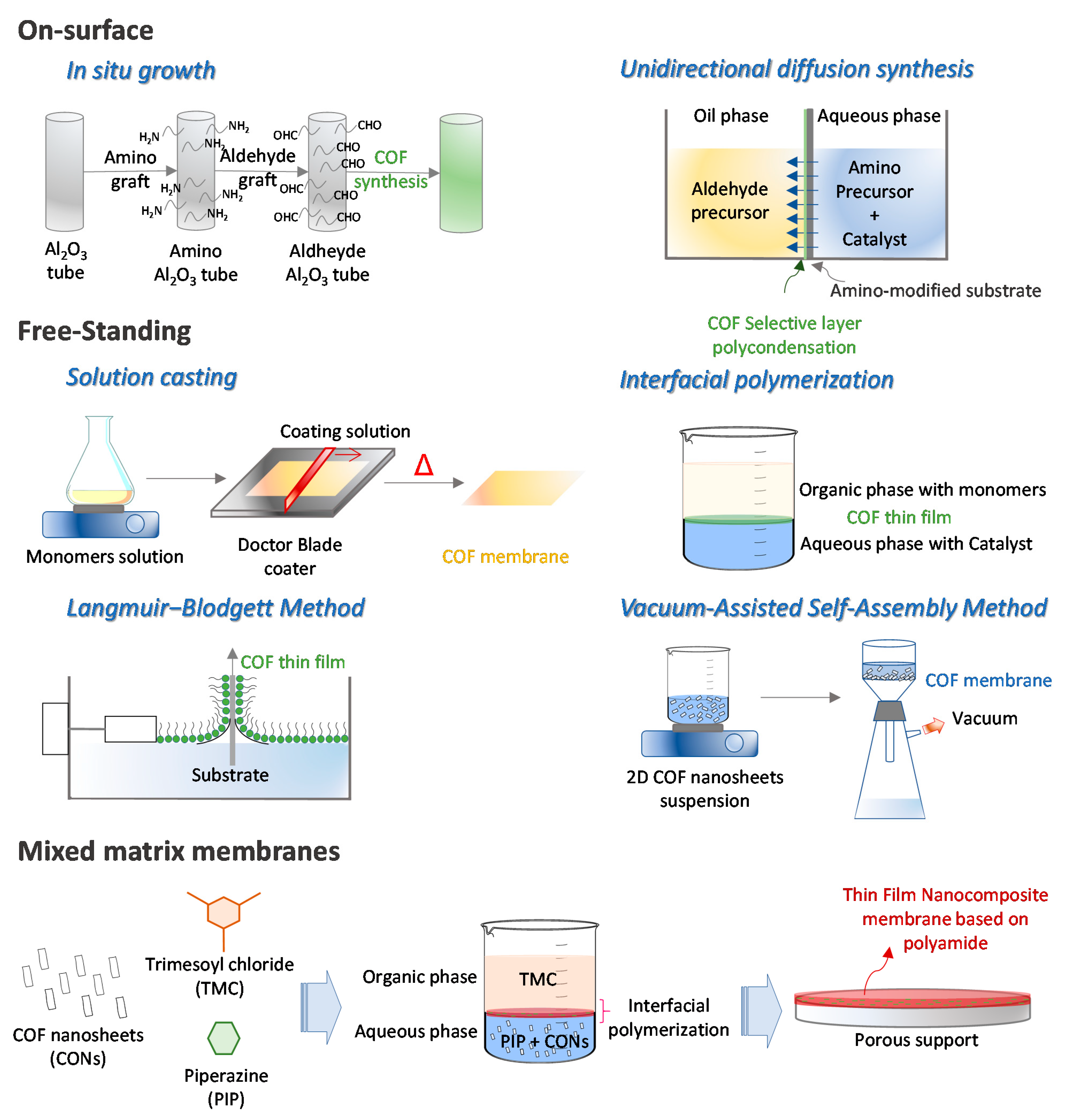
2.1.1. On Surface
- In situ growth
- Unidirectional diffusion synthesis
2.1.2. Free-Standing
- Solution Casting
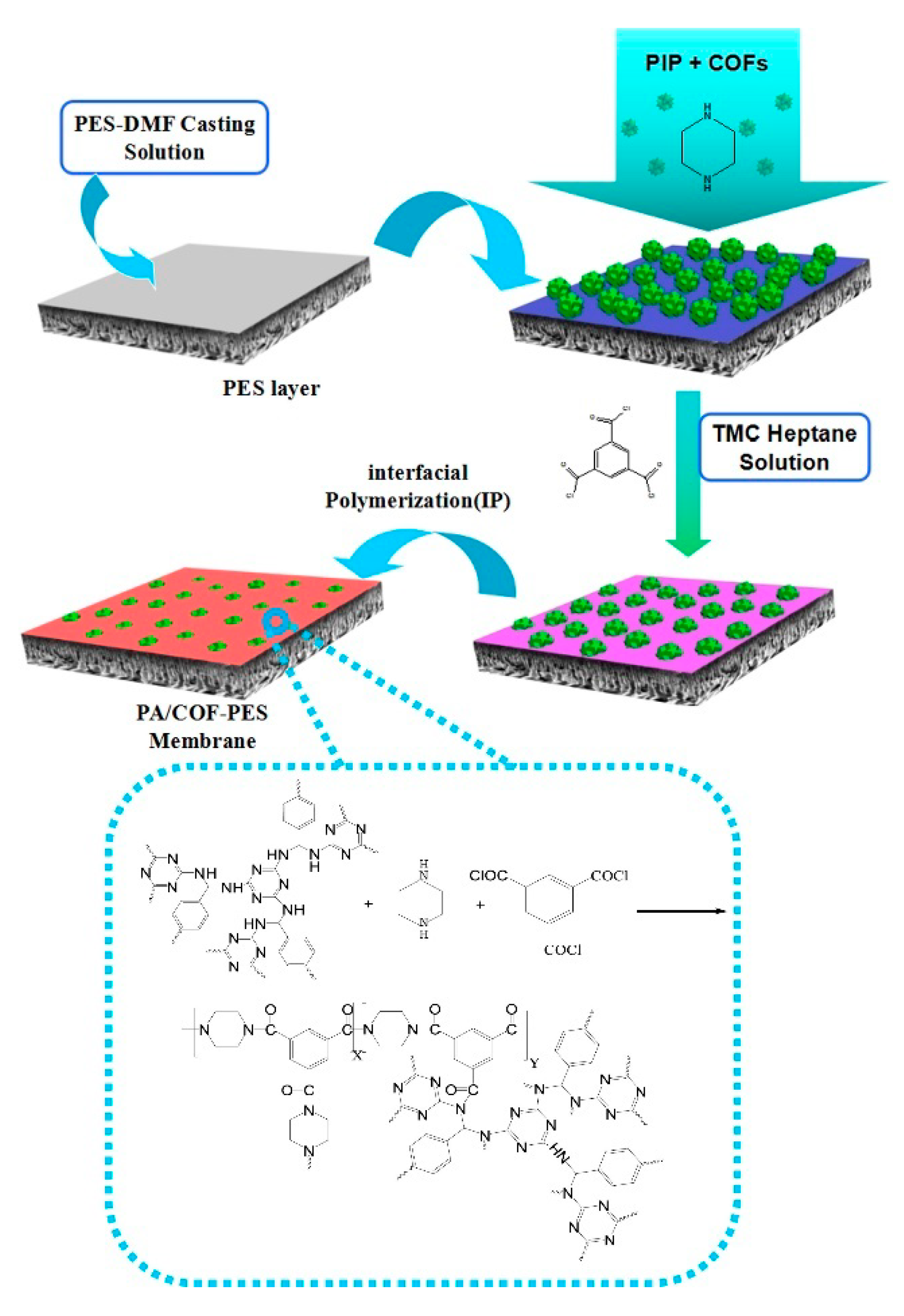
- Interfacial Polymerization
- Langmuir−Blodgett (LB) Method
- Vacuum-Assisted Self-Assembly Method
2.1.3. Mixed Matrix Membranes
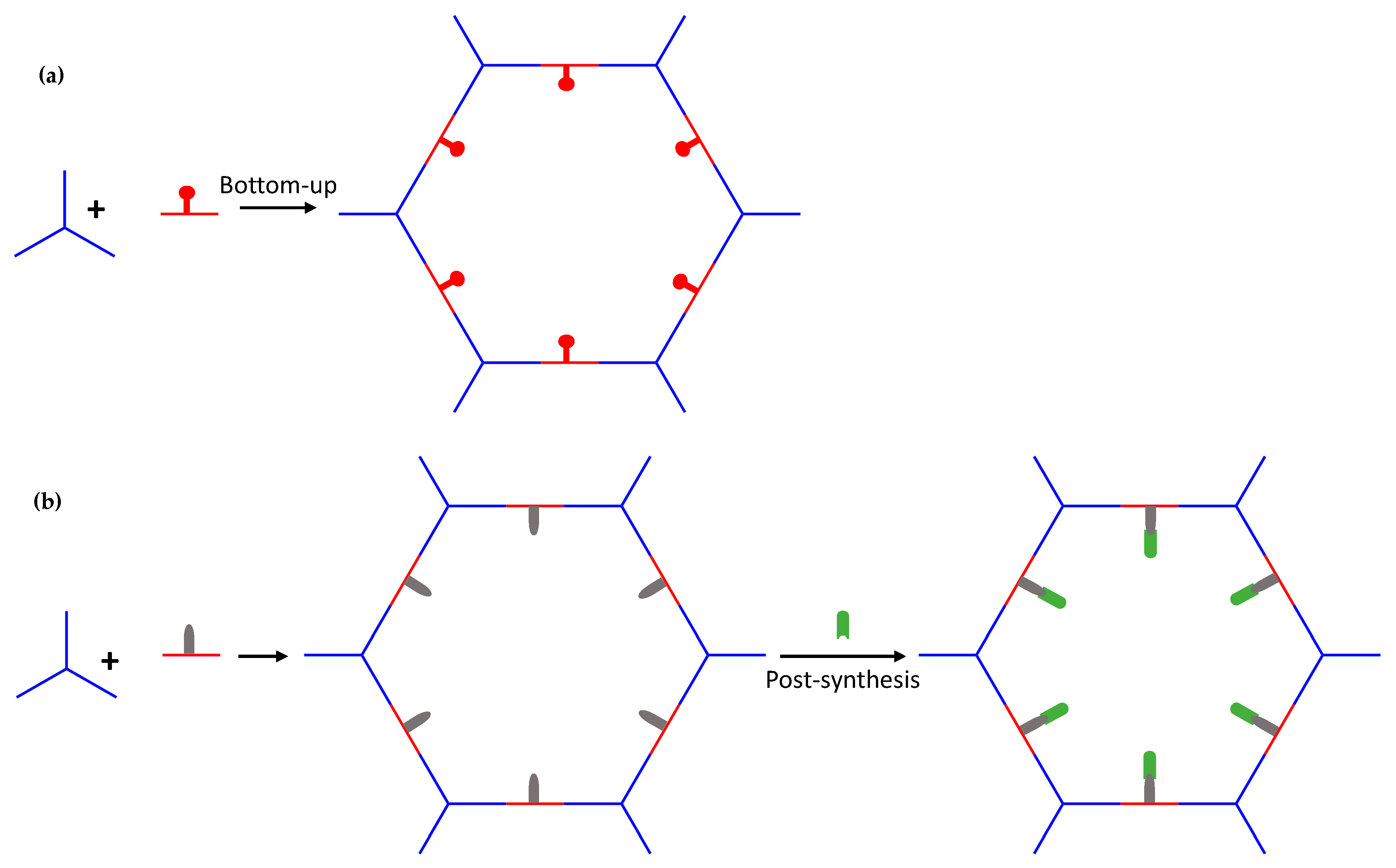
2.2. Nanoprocessed COFs
3. COFs for Water Treatment
3.1. COF-Based Membranes for Waste Water Treatment
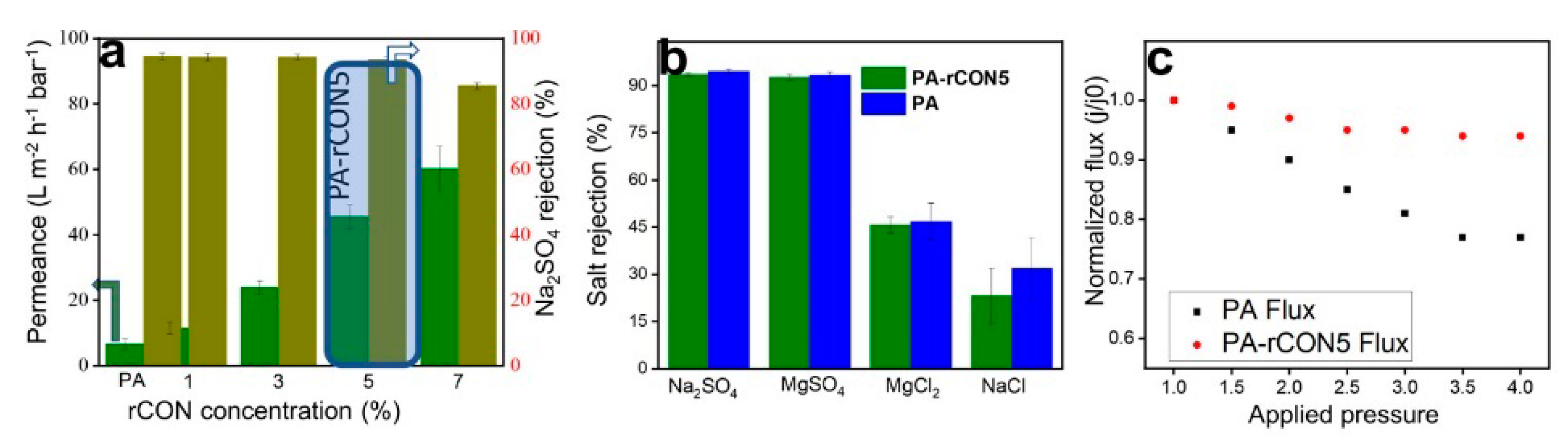
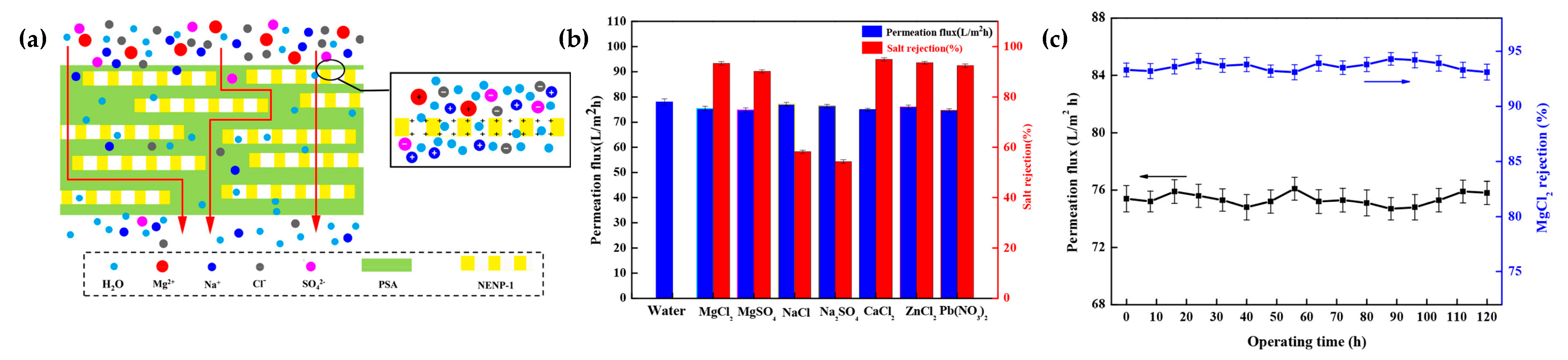
3.2. COF-Based Membranes for Water Desalination
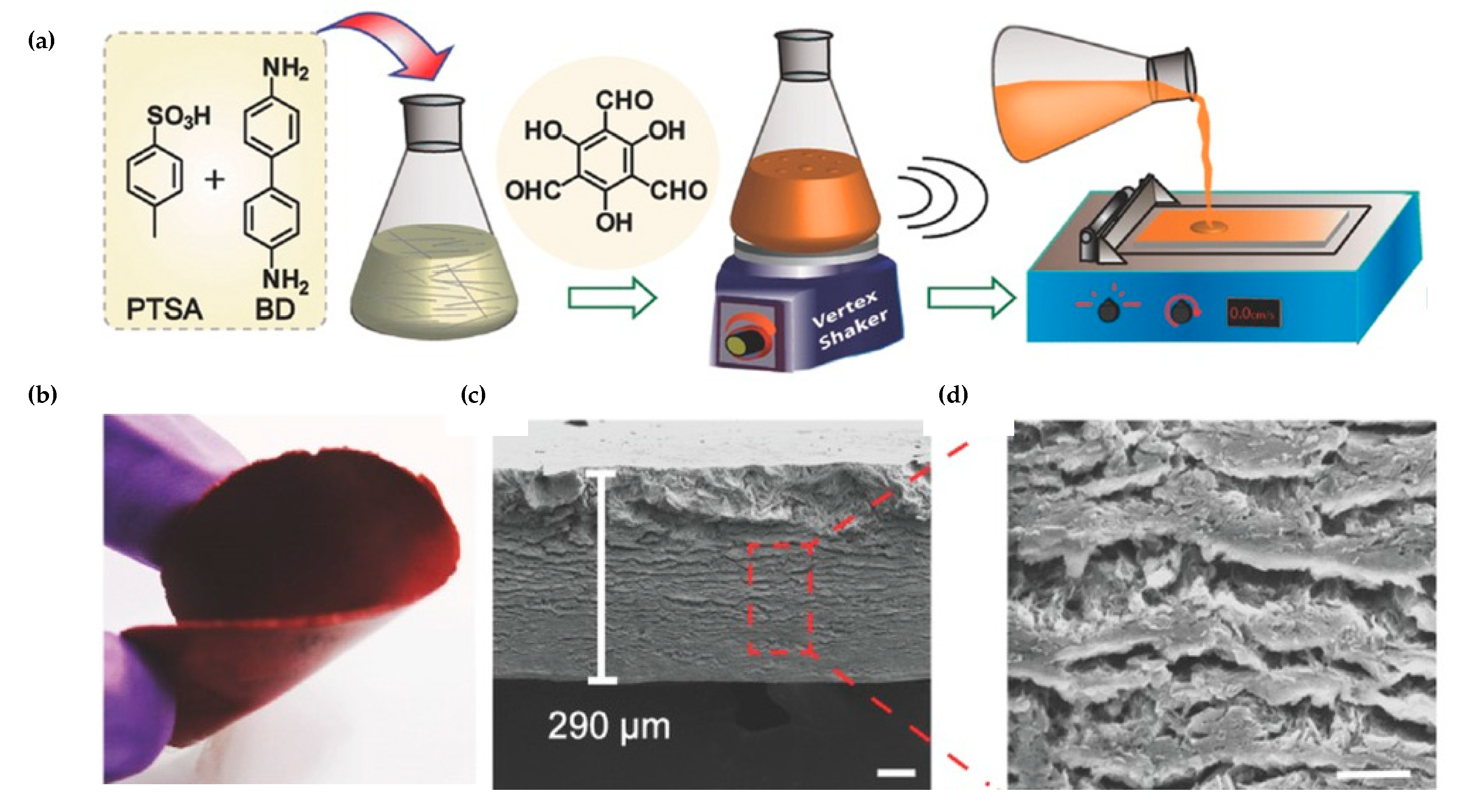
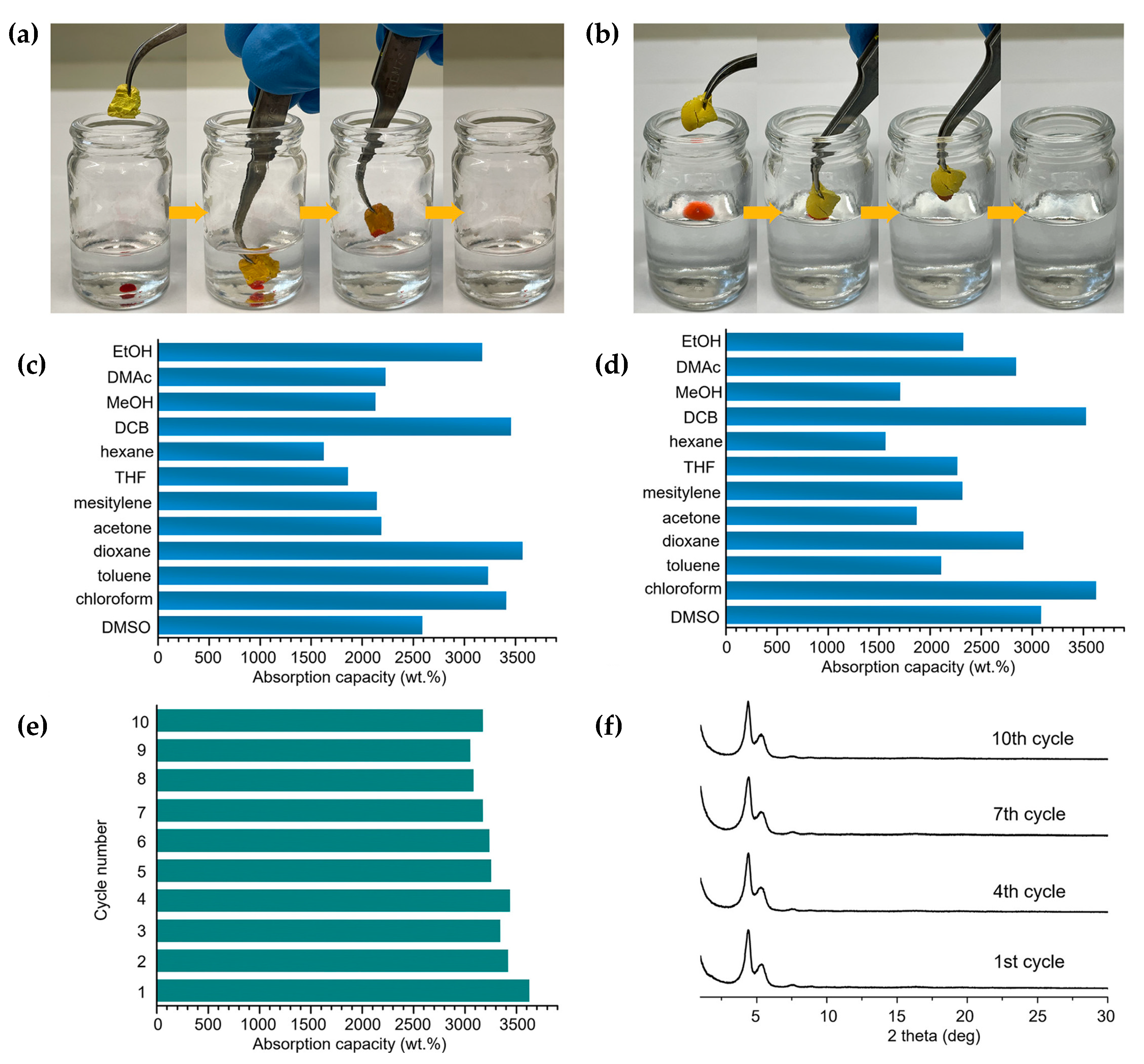
3.3. COF Monoliths for Decontamination of Organic Pollutants
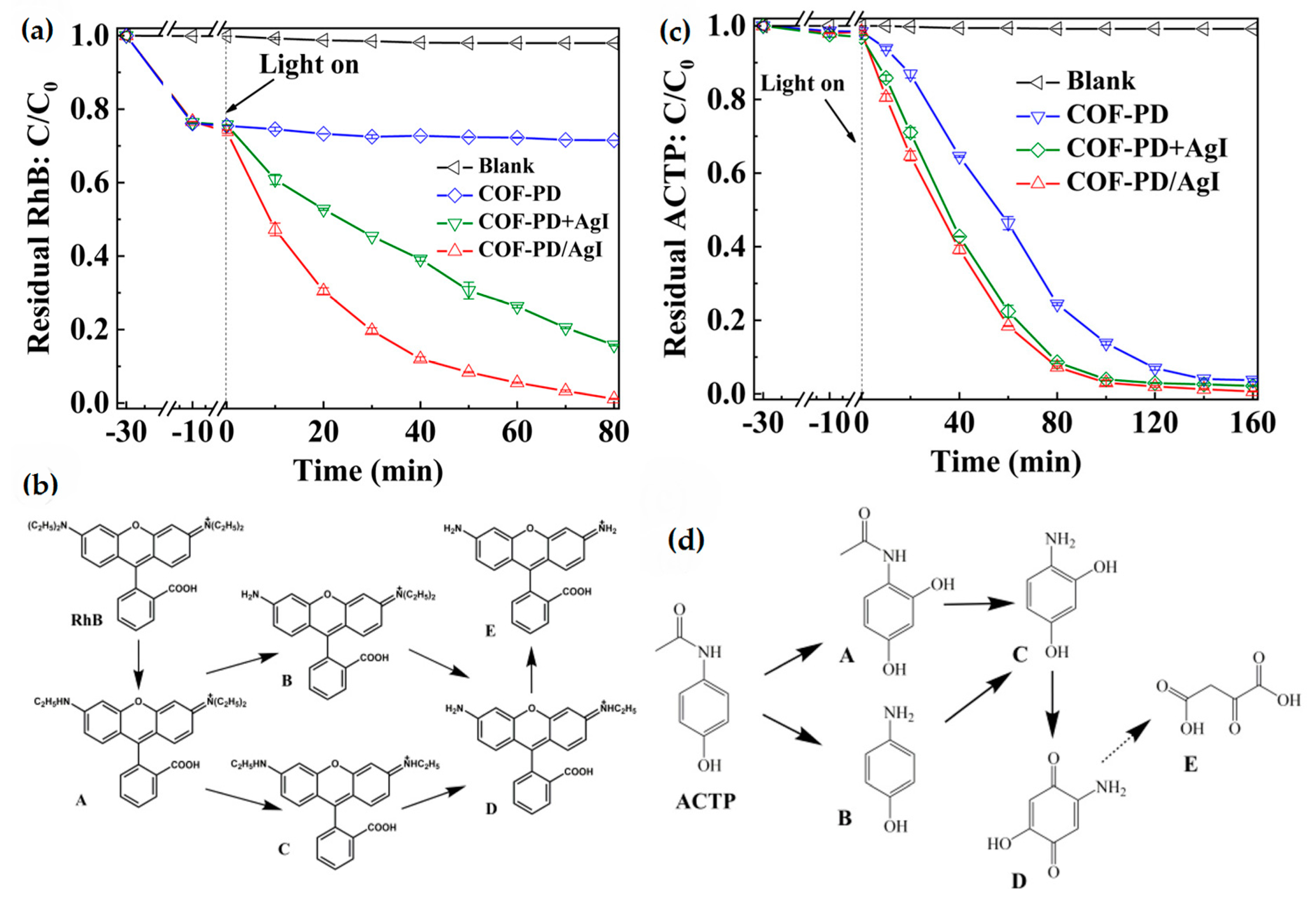
3.4. COFs for Degradation
3.5. COFs for Capture and Detection
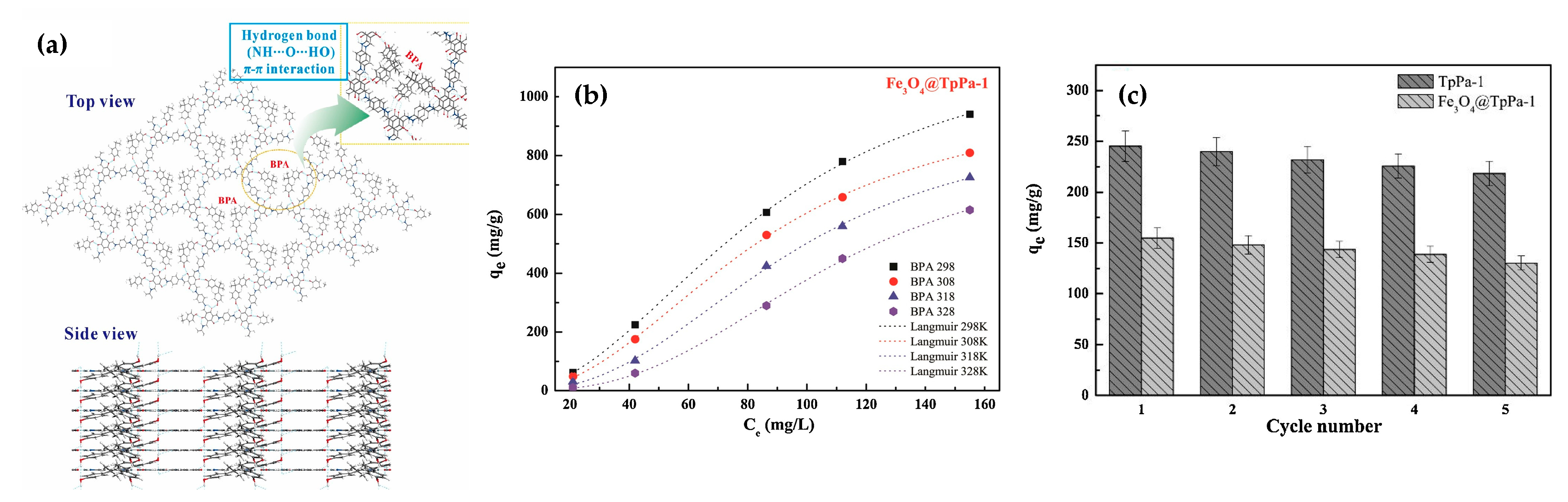
- Bisphenol A
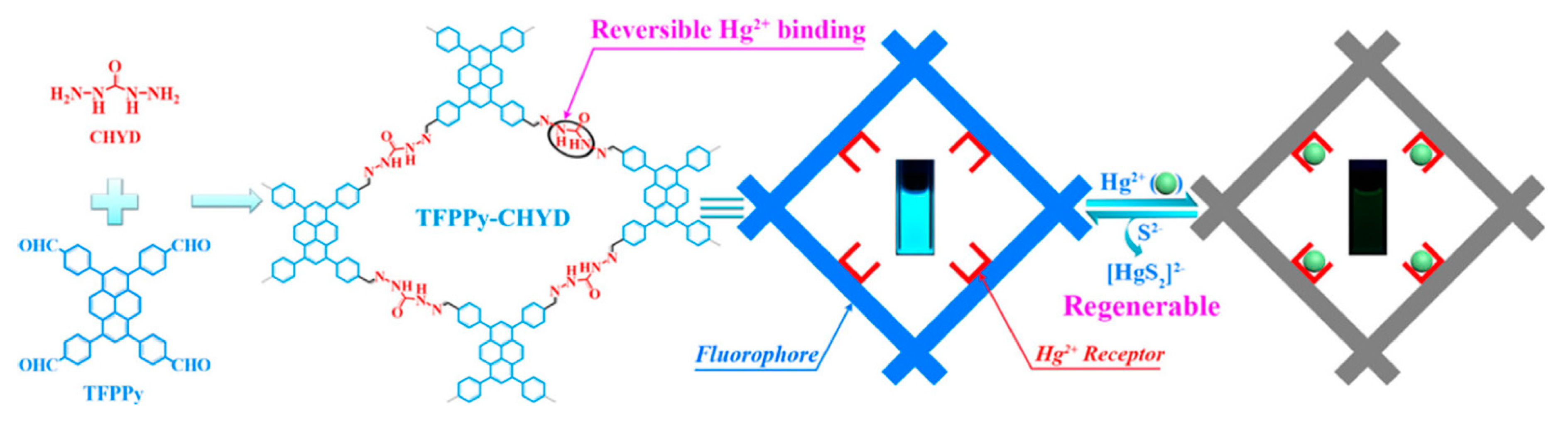
- Hg(II) detection and removal
- Pb(II) detection and removal
- Non-steroidal anti-inflammatory drugs
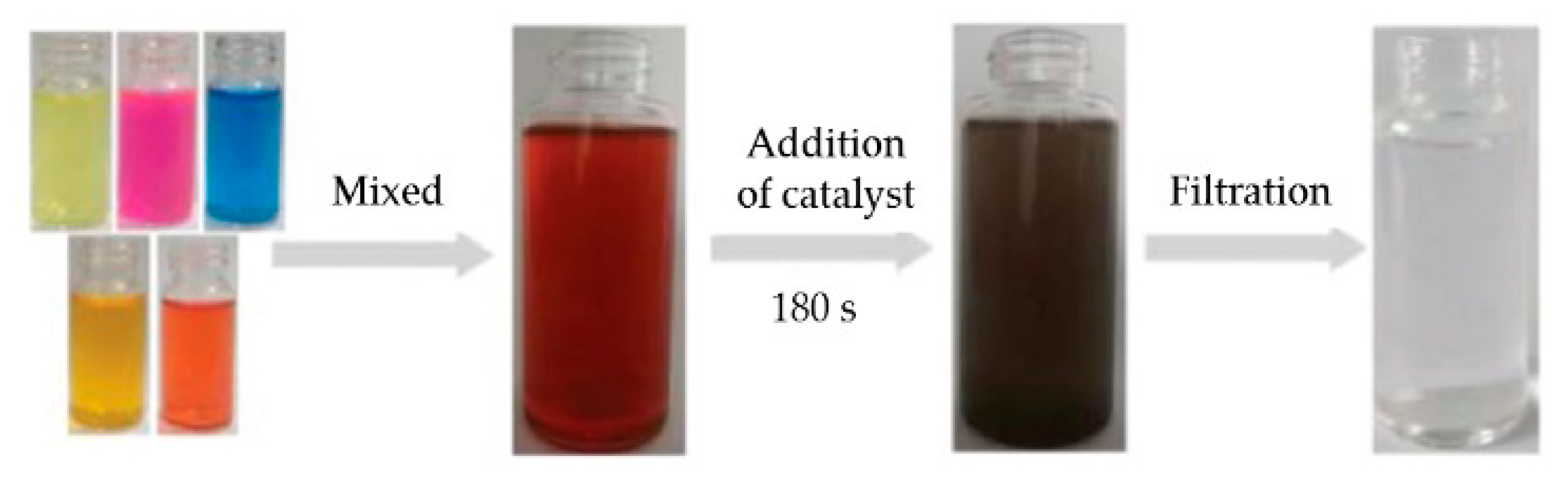
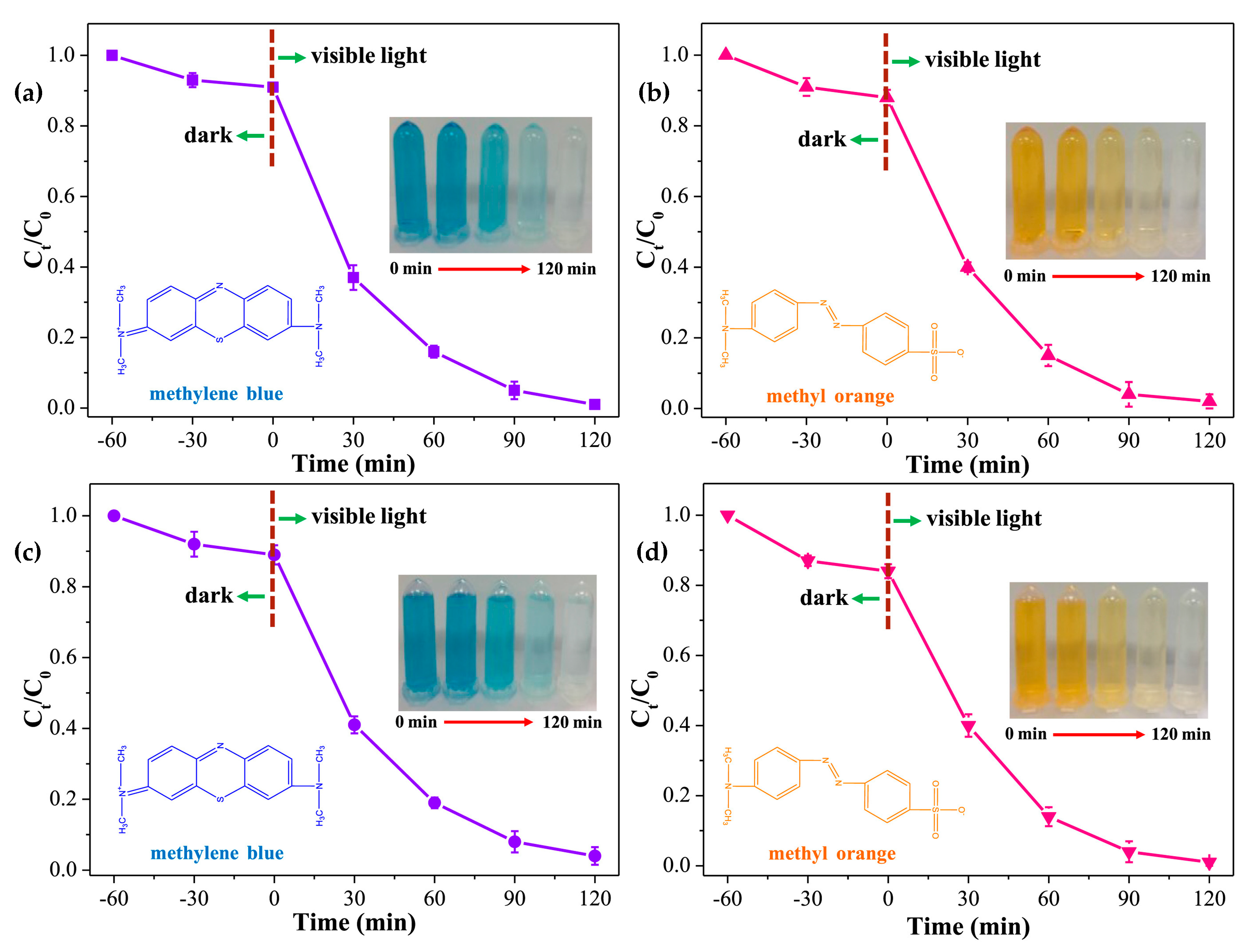
- Dye compounds
- Detection and removal of antibiotics
| COFs | Adsorbate | Capacity (mg g−1) | Active Sites | Reference |
|---|---|---|---|---|
| Fe3O4@TpBD | BPA | 160 | Carboxyl groups | [57] |
| Hierarchically porous monolith-COF (M16) | BPA | 22 | Aldehyde and hydrazine groups | [48] |
| Fe3O4@TpPa-1 | BPA | 1220 | Amine and carbonyl groups | [58] |
| TPB-DMTP-COF-SH | Hg(II) | 4395 | Thiol and triazole functional groups | [59] |
| COOH@COF | Hg(II) | 99 | Carboxyl and thioether groups | [60] |
| TFPPy-CHYD COF | Hg(II) | 758 | Secondary amine group | [61] |
| AgNPs@COF-LZU1 | Hg(II) | 113 | - | [62] |
| T-COF | Hg(II) | 1826 | Nitrogen and oxygen atoms | [63] |
| COOH@COF | Pb(II) | 124 | Carboxyl and thioether groups | [60] |
| COF-Tz-OH | Pb(II) | 476 | Triazine and hydroxyl groups | [64] |
| COF-SH | Pb(II) | 239 | Sulfhydryl grupos | [65] |
| COF-NO2 | KTP IBP NPX | 70 94 80 | Amino groups | [66] |
| COF-NH2 | KTP IBP NPX | 33 18 16 | Amino groups | [66] |
| TS-COF-1 | MB RhB CR 4-NP 3-NP | 1691 625 319 369 424 | Triazine groups | [67] |
| TS-COF-2 | MB | 377 | Triazine groups | [67] |
| F-CTF-1 | NZF NFT FZD | 351 240 196 | Triazine groups | [68] |
| F-CTF-1 | NZF NFT FZD | 298 200 154 | Triazine groups | [68] |
| MIL-53 (Cr) (MOF) | BPA | 421 | π-π interaction, hydrogen bonding | [69] |
| ED-MIL-101(Cr) (MOF) | NPX | 154 | Acid-base interaction | [70] |
| NH2-MIL-101(Al) (MOF) | 4-NP MB | 193 1409 | Hydrogen bonding Electrostatic interaction | [71,72] |
| MnO2 nanotubes@rGO (MNGH) | Pb(II) | 356 | - | [73] |
| Na+ modified rGO-Fe3O4(SMGI) | Pb(II) | 1666 | - | [74] |
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lohse, M.S.; Bein, T. Covalent Organic Frameworks: Structures, Synthesis, and Applications. Adv. Funct. Mater. 2018, 28, 1705553–1705624. [Google Scholar] [CrossRef] [Green Version]
- Ongari, D.; Yakutovich, A.V.; Talirz, L.; Smit, B. Building a consistent and reproducible database for adsorp-tion evaluation in Covalent-Organic Frameworks. Mater. Cloud Arch. 2020, 133. [Google Scholar] [CrossRef]
- Segura, J.L.; Royuela, S.; Mar Ramos, M. Post-synthetic modification of covalent organic frameworks. Chem. Soc. Rev. 2019, 48, 3903–3945. [Google Scholar] [CrossRef]
- Waller, P.J.; Gándara, F.; Yaghi, O.M. Chemistry of Covalent Organic Frameworks. Acc. Chem. Res. 2015, 48, 3053–3063. [Google Scholar] [CrossRef]
- Xia, Z.; Zhao, Y.; Darling, S.B. Covalent Organic Frameworks for Water Treatment. Adv. Mater. Interfaces 2021, 8, 2001507–2001524. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [Green Version]
- Côté, A.P.; El-Kaderi, H.M.; Furukawa, H.; Hunt, J.R.; Yaghi, O.M. Reticular synthesis of microporous and mesoporous 2D covalent organic frameworks. J. Am. Chem. Soc. 2007, 129, 12914–12915. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W.-G.; Su, C.-Y.; Wang, W. Construction of Covalent Organic Framework for Catalysis: Pd/COF-LZU1 in Suzuki–Miyaura Coupling Reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822. [Google Scholar] [CrossRef]
- Uribe-Romo, F.J.; Doonan, C.J.; Furukawa, H.; Oisaki, K.; Yaghi, O.M. Crystalline covalent organic frameworks with hydrazone linkages. J. Am. Chem. Soc. 2011, 133, 11478–11481. [Google Scholar] [CrossRef]
- Kandambeth, S.; Mallick, A.; Lukose, B.; Mane, M.V.; Heine, T.; Banerjee, R. Construction of Crystalline 2D Covalent Organic Frameworks with Remarkable Chemical (Acid/Base) Stability via a Combined Reversible and Irreversible Route. J. Am. Chem. Soc. 2012, 134, 19524–19527. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Ma, S.; Zhang, H.; Ou, J.; Wei, Y.; Ye, M. Fabrication of Hydrazone-Linked Covalent Organic Frameworks Using Alkyl Amine as Building Block for High Adsorption Capacity of Metal Ions. ACS Appl. Mater. Interfaces 2019, 11, 11706–11714. [Google Scholar] [CrossRef]
- Kandambeth, S.; Dey, K.; Banerjee, R. Covalent Organic Frameworks: Chemistry beyond the Structure. J. Am. Chem. Soc. 2019, 141, 1807–1822. [Google Scholar] [CrossRef]
- Carrington, M.; Rampal, N.; Madden, D.; O’Nolan, D.; Casati, N.P.M.; Divitini, G.; Cepitis, R.; Ilan, J.M.; Camur, C.; Silvestre-Albero, J.; et al. Sol-Gel Processing of a Covalent Organic Framework for the Generation of Hierarchically Porous Monolithic Adsorbents. ChemRxiv 2021. Preprint. [Google Scholar] [CrossRef]
- Rodríguez-San-Miguel, D.; Zamora, F. Processing of covalent organic frameworks: An ingredient for a material to succeed. Chem. Soc. Rev. 2019, 48, 4375–4386. [Google Scholar] [CrossRef]
- Martín-Illán, J.Á.; Rodríguez-San-Miguel, D.; Castillo, O.; Beobide, G.; Perez-Carvajal, J.; Imaz, I.; Maspoch, D.; Zamora, F. Macroscopic Ultralight Aerogel Monoliths of Imine-based Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2021, 13969–13977. [Google Scholar] [CrossRef]
- Zhu, D.; Zhu, Y.; Yan, Q.; Barnes, M.; Liu, F.; Yu, P.; Tseng, C.-P.; Tjahjono, N.; Huang, P.-C.; Rahman, M.M.; et al. Pure Crystalline Covalent Organic Framework Aerogels. Chem. Mater. 2021. [Google Scholar] [CrossRef]
- Yan, T.; Lan, Y.; Tong, M.; Zhong, C. Screening and Design of Covalent Organic Framework Membranes for CO2/CH4 Separation. ACS Sustain. Chem. Eng. 2019, 7, 1220–1227. [Google Scholar] [CrossRef]
- Duan, K.; Wang, J.; Zhang, Y.; Liu, J. Covalent organic frameworks (COFs) functionalized mixed matrix membrane for effective CO2/N2 separation. J. Memb. Sci. 2019, 572, 588–595. [Google Scholar] [CrossRef]
- Cao, X.; Qiao, Z.; Wang, Z.; Zhao, S.; Li, P.; Wang, J.; Wang, S. Enhanced performance of mixed matrix membrane by incorporating a highly compatible covalent organic framework into poly(vinylamine) for hydrogen purification. Int. J. Hydrogen Energy 2016, 41, 9167–9174. [Google Scholar] [CrossRef]
- Fan, H.; Mundstock, A.; Feldhoff, A.; Knebel, A.; Gu, J.; Meng, H.; Caro, J. Covalent Organic Framework-Covalent Organic Framework Bilayer Membranes for Highly Selective Gas Separation. J. Am. Chem. Soc. 2018, 140, 10094–10098. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Li, Z.; Yang, X.; Cao, L.; Wang, C.; Zhang, B.; Wu, H.; Jiang, Z. Enhanced proton conductivity of Nafion composite membrane by incorporating phosphoric acid-loaded covalent organic framework. J. Power Sources 2016, 332, 265–273. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; Yin, Y.; Cao, L.; He, X.; Shi, B.; Li, J.; Xu, M.; Jiang, Z. Fabrication of Nafion/zwitterion-functionalized covalent organic framework composite membranes with improved proton conductivity. J. Memb. Sci. 2018, 568, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Chen, Y.; Zhang, Z.; Ma, S. Covalent organic frameworks for separation applications. Chem. Soc. Rev. 2020, 49, 708–735. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Li, X.; Zhu, J.; Zhang, G.; Van Puyvelde, P.; Van der Bruggen, B. Covalent organic frameworks for membrane separation. Chem. Soc. Rev. 2019, 48, 2665–2681. [Google Scholar] [CrossRef]
- Fang, M.; Montoro, C.; Semsarilar, M. Metal and covalent organic frameworks for membrane applications. Membranes 2020, 10, 107. [Google Scholar] [CrossRef]
- Fan, H.; Gu, J.; Meng, H.; Knebel, A.; Caro, J. High-Flux Membranes Based on the Covalent Organic Framework COF-LZU1 for Selective Dye Separation by Nanofiltration. Angew. Chem.-Int. Ed. 2018, 57, 4083–4087. [Google Scholar] [CrossRef]
- Hao, D.; Zhang, J.; Lu, H.; Leng, W.; Ge, R.; Dai, X.; Gao, Y. Fabrication of a COF-5 membrane on a functionalized α-Al 2O3 ceramic support using a microwave irradiation method. Chem. Commun. 2014, 50, 1462–1464. [Google Scholar] [CrossRef]
- Wang, Z.; Si, Z.; Cai, D.; Li, G.; Li, S.; Qin, P. Synthesis of stable COF-300 nanofiltration membrane via in situ growth with ultrahigh flux for selective dye separation. J. Memb. Sci. 2020, 615, 118466–118472. [Google Scholar] [CrossRef]
- Pan, F.; Guo, W.; Su, Y.; Khan, N.A.; Yang, H.; Jiang, Z. Direct growth of covalent organic framework nanofiltration membranes on modified porous substrates for dyes separation. Sep. Purif. Technol. 2019, 215, 582–589. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, Y.; Nalaparaju, A.; Jiang, J.; Huang, A. Post-synthesis of a covalent organic framework nanofiltration membrane for highly efficient water treatment. J. Mater. Chem. A 2019, 7, 24205–24210. [Google Scholar] [CrossRef]
- Wang, R.; Shi, X.; Zhang, Z.; Xiao, A.; Sun, S.; Cui, Z.; Wang, Y. Unidirectional di ff usion synthesis of covalent organic frameworks ( COFs ) on polymeric substrates for dye separation. J. Memb. Sci. 2019, 586, 274–280. [Google Scholar] [CrossRef]
- Wang, R.; Wei, M.; Wang, Y. Secondary growth of covalent organic frameworks (COFs) on porous substrates for fast desalination. J. Memb. Sci. 2020, 604, 118090–118097. [Google Scholar] [CrossRef]
- Kandambeth, S.; Biswal, B.P.; Chaudhari, H.D.; Rout, K.C.; H., S.K.; Mitra, S.; Karak, S.; Das, A.; Mukherjee, R.; Kharul, U.K.; et al. Selective Molecular Sieving in Self-Standing Porous Covalent-Organic-Framework Membranes. Adv. Mater. 2017, 29, 1603945–1603954. [Google Scholar] [CrossRef]
- Jiang, Z.; Livingston, A.G.; Santanu, K. Sub-10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 2015, 348, 1347–1351. [Google Scholar] [CrossRef]
- Jimenez-Solomon, M.F.; Song, Q.; Jelfs, K.E.; Munoz-Ibanez, M.; Livingston, A.G. Polymer nanofilms with enhanced microporosity by interfacial polymerization. Nat. Mater. 2016, 15, 760–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, K.; Pal, M.; Rout, K.C.; Kunjattu, S.S.; Das, A.; Mukherjee, R.; Kharul, U.K.; Banerjee, R. Selective Molecular Separation by Interfacially Crystallized Covalent Organic Framework Thin Films. J. Am. Chem. Soc. 2017, 139, 13083–13091. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Valentino, L.; Stiehl, G.M.; Balch, H.B.; Corcos, A.R.; Wang, F.; Ralph, D.C.; Mariñas, B.J.; Dichtel, W.R. Lewis-Acid-Catalyzed Interfacial Polymerization of Covalent Organic Framework Films. Chem 2018, 4, 308–317. [Google Scholar] [CrossRef] [Green Version]
- Valentino, L.; Matsumoto, M.; Dichtel, W.R.; Marinas, B.J. Development and Performance Characterization of a Polyimine Covalent Organic Framework Thin-Film Composite Nanofiltration Membrane. Environ. Sci. Technol. 2017, 51, 14352–14359. [Google Scholar] [CrossRef]
- Wang, R.; Shi, X.; Xiao, A.; Zhou, W.; Wang, Y. Interfacial polymerization of covalent organic frameworks (COFs) on polymeric substrates for molecular separations. J. Memb. Sci. 2018, 566, 197–204. [Google Scholar] [CrossRef]
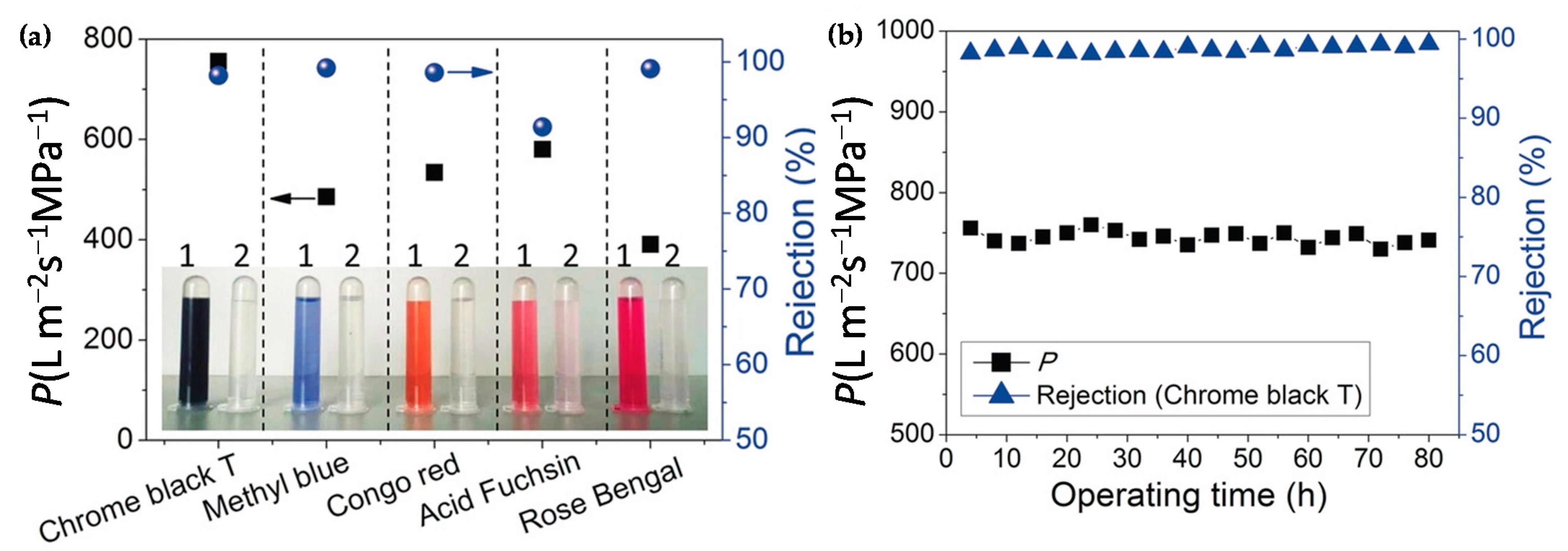
- Su, Y.Y.; Yan, X.; Chen, Y.; Guo, X.J.; Chen, X.F.; Lang, W.Z. Facile fabrication of COF-LZU1/PES composite membrane via interfacial polymerization on microfiltration substrate for dye/salt separation. J. Memb. Sci. 2021, 618, 118706–118714. [Google Scholar] [CrossRef]
- Shinde, D.B.; Sheng, G.; Li, X.; Ostwal, M.; Emwas, A.-H.; Huang, K.-W.; Lai, Z. Crystalline 2D Covalent Organic Framework Membranes for High-Flux Organic Solvent Nanofiltration. J. Am. Chem. Soc. 2018, 140, 14342–14349. [Google Scholar] [CrossRef] [Green Version]
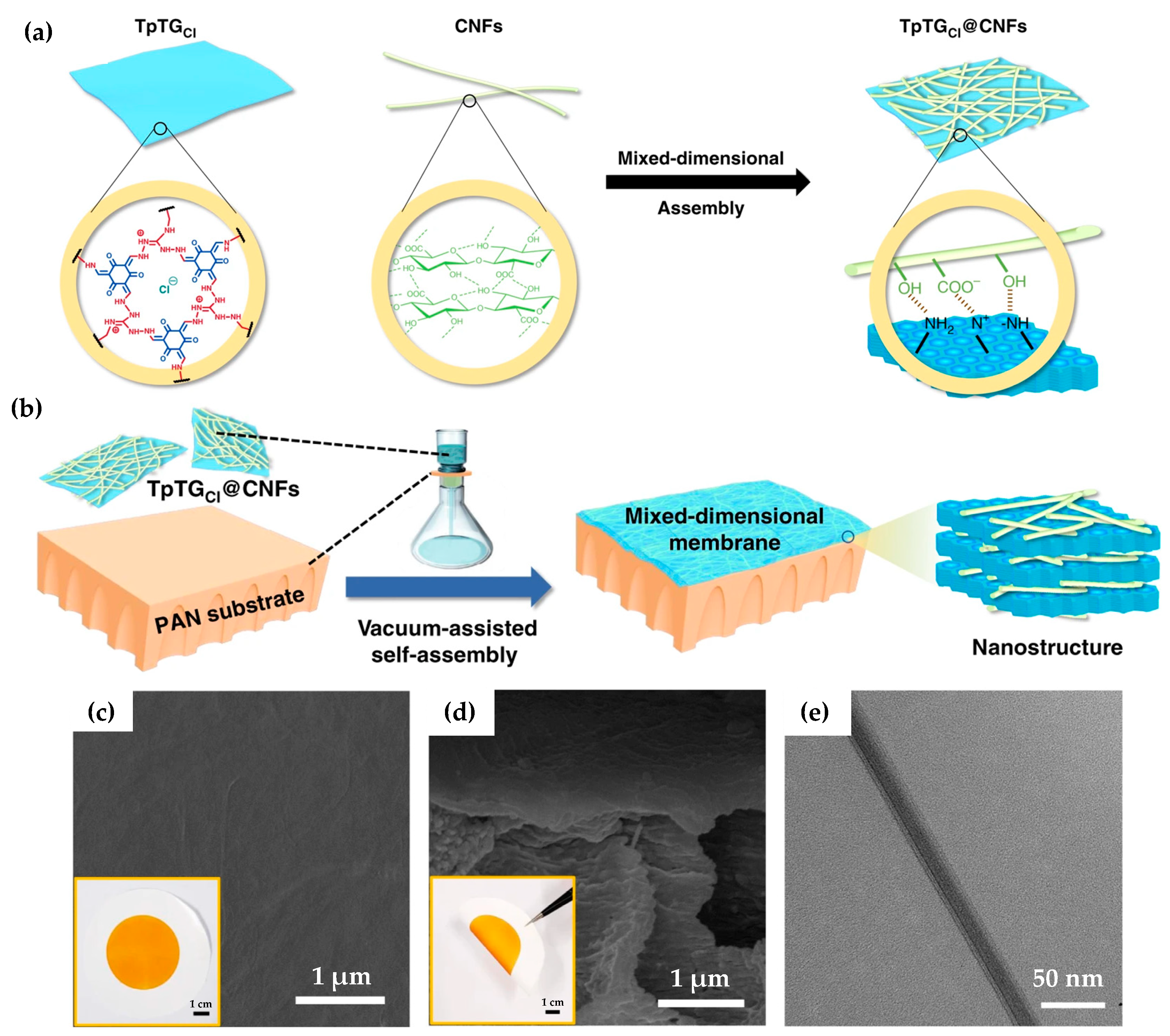
- Yang, H.; Yang, L.; Wang, H.; Xu, Z.; Zhao, Y.; Luo, Y.; Nasir, N.; Song, Y.; Wu, H.; Pan, F.; et al. Covalent organic framework membranes through a mixed-dimensional assembly for molecular separations. Nat. Commun. 2019, 10, 2101–2111. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Li, Z.; Chen, J.; Li, Z.; Yin, Y.; Cao, L.; Zhong, Y.; Wu, H. Covalent organic framework modified polyamide nanofiltration membrane with enhanced performance for desalination. J. Memb. Sci. 2017, 523, 273–281. [Google Scholar] [CrossRef]
- Khan, N.A.; Khan, N.A.; Khan, N.A.; Yuan, J.; Yuan, J.; Wu, H.; Wu, H.; Wu, H.; Huang, T.; Huang, T.; et al. Covalent Organic Framework Nanosheets as Reactive Fillers to Fabricate Free-Standing Polyamide Membranes for Efficient Desalination. ACS Appl. Mater. Interfaces 2020, 12, 27777–27785. [Google Scholar] [CrossRef]
- Xu, L.; Shan, B.; Gao, C.; Xu, J. Multifunctional thin-film nanocomposite membranes comprising covalent organic nanosheets with high crystallinity for efficient reverse osmosis desalination. J. Memb. Sci. 2020, 593, 117398–117408. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Jiang, H.; Sheng, A.; Wei, Z.; Li, Y.; Wu, C.; Li, H. Positively Charged Polysulfonamide Nanocomposite Membranes Incorporating Hydrophilic Triazine-Structured COFs for Highly Efficient Nanofiltration. ACS Appl. Nano Mater. 2020, 3, 9329–9339. [Google Scholar] [CrossRef]
- Wu, C.; Wang, X.; Zhu, T.; Li, P.; Xia, S. Covalent organic frameworks embedded membrane via acetic-acid-catalyzed interfacial polymerization for dyes separation: Enhanced permeability and selectivity. Chemosphere 2020, 261, 127580–127590. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, H.; Ou, J.; Chen, L.; Ye, M. Construction of hierarchically porous monoliths from covalent organic frameworks (COFs) and their application for bisphenol A removal. J. Hazard. Mater. 2018, 355, 145–153. [Google Scholar] [CrossRef] [PubMed]
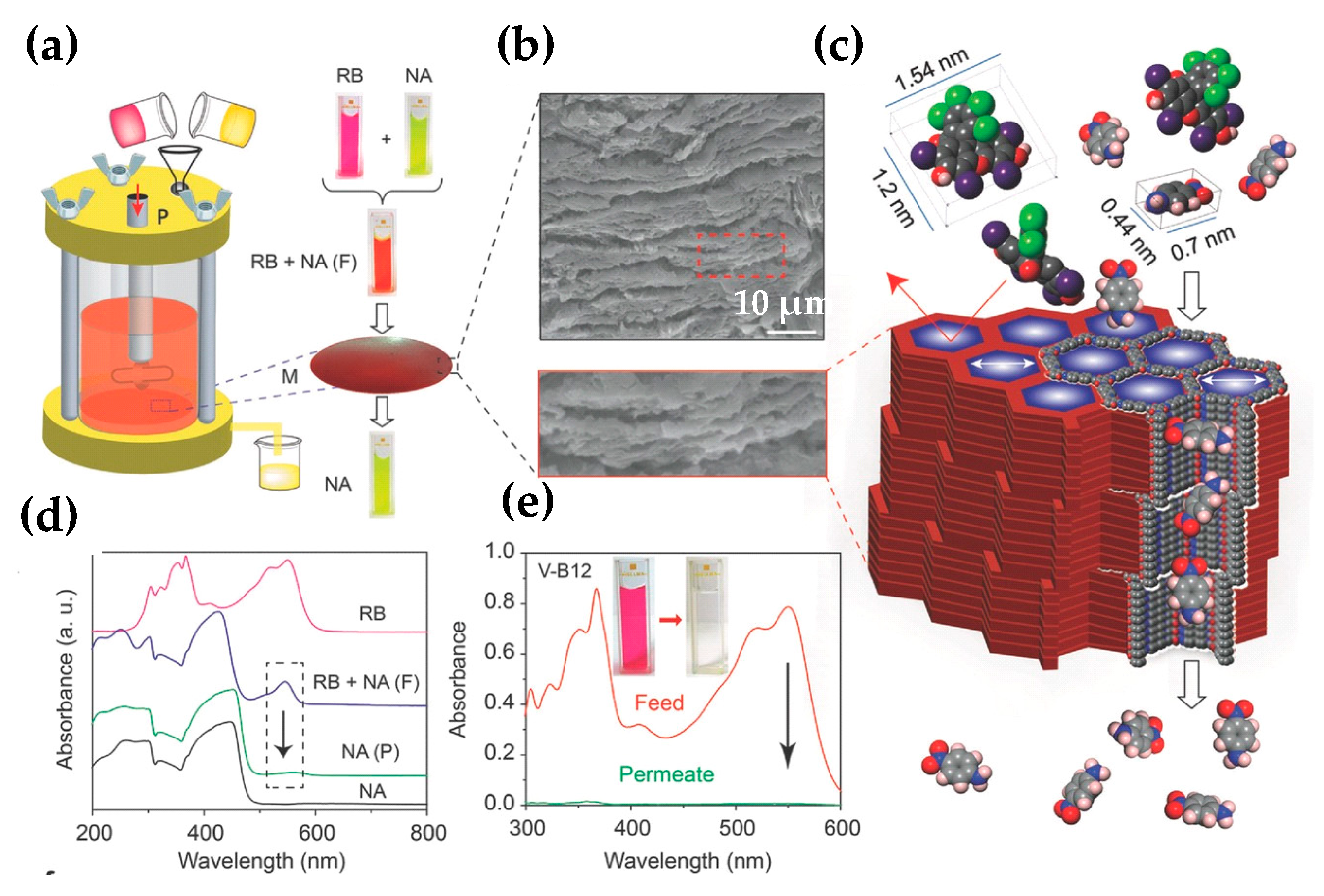
- Li, Y.; Wu, Q.; Guo, X.; Zhang, M.; Chen, B.; Wei, G.; Li, X.; Li, X.; Li, S.; Ma, L. Laminated self-standing covalent organic framework membrane with uniformly distributed subnanopores for ionic and molecular sieving. Nat. Commun. 2020, 11, 599–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.; Wu, M.; Wu, H.; Liu, Y.; You, X.; Zhang, R.; Su, Y.; Yang, H.; Jiang, Z. Covalent organic framework-modulated interfacial polymerization for ultrathin desalination membranes. J. Mater. Chem. A 2019, 7, 25641–25649. [Google Scholar] [CrossRef]
- Li, N.; Du, J.; Wu, D.; Liu, J.; Li, N.; Sun, Z.; Li, G.; Wu, Y. Recent advances in facile synthesis and applications of covalent organic framework materials as superior adsorbents in sample pretreatment. TrAC-Trends Anal. Chem. 2018, 108, 154–166. [Google Scholar] [CrossRef]
- Liang, J.; Liu, F.; Li, M.; Liu, W.; Tong, M. Facile synthesis of magnetic Fe3O4@BiOI@AgI for water decontamination with visible light irradiation: Different mechanisms for different organic pollutants degradation and bacterial disinfection. Water Res. 2018, 137, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Wang, R.L.; Li, D.P.; Wang, L.J.; Zhang, X.; Zhou, Z.Y.; Mu, J.L.; Su, Z.M. The preparation of new covalent organic framework embedded with silver nanoparticles and its applications in degradation of organic pollutants from waste water. Dalt. Trans. 2019, 48, 1051–1059. [Google Scholar] [CrossRef]
- Liu, F.; Nie, C.; Dong, Q.; Ma, Z.; Liu, W.; Tong, M. AgI modified covalent organic frameworks for effective bacterial disinfection and organic pollutant degradation under visible light irradiation. J. Hazard. Mater. 2020, 398, 122865–122876. [Google Scholar] [CrossRef]
- Lv, S.W.; Liu, J.M.; Li, C.Y.; Zhao, N.; Wang, Z.H.; Wang, S. Two novel MOFs@COFs hybrid-based photocatalytic platforms coupling with sulfate radical-involved advanced oxidation processes for enhanced degradation of bisphenol A. Chemosphere 2020, 243, 125378–125388. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.X.; Yan, X.P. Controllable preparation of core-shell magnetic covalent-organic framework nanospheres for efficient adsorption and removal of bisphenols in aqueous solution. Chem. Commun. 2017, 53, 2511–2514. [Google Scholar] [CrossRef]
- Zhong, X.; Lu, Z.; Liang, W.; Hu, B. The magnetic covalent organic framework as a platform for high-performance extraction of Cr(VI) and bisphenol a from aqueous solution. J. Hazard. Mater. 2020, 393, 122353–122367. [Google Scholar] [CrossRef]
- Merí-Bofí, L.; Royuela, S.; Zamora, F.; Ruiz-González, M.L.; Segura, J.L.; Muñoz-Olivas, R.; Mancheño, M.J. Thiol grafted imine-based covalent organic frameworks for water remediation through selective removal of Hg(II). J. Mater. Chem. A 2017, 5, 17973–17981. [Google Scholar] [CrossRef]
- Lu, X.F.; Ji, W.H.; Yuan, L.; Yu, S.; Guo, D.S. Preparation of Carboxy-Functionalized Covalent Organic Framework for Efficient Removal of Hg2+ and Pb2+ from Water. Ind. Eng. Chem. Res. 2019, 58, 17660–17667. [Google Scholar] [CrossRef]
- Cui, W.R.; Jiang, W.; Zhang, C.R.; Liang, R.P.; Liu, J.; Qiu, J.D. Regenerable Carbohydrazide-Linked Fluorescent Covalent Organic Frameworks for Ultrasensitive Detection and Removal of Mercury. ACS Sustain. Chem. Eng. 2020, 8, 445–451. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Qiu, Y.; Liu, X.; Huang, W.; Yan, N.; Qu, Z. Utilization of Ag nanoparticles anchored in covalent organic frameworks for mercury removal from acidic waste water. J. Hazard. Mater. 2020, 389, 121824–121834. [Google Scholar] [CrossRef] [PubMed]
- Afshari, M.; Dinari, M.; Zargoosh, K.; Moradi, H. Novel Triazine-Based Covalent Organic Framework as a Superadsorbent for the Removal of Mercury(II) from Aqueous Solutions. Ind. Eng. Chem. Res. 2020, 59, 9116–9126. [Google Scholar] [CrossRef]
- Xu, T.; Zhou, L.; He, Y.; An, S.; Peng, C.; Hu, J.; Liu, H. Covalent Organic Framework with Triazine and Hydroxyl Bifunctional Groups for Efficient Removal of Lead(II) Ions. Ind. Eng. Chem. Res. 2019, 58, 19642–19648. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, X.; Zhu, C.; Zhou, S.; Li, R.; Shi, H.; Miao, S.; Vakili, M.; Wang, W.; Qi, D. Sulfhydryl functionalized covalent organic framework as an efficient adsorbent for selective Pb (II) removal. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 600, 125004–125012. [Google Scholar] [CrossRef]
- Liang, Y.; Feng, L.; Liu, X.; Zhao, Y.; Chen, Q.; Sui, Z.; Wang, N. Enhanced selective adsorption of NSAIDs by covalent organic frameworks via functional group tuning. Chem. Eng. J. 2021, 404, 127095–127102. [Google Scholar] [CrossRef]
- Zhu, X.; An, S.; Liu, Y.; Hu, J.; Liu, H.; Tian, C.; Dai, S.; Yang, X.; Wang, H.; Abney, C.W. Efficient removal of organic dye pollutants using covalent organic frameworks. AIChE J. 2017, 63, 3470–3478. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, H.; Xue, W.; Chang, Y.; Li, Y.; Guo, X.; Zhong, C. Rigidifying induced fluorescence enhancement in 2D porous covalent triazine framework nanosheets for the simultaneously luminous detection and adsorption removal of antibiotics. Chem. Eng. J. 2020, 384, 123382–123391. [Google Scholar] [CrossRef]
- Park, E.Y.; Hasan, Z.; Khan, N.A.; Jhung, S.H. Adsorptive removal of bisphenol-a from water with a metal-organic framework, a porous chromium-benzenedicarboxylate. J. Nanosci. Nanotechnol. 2013, 13, 2789–2794. [Google Scholar] [CrossRef]
- Hasan, Z.; Choi, E.J.; Jhung, S.H. Adsorption of naproxen and clofibric acid over a metal–organic framework MIL-101 functionalized with acidic and basic groups. Chem. Eng. J. 2013, 219, 537–544. [Google Scholar] [CrossRef]
- Liu, B.; Yang, F.; Zou, Y.; Peng, Y. Adsorption of phenol and p -nitrophenol from aqueous solutions on metal-organic frameworks: Effect of hydrogen bonding. J. Chem. Eng. Data 2014, 59, 1476–1482. [Google Scholar] [CrossRef]
- Haque, E.; Lo, V.; Minett, A.I.; Harris, A.T.; Church, T.L. Dichotomous adsorption behaviour of dyes on an amino-functionalised metal-organic framework, amino-MIL-101(Al). J. Mater. Chem. A 2014, 2, 193–203. [Google Scholar] [CrossRef]
- Zeng, T.; Yu, Y.; Li, Z.; Zuo, J.; Kuai, Z.; Jin, Y.; Wang, Y.; Wu, A.; Peng, C. 3D MnO2 nanotubes@reduced graphene oxide hydrogel as reusable adsorbent for the removal of heavy metal ions. Mater. Chem. Phys. 2019, 231, 105–108. [Google Scholar] [CrossRef]
- Kireeti, K.V.M.K.; Chandrakanth, G.; Kadam, M.M.; Jha, N. A sodium modified reduced graphene oxide-Fe3O4 nanocomposite for efficient lead(II) adsorption. RSC Adv. 2016, 6, 84825–84836. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Slater, A.G.; Cooper, A.I. Function-led design of new porous materials. Science 2015, 348, aaa8075. [Google Scholar] [CrossRef] [PubMed]
- Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef] [PubMed]
- Mitch Jacoby 2-D materials go beyond graphene. Chem. Eng. News 2017, 95, 36–40.
- Feriante, C.H.; Jhulki, S.; Evans, A.M.; Dasari, R.R.; Slicker, K.; Dichtel, W.R.; Marder, S.R. Rapid Synthesis of High Surface Area Imine-Linked 2D Covalent Organic Frameworks by Avoiding Pore Collapse During Isolation. Adv. Mater. 2020, 32, 1–5. [Google Scholar] [CrossRef] [PubMed]




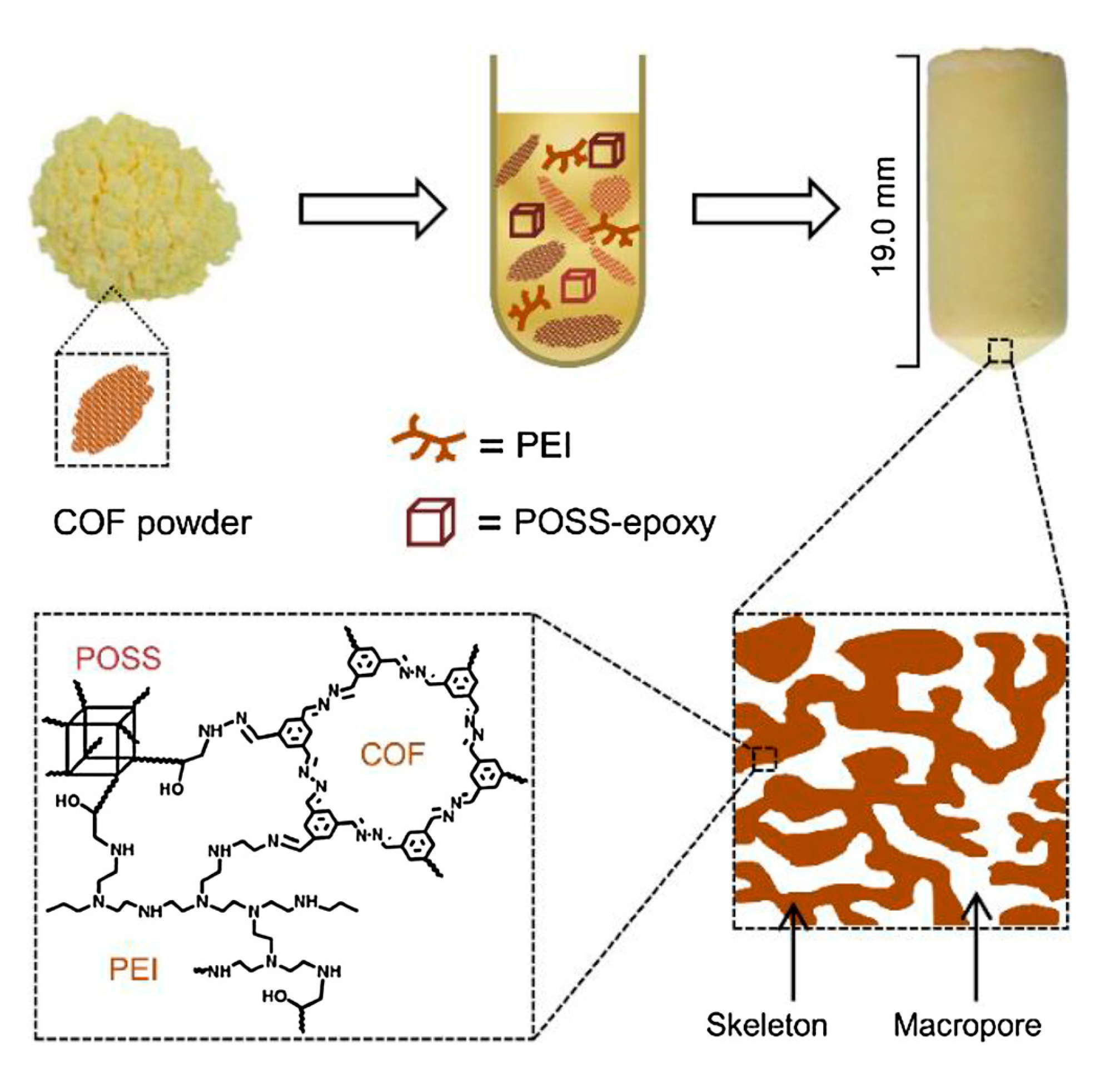


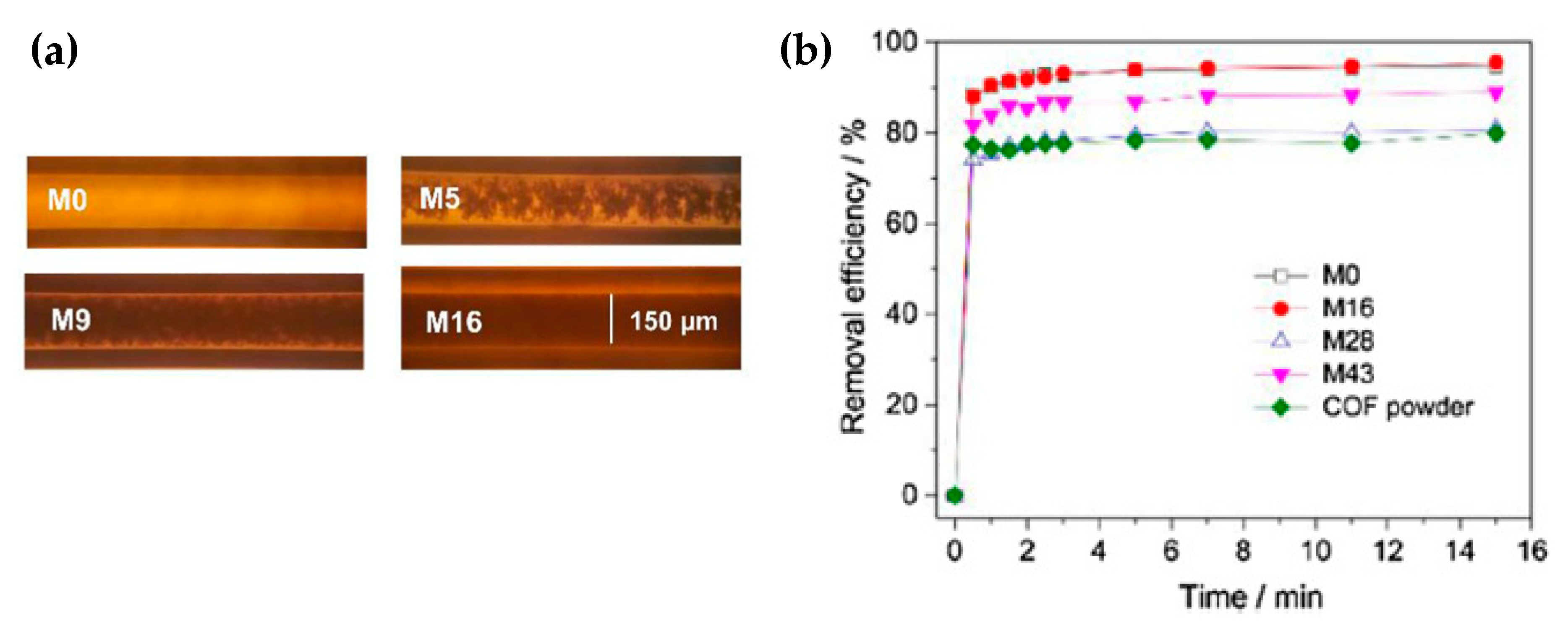

| Sample | K2/(g mg−1 min−1) | Qe/(mg g−1) |
|---|---|---|
| M0 | 0.82 | 21.7 |
| M16 | 0.58 | 21.9 |
| M28 | 0.65 | 18.5 |
| M43 | 0.63 | 20.4 |
| COF powder | 0.90 | 18.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arqueros, C.; Zamora, F.; Montoro, C. A Perspective on the Application of Covalent Organic Frameworks for Detection and Water Treatment. Nanomaterials 2021, 11, 1651. https://doi.org/10.3390/nano11071651
Arqueros C, Zamora F, Montoro C. A Perspective on the Application of Covalent Organic Frameworks for Detection and Water Treatment. Nanomaterials. 2021; 11(7):1651. https://doi.org/10.3390/nano11071651
Chicago/Turabian StyleArqueros, Cristina, Félix Zamora, and Carmen Montoro. 2021. "A Perspective on the Application of Covalent Organic Frameworks for Detection and Water Treatment" Nanomaterials 11, no. 7: 1651. https://doi.org/10.3390/nano11071651
APA StyleArqueros, C., Zamora, F., & Montoro, C. (2021). A Perspective on the Application of Covalent Organic Frameworks for Detection and Water Treatment. Nanomaterials, 11(7), 1651. https://doi.org/10.3390/nano11071651








